 Found 182 hits with Last Name = 'morgan' and Initial = 'ca'
Found 182 hits with Last Name = 'morgan' and Initial = 'ca' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Substance-P receptor
(Homo sapiens (Human)) | BDBM50186527

((R)-3-((1-(3,5-bis(trifluoromethyl)phenyl)ethoxy)m...)Show SMILES C[C@@H](OCC1(CC(N)C1)c1ccccc1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |wU:1.0,(-2.49,.49,;-2.49,-1.05,;-1.15,-1.81,;.18,-1.04,;1.52,-1.8,;.45,-2.91,;1.56,-3.98,;1.59,-5.52,;2.63,-2.87,;2.86,-1.02,;4.18,-1.79,;5.51,-1.03,;5.51,.51,;4.18,1.27,;2.85,.51,;-3.82,-1.82,;-5.15,-1.06,;-6.48,-1.83,;-6.48,-3.37,;-5.15,-4.14,;-3.81,-3.37,;-5.15,-5.68,;-6.7,-5.67,;-3.62,-5.68,;-5.14,-7.22,;-7.82,-1.06,;-7.05,.28,;-8.59,-2.39,;-9.15,-.29,)| Show InChI InChI=1S/C21H21F6NO/c1-13(14-7-16(20(22,23)24)9-17(8-14)21(25,26)27)29-12-19(10-18(28)11-19)15-5-3-2-4-6-15/h2-9,13,18H,10-12,28H2,1H3/t13-,18?,19?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]Sar-Met substance P from human recombinant NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 16: 3859-63 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.031
BindingDB Entry DOI: 10.7270/Q2QV3M44 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50186522
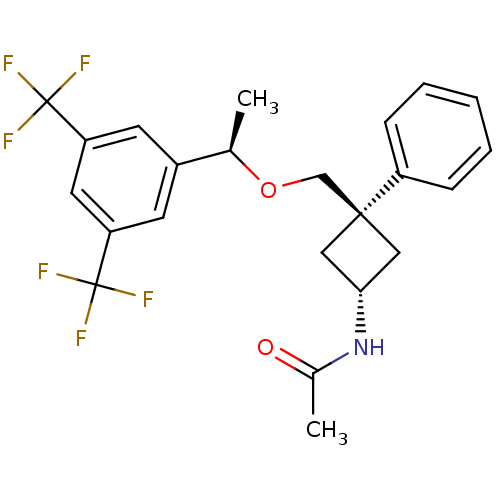
(CHEMBL379072 | trans-N-{3-[(R)-1-(3,5-bis-trifluor...)Show SMILES C[C@@H](OC[C@]1(C[C@@H](C1)NC(C)=O)c1ccccc1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |wU:1.0,6.8,wD:4.3,(.79,-3.02,;.8,-4.56,;2.13,-5.33,;3.46,-4.55,;4.8,-5.32,;3.73,-6.43,;4.84,-7.49,;5.91,-6.38,;4.88,-9.03,;3.56,-9.83,;3.59,-11.37,;2.21,-9.09,;6.13,-4.54,;7.46,-5.31,;8.79,-4.54,;8.78,-2.99,;7.44,-2.23,;6.11,-3.01,;-.54,-5.34,;-1.87,-4.57,;-3.2,-5.34,;-3.2,-6.89,;-1.87,-7.66,;-.53,-6.89,;-1.87,-9.2,;-3.41,-9.19,;-.33,-9.19,;-1.85,-10.74,;-4.53,-4.57,;-3.77,-3.24,;-5.31,-5.9,;-5.87,-3.8,)| Show InChI InChI=1S/C23H23F6NO2/c1-14(16-8-18(22(24,25)26)10-19(9-16)23(27,28)29)32-13-21(17-6-4-3-5-7-17)11-20(12-21)30-15(2)31/h3-10,14,20H,11-13H2,1-2H3,(H,30,31)/t14-,20-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]Sar-Met substance P from human recombinant NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 16: 3859-63 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.031
BindingDB Entry DOI: 10.7270/Q2QV3M44 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50186528

(CHEMBL212112 | cis-1-{3-[(R)-1-(3,5-bis-trifluorom...)Show SMILES C[C@@H](OC[C@@]1(C[C@@H](C1)N1CCCC1=O)c1ccccc1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |wU:1.0,wD:6.8,4.3,(22.41,-39.62,;22.42,-41.16,;23.75,-41.93,;25.09,-41.15,;26.42,-41.92,;27.53,-42.98,;26.47,-44.1,;25.36,-43.03,;26.5,-45.64,;25.28,-46.57,;25.78,-48.02,;27.32,-47.99,;27.77,-46.52,;29.22,-46.01,;27.75,-41.14,;29.08,-41.91,;30.41,-41.14,;30.41,-39.6,;29.06,-38.83,;27.74,-39.61,;21.09,-41.94,;19.75,-41.17,;18.42,-41.95,;18.42,-43.49,;19.76,-44.26,;21.09,-43.49,;19.75,-45.8,;18.21,-45.79,;21.29,-45.8,;19.77,-47.34,;17.09,-41.18,;17.85,-39.84,;16.31,-42.51,;15.76,-40.4,)| Show InChI InChI=1S/C25H25F6NO2/c1-16(17-10-19(24(26,27)28)12-20(11-17)25(29,30)31)34-15-23(18-6-3-2-4-7-18)13-21(14-23)32-9-5-8-22(32)33/h2-4,6-7,10-12,16,21H,5,8-9,13-15H2,1H3/t16-,21-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]Sar-Met substance P from human recombinant NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 16: 3859-63 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.031
BindingDB Entry DOI: 10.7270/Q2QV3M44 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50186525
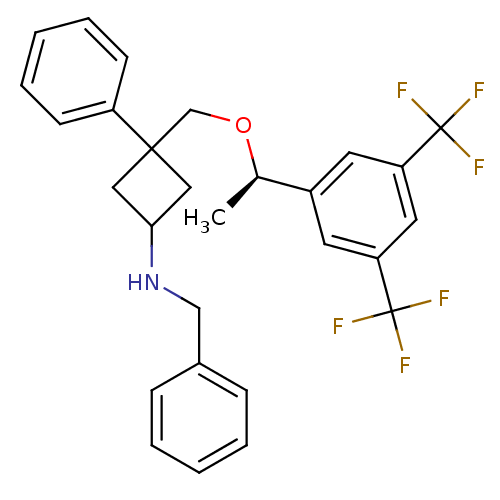
((R)-3-((1-(3,5-bis(trifluoromethyl)phenyl)ethoxy)m...)Show SMILES C[C@@H](OCC1(CC(C1)NCc1ccccc1)c1ccccc1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |wU:1.0,(17.4,-36.54,;17.4,-38.08,;18.74,-38.84,;20.07,-38.07,;21.41,-38.83,;20.34,-39.94,;21.45,-41.01,;22.52,-39.9,;21.48,-42.55,;20.17,-43.35,;20.2,-44.89,;18.88,-45.68,;18.91,-47.22,;20.26,-47.96,;21.59,-47.15,;21.55,-45.62,;22.74,-38.06,;24.06,-38.82,;25.39,-38.05,;25.39,-36.51,;24.04,-35.74,;22.72,-36.52,;16.07,-38.85,;14.74,-38.09,;13.41,-38.86,;13.4,-40.4,;14.74,-41.17,;16.08,-40.4,;14.74,-42.71,;13.19,-42.7,;16.27,-42.71,;14.75,-44.25,;12.07,-38.09,;12.83,-36.75,;11.29,-39.42,;10.74,-37.32,)| Show InChI InChI=1S/C28H27F6NO/c1-19(21-12-23(27(29,30)31)14-24(13-21)28(32,33)34)36-18-26(22-10-6-3-7-11-22)15-25(16-26)35-17-20-8-4-2-5-9-20/h2-14,19,25,35H,15-18H2,1H3/t19-,25?,26?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]Sar-Met substance P from human recombinant NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 16: 3859-63 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.031
BindingDB Entry DOI: 10.7270/Q2QV3M44 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50186531

(CHEMBL379073 | cis-N-{3-[(R)-1-(3,5-bis-trifluorom...)Show SMILES C[C@@H](OC[C@@]1(C[C@@H](C1)NC(C)=O)c1ccccc1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |wU:1.0,wD:6.8,4.3,(3.11,.86,;3.11,-.68,;4.45,-1.45,;5.78,-.67,;7.12,-1.44,;8.23,-2.5,;7.16,-3.61,;6.05,-2.55,;7.19,-5.15,;5.88,-5.95,;5.91,-7.49,;4.53,-5.21,;8.45,-.66,;9.78,-1.43,;11.11,-.65,;11.1,.89,;9.76,1.65,;8.43,.87,;1.78,-1.46,;.45,-.69,;-.88,-1.46,;-.88,-3.01,;.45,-3.78,;1.79,-3.01,;.45,-5.32,;-1.1,-5.3,;1.98,-5.31,;.46,-6.86,;-2.22,-.69,;-1.45,.65,;-2.99,-2.02,;-3.55,.08,)| Show InChI InChI=1S/C23H23F6NO2/c1-14(16-8-18(22(24,25)26)10-19(9-16)23(27,28)29)32-13-21(17-6-4-3-5-7-17)11-20(12-21)30-15(2)31/h3-10,14,20H,11-13H2,1-2H3,(H,30,31)/t14-,20-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]Sar-Met substance P from human recombinant NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 16: 3859-63 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.031
BindingDB Entry DOI: 10.7270/Q2QV3M44 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50177371

(2-((R)-4-(((R)-1-(3,5-bis(trifluoromethyl)phenyl)e...)Show SMILES C[C@@H](OC[C@]1(CN(CC(N)=O)C(=O)N1)c1ccc(F)cc1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C22H20F7N3O3/c1-12(13-6-15(21(24,25)26)8-16(7-13)22(27,28)29)35-11-20(14-2-4-17(23)5-3-14)10-32(9-18(30)33)19(34)31-20/h2-8,12H,9-11H2,1H3,(H2,30,33)(H,31,34)/t12-,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]-Sar-Met Substance P from recombinant human NK1 receptor in CHO cells |
Bioorg Med Chem Lett 16: 1065-9 (2006)
Article DOI: 10.1016/j.bmcl.2005.10.072
BindingDB Entry DOI: 10.7270/Q2K073TJ |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50177376

(4-((3,5-bis(trifluoromethyl)benzyloxy)methyl)-4-(4...)Show SMILES CN1CCC(CC1)N1CC(COCc2cc(cc(c2)C(F)(F)F)C(F)(F)F)(NC1=O)c1ccc(F)cc1 Show InChI InChI=1S/C25H26F7N3O2/c1-34-8-6-21(7-9-34)35-14-23(33-22(35)36,17-2-4-20(26)5-3-17)15-37-13-16-10-18(24(27,28)29)12-19(11-16)25(30,31)32/h2-5,10-12,21H,6-9,13-15H2,1H3,(H,33,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]-Sar-Met Substance P from recombinant human NK1 receptor in CHO cells |
Bioorg Med Chem Lett 16: 1065-9 (2006)
Article DOI: 10.1016/j.bmcl.2005.10.072
BindingDB Entry DOI: 10.7270/Q2K073TJ |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50186520

(CHEMBL379520 | trans-1-{3-[(R)-1-(3,5-bis-trifluor...)Show SMILES C[C@@H](OC[C@]1(C[C@@H](C1)N1CCCC1=O)c1ccccc1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |wU:1.0,6.8,wD:4.3,(-2.23,-39.04,;-2.22,-40.58,;-.89,-41.35,;.45,-40.57,;1.78,-41.34,;.72,-42.45,;1.83,-43.51,;2.89,-42.4,;1.86,-45.05,;.64,-45.99,;1.14,-47.44,;2.68,-47.41,;3.13,-45.93,;4.58,-45.43,;3.11,-40.56,;4.44,-41.33,;5.77,-40.55,;5.77,-39.01,;4.42,-38.25,;3.1,-39.03,;-3.55,-41.36,;-4.89,-40.59,;-6.22,-41.36,;-6.22,-42.91,;-4.88,-43.68,;-3.55,-42.91,;-4.89,-45.22,;-6.43,-45.2,;-3.35,-45.21,;-4.87,-46.76,;-7.55,-40.59,;-6.79,-39.25,;-8.33,-41.92,;-8.88,-39.82,)| Show InChI InChI=1S/C25H25F6NO2/c1-16(17-10-19(24(26,27)28)12-20(11-17)25(29,30)31)34-15-23(18-6-3-2-4-7-18)13-21(14-23)32-9-5-8-22(32)33/h2-4,6-7,10-12,16,21H,5,8-9,13-15H2,1H3/t16-,21-,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]Sar-Met substance P from human recombinant NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 16: 3859-63 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.031
BindingDB Entry DOI: 10.7270/Q2QV3M44 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50186518

(CHEMBL210641 | cis-3-(((R)-1-(3,5-bis(trifluoromet...)Show SMILES C[C@@H](OC[C@@]1(C[C@](N)(C1)C(N)=O)c1ccccc1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |wU:1.0,6.9,wD:6.6,4.3,(6.13,1.85,;6.13,.31,;7.47,-.46,;8.8,.32,;10.14,-.45,;11.24,-1.52,;10.17,-2.63,;11.5,-3.39,;9.07,-1.56,;8.81,-3.36,;7.5,-2.54,;8.76,-4.9,;11.47,.33,;12.8,-.44,;14.12,.33,;14.12,1.87,;12.77,2.64,;11.45,1.86,;4.8,-.47,;4.81,-2.02,;3.47,-2.79,;2.13,-2.02,;2.14,-.47,;3.47,.3,;.8,.3,;1.56,1.63,;.02,-1.03,;-.54,1.07,;3.47,-4.33,;5,-4.32,;1.92,-4.32,;3.48,-5.87,)| Show InChI InChI=1S/C22H22F6N2O2/c1-13(14-7-16(21(23,24)25)9-17(8-14)22(26,27)28)32-12-19(15-5-3-2-4-6-15)10-20(30,11-19)18(29)31/h2-9,13H,10-12,30H2,1H3,(H2,29,31)/t13-,19-,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]Sar-Met substance P from human recombinant NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 16: 3859-63 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.031
BindingDB Entry DOI: 10.7270/Q2QV3M44 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50177385
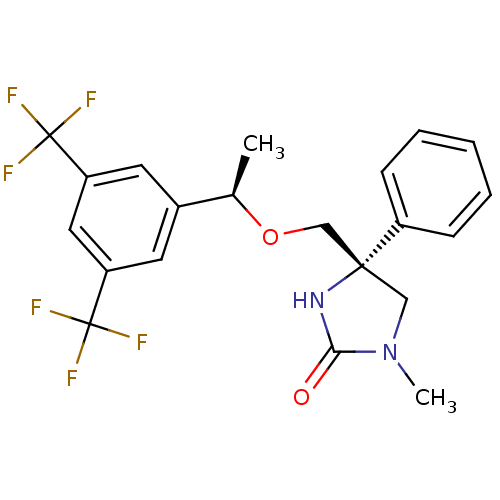
((R)-4-(((R)-1-(3,5-bis(trifluoromethyl)phenyl)etho...)Show SMILES C[C@@H](OC[C@]1(CN(C)C(=O)N1)c1ccccc1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C21H20F6N2O2/c1-13(14-8-16(20(22,23)24)10-17(9-14)21(25,26)27)31-12-19(11-29(2)18(30)28-19)15-6-4-3-5-7-15/h3-10,13H,11-12H2,1-2H3,(H,28,30)/t13-,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]-Sar-Met Substance P from recombinant human NK1 receptor in CHO cells |
Bioorg Med Chem Lett 16: 1065-9 (2006)
Article DOI: 10.1016/j.bmcl.2005.10.072
BindingDB Entry DOI: 10.7270/Q2K073TJ |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50186530
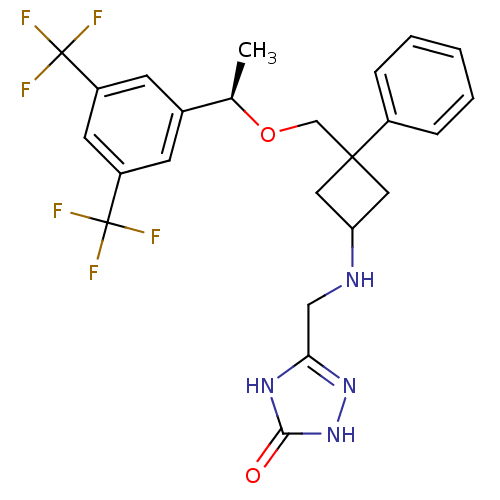
((R)-5-((3-((1-(3,5-bis(trifluoromethyl)phenyl)etho...)Show SMILES C[C@@H](OCC1(CC(C1)NCc1n[nH]c(=O)[nH]1)c1ccccc1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |wU:1.0,(6.12,-4.05,;6.12,-5.59,;7.46,-6.35,;8.79,-5.58,;10.13,-6.34,;9.06,-7.45,;10.17,-8.52,;11.24,-7.41,;10.2,-10.06,;8.89,-10.86,;8.92,-12.4,;7.68,-13.32,;8.19,-14.78,;9.73,-14.74,;10.67,-15.96,;10.17,-13.26,;11.46,-5.57,;12.79,-6.34,;14.12,-5.56,;14.11,-4.02,;12.77,-3.26,;11.44,-4.03,;4.79,-6.36,;3.46,-5.6,;2.13,-6.37,;2.13,-7.91,;3.46,-8.69,;4.8,-7.91,;3.46,-10.23,;1.91,-10.21,;4.99,-10.22,;3.47,-11.77,;.79,-5.6,;1.56,-4.26,;.02,-6.93,;-.54,-4.83,)| Show InChI InChI=1S/C24H24F6N4O2/c1-14(15-7-17(23(25,26)27)9-18(8-15)24(28,29)30)36-13-22(16-5-3-2-4-6-16)10-19(11-22)31-12-20-32-21(35)34-33-20/h2-9,14,19,31H,10-13H2,1H3,(H2,32,33,34,35)/t14-,19?,22?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]Sar-Met substance P from human recombinant NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 16: 3859-63 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.031
BindingDB Entry DOI: 10.7270/Q2QV3M44 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50186523
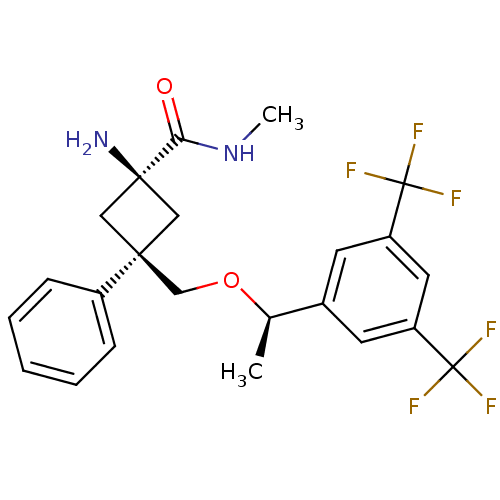
(CHEMBL214194 | cis-3-(((R)-1-(3,5-bis(trifluoromet...)Show SMILES CNC(=O)[C@]1(N)C[C@@](CO[C@H](C)c2cc(cc(c2)C(F)(F)F)C(F)(F)F)(C1)c1ccccc1 |wU:10.10,4.3,wD:4.4,7.7,(-.65,-7.07,;.66,-6.27,;2.02,-7.01,;2.05,-8.55,;3.34,-6.21,;4.64,-7.01,;4.44,-5.13,;3.36,-4.03,;2.05,-3.23,;.7,-3.96,;-.61,-3.16,;-.58,-1.62,;-1.97,-3.89,;-2.01,-5.44,;-3.37,-6.18,;-4.68,-5.37,;-4.63,-3.82,;-3.28,-3.09,;-5.94,-3.01,;-5.14,-1.7,;-6.76,-4.32,;-7.26,-2.2,;-3.41,-7.71,;-1.88,-7.75,;-4.96,-7.66,;-3.44,-9.25,;2.26,-5.11,;4.72,-3.29,;6.02,-4.1,;7.38,-3.37,;7.42,-1.83,;6.09,-1.02,;4.75,-1.76,)| Show InChI InChI=1S/C23H24F6N2O2/c1-14(15-8-17(22(24,25)26)10-18(9-15)23(27,28)29)33-13-20(16-6-4-3-5-7-16)11-21(30,12-20)19(32)31-2/h3-10,14H,11-13,30H2,1-2H3,(H,31,32)/t14-,20-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]Sar-Met substance P from human recombinant NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 16: 3859-63 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.031
BindingDB Entry DOI: 10.7270/Q2QV3M44 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50177387

((R)-4-(((R)-1-(3,5-bis(trifluoromethyl)phenyl)etho...)Show SMILES C[C@@H](OC[C@]1(CNC(=O)N1)c1ccccc1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C20H18F6N2O2/c1-12(13-7-15(19(21,22)23)9-16(8-13)20(24,25)26)30-11-18(10-27-17(29)28-18)14-5-3-2-4-6-14/h2-9,12H,10-11H2,1H3,(H2,27,28,29)/t12-,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]-Sar-Met Substance P from recombinant human NK1 receptor in CHO cells |
Bioorg Med Chem Lett 16: 1065-9 (2006)
Article DOI: 10.1016/j.bmcl.2005.10.072
BindingDB Entry DOI: 10.7270/Q2K073TJ |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50177379

((R)-4-(((R)-1-(3,5-bis(trifluoromethyl)phenyl)etho...)Show SMILES CCN1C[C@](CO[C@H](C)c2cc(cc(c2)C(F)(F)F)C(F)(F)F)(NC1=O)c1ccc(F)cc1 Show InChI InChI=1S/C22H21F7N2O2/c1-3-31-11-20(30-19(31)32,15-4-6-18(23)7-5-15)12-33-13(2)14-8-16(21(24,25)26)10-17(9-14)22(27,28)29/h4-10,13H,3,11-12H2,1-2H3,(H,30,32)/t13-,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]-Sar-Met Substance P from recombinant human NK1 receptor in CHO cells |
Bioorg Med Chem Lett 16: 1065-9 (2006)
Article DOI: 10.1016/j.bmcl.2005.10.072
BindingDB Entry DOI: 10.7270/Q2K073TJ |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50177386

(4-((3,5-bis(trifluoromethyl)benzyloxy)methyl)-4-(4...)Show SMILES Fc1ccc(cc1)C1(COCc2cc(cc(c2)C(F)(F)F)C(F)(F)F)CN(C2CCNCC2)C(=O)N1 Show InChI InChI=1S/C24H24F7N3O2/c25-19-3-1-16(2-4-19)22(13-34(21(35)33-22)20-5-7-32-8-6-20)14-36-12-15-9-17(23(26,27)28)11-18(10-15)24(29,30)31/h1-4,9-11,20,32H,5-8,12-14H2,(H,33,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]-Sar-Met Substance P from recombinant human NK1 receptor in CHO cells |
Bioorg Med Chem Lett 16: 1065-9 (2006)
Article DOI: 10.1016/j.bmcl.2005.10.072
BindingDB Entry DOI: 10.7270/Q2K073TJ |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50186519
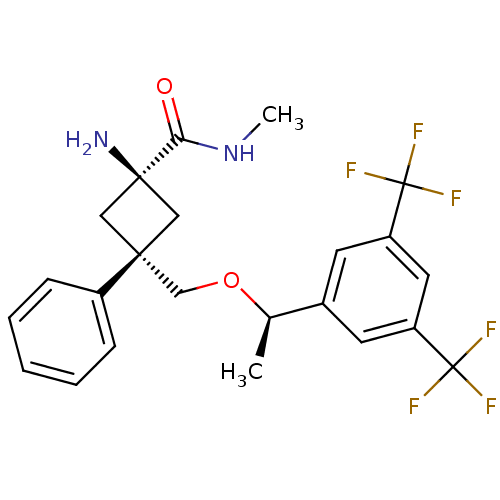
(CHEMBL214388 | trans-3-(((R)-1-(3,5-bis(trifluorom...)Show SMILES CNC(=O)[C@]1(N)C[C@](CO[C@H](C)c2cc(cc(c2)C(F)(F)F)C(F)(F)F)(C1)c1ccccc1 |wU:10.10,4.4,wD:7.7,4.3,(1.54,-7.56,;2.89,-6.83,;4.2,-7.65,;4.16,-9.18,;5.57,-6.91,;6.89,-7.68,;4.46,-5.84,;5.53,-4.73,;4.19,-3.97,;2.86,-4.74,;1.53,-3.98,;1.52,-2.44,;.2,-4.75,;.2,-6.3,;-1.14,-7.08,;-2.47,-6.3,;-2.47,-4.76,;-1.14,-3.99,;-3.8,-3.99,;-3.04,-2.65,;-4.58,-5.32,;-5.14,-3.22,;-1.14,-8.62,;.4,-8.61,;-2.68,-8.61,;-1.12,-10.16,;6.64,-5.8,;6.86,-3.96,;8.19,-4.73,;9.52,-3.95,;9.52,-2.41,;8.17,-1.65,;6.85,-2.42,)| Show InChI InChI=1S/C23H24F6N2O2/c1-14(15-8-17(22(24,25)26)10-18(9-15)23(27,28)29)33-13-20(16-6-4-3-5-7-16)11-21(30,12-20)19(32)31-2/h3-10,14H,11-13,30H2,1-2H3,(H,31,32)/t14-,20-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]Sar-Met substance P from human recombinant NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 16: 3859-63 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.031
BindingDB Entry DOI: 10.7270/Q2QV3M44 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50186521
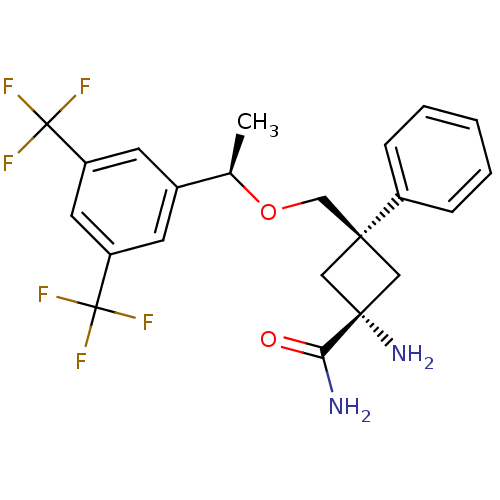
(CHEMBL210591 | trans-3-(((R)-1-(3,5-bis(trifluorom...)Show SMILES C[C@@H](OC[C@]1(C[C@](N)(C1)C(N)=O)c1ccccc1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |wU:1.0,6.6,wD:4.3,6.9,(-.21,1.61,;-.21,.07,;1.13,-.69,;2.46,.08,;3.8,-.68,;2.73,-1.79,;3.83,-2.86,;5.16,-3.62,;4.9,-1.75,;2.47,-3.59,;1.16,-2.77,;2.41,-5.12,;5.13,.09,;6.46,-.67,;7.79,.1,;7.78,1.64,;6.44,2.41,;5.11,1.63,;-1.54,-.7,;-1.53,-2.25,;-2.87,-3.02,;-4.2,-2.25,;-4.2,-.71,;-2.87,.06,;-5.54,.06,;-4.78,1.4,;-6.32,-1.27,;-6.88,.83,;-2.87,-4.56,;-1.34,-4.56,;-4.42,-4.56,;-2.86,-6.1,)| Show InChI InChI=1S/C22H22F6N2O2/c1-13(14-7-16(21(23,24)25)9-17(8-14)22(26,27)28)32-12-19(15-5-3-2-4-6-15)10-20(30,11-19)18(29)31/h2-9,13H,10-12,30H2,1H3,(H2,29,31)/t13-,19-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]Sar-Met substance P from human recombinant NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 16: 3859-63 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.031
BindingDB Entry DOI: 10.7270/Q2QV3M44 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50186524
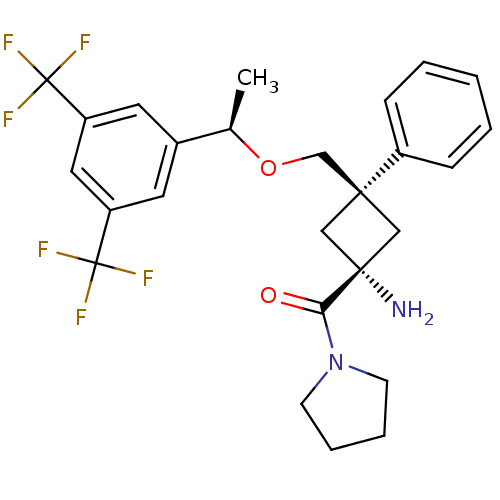
(CHEMBL378027 | trans-3-(((R)-1-(3,5-bis(trifluorom...)Show SMILES C[C@@H](OC[C@]1(C[C@](N)(C1)C(=O)N1CCCC1)c1ccccc1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |wU:1.0,6.6,wD:4.3,6.9,(1.06,-4.39,;1.07,-5.93,;2.4,-6.7,;3.74,-5.92,;5.07,-6.69,;4,-7.79,;5.11,-8.86,;6.43,-9.63,;6.18,-7.76,;3.77,-9.64,;3.77,-11.18,;2.43,-8.87,;2.26,-7.35,;.76,-7.03,;-.01,-8.37,;1.02,-9.51,;6.4,-5.91,;7.73,-6.68,;9.06,-5.9,;9.06,-4.36,;7.71,-3.6,;6.39,-4.38,;-.26,-6.71,;-.26,-8.26,;-1.59,-9.03,;-2.93,-8.26,;-2.93,-6.71,;-1.6,-5.94,;-4.26,-5.94,;-3.5,-4.6,;-5.04,-7.27,;-5.6,-5.17,;-1.6,-10.57,;-.06,-10.56,;-3.14,-10.56,;-1.58,-12.11,)| Show InChI InChI=1S/C26H28F6N2O2/c1-17(18-11-20(25(27,28)29)13-21(12-18)26(30,31)32)36-16-23(19-7-3-2-4-8-19)14-24(33,15-23)22(35)34-9-5-6-10-34/h2-4,7-8,11-13,17H,5-6,9-10,14-16,33H2,1H3/t17-,23-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]Sar-Met substance P from human recombinant NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 16: 3859-63 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.031
BindingDB Entry DOI: 10.7270/Q2QV3M44 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50186526

(CHEMBL377242 | trans-3-(((R)-1-(3,5-bis(trifluorom...)Show SMILES C[C@@H](OC[C@]1(C[C@](N)(C1)C(=O)N1CCCCC1)c1ccccc1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |wU:1.0,6.6,wD:4.3,6.9,(1.32,-2.59,;1.32,-4.13,;2.66,-4.89,;3.99,-4.12,;5.33,-4.88,;4.26,-5.99,;5.37,-7.06,;4.26,-8.14,;6.44,-5.95,;6.71,-7.81,;6.72,-9.35,;8.03,-7.03,;9.37,-7.79,;10.69,-7.02,;10.68,-5.48,;9.34,-4.72,;8.01,-5.5,;6.66,-4.11,;7.99,-4.87,;9.32,-4.1,;9.31,-2.56,;7.97,-1.79,;6.64,-2.57,;-.01,-4.9,;-0,-6.45,;-1.34,-7.22,;-2.67,-6.45,;-2.67,-4.91,;-1.34,-4.14,;-4,-4.14,;-3.24,-2.8,;-4.78,-5.47,;-5.34,-3.37,;-1.34,-8.76,;.19,-8.76,;-2.89,-8.76,;-1.33,-10.3,)| Show InChI InChI=1S/C27H30F6N2O2/c1-18(19-12-21(26(28,29)30)14-22(13-19)27(31,32)33)37-17-24(20-8-4-2-5-9-20)15-25(34,16-24)23(36)35-10-6-3-7-11-35/h2,4-5,8-9,12-14,18H,3,6-7,10-11,15-17,34H2,1H3/t18-,24-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]Sar-Met substance P from human recombinant NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 16: 3859-63 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.031
BindingDB Entry DOI: 10.7270/Q2QV3M44 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50177384
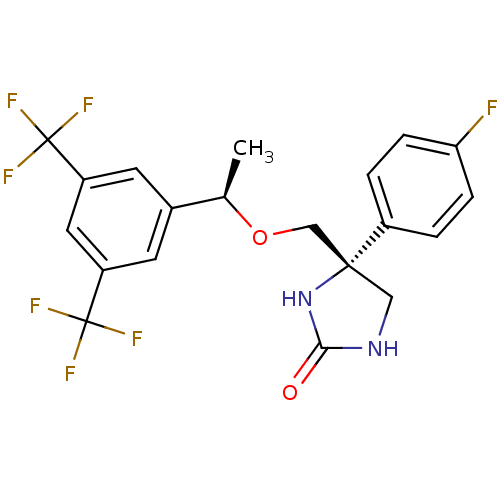
((R)-4-(((R)-1-(3,5-bis(trifluoromethyl)phenyl)etho...)Show SMILES C[C@@H](OC[C@]1(CNC(=O)N1)c1ccc(F)cc1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C20H17F7N2O2/c1-11(12-6-14(19(22,23)24)8-15(7-12)20(25,26)27)31-10-18(9-28-17(30)29-18)13-2-4-16(21)5-3-13/h2-8,11H,9-10H2,1H3,(H2,28,29,30)/t11-,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]-Sar-Met Substance P from recombinant human NK1 receptor in CHO cells |
Bioorg Med Chem Lett 16: 1065-9 (2006)
Article DOI: 10.1016/j.bmcl.2005.10.072
BindingDB Entry DOI: 10.7270/Q2K073TJ |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50177383

(4-((3,5-bis(trifluoromethyl)benzyloxy)methyl)-4-(4...)Show SMILES Fc1ccc(cc1)C1(COCc2cc(cc(c2)C(F)(F)F)C(F)(F)F)CN(CCN2CCCC2)C(=O)N1 Show InChI InChI=1S/C25H26F7N3O2/c26-21-5-3-18(4-6-21)23(15-35(22(36)33-23)10-9-34-7-1-2-8-34)16-37-14-17-11-19(24(27,28)29)13-20(12-17)25(30,31)32/h3-6,11-13H,1-2,7-10,14-16H2,(H,33,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]-Sar-Met Substance P from recombinant human NK1 receptor in CHO cells |
Bioorg Med Chem Lett 16: 1065-9 (2006)
Article DOI: 10.1016/j.bmcl.2005.10.072
BindingDB Entry DOI: 10.7270/Q2K073TJ |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50177380
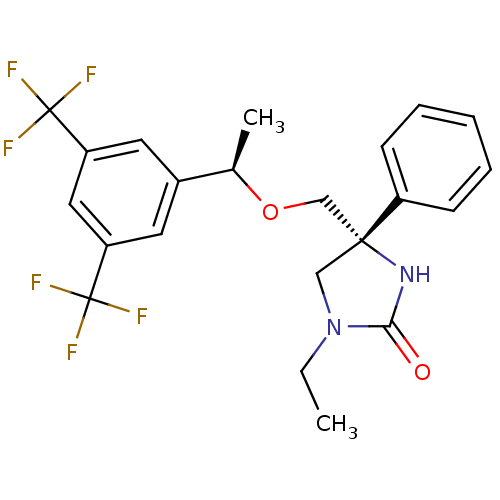
((R)-4-(((R)-1-(3,5-bis(trifluoromethyl)phenyl)etho...)Show SMILES CCN1C[C@](CO[C@H](C)c2cc(cc(c2)C(F)(F)F)C(F)(F)F)(NC1=O)c1ccccc1 Show InChI InChI=1S/C22H22F6N2O2/c1-3-30-12-20(29-19(30)31,16-7-5-4-6-8-16)13-32-14(2)15-9-17(21(23,24)25)11-18(10-15)22(26,27)28/h4-11,14H,3,12-13H2,1-2H3,(H,29,31)/t14-,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]-Sar-Met Substance P from recombinant human NK1 receptor in CHO cells |
Bioorg Med Chem Lett 16: 1065-9 (2006)
Article DOI: 10.1016/j.bmcl.2005.10.072
BindingDB Entry DOI: 10.7270/Q2K073TJ |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50186532
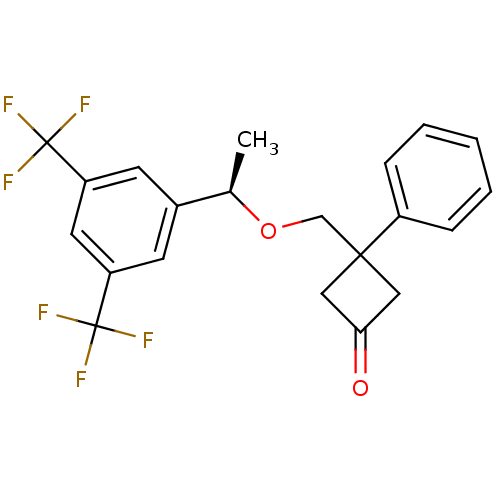
((R)-3-((1-(3,5-bis(trifluoromethyl)phenyl)ethoxy)m...)Show SMILES C[C@@H](OCC1(CC(=O)C1)c1ccccc1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C21H18F6O2/c1-13(14-7-16(20(22,23)24)9-17(8-14)21(25,26)27)29-12-19(10-18(28)11-19)15-5-3-2-4-6-15/h2-9,13H,10-12H2,1H3/t13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]Sar-Met substance P from human recombinant NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 16: 3859-63 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.031
BindingDB Entry DOI: 10.7270/Q2QV3M44 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50177375
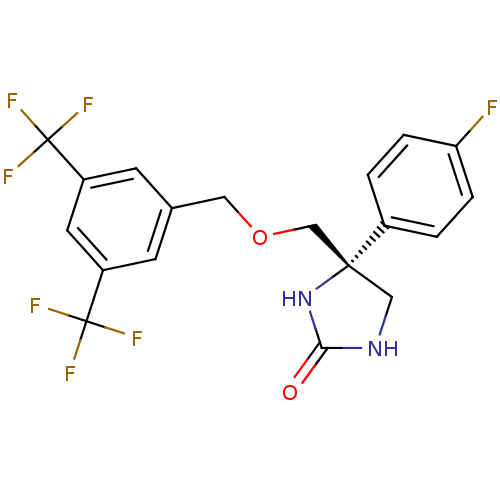
((R)-4-((3,5-bis(trifluoromethyl)benzyloxy)methyl)-...)Show SMILES Fc1ccc(cc1)[C@]1(COCc2cc(cc(c2)C(F)(F)F)C(F)(F)F)CNC(=O)N1 Show InChI InChI=1S/C19H15F7N2O2/c20-15-3-1-12(2-4-15)17(9-27-16(29)28-17)10-30-8-11-5-13(18(21,22)23)7-14(6-11)19(24,25)26/h1-7H,8-10H2,(H2,27,28,29)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]-Sar-Met Substance P from recombinant human NK1 receptor in CHO cells |
Bioorg Med Chem Lett 16: 1065-9 (2006)
Article DOI: 10.1016/j.bmcl.2005.10.072
BindingDB Entry DOI: 10.7270/Q2K073TJ |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50177370

((R)-4-(((R)-1-(3,5-bis(trifluoromethyl)phenyl)etho...)Show SMILES CC(C)N1C[C@](CO[C@H](C)c2cc(cc(c2)C(F)(F)F)C(F)(F)F)(NC1=O)c1ccccc1 Show InChI InChI=1S/C23H24F6N2O2/c1-14(2)31-12-21(30-20(31)32,17-7-5-4-6-8-17)13-33-15(3)16-9-18(22(24,25)26)11-19(10-16)23(27,28)29/h4-11,14-15H,12-13H2,1-3H3,(H,30,32)/t15-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]-Sar-Met Substance P from recombinant human NK1 receptor in CHO cells |
Bioorg Med Chem Lett 16: 1065-9 (2006)
Article DOI: 10.1016/j.bmcl.2005.10.072
BindingDB Entry DOI: 10.7270/Q2K073TJ |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50186529

(CHEMBL211888 | trans-3-(((R)-1-(3,5-bis(trifluorom...)Show SMILES C[C@@H](OC[C@]1(C[C@](N)(C1)C(=O)N(C)C)c1ccccc1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |wU:1.0,6.6,wD:4.3,6.9,(10.5,-8.16,;10.47,-9.7,;11.78,-10.5,;13.13,-9.76,;14.45,-10.56,;13.35,-11.64,;14.43,-12.73,;15.74,-13.53,;15.53,-11.65,;13.12,-13.54,;13.16,-15.08,;11.76,-12.81,;10.45,-13.62,;11.72,-11.27,;15.8,-9.81,;17.11,-10.61,;18.46,-9.87,;18.49,-8.33,;17.17,-7.53,;15.82,-8.28,;9.12,-10.44,;9.09,-11.99,;7.73,-12.73,;6.41,-11.93,;6.45,-10.39,;7.8,-9.65,;5.14,-9.58,;5.93,-8.26,;4.33,-10.89,;3.82,-8.78,;7.69,-14.27,;9.22,-14.31,;6.14,-14.23,;7.67,-15.81,)| Show InChI InChI=1S/C24H26F6N2O2/c1-15(16-9-18(23(25,26)27)11-19(10-16)24(28,29)30)34-14-21(17-7-5-4-6-8-17)12-22(31,13-21)20(33)32(2)3/h4-11,15H,12-14,31H2,1-3H3/t15-,21-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]Sar-Met substance P from human recombinant NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 16: 3859-63 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.031
BindingDB Entry DOI: 10.7270/Q2QV3M44 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50177374

((R)-4-(((R)-1-(3,5-bis(trifluoromethyl)phenyl)etho...)Show SMILES C[C@@H](OC[C@]1(CN(C)C(=O)N1)c1ccc(F)cc1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C21H19F7N2O2/c1-12(13-7-15(20(23,24)25)9-16(8-13)21(26,27)28)32-11-19(10-30(2)18(31)29-19)14-3-5-17(22)6-4-14/h3-9,12H,10-11H2,1-2H3,(H,29,31)/t12-,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]-Sar-Met Substance P from recombinant human NK1 receptor in CHO cells |
Bioorg Med Chem Lett 16: 1065-9 (2006)
Article DOI: 10.1016/j.bmcl.2005.10.072
BindingDB Entry DOI: 10.7270/Q2K073TJ |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50177378

(4-((3,5-bis(trifluoromethyl)benzyloxy)methyl)-4-(4...)Show SMILES Fc1ccc(cc1)C1(COCc2cc(cc(c2)C(F)(F)F)C(F)(F)F)CNC(=O)N1 Show InChI InChI=1S/C19H15F7N2O2/c20-15-3-1-12(2-4-15)17(9-27-16(29)28-17)10-30-8-11-5-13(18(21,22)23)7-14(6-11)19(24,25)26/h1-7H,8-10H2,(H2,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]-Sar-Met Substance P from recombinant human NK1 receptor in CHO cells |
Bioorg Med Chem Lett 16: 1065-9 (2006)
Article DOI: 10.1016/j.bmcl.2005.10.072
BindingDB Entry DOI: 10.7270/Q2K073TJ |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50177377

((R)-4-((3,5-bis(trifluoromethyl)benzyloxy)methyl)-...)Show SMILES FC(F)(F)c1cc(COC[C@]2(CNC(=O)N2)c2ccccc2)cc(c1)C(F)(F)F Show InChI InChI=1S/C19H16F6N2O2/c20-18(21,22)14-6-12(7-15(8-14)19(23,24)25)9-29-11-17(10-26-16(28)27-17)13-4-2-1-3-5-13/h1-8H,9-11H2,(H2,26,27,28)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]-Sar-Met Substance P from recombinant human NK1 receptor in CHO cells |
Bioorg Med Chem Lett 16: 1065-9 (2006)
Article DOI: 10.1016/j.bmcl.2005.10.072
BindingDB Entry DOI: 10.7270/Q2K073TJ |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50177382
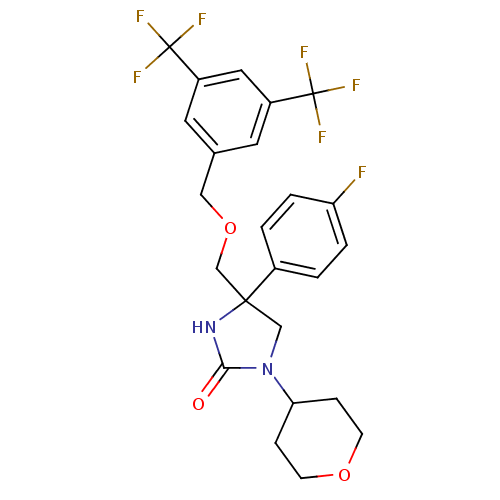
(4-((3,5-bis(trifluoromethyl)benzyloxy)methyl)-4-(4...)Show SMILES Fc1ccc(cc1)C1(COCc2cc(cc(c2)C(F)(F)F)C(F)(F)F)CN(C2CCOCC2)C(=O)N1 Show InChI InChI=1S/C24H23F7N2O3/c25-19-3-1-16(2-4-19)22(13-33(21(34)32-22)20-5-7-35-8-6-20)14-36-12-15-9-17(23(26,27)28)11-18(10-15)24(29,30)31/h1-4,9-11,20H,5-8,12-14H2,(H,32,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]-Sar-Met Substance P from recombinant human NK1 receptor in CHO cells |
Bioorg Med Chem Lett 16: 1065-9 (2006)
Article DOI: 10.1016/j.bmcl.2005.10.072
BindingDB Entry DOI: 10.7270/Q2K073TJ |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50177372
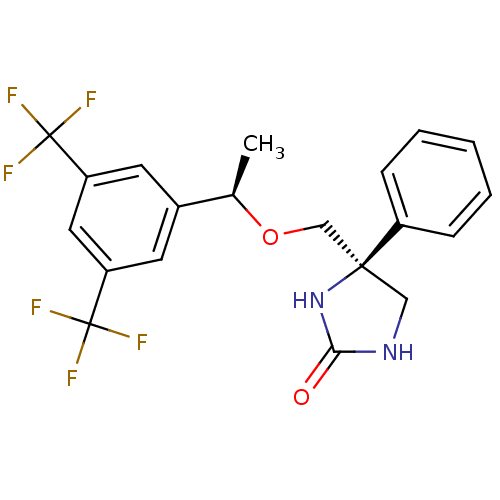
((S)-4-(((R)-1-(3,5-bis(trifluoromethyl)phenyl)etho...)Show SMILES C[C@@H](OC[C@@]1(CNC(=O)N1)c1ccccc1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C20H18F6N2O2/c1-12(13-7-15(19(21,22)23)9-16(8-13)20(24,25)26)30-11-18(10-27-17(29)28-18)14-5-3-2-4-6-14/h2-9,12H,10-11H2,1H3,(H2,27,28,29)/t12-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]-Sar-Met Substance P from recombinant human NK1 receptor in CHO cells |
Bioorg Med Chem Lett 16: 1065-9 (2006)
Article DOI: 10.1016/j.bmcl.2005.10.072
BindingDB Entry DOI: 10.7270/Q2K073TJ |
More data for this
Ligand-Target Pair | |
Retinal dehydrogenase 2
(Homo sapiens (Human)) | BDBM50459608
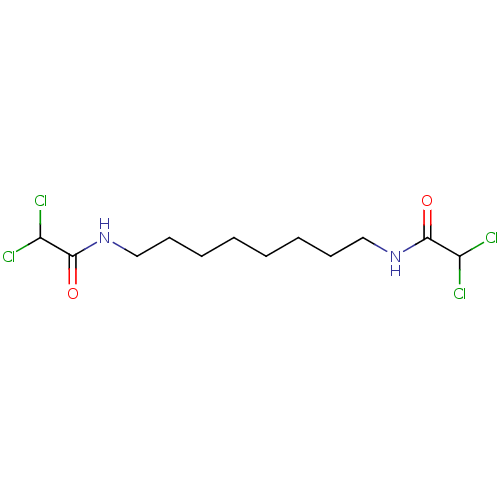
(CHEBI:90441 | CHEMBL3276621)Show InChI InChI=1S/C12H20Cl4N2O2/c13-9(14)11(19)17-7-5-3-1-2-4-6-8-18-12(20)10(15)16/h9-10H,1-8H2,(H,17,19)(H,18,20) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human ALDH1A2 |
J Med Chem 61: 8754-8773 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00930
BindingDB Entry DOI: 10.7270/Q2V127F6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50177373

((S)-4-((3,5-bis(trifluoromethyl)benzyloxy)methyl)-...)Show SMILES Fc1ccc(cc1)[C@@]1(COCc2cc(cc(c2)C(F)(F)F)C(F)(F)F)CNC(=O)N1 Show InChI InChI=1S/C19H15F7N2O2/c20-15-3-1-12(2-4-15)17(9-27-16(29)28-17)10-30-8-11-5-13(18(21,22)23)7-14(6-11)19(24,25)26/h1-7H,8-10H2,(H2,27,28,29)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]-Sar-Met Substance P from recombinant human NK1 receptor in CHO cells |
Bioorg Med Chem Lett 16: 1065-9 (2006)
Article DOI: 10.1016/j.bmcl.2005.10.072
BindingDB Entry DOI: 10.7270/Q2K073TJ |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50177381
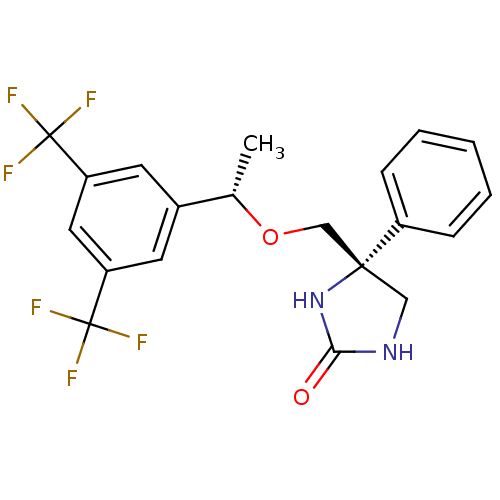
((R)-4-(((S)-1-(3,5-bis(trifluoromethyl)phenyl)etho...)Show SMILES C[C@H](OC[C@]1(CNC(=O)N1)c1ccccc1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C20H18F6N2O2/c1-12(13-7-15(19(21,22)23)9-16(8-13)20(24,25)26)30-11-18(10-27-17(29)28-18)14-5-3-2-4-6-14/h2-9,12H,10-11H2,1H3,(H2,27,28,29)/t12-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]-Sar-Met Substance P from recombinant human NK1 receptor in CHO cells |
Bioorg Med Chem Lett 16: 1065-9 (2006)
Article DOI: 10.1016/j.bmcl.2005.10.072
BindingDB Entry DOI: 10.7270/Q2K073TJ |
More data for this
Ligand-Target Pair | |
Aldehyde dehydrogenase 1A1
(Homo sapiens (Human)) | BDBM50076550
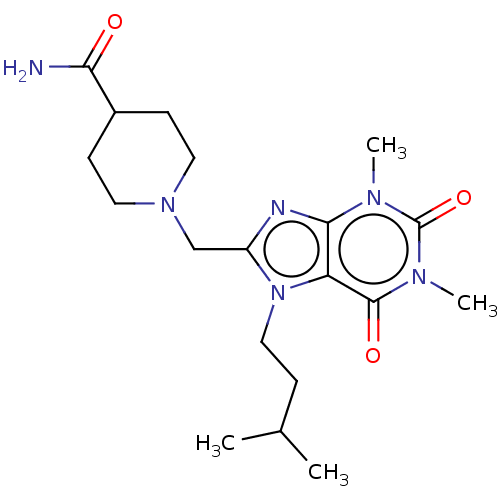
(CHEMBL3416554)Show SMILES CC(C)CCn1c(CN2CCC(CC2)C(N)=O)nc2n(C)c(=O)n(C)c(=O)c12 Show InChI InChI=1S/C19H30N6O3/c1-12(2)5-10-25-14(11-24-8-6-13(7-9-24)16(20)26)21-17-15(25)18(27)23(4)19(28)22(17)3/h12-13H,5-11H2,1-4H3,(H2,20,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 96 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University
Curated by ChEMBL
| Assay Description
Non-competitive tight inhibition of recombinant human ALDH1A1 using 1000 uM NAD+ as cofactor by Lineweaver-Burk plot analysis in presence of 100 to 8... |
J Med Chem 58: 1964-75 (2015)
Article DOI: 10.1021/jm501900s
BindingDB Entry DOI: 10.7270/Q22V2HTK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aldehyde dehydrogenase 1A1
(Homo sapiens (Human)) | BDBM50076741
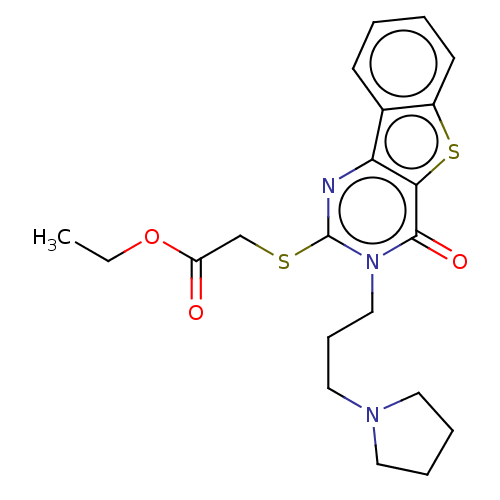
(CHEMBL3416562)Show SMILES CCOC(=O)CSc1nc2c3ccccc3sc2c(=O)n1CCCN1CCCC1 Show InChI InChI=1S/C21H25N3O3S2/c1-2-27-17(25)14-28-21-22-18-15-8-3-4-9-16(15)29-19(18)20(26)24(21)13-7-12-23-10-5-6-11-23/h3-4,8-9H,2,5-7,10-14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University
Curated by ChEMBL
| Assay Description
Competitive inhibition of recombinant human ALDH1A1 using 800 uM NAD+ as cofactor by Lineweaver-Burk plot analysis in presence of 100 to 800 uM aceta... |
J Med Chem 58: 1964-75 (2015)
Article DOI: 10.1021/jm501900s
BindingDB Entry DOI: 10.7270/Q22V2HTK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aldehyde dehydrogenase 1A1
(Homo sapiens (Human)) | BDBM50076740
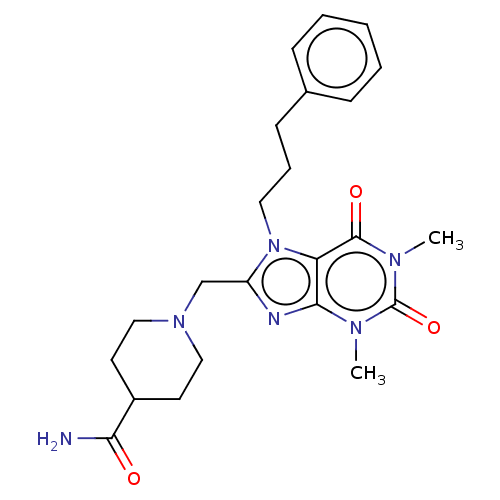
(CHEMBL1382328)Show SMILES Cn1c2nc(CN3CCC(CC3)C(N)=O)n(CCCc3ccccc3)c2c(=O)n(C)c1=O Show InChI InChI=1S/C23H30N6O3/c1-26-21-19(22(31)27(2)23(26)32)29(12-6-9-16-7-4-3-5-8-16)18(25-21)15-28-13-10-17(11-14-28)20(24)30/h3-5,7-8,17H,6,9-15H2,1-2H3,(H2,24,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University
Curated by ChEMBL
| Assay Description
Competitive tight inhibition of recombinant human ALDH1A1 using 1000 uM NAD+ as cofactor by Lineweaver-Burk plot analysis in presence of 100 to 800 u... |
J Med Chem 58: 1964-75 (2015)
Article DOI: 10.1021/jm501900s
BindingDB Entry DOI: 10.7270/Q22V2HTK |
More data for this
Ligand-Target Pair | |
Aldehyde dehydrogenase family 1 member A3
(Homo sapiens (Human)) | BDBM50459608

(CHEBI:90441 | CHEMBL3276621)Show InChI InChI=1S/C12H20Cl4N2O2/c13-9(14)11(19)17-7-5-3-1-2-4-6-8-18-12(20)10(15)16/h9-10H,1-8H2,(H,17,19)(H,18,20) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 261 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human ALDH1A3 |
J Med Chem 61: 8754-8773 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00930
BindingDB Entry DOI: 10.7270/Q2V127F6 |
More data for this
Ligand-Target Pair | |
Aldehyde dehydrogenase 1A1
(Homo sapiens (Human)) | BDBM50459608

(CHEBI:90441 | CHEMBL3276621)Show InChI InChI=1S/C12H20Cl4N2O2/c13-9(14)11(19)17-7-5-3-1-2-4-6-8-18-12(20)10(15)16/h9-10H,1-8H2,(H,17,19)(H,18,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 285 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human ALDH1A1 |
J Med Chem 61: 8754-8773 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00930
BindingDB Entry DOI: 10.7270/Q2V127F6 |
More data for this
Ligand-Target Pair | |
Aldehyde dehydrogenase 1A1
(Homo sapiens (Human)) | BDBM50459601
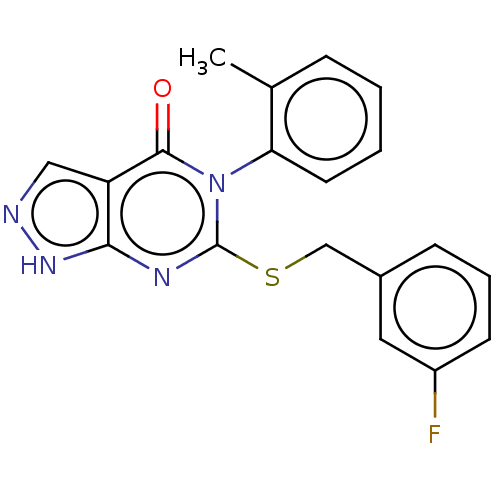
(CHEMBL4217738)Show SMILES Cc1ccccc1-n1c(SCc2cccc(F)c2)nc2[nH]ncc2c1=O |(15.24,-30.22,;16.57,-30.99,;17.9,-30.22,;19.24,-30.99,;19.24,-32.53,;17.9,-33.3,;16.57,-32.52,;15.24,-33.28,;15.24,-34.82,;16.58,-35.59,;16.57,-37.13,;17.91,-37.91,;17.9,-39.45,;19.23,-40.22,;20.57,-39.45,;20.56,-37.9,;21.9,-37.13,;19.23,-37.14,;13.91,-35.59,;12.59,-34.82,;11.12,-35.31,;10.21,-34.07,;11.11,-32.82,;12.58,-33.28,;13.91,-32.51,;13.92,-30.96,)| Show InChI InChI=1S/C19H15FN4OS/c1-12-5-2-3-8-16(12)24-18(25)15-10-21-23-17(15)22-19(24)26-11-13-6-4-7-14(20)9-13/h2-10H,11H2,1H3,(H,21,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of human recombinant ALDH1A1 assessed as reduction of NAD(P)H formation using varying levels of NAD+ |
J Med Chem 61: 8754-8773 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00930
BindingDB Entry DOI: 10.7270/Q2V127F6 |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM50186522

(CHEMBL379072 | trans-N-{3-[(R)-1-(3,5-bis-trifluor...)Show SMILES C[C@@H](OC[C@]1(C[C@@H](C1)NC(C)=O)c1ccccc1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |wU:1.0,6.8,wD:4.3,(.79,-3.02,;.8,-4.56,;2.13,-5.33,;3.46,-4.55,;4.8,-5.32,;3.73,-6.43,;4.84,-7.49,;5.91,-6.38,;4.88,-9.03,;3.56,-9.83,;3.59,-11.37,;2.21,-9.09,;6.13,-4.54,;7.46,-5.31,;8.79,-4.54,;8.78,-2.99,;7.44,-2.23,;6.11,-3.01,;-.54,-5.34,;-1.87,-4.57,;-3.2,-5.34,;-3.2,-6.89,;-1.87,-7.66,;-.53,-6.89,;-1.87,-9.2,;-3.41,-9.19,;-.33,-9.19,;-1.85,-10.74,;-4.53,-4.57,;-3.77,-3.24,;-5.31,-5.9,;-5.87,-3.8,)| Show InChI InChI=1S/C23H23F6NO2/c1-14(16-8-18(22(24,25)26)10-19(9-16)23(27,28)29)32-13-21(17-6-4-3-5-7-17)11-20(12-21)30-15(2)31/h3-10,14,20H,11-13H2,1-2H3,(H,30,31)/t14-,20-,21+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]neurokinin B from human recombinant NK3 receptor in CHO cells |
Bioorg Med Chem Lett 16: 3859-63 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.031
BindingDB Entry DOI: 10.7270/Q2QV3M44 |
More data for this
Ligand-Target Pair | |
Aldehyde dehydrogenase 1A1
(Homo sapiens (Human)) | BDBM30972
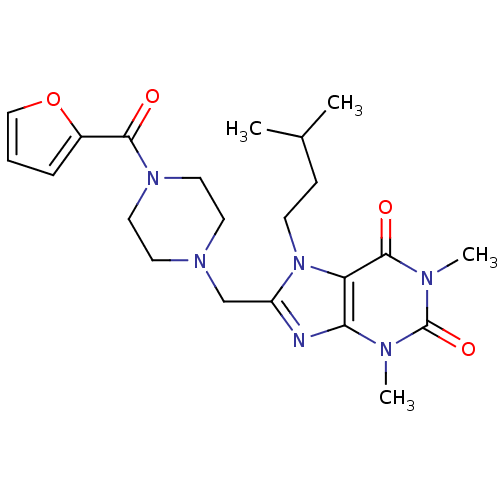
(8-[[4-(2-furoyl)piperazino]methyl]-7-isoamyl-1,3-d...)Show SMILES CC(C)CCn1c(CN2CCN(CC2)C(=O)c2ccco2)nc2n(C)c(=O)n(C)c(=O)c12 Show InChI InChI=1S/C22H30N6O4/c1-15(2)7-8-28-17(23-19-18(28)21(30)25(4)22(31)24(19)3)14-26-9-11-27(12-10-26)20(29)16-6-5-13-32-16/h5-6,13,15H,7-12,14H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University
Curated by ChEMBL
| Assay Description
Uncompetitive partial inhibition of recombinant human ALDH1A1 using 200 uM propionaldehyde as substrate by Lineweaver-Burk plot analysis in presence ... |
J Med Chem 58: 1964-75 (2015)
Article DOI: 10.1021/jm501900s
BindingDB Entry DOI: 10.7270/Q22V2HTK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aldehyde dehydrogenase 1A1
(Homo sapiens (Human)) | BDBM30972

(8-[[4-(2-furoyl)piperazino]methyl]-7-isoamyl-1,3-d...)Show SMILES CC(C)CCn1c(CN2CCN(CC2)C(=O)c2ccco2)nc2n(C)c(=O)n(C)c(=O)c12 Show InChI InChI=1S/C22H30N6O4/c1-15(2)7-8-28-17(23-19-18(28)21(30)25(4)22(31)24(19)3)14-26-9-11-27(12-10-26)20(29)16-6-5-13-32-16/h5-6,13,15H,7-12,14H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University
Curated by ChEMBL
| Assay Description
Non-competitive partial inhibition of recombinant human ALDH1A1 using 800 uM NAD+ as cofactor by Lineweaver-Burk plot analysis in presence of 100 to ... |
J Med Chem 58: 1964-75 (2015)
Article DOI: 10.1021/jm501900s
BindingDB Entry DOI: 10.7270/Q22V2HTK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glycogen [starch] synthase, muscle
(Homo sapiens (Human)) | BDBM50527393
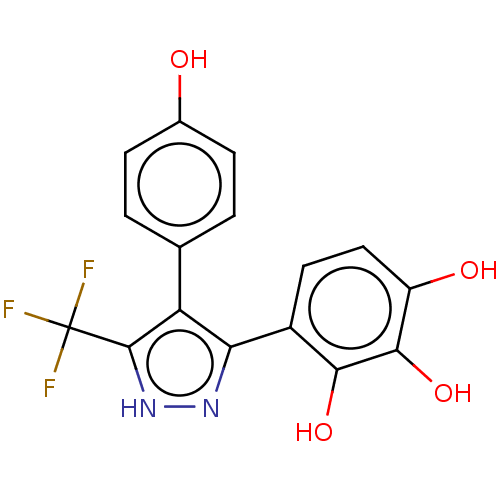
(CHEMBL4446895)Show SMILES Oc1ccc(cc1)-c1c(n[nH]c1C(F)(F)F)-c1ccc(O)c(O)c1O Show InChI InChI=1S/C16H11F3N2O4/c17-16(18,19)15-11(7-1-3-8(22)4-2-7)12(20-21-15)9-5-6-10(23)14(25)13(9)24/h1-6,22-25H,(H,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 1.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine
Curated by ChEMBL
| Assay Description
Competitive inhibition of C-terminal 6His-tagged human GYS1 using UDPG as substrate in presence of G-6-P by 14C-glucose incorporation based Michaelis... |
J Med Chem 63: 3538-3551 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01851
BindingDB Entry DOI: 10.7270/Q2MS3X64 |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM50186519

(CHEMBL214388 | trans-3-(((R)-1-(3,5-bis(trifluorom...)Show SMILES CNC(=O)[C@]1(N)C[C@](CO[C@H](C)c2cc(cc(c2)C(F)(F)F)C(F)(F)F)(C1)c1ccccc1 |wU:10.10,4.4,wD:7.7,4.3,(1.54,-7.56,;2.89,-6.83,;4.2,-7.65,;4.16,-9.18,;5.57,-6.91,;6.89,-7.68,;4.46,-5.84,;5.53,-4.73,;4.19,-3.97,;2.86,-4.74,;1.53,-3.98,;1.52,-2.44,;.2,-4.75,;.2,-6.3,;-1.14,-7.08,;-2.47,-6.3,;-2.47,-4.76,;-1.14,-3.99,;-3.8,-3.99,;-3.04,-2.65,;-4.58,-5.32,;-5.14,-3.22,;-1.14,-8.62,;.4,-8.61,;-2.68,-8.61,;-1.12,-10.16,;6.64,-5.8,;6.86,-3.96,;8.19,-4.73,;9.52,-3.95,;9.52,-2.41,;8.17,-1.65,;6.85,-2.42,)| Show InChI InChI=1S/C23H24F6N2O2/c1-14(15-8-17(22(24,25)26)10-18(9-15)23(27,28)29)33-13-20(16-6-4-3-5-7-16)11-21(30,12-20)19(32)31-2/h3-10,14H,11-13,30H2,1-2H3,(H,31,32)/t14-,20-,21+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]neurokinin B from human recombinant NK3 receptor in CHO cells |
Bioorg Med Chem Lett 16: 3859-63 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.031
BindingDB Entry DOI: 10.7270/Q2QV3M44 |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM50186531

(CHEMBL379073 | cis-N-{3-[(R)-1-(3,5-bis-trifluorom...)Show SMILES C[C@@H](OC[C@@]1(C[C@@H](C1)NC(C)=O)c1ccccc1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |wU:1.0,wD:6.8,4.3,(3.11,.86,;3.11,-.68,;4.45,-1.45,;5.78,-.67,;7.12,-1.44,;8.23,-2.5,;7.16,-3.61,;6.05,-2.55,;7.19,-5.15,;5.88,-5.95,;5.91,-7.49,;4.53,-5.21,;8.45,-.66,;9.78,-1.43,;11.11,-.65,;11.1,.89,;9.76,1.65,;8.43,.87,;1.78,-1.46,;.45,-.69,;-.88,-1.46,;-.88,-3.01,;.45,-3.78,;1.79,-3.01,;.45,-5.32,;-1.1,-5.3,;1.98,-5.31,;.46,-6.86,;-2.22,-.69,;-1.45,.65,;-2.99,-2.02,;-3.55,.08,)| Show InChI InChI=1S/C23H23F6NO2/c1-14(16-8-18(22(24,25)26)10-19(9-16)23(27,28)29)32-13-21(17-6-4-3-5-7-17)11-20(12-21)30-15(2)31/h3-10,14,20H,11-13H2,1-2H3,(H,30,31)/t14-,20-,21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]neurokinin B from human recombinant NK3 receptor in CHO cells |
Bioorg Med Chem Lett 16: 3859-63 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.031
BindingDB Entry DOI: 10.7270/Q2QV3M44 |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM50186527

((R)-3-((1-(3,5-bis(trifluoromethyl)phenyl)ethoxy)m...)Show SMILES C[C@@H](OCC1(CC(N)C1)c1ccccc1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |wU:1.0,(-2.49,.49,;-2.49,-1.05,;-1.15,-1.81,;.18,-1.04,;1.52,-1.8,;.45,-2.91,;1.56,-3.98,;1.59,-5.52,;2.63,-2.87,;2.86,-1.02,;4.18,-1.79,;5.51,-1.03,;5.51,.51,;4.18,1.27,;2.85,.51,;-3.82,-1.82,;-5.15,-1.06,;-6.48,-1.83,;-6.48,-3.37,;-5.15,-4.14,;-3.81,-3.37,;-5.15,-5.68,;-6.7,-5.67,;-3.62,-5.68,;-5.14,-7.22,;-7.82,-1.06,;-7.05,.28,;-8.59,-2.39,;-9.15,-.29,)| Show InChI InChI=1S/C21H21F6NO/c1-13(14-7-16(20(22,23)24)9-17(8-14)21(25,26)27)29-12-19(10-18(28)11-19)15-5-3-2-4-6-15/h2-9,13,18H,10-12,28H2,1H3/t13-,18?,19?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]neurokinin B from human recombinant NK3 receptor in CHO cells |
Bioorg Med Chem Lett 16: 3859-63 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.031
BindingDB Entry DOI: 10.7270/Q2QV3M44 |
More data for this
Ligand-Target Pair | |
Aldehyde dehydrogenase 1A1
(Homo sapiens (Human)) | BDBM50076742
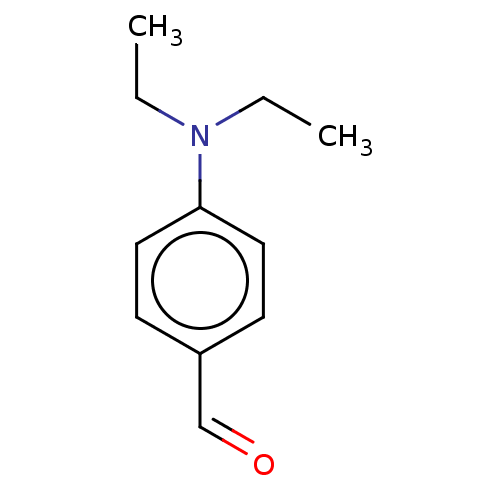
(CHEBI:86194 | CHEMBL3416563)Show InChI InChI=1S/C11H15NO/c1-3-12(4-2)11-7-5-10(9-13)6-8-11/h5-9H,3-4H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human ALDH1A1 |
J Med Chem 61: 8754-8773 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00930
BindingDB Entry DOI: 10.7270/Q2V127F6 |
More data for this
Ligand-Target Pair | |
Aldehyde dehydrogenase 1A1
(Homo sapiens (Human)) | BDBM50076742

(CHEBI:86194 | CHEMBL3416563)Show InChI InChI=1S/C11H15NO/c1-3-12(4-2)11-7-5-10(9-13)6-8-11/h5-9H,3-4H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human ALDH1A1 using propionaldehyde as substrate preincubated for 2 mins with NAD+ followed by substrate addition by UV-Vis... |
J Med Chem 58: 1964-75 (2015)
Article DOI: 10.1021/jm501900s
BindingDB Entry DOI: 10.7270/Q22V2HTK |
More data for this
Ligand-Target Pair | |
Aldehyde dehydrogenase 1A1
(Homo sapiens (Human)) | BDBM50459603
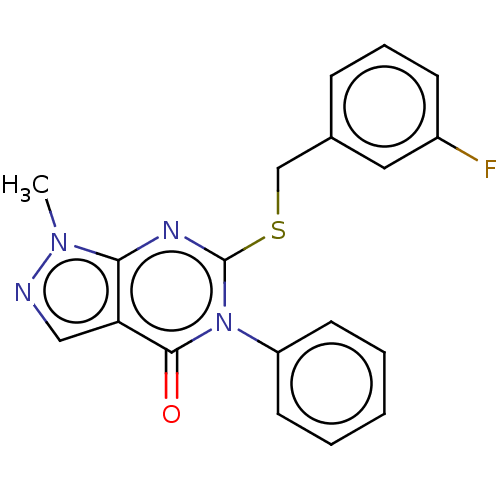
(CHEMBL4213331)Show InChI InChI=1S/C19H15FN4OS/c1-23-17-16(11-21-23)18(25)24(15-8-3-2-4-9-15)19(22-17)26-12-13-6-5-7-14(20)10-13/h2-11H,12H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ALDH1A1 assessed as reduction in of NAD(P)H formation incubated for 2 mins by spectrophotometry |
J Med Chem 61: 8754-8773 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00930
BindingDB Entry DOI: 10.7270/Q2V127F6 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data