

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492156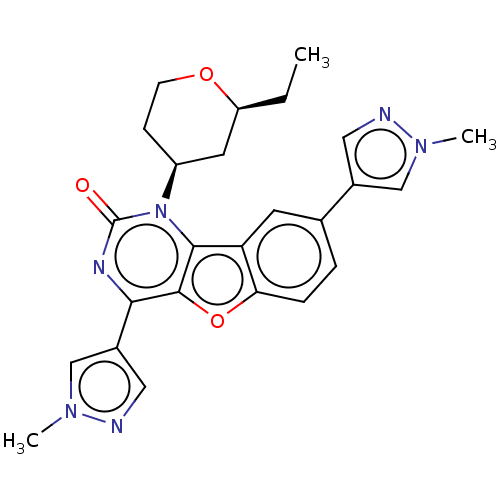 (CHEMBL2397560) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492164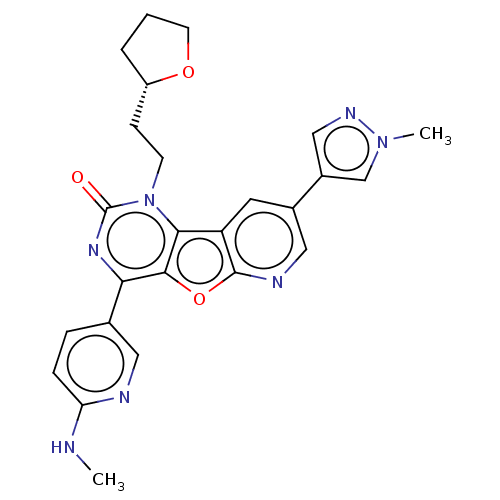 (CHEMBL2381561) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492155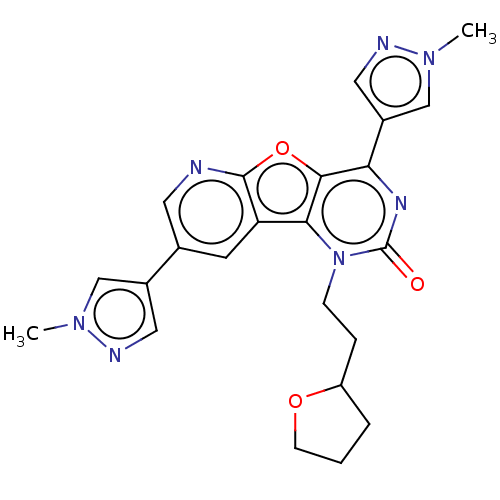 (CHEMBL2397554) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492176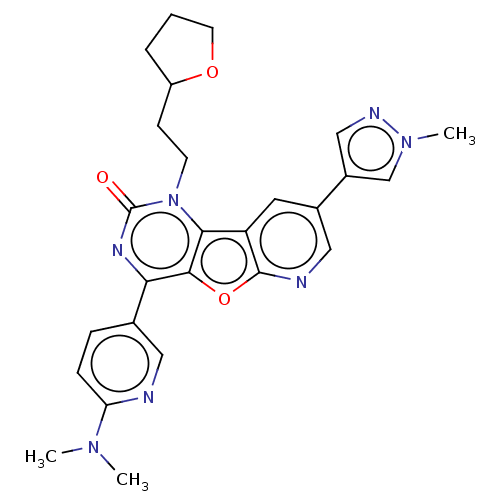 (CHEMBL2397567) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492175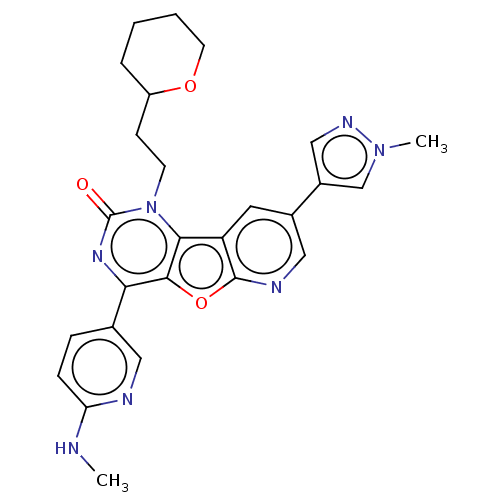 (CHEMBL2397583) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492163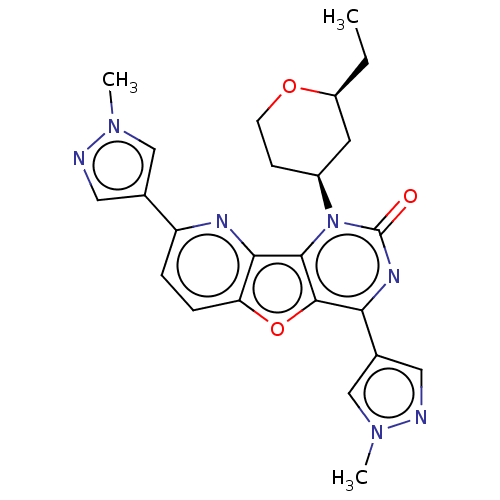 (CHEMBL2397557) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492154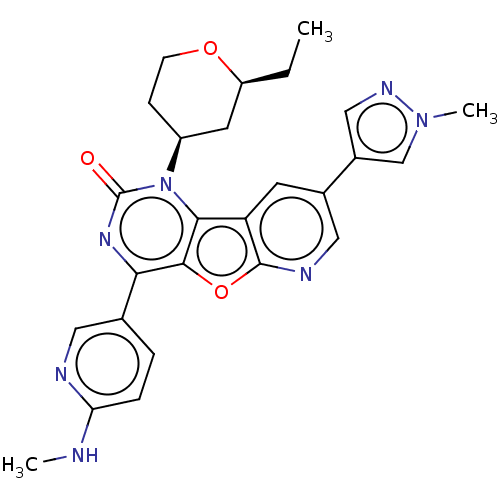 (CHEMBL2397572) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492168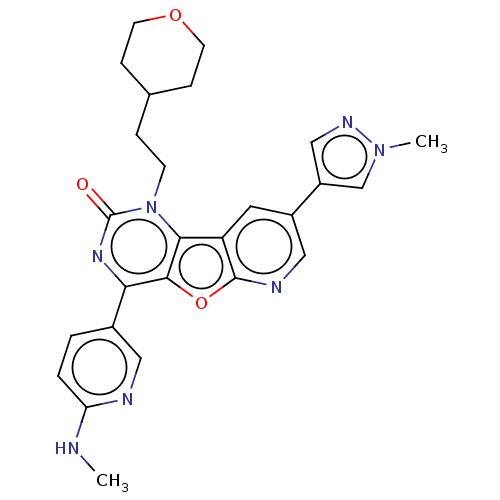 (CHEMBL2397584) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492153 (CHEMBL2397563) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492174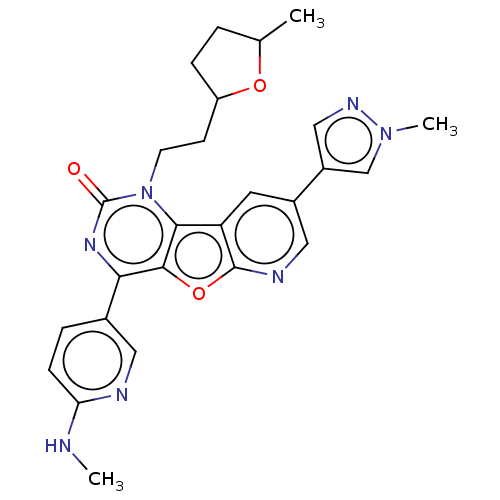 (CHEMBL2397586) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492179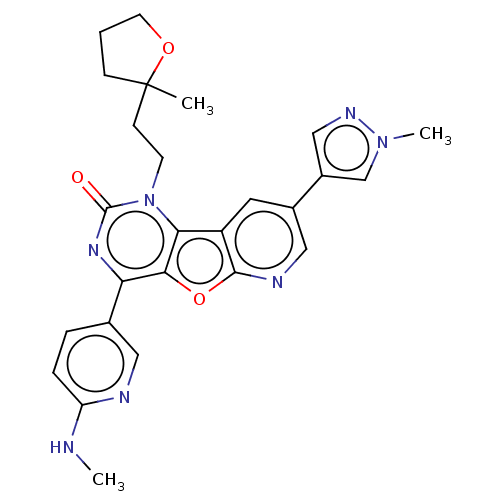 (CHEMBL2397587) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492169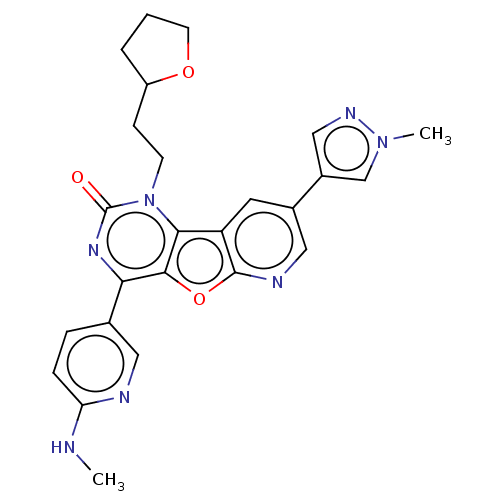 (CHEMBL2397582) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492152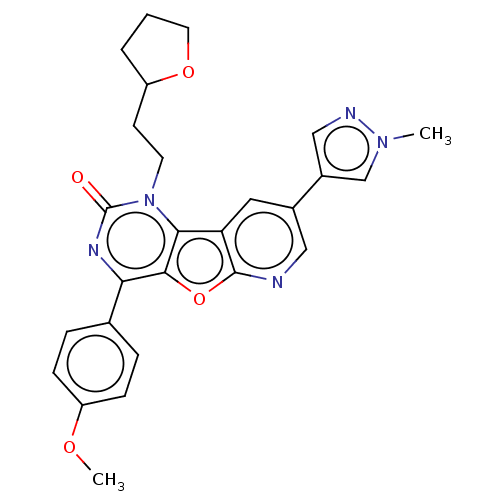 (CHEMBL2397565) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492151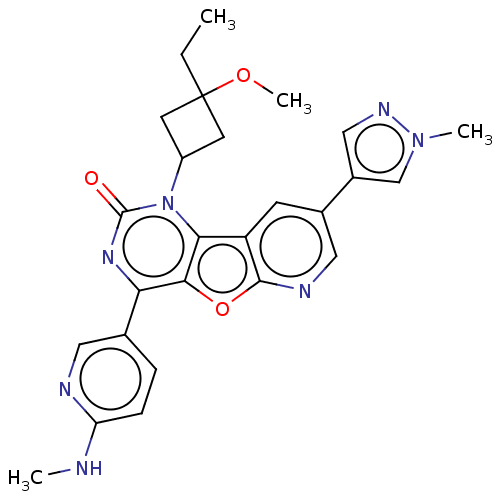 (CHEMBL2397588) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492162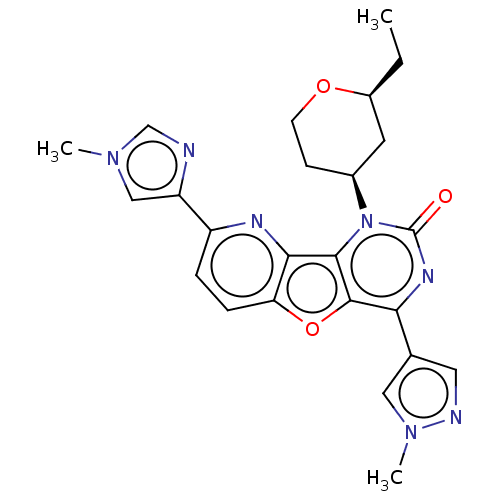 (CHEMBL2397549) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492158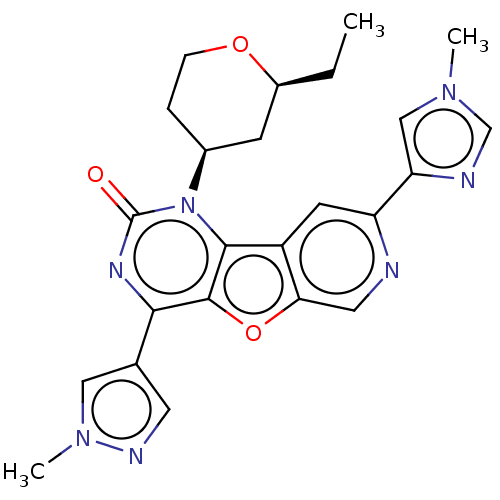 (CHEMBL2397548) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492173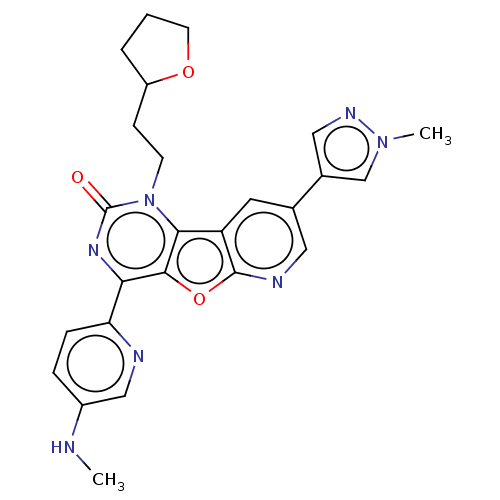 (CHEMBL2397573) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492150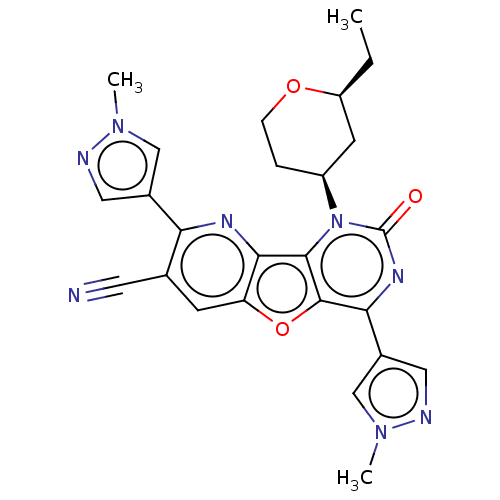 (CHEMBL2397559) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492149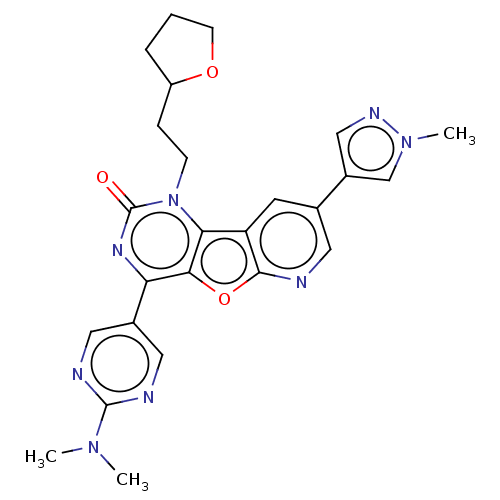 (CHEMBL2397569) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492172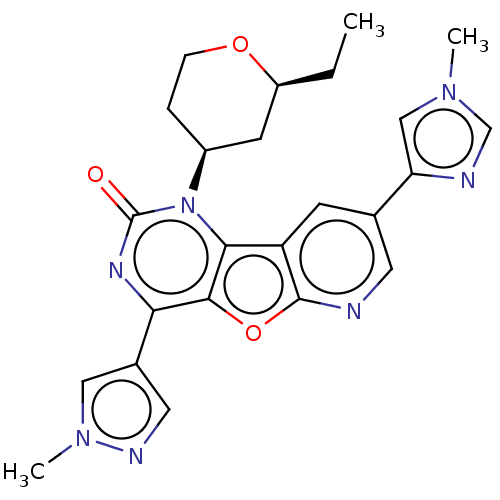 (CHEMBL2397547) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492167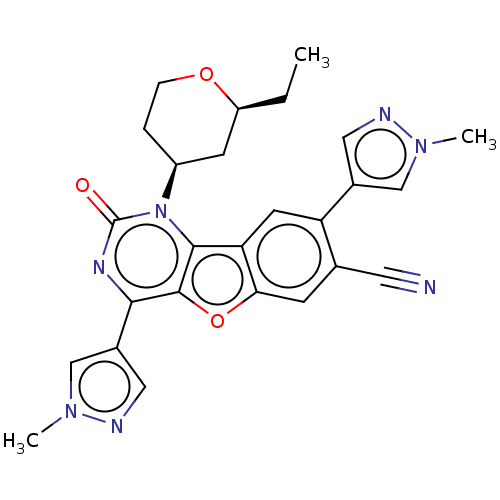 (CHEMBL2397558) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492148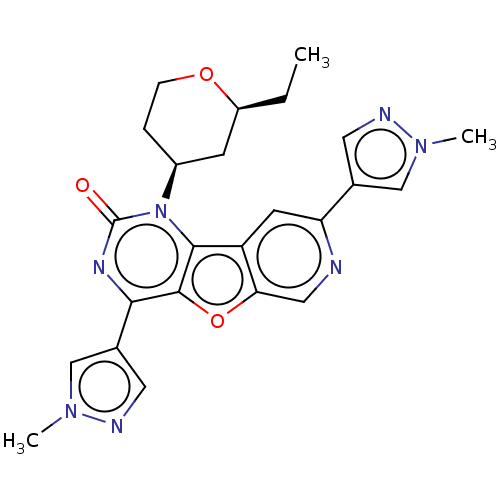 (CHEMBL2397556) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492161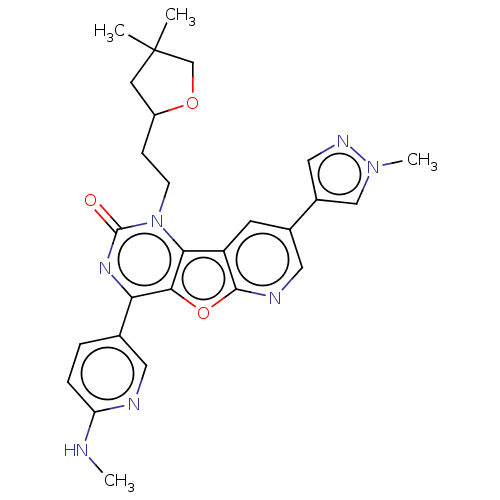 (CHEMBL2396665) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492147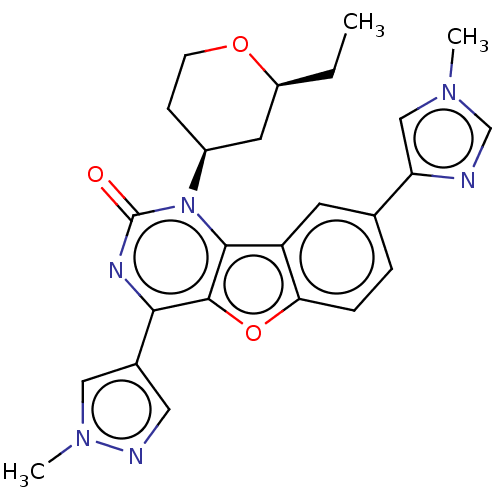 (CHEMBL2397562) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492146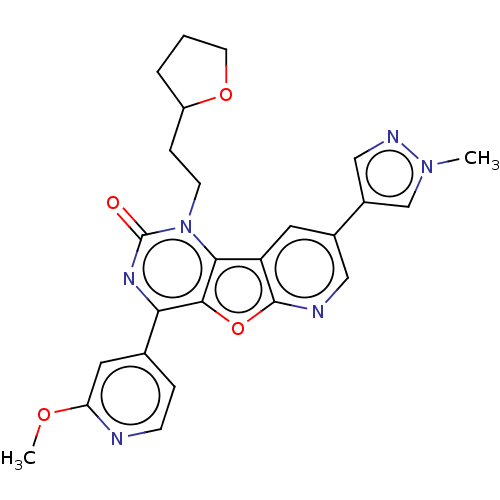 (CHEMBL2397576) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492145 (CHEMBL2397570) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492180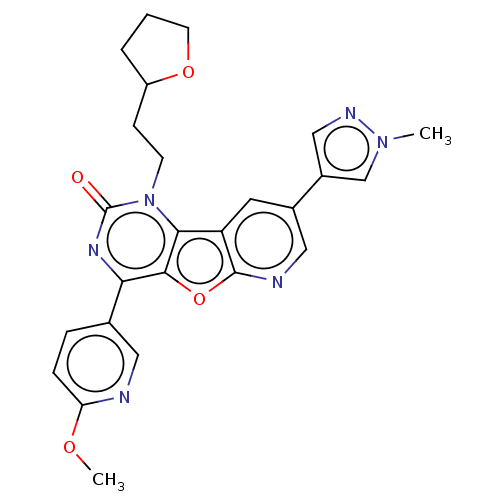 (CHEMBL2397566) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492178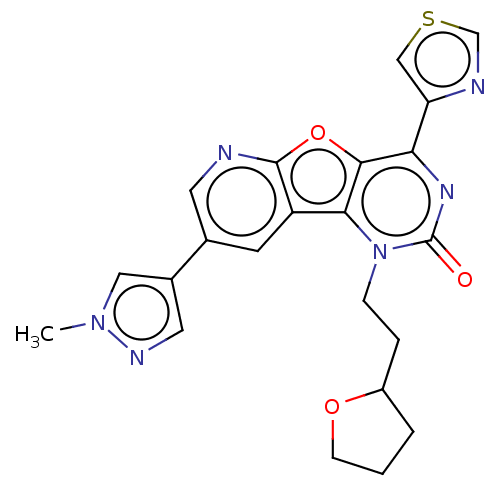 (CHEMBL2397551) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492171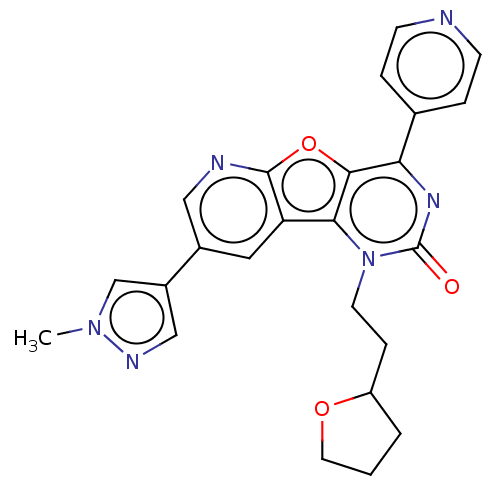 (CHEMBL2397575) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492160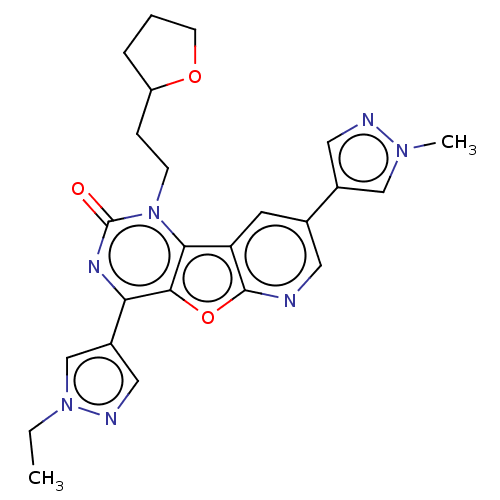 (CHEMBL2397555) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492166 (CHEMBL2397568) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492144 (CHEMBL2397574) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492142 (CHEMBL2397550) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492143 (CHEMBL2397571) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492157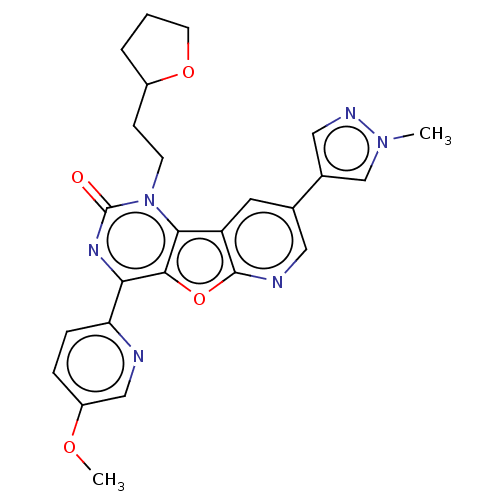 (CHEMBL2397577) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492177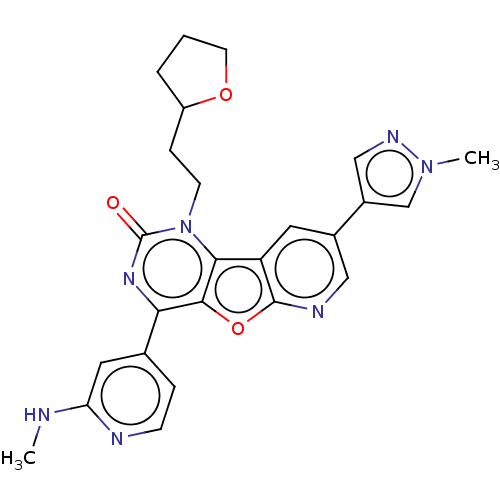 (CHEMBL2397581) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492141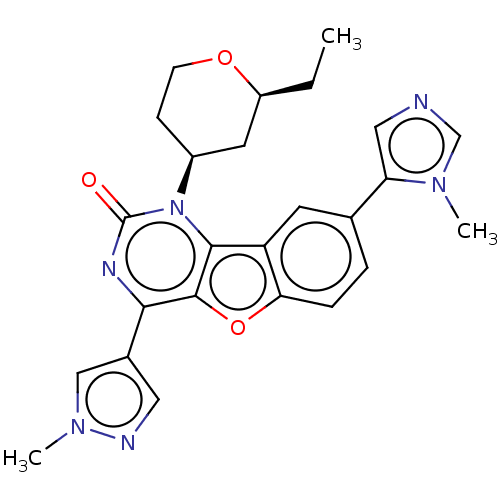 (CHEMBL2397561) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50175297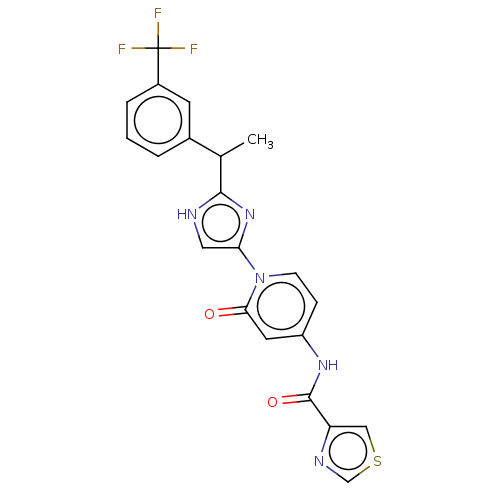 (CHEMBL3810245) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP2C9 (unknown origin) | ACS Med Chem Lett 7: 525-30 (2016) Article DOI: 10.1021/acsmedchemlett.6b00064 BindingDB Entry DOI: 10.7270/Q24B337T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492140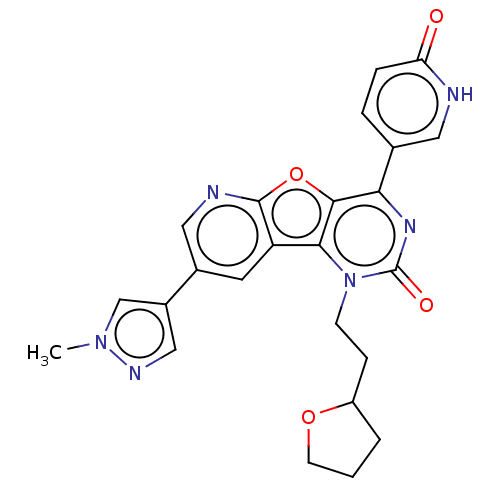 (CHEMBL2397578) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492170 (CHEMBL2397585) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492159 (CHEMBL2397564) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492139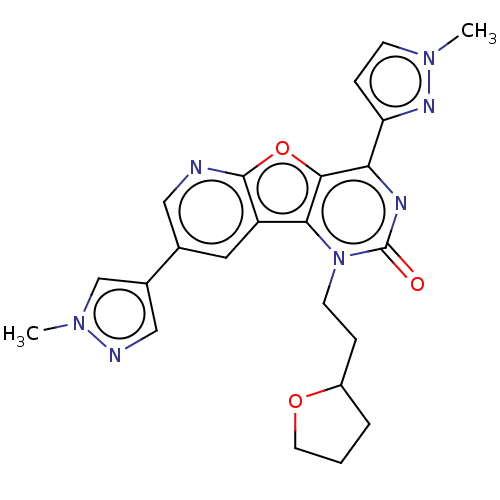 (CHEMBL2397552) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50175302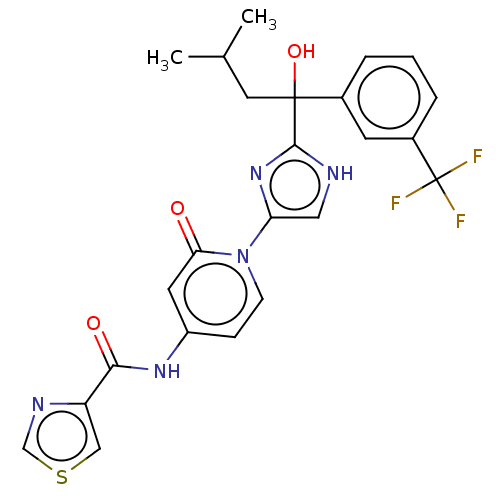 (CHEMBL3808401) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP2C9 (unknown origin) | ACS Med Chem Lett 7: 525-30 (2016) Article DOI: 10.1021/acsmedchemlett.6b00064 BindingDB Entry DOI: 10.7270/Q24B337T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM50175303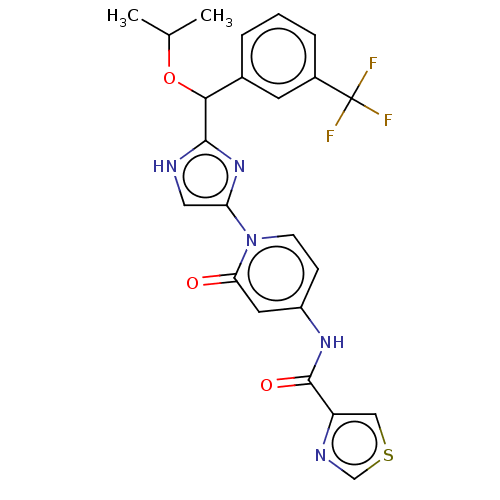 (CHEMBL3810042) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP2C8 (unknown origin) | ACS Med Chem Lett 7: 525-30 (2016) Article DOI: 10.1021/acsmedchemlett.6b00064 BindingDB Entry DOI: 10.7270/Q24B337T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492137 (CHEMBL2397580) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >410 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492138 (CHEMBL2397579) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50175303 (CHEMBL3810042) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP2C9 (unknown origin) | ACS Med Chem Lett 7: 525-30 (2016) Article DOI: 10.1021/acsmedchemlett.6b00064 BindingDB Entry DOI: 10.7270/Q24B337T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM50175304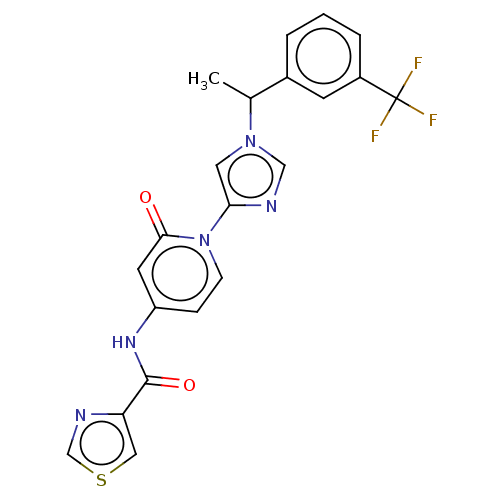 (CHEMBL3808842) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP2C8 (unknown origin) | ACS Med Chem Lett 7: 525-30 (2016) Article DOI: 10.1021/acsmedchemlett.6b00064 BindingDB Entry DOI: 10.7270/Q24B337T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50175296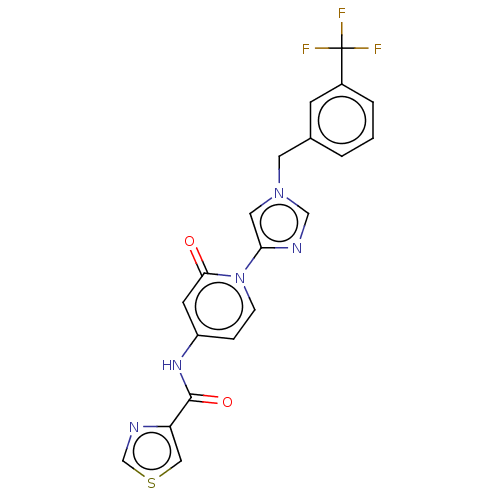 (CHEMBL3809896) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) | ACS Med Chem Lett 7: 525-30 (2016) Article DOI: 10.1021/acsmedchemlett.6b00064 BindingDB Entry DOI: 10.7270/Q24B337T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50175304 (CHEMBL3808842) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP2C9 (unknown origin) | ACS Med Chem Lett 7: 525-30 (2016) Article DOI: 10.1021/acsmedchemlett.6b00064 BindingDB Entry DOI: 10.7270/Q24B337T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 70 total ) | Next | Last >> |