 Found 18186 hits with Last Name = 'moreno' and Initial = 'da'
Found 18186 hits with Last Name = 'moreno' and Initial = 'da' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Proto-oncogene tyrosine-protein kinase receptor Ret [G810S]
(Homo sapiens (Human)) | BDBM576988
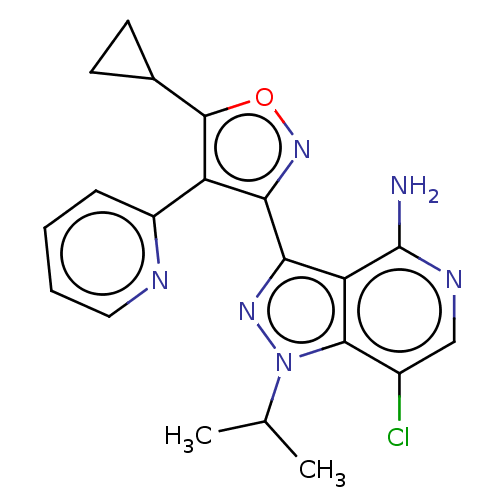
(US11472802, Example 8)Show SMILES CC(C)n1nc(-c2noc(C3CC3)c2-c2ccccn2)c2c(N)ncc(Cl)c12 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22N55HG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM576988

(US11472802, Example 8)Show SMILES CC(C)n1nc(-c2noc(C3CC3)c2-c2ccccn2)c2c(N)ncc(Cl)c12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The potency of compounds inhibiting several different RET kinase forms (Wild Type, V804M, M918T, G810R, & G810S) were determined using CisBio's H... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22N55HG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret [M918T]
(Homo sapiens (Human)) | BDBM576988

(US11472802, Example 8)Show SMILES CC(C)n1nc(-c2noc(C3CC3)c2-c2ccccn2)c2c(N)ncc(Cl)c12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22N55HG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret [V804M]
(Homo sapiens (Human)) | BDBM576988

(US11472802, Example 8)Show SMILES CC(C)n1nc(-c2noc(C3CC3)c2-c2ccccn2)c2c(N)ncc(Cl)c12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22N55HG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret [G810R]
(Homo sapiens (Human)) | BDBM576988

(US11472802, Example 8)Show SMILES CC(C)n1nc(-c2noc(C3CC3)c2-c2ccccn2)c2c(N)ncc(Cl)c12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22N55HG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret [M918T]
(Homo sapiens (Human)) | BDBM577053

(US11472802, Example 58)Show SMILES CC(C)n1nc(-c2noc(C3CC3)c2-c2c[nH]cn2)c2c(N)nccc12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22N55HG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM577053

(US11472802, Example 58)Show SMILES CC(C)n1nc(-c2noc(C3CC3)c2-c2c[nH]cn2)c2c(N)nccc12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The potency of compounds inhibiting several different RET kinase forms (Wild Type, V804M, M918T, G810R, & G810S) were determined using CisBio's H... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22N55HG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM577046
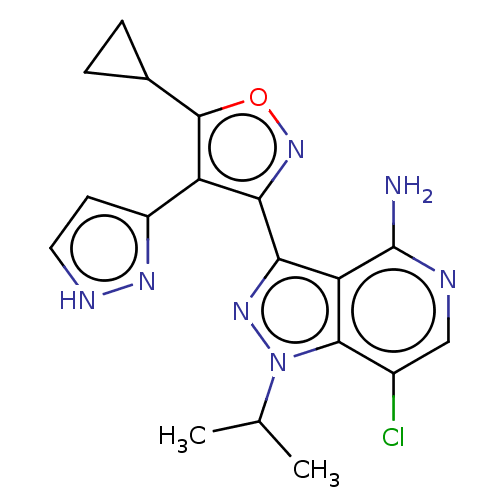
(7-chloro-3-(5-cyclopropyl- 4-(1H-pyrazol-3- yl)iso...)Show SMILES CC(C)n1nc(-c2noc(C3CC3)c2-c2cc[nH]n2)c2c(N)ncc(Cl)c12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The potency of compounds inhibiting several different RET kinase forms (Wild Type, V804M, M918T, G810R, & G810S) were determined using CisBio's H... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22N55HG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret [M918T]
(Homo sapiens (Human)) | BDBM577046

(7-chloro-3-(5-cyclopropyl- 4-(1H-pyrazol-3- yl)iso...)Show SMILES CC(C)n1nc(-c2noc(C3CC3)c2-c2cc[nH]n2)c2c(N)ncc(Cl)c12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22N55HG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret [M918T]
(Homo sapiens (Human)) | BDBM577063
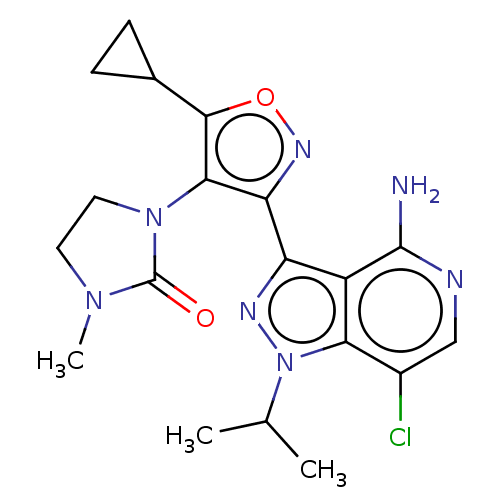
(1-(3-(4-amino-7-chloro-1- isopropyl-1H-pyrazolo[4,...)Show SMILES CC(C)n1nc(-c2noc(C3CC3)c2N2CCN(C)C2=O)c2c(N)ncc(Cl)c12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22N55HG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret [M918T]
(Homo sapiens (Human)) | BDBM577049

(US11472802, Example 55)Show SMILES CC(C)n1nc(-c2noc(C3CC3)c2-c2cn(C)cn2)c2c(N)ncc(Cl)c12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22N55HG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret [M918T]
(Homo sapiens (Human)) | BDBM577050

(7-chloro-3-(5- cyclopropyl-4-(1H- imidazol-4- yl)i...)Show SMILES CC(C)n1nc(-c2noc(C3CC3)c2-c2c[nH]cn2)c2c(N)ncc(Cl)c12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22N55HG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM577049

(US11472802, Example 55)Show SMILES CC(C)n1nc(-c2noc(C3CC3)c2-c2cn(C)cn2)c2c(N)ncc(Cl)c12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The potency of compounds inhibiting several different RET kinase forms (Wild Type, V804M, M918T, G810R, & G810S) were determined using CisBio's H... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22N55HG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret [M918T]
(Homo sapiens (Human)) | BDBM577045

(US11472802, Example 51)Show SMILES CC(C)n1nc(-c2noc(C3CC3)c2-c2cc(C)[nH]n2)c2c(N)ncc(Cl)c12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22N55HG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret [V804M]
(Homo sapiens (Human)) | BDBM577049

(US11472802, Example 55)Show SMILES CC(C)n1nc(-c2noc(C3CC3)c2-c2cn(C)cn2)c2c(N)ncc(Cl)c12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22N55HG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM577050

(7-chloro-3-(5- cyclopropyl-4-(1H- imidazol-4- yl)i...)Show SMILES CC(C)n1nc(-c2noc(C3CC3)c2-c2c[nH]cn2)c2c(N)ncc(Cl)c12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The potency of compounds inhibiting several different RET kinase forms (Wild Type, V804M, M918T, G810R, & G810S) were determined using CisBio's H... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22N55HG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret [M918T]
(Homo sapiens (Human)) | BDBM597662

(US11603374, Example 17) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The potency of compounds inhibiting several different RET kinase forms (Wild Type, V804M, M918T, G810R, & G810S) were determined using CisBio's H... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2Z323KZ |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM576996

(US11472802, Example 11)Show SMILES CC(C)n1nc(-c2noc(C3CC3)c2-c2ccccn2)c2c(N)ncc(F)c12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The potency of compounds inhibiting several different RET kinase forms (Wild Type, V804M, M918T, G810R, & G810S) were determined using CisBio's H... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22N55HG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret [M918T]
(Homo sapiens (Human)) | BDBM576996

(US11472802, Example 11)Show SMILES CC(C)n1nc(-c2noc(C3CC3)c2-c2ccccn2)c2c(N)ncc(F)c12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22N55HG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret [M918T]
(Homo sapiens (Human)) | BDBM577026

(7-chloro-3-(5-cyclopropyl-4-(4- methylpyridin-2-yl...)Show SMILES CC(C)n1nc(-c2noc(C3CC3)c2-c2cc(C)ccn2)c2c(N)ncc(Cl)c12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22N55HG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret [M918T]
(Homo sapiens (Human)) | BDBM577061

(US11472802, Example 65)Show SMILES CC(C)n1nc(-c2noc(C3CC3)c2N2CCC2=O)c2c(N)ncc(Cl)c12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22N55HG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM577063

(1-(3-(4-amino-7-chloro-1- isopropyl-1H-pyrazolo[4,...)Show SMILES CC(C)n1nc(-c2noc(C3CC3)c2N2CCN(C)C2=O)c2c(N)ncc(Cl)c12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The potency of compounds inhibiting several different RET kinase forms (Wild Type, V804M, M918T, G810R, & G810S) were determined using CisBio's H... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22N55HG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM577064
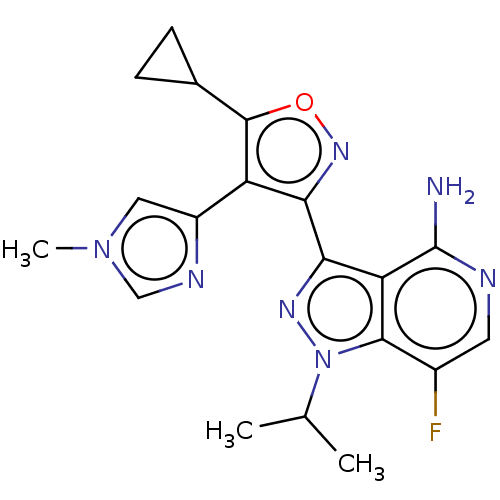
(US11472802, Example 68)Show SMILES CC(C)n1nc(-c2noc(C3CC3)c2-c2cn(C)cn2)c2c(N)ncc(F)c12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The potency of compounds inhibiting several different RET kinase forms (Wild Type, V804M, M918T, G810R, & G810S) were determined using CisBio's H... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22N55HG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret [M918T]
(Homo sapiens (Human)) | BDBM577064

(US11472802, Example 68)Show SMILES CC(C)n1nc(-c2noc(C3CC3)c2-c2cn(C)cn2)c2c(N)ncc(F)c12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22N55HG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret [M918T]
(Homo sapiens (Human)) | BDBM577066
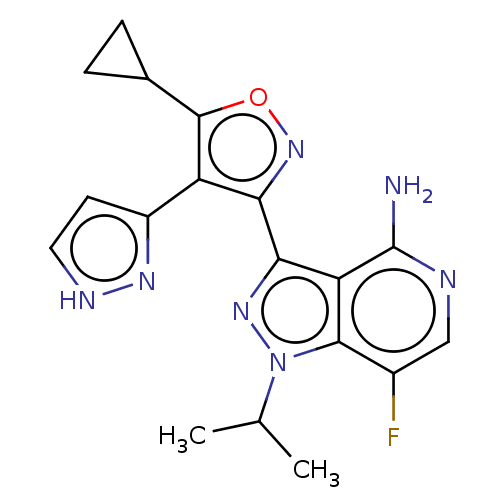
(3-(5-cyclopropyl- 4-(1H-pyrazol-3- yl)isoxazol-3- ...)Show SMILES CC(C)n1nc(-c2noc(C3CC3)c2-c2cc[nH]n2)c2c(N)ncc(F)c12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22N55HG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret [M918T]
(Homo sapiens (Human)) | BDBM577025
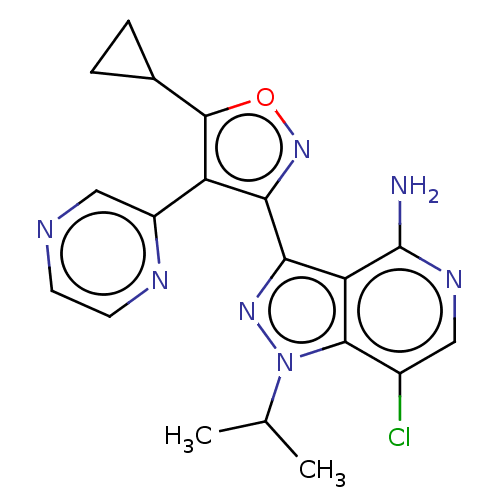
(7-chloro-3-(5-cyclopropyl-4- (pyrazin-2-yl)isoxazo...)Show SMILES CC(C)n1nc(-c2noc(C3CC3)c2-c2cnccn2)c2c(N)ncc(Cl)c12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22N55HG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM577061

(US11472802, Example 65)Show SMILES CC(C)n1nc(-c2noc(C3CC3)c2N2CCC2=O)c2c(N)ncc(Cl)c12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The potency of compounds inhibiting several different RET kinase forms (Wild Type, V804M, M918T, G810R, & G810S) were determined using CisBio's H... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22N55HG |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM565148

(US11414404, Ex. # 25 | US11634409, Example 25)Show SMILES Cc1c(Nc2c(F)ccc(NS(=O)(=O)c3cc(F)ccc3F)c2Cl)ccc2ncn(C)c(=O)c12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
A competitive displacement assay was configured for B-Raf that monitors the amount of a fluorescently-tagged “tracer” bound to B-Raf via TR-FRET from... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2JD511G |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM565148

(US11414404, Ex. # 25 | US11634409, Example 25)Show SMILES Cc1c(Nc2c(F)ccc(NS(=O)(=O)c3cc(F)ccc3F)c2Cl)ccc2ncn(C)c(=O)c12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2FT8R02 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM577045

(US11472802, Example 51)Show SMILES CC(C)n1nc(-c2noc(C3CC3)c2-c2cc(C)[nH]n2)c2c(N)ncc(Cl)c12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The potency of compounds inhibiting several different RET kinase forms (Wild Type, V804M, M918T, G810R, & G810S) were determined using CisBio's H... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22N55HG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM577067
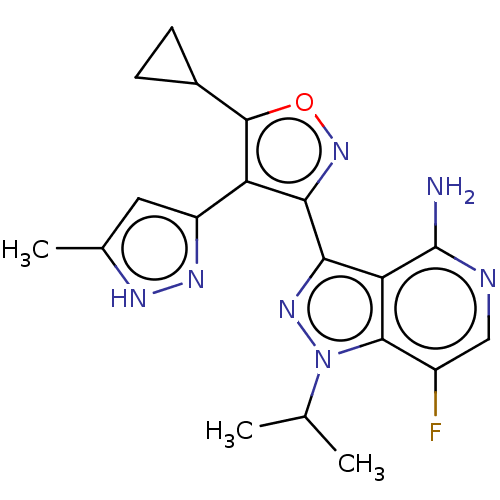
(3-(5-cyclopropyl- 4-(5-methyl-1H- pyrazol-3-yl) is...)Show SMILES CC(C)n1nc(-c2noc(C3CC3)c2-c2cc(C)[nH]n2)c2c(N)ncc(F)c12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The potency of compounds inhibiting several different RET kinase forms (Wild Type, V804M, M918T, G810R, & G810S) were determined using CisBio's H... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22N55HG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM597647
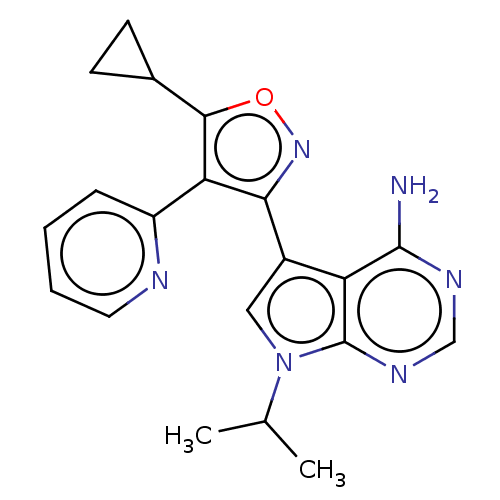
(US11603374, Example 2)Show SMILES CC(C)n1cc(-c2noc(C3CC3)c2-c2ccccn2)c2c(N)ncnc12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The potency of compounds inhibiting several different RET kinase forms (Wild Type, V804M, M918T, G810R, & G810S) were determined using CisBio's H... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2Z323KZ |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret [M918T]
(Homo sapiens (Human)) | BDBM597647

(US11603374, Example 2)Show SMILES CC(C)n1cc(-c2noc(C3CC3)c2-c2ccccn2)c2c(N)ncnc12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The potency of compounds inhibiting several different RET kinase forms (Wild Type, V804M, M918T, G810R, & G810S) were determined using CisBio's H... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2Z323KZ |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1 [8-360]
(Homo sapiens (Human)) | BDBM193676

(US9670208, Example 295 4-(3-(cyclopropylmethyl)-[1...)Show InChI InChI=1S/C19H19N7/c1-25-17(5-8-21-25)22-16-11-14(4-7-20-16)15-6-9-26-18(10-13-2-3-13)23-24-19(26)12-15/h4-9,11-13H,2-3,10H2,1H3,(H,20,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.3 | n/a |
Array BioPharma Inc.; Genentech, Inc.
US Patent
| Assay Description
Compounds were tested in an enzymatic assay using human ERK-2 (Mitogen Activated Kinase 1), recombinantly expressed as an n-terminal 6-His fusion pro... |
US Patent US9670208 (2017)
BindingDB Entry DOI: 10.7270/Q2RJ4GMB |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret [M918T]
(Homo sapiens (Human)) | BDBM577029

(7-chloro-3-(5-cyclopropyl-4-(5- methylpyridin-2-yl...)Show SMILES CC(C)n1nc(-c2noc(C3CC3)c2-c2ccc(C)cn2)c2c(N)ncc(Cl)c12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22N55HG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret [M918T]
(Homo sapiens (Human)) | BDBM577062
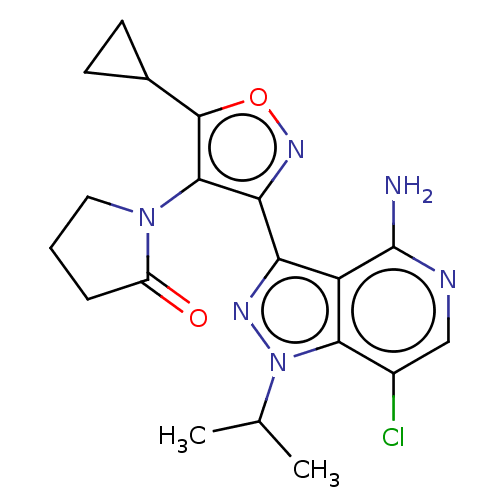
(1-(3-(4-amino-7-chloro-1- isopropyl-1H-pyrazolo[4,...)Show SMILES CC(C)n1nc(-c2noc(C3CC3)c2N2CCCC2=O)c2c(N)ncc(Cl)c12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22N55HG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret [V804M]
(Homo sapiens (Human)) | BDBM577063

(1-(3-(4-amino-7-chloro-1- isopropyl-1H-pyrazolo[4,...)Show SMILES CC(C)n1nc(-c2noc(C3CC3)c2N2CCN(C)C2=O)c2c(N)ncc(Cl)c12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22N55HG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM577066

(3-(5-cyclopropyl- 4-(1H-pyrazol-3- yl)isoxazol-3- ...)Show SMILES CC(C)n1nc(-c2noc(C3CC3)c2-c2cc[nH]n2)c2c(N)ncc(F)c12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The potency of compounds inhibiting several different RET kinase forms (Wild Type, V804M, M918T, G810R, & G810S) were determined using CisBio's H... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22N55HG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM577025

(7-chloro-3-(5-cyclopropyl-4- (pyrazin-2-yl)isoxazo...)Show SMILES CC(C)n1nc(-c2noc(C3CC3)c2-c2cnccn2)c2c(N)ncc(Cl)c12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The potency of compounds inhibiting several different RET kinase forms (Wild Type, V804M, M918T, G810R, & G810S) were determined using CisBio's H... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22N55HG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM577026

(7-chloro-3-(5-cyclopropyl-4-(4- methylpyridin-2-yl...)Show SMILES CC(C)n1nc(-c2noc(C3CC3)c2-c2cc(C)ccn2)c2c(N)ncc(Cl)c12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The potency of compounds inhibiting several different RET kinase forms (Wild Type, V804M, M918T, G810R, & G810S) were determined using CisBio's H... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22N55HG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret [V804M]
(Homo sapiens (Human)) | BDBM576996

(US11472802, Example 11)Show SMILES CC(C)n1nc(-c2noc(C3CC3)c2-c2ccccn2)c2c(N)ncc(F)c12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22N55HG |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM565192
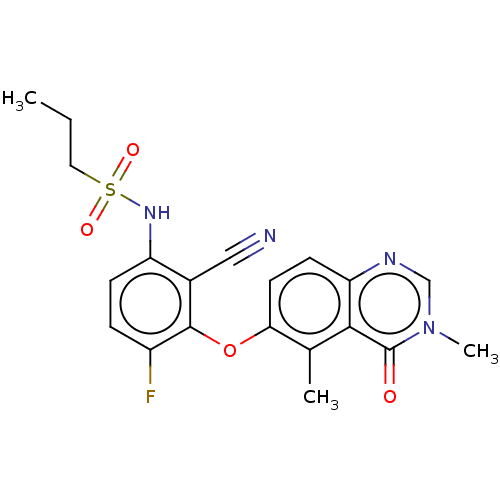
(US11414404, Ex. # 69 | US11634409, Example 69)Show SMILES CCCS(=O)(=O)Nc1ccc(F)c(Oc2ccc3ncn(C)c(=O)c3c2C)c1C#N | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
A competitive displacement assay was configured for B-Raf that monitors the amount of a fluorescently-tagged “tracer” bound to B-Raf via TR-FRET from... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2JD511G |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM565192

(US11414404, Ex. # 69 | US11634409, Example 69)Show SMILES CCCS(=O)(=O)Nc1ccc(F)c(Oc2ccc3ncn(C)c(=O)c3c2C)c1C#N | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2FT8R02 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM577047

(7-chloro-3-(5-cyclopropyl- 4-(1-methyl-1H-pyrazol-...)Show SMILES CC(C)n1nc(-c2noc(C3CC3)c2-c2ccn(C)n2)c2c(N)ncc(Cl)c12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The potency of compounds inhibiting several different RET kinase forms (Wild Type, V804M, M918T, G810R, & G810S) were determined using CisBio's H... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22N55HG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret [M918T]
(Homo sapiens (Human)) | BDBM577047

(7-chloro-3-(5-cyclopropyl- 4-(1-methyl-1H-pyrazol-...)Show SMILES CC(C)n1nc(-c2noc(C3CC3)c2-c2ccn(C)n2)c2c(N)ncc(Cl)c12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22N55HG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM577048

(7-chloro-3-(5-cyclopropyl- 4-(1-ethyl-1H-pyrazol-3...)Show SMILES CCn1ccc(n1)-c1c(noc1C1CC1)-c1nn(C(C)C)c2c(Cl)cnc(N)c12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The potency of compounds inhibiting several different RET kinase forms (Wild Type, V804M, M918T, G810R, & G810S) were determined using CisBio's H... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22N55HG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM597673
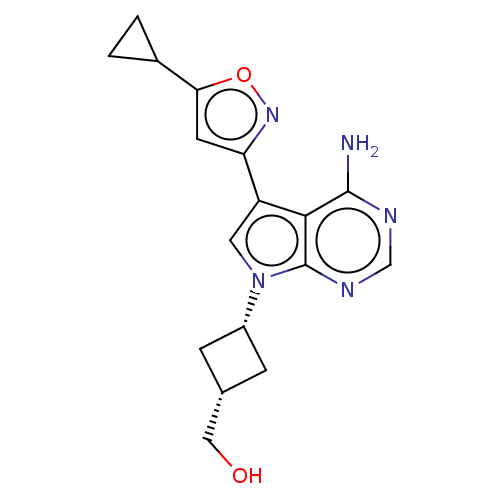
(US11603374, Example 28)Show SMILES Nc1ncnc2n(cc(-c3cc(on3)C3CC3)c12)[C@@H]1C[C@H](CO)C1 |r,wU:18.21,20.24,(-2.78,4.69,;-3.17,3.2,;-4.68,2.88,;-5.16,1.42,;-4.13,.27,;-2.62,.59,;-1.37,-.31,;-.13,.59,;-.6,2.06,;.17,3.39,;1.71,3.39,;2.18,4.85,;.94,5.76,;-.31,4.85,;3.67,5.25,;5.16,4.85,;4.76,6.34,;-2.14,2.06,;-1.37,-1.85,;-2.46,-2.94,;-1.37,-4.03,;-1.37,-5.57,;-.04,-6.34,;-.29,-2.94,)| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The potency of compounds inhibiting several different RET kinase forms (Wild Type, V804M, M918T, G810R, & G810S) were determined using CisBio's H... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2Z323KZ |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret [M918T]
(Homo sapiens (Human)) | BDBM597673

(US11603374, Example 28)Show SMILES Nc1ncnc2n(cc(-c3cc(on3)C3CC3)c12)[C@@H]1C[C@H](CO)C1 |r,wU:18.21,20.24,(-2.78,4.69,;-3.17,3.2,;-4.68,2.88,;-5.16,1.42,;-4.13,.27,;-2.62,.59,;-1.37,-.31,;-.13,.59,;-.6,2.06,;.17,3.39,;1.71,3.39,;2.18,4.85,;.94,5.76,;-.31,4.85,;3.67,5.25,;5.16,4.85,;4.76,6.34,;-2.14,2.06,;-1.37,-1.85,;-2.46,-2.94,;-1.37,-4.03,;-1.37,-5.57,;-.04,-6.34,;-.29,-2.94,)| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The potency of compounds inhibiting several different RET kinase forms (Wild Type, V804M, M918T, G810R, & G810S) were determined using CisBio's H... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2Z323KZ |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret [M918T]
(Homo sapiens (Human)) | BDBM597674

(US11603374, Example 29)Show SMILES Nc1ncnc2n(cc(-c3cc(on3)C3CC3)c12)[C@H]1C[C@H](CO)C1 |r,wU:18.21,wD:20.24,(-2.78,4.69,;-3.17,3.2,;-4.68,2.88,;-5.16,1.42,;-4.13,.27,;-2.62,.59,;-1.37,-.31,;-.13,.59,;-.6,2.06,;.17,3.39,;1.71,3.39,;2.18,4.85,;.94,5.76,;-.31,4.85,;3.67,5.25,;5.16,4.85,;4.76,6.34,;-2.14,2.06,;-1.37,-1.85,;-.29,-2.94,;-1.37,-4.03,;-1.37,-5.57,;-.04,-6.34,;-2.46,-2.94,)| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The potency of compounds inhibiting several different RET kinase forms (Wild Type, V804M, M918T, G810R, & G810S) were determined using CisBio's H... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2Z323KZ |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM577029

(7-chloro-3-(5-cyclopropyl-4-(5- methylpyridin-2-yl...)Show SMILES CC(C)n1nc(-c2noc(C3CC3)c2-c2ccc(C)cn2)c2c(N)ncc(Cl)c12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The potency of compounds inhibiting several different RET kinase forms (Wild Type, V804M, M918T, G810R, & G810S) were determined using CisBio's H... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22N55HG |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data