

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Substance-P receptor (Homo sapiens (Human)) | BDBM50364189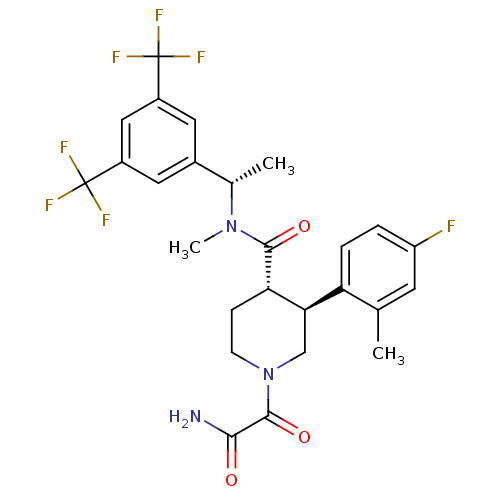 (CHEMBL1951813) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]BH-SP from tachykinin NK1 receptor in human IM9 cells after 30 mins by scintillation counting | Bioorg Med Chem 20: 962-77 (2012) Article DOI: 10.1016/j.bmc.2011.11.048 BindingDB Entry DOI: 10.7270/Q2NP24WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50364190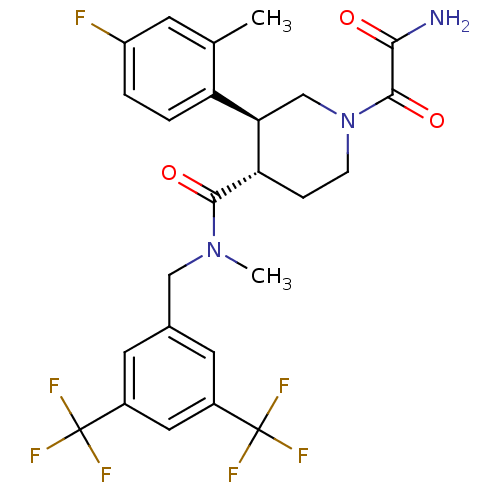 (CHEMBL1951810) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]BH-SP from tachykinin NK1 receptor in human IM9 cells after 30 mins by scintillation counting | Bioorg Med Chem 20: 962-77 (2012) Article DOI: 10.1016/j.bmc.2011.11.048 BindingDB Entry DOI: 10.7270/Q2NP24WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50364190 (CHEMBL1951810) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]BH-SP from tachykinin NK1 receptor in human IM9 cells after 30 mins by scintillation counting | Bioorg Med Chem 20: 962-77 (2012) Article DOI: 10.1016/j.bmc.2011.11.048 BindingDB Entry DOI: 10.7270/Q2NP24WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50364202 (CHEMBL1951626) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0320 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]BH-SP from tachykinin NK1 receptor in human IM9 cells after 30 mins by scintillation counting | Bioorg Med Chem 20: 962-77 (2012) Article DOI: 10.1016/j.bmc.2011.11.048 BindingDB Entry DOI: 10.7270/Q2NP24WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50364195 (CHEMBL1951633) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]BH-SP from tachykinin NK1 receptor in human IM9 cells after 30 mins by scintillation counting | Bioorg Med Chem 20: 962-77 (2012) Article DOI: 10.1016/j.bmc.2011.11.048 BindingDB Entry DOI: 10.7270/Q2NP24WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50364197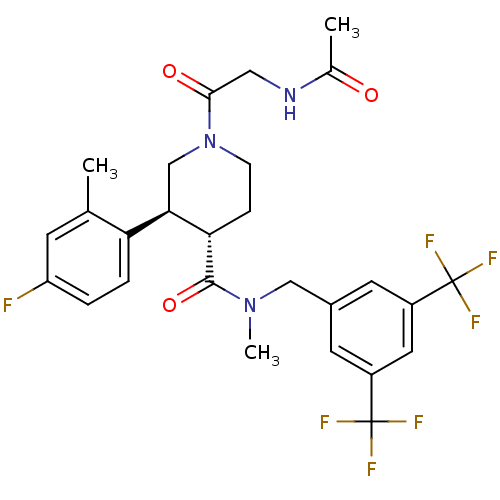 (CHEMBL1951631) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]BH-SP from tachykinin NK1 receptor in human IM9 cells after 30 mins by scintillation counting | Bioorg Med Chem 20: 962-77 (2012) Article DOI: 10.1016/j.bmc.2011.11.048 BindingDB Entry DOI: 10.7270/Q2NP24WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50364192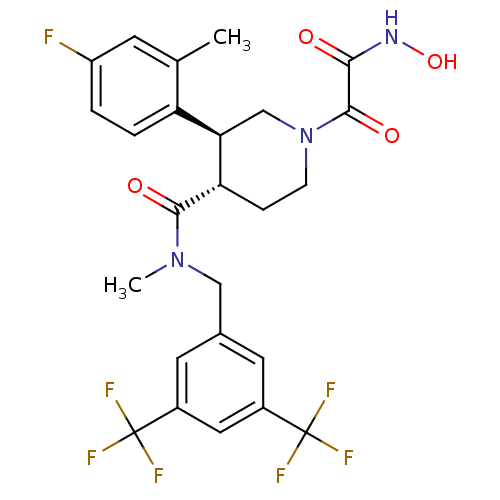 (CHEMBL1951811) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0460 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]BH-SP from tachykinin NK1 receptor in human IM9 cells after 30 mins by scintillation counting | Bioorg Med Chem 20: 962-77 (2012) Article DOI: 10.1016/j.bmc.2011.11.048 BindingDB Entry DOI: 10.7270/Q2NP24WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50364198 (CHEMBL1951630) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0470 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]BH-SP from tachykinin NK1 receptor in human IM9 cells after 30 mins by scintillation counting | Bioorg Med Chem 20: 962-77 (2012) Article DOI: 10.1016/j.bmc.2011.11.048 BindingDB Entry DOI: 10.7270/Q2NP24WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50364196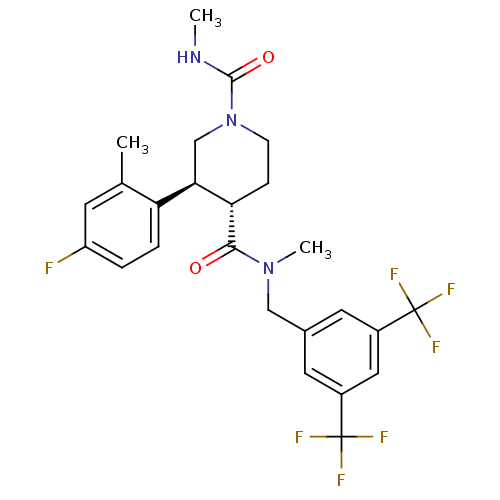 (CHEMBL1951632) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0480 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]BH-SP from tachykinin NK1 receptor in human IM9 cells after 30 mins by scintillation counting | Bioorg Med Chem 20: 962-77 (2012) Article DOI: 10.1016/j.bmc.2011.11.048 BindingDB Entry DOI: 10.7270/Q2NP24WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50364199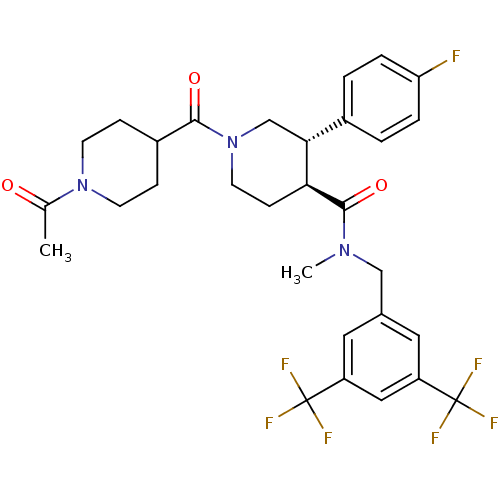 (CHEMBL1951629) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0520 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]BH-SP from tachykinin NK1 receptor in human IM9 cells after 30 mins by scintillation counting | Bioorg Med Chem 20: 962-77 (2012) Article DOI: 10.1016/j.bmc.2011.11.048 BindingDB Entry DOI: 10.7270/Q2NP24WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50364201 (CHEMBL1951627) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0640 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]BH-SP from tachykinin NK1 receptor in human IM9 cells after 30 mins by scintillation counting | Bioorg Med Chem 20: 962-77 (2012) Article DOI: 10.1016/j.bmc.2011.11.048 BindingDB Entry DOI: 10.7270/Q2NP24WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50364194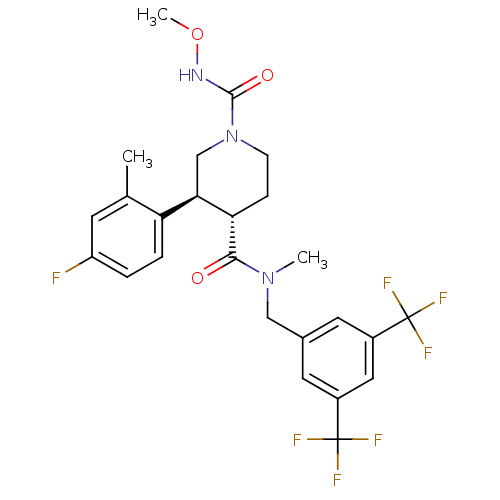 (CHEMBL1951634) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0730 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]BH-SP from tachykinin NK1 receptor in human IM9 cells after 30 mins by scintillation counting | Bioorg Med Chem 20: 962-77 (2012) Article DOI: 10.1016/j.bmc.2011.11.048 BindingDB Entry DOI: 10.7270/Q2NP24WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50364207 (CHEMBL1951621) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0980 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]BH-SP from tachykinin NK1 receptor in human IM9 cells after 30 mins by scintillation counting | Bioorg Med Chem 20: 962-77 (2012) Article DOI: 10.1016/j.bmc.2011.11.048 BindingDB Entry DOI: 10.7270/Q2NP24WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50364193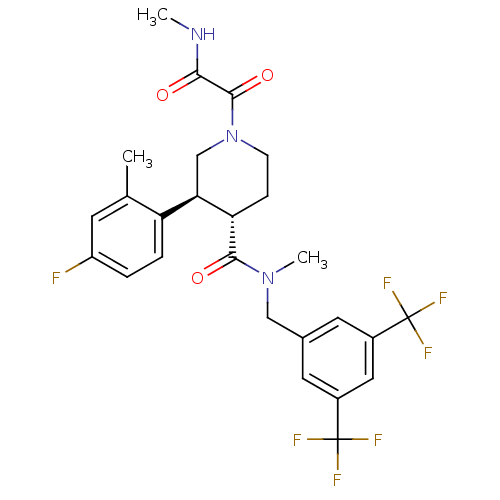 (CHEMBL1951635) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]BH-SP from tachykinin NK1 receptor in human IM9 cells after 30 mins by scintillation counting | Bioorg Med Chem 20: 962-77 (2012) Article DOI: 10.1016/j.bmc.2011.11.048 BindingDB Entry DOI: 10.7270/Q2NP24WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50364203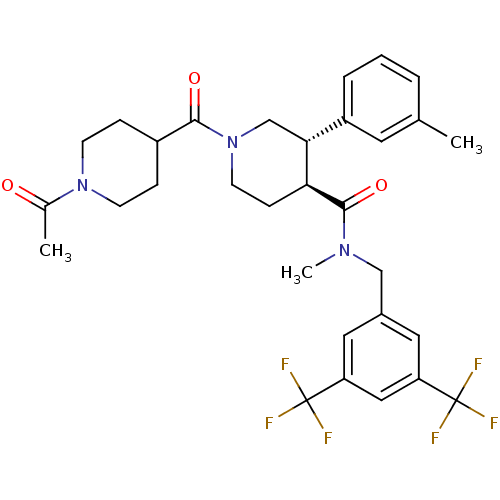 (CHEMBL1951625) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]BH-SP from tachykinin NK1 receptor in human IM9 cells after 30 mins by scintillation counting | Bioorg Med Chem 20: 962-77 (2012) Article DOI: 10.1016/j.bmc.2011.11.048 BindingDB Entry DOI: 10.7270/Q2NP24WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50364205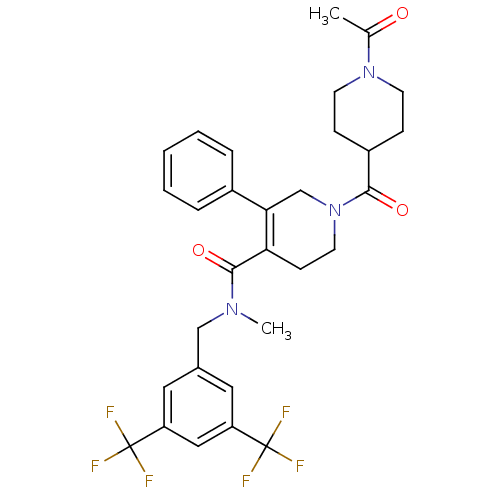 (CHEMBL1951623) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]BH-SP from tachykinin NK1 receptor in human IM9 cells after 30 mins by scintillation counting | Bioorg Med Chem 20: 962-77 (2012) Article DOI: 10.1016/j.bmc.2011.11.048 BindingDB Entry DOI: 10.7270/Q2NP24WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50364204 (CHEMBL1951624) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]BH-SP from tachykinin NK1 receptor in human IM9 cells after 30 mins by scintillation counting | Bioorg Med Chem 20: 962-77 (2012) Article DOI: 10.1016/j.bmc.2011.11.048 BindingDB Entry DOI: 10.7270/Q2NP24WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50364188 (CHEMBL1951814) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]BH-SP from tachykinin NK1 receptor in human IM9 cells after 30 mins by scintillation counting | Bioorg Med Chem 20: 962-77 (2012) Article DOI: 10.1016/j.bmc.2011.11.048 BindingDB Entry DOI: 10.7270/Q2NP24WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50364200 (CHEMBL1951628) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]BH-SP from tachykinin NK1 receptor in human IM9 cells after 30 mins by scintillation counting | Bioorg Med Chem 20: 962-77 (2012) Article DOI: 10.1016/j.bmc.2011.11.048 BindingDB Entry DOI: 10.7270/Q2NP24WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Homo sapiens (Human)) | BDBM50339725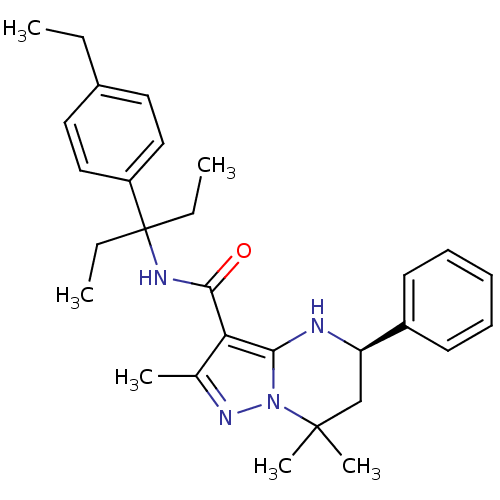 ((5R)-N-[1-Ethyl-1-(4-ethylphenyl)propyl]-2,7,7-tri...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Antagonist activity at CaSR expressed in CHO cells assessed as inhibition of [35S]GTPgammaS binding after 10 mins by scintillation counting | Bioorg Med Chem 19: 1881-94 (2011) Article DOI: 10.1016/j.bmc.2011.02.001 BindingDB Entry DOI: 10.7270/Q2MW2HFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase (Homo sapiens (Human)) | BDBM50090510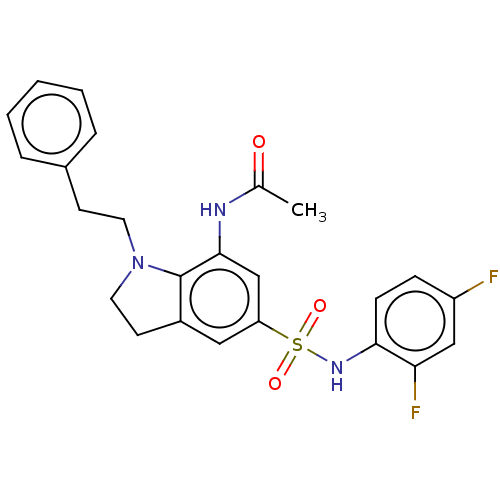 (CHEMBL3581716) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of full length human MGAT2 transfected in FreeStyle293 cells assessed as dioleoylglycerol by RapidFire/MS assay | J Med Chem 58: 3892-909 (2015) Article DOI: 10.1021/acs.jmedchem.5b00178 BindingDB Entry DOI: 10.7270/Q2XK8H94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase (Homo sapiens (Human)) | BDBM50090666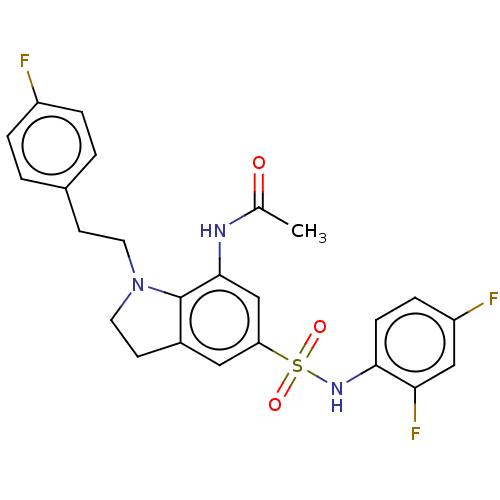 (CHEMBL3581717) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of full length human MGAT2 transfected in FreeStyle293 cells assessed as dioleoylglycerol by RapidFire/MS assay | J Med Chem 58: 3892-909 (2015) Article DOI: 10.1021/acs.jmedchem.5b00178 BindingDB Entry DOI: 10.7270/Q2XK8H94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Homo sapiens (Human)) | BDBM50339727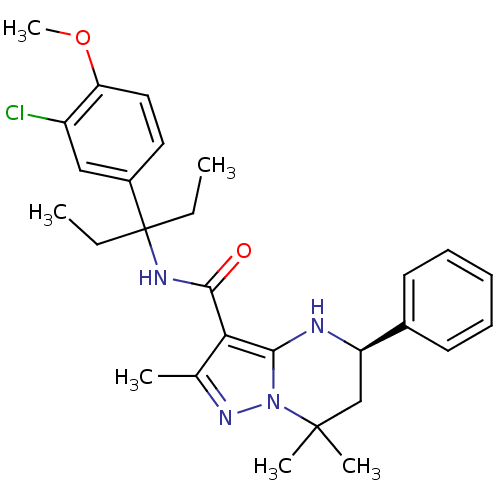 ((5R)-N-[1-(3-Chloro-4-methoxyphenyl)-1-ethylpropyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Antagonist activity at CaSR expressed in CHO cells assessed as inhibition of [35S]GTPgammaS binding after 10 mins by scintillation counting | Bioorg Med Chem 19: 1881-94 (2011) Article DOI: 10.1016/j.bmc.2011.02.001 BindingDB Entry DOI: 10.7270/Q2MW2HFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Homo sapiens (Human)) | BDBM50339726 ((5R)-N-[1-Ethyl-1-(4-isopropylphenyl)propyl]-2,7,7...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Antagonist activity at CaSR expressed in CHO cells assessed as inhibition of [35S]GTPgammaS binding after 10 mins by scintillation counting | Bioorg Med Chem 19: 1881-94 (2011) Article DOI: 10.1016/j.bmc.2011.02.001 BindingDB Entry DOI: 10.7270/Q2MW2HFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Homo sapiens (Human)) | BDBM50339728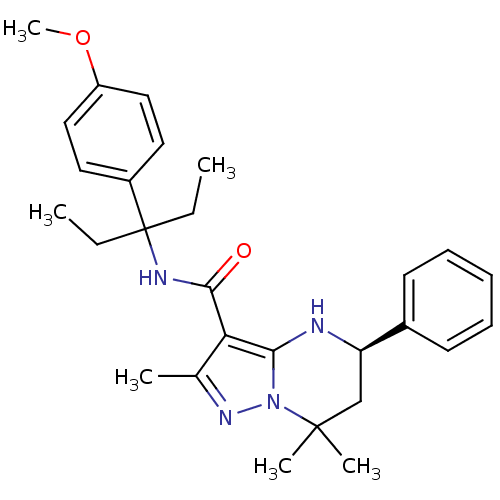 ((5R)-N-[1-Ethyl-1-(4-methoxyphenyl)propyl]-2,7,7-t...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Antagonist activity at CaSR expressed in CHO cells assessed as inhibition of [35S]GTPgammaS binding after 10 mins by scintillation counting | Bioorg Med Chem 19: 1881-94 (2011) Article DOI: 10.1016/j.bmc.2011.02.001 BindingDB Entry DOI: 10.7270/Q2MW2HFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM50268236 (CHEMBL4101718) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Division, Takeda Pharmaceutical Co., Ltd., 26-1, Muraoka-Higashi 2-Chome, Fujisawa, Kanagawa 251-8555, Japan. Electronic address: takeshi.yamasaki@takeda.com. Curated by ChEMBL | Assay Description Antagonist activity at human SSTR5 expressed in CHO cells assessed as inhibition of SST14-induced forskolin-stimulated intracellular cAMP level incub... | Bioorg Med Chem 25: 4153-4162 (2017) Article DOI: 10.1016/j.bmc.2017.06.003 BindingDB Entry DOI: 10.7270/Q28G8P5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase (Homo sapiens (Human)) | BDBM50090665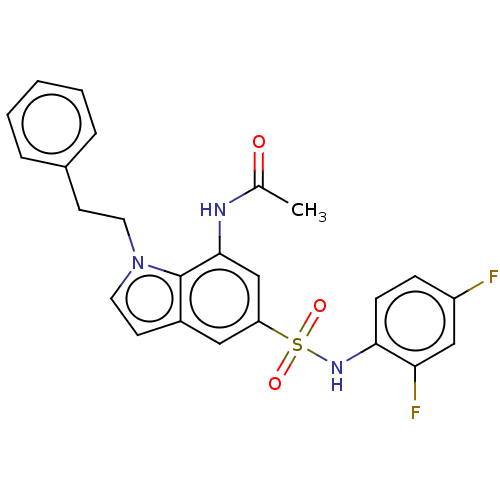 (CHEMBL3581718) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of full length human MGAT2 transfected in FreeStyle293 cells assessed as dioleoylglycerol by RapidFire/MS assay | J Med Chem 58: 3892-909 (2015) Article DOI: 10.1021/acs.jmedchem.5b00178 BindingDB Entry DOI: 10.7270/Q2XK8H94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Homo sapiens (Human)) | BDBM50339729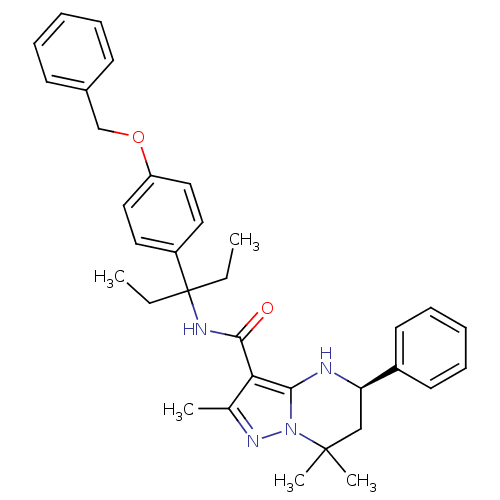 ((5R)-N-{1-[4-(Benzyloxy)phenyl]-1-ethylpropyl}-2,7...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Antagonist activity at CaSR expressed in CHO cells assessed as inhibition of [35S]GTPgammaS binding after 10 mins by scintillation counting | Bioorg Med Chem 19: 1881-94 (2011) Article DOI: 10.1016/j.bmc.2011.02.001 BindingDB Entry DOI: 10.7270/Q2MW2HFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase (Homo sapiens (Human)) | BDBM50090687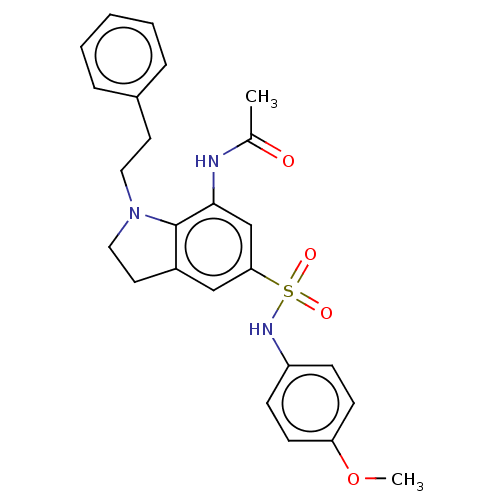 (CHEMBL3581715) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of full length human MGAT2 transfected in FreeStyle293 cells assessed as dioleoylglycerol by RapidFire/MS assay | J Med Chem 58: 3892-909 (2015) Article DOI: 10.1021/acs.jmedchem.5b00178 BindingDB Entry DOI: 10.7270/Q2XK8H94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase (Homo sapiens (Human)) | BDBM50090648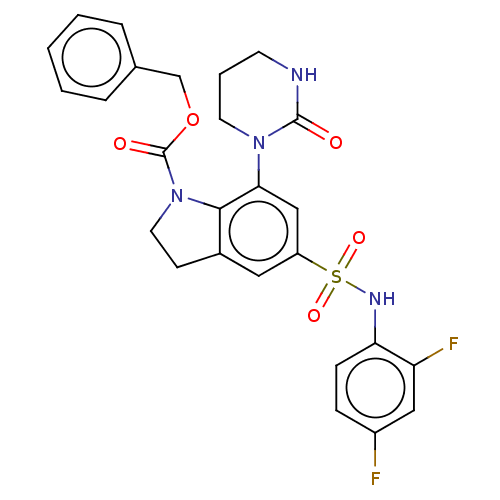 (CHEMBL3581732) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of full length human MGAT2 transfected in FreeStyle293 cells assessed as dioleoylglycerol by RapidFire/MS assay | J Med Chem 58: 3892-909 (2015) Article DOI: 10.1021/acs.jmedchem.5b00178 BindingDB Entry DOI: 10.7270/Q2XK8H94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Homo sapiens (Human)) | BDBM50339341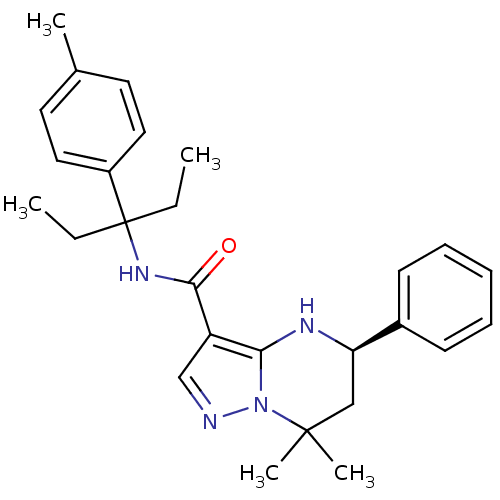 ((5R)-(-)-N-[1-Ethyl-1-(4-methylphenyl)propyl]-7,7-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Antagonist activity at CaSR expressed in CHO cells assessed as inhibition of [35S]GTPgammaS binding after 10 mins by scintillation counting | Bioorg Med Chem 19: 1881-94 (2011) Article DOI: 10.1016/j.bmc.2011.02.001 BindingDB Entry DOI: 10.7270/Q2MW2HFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Homo sapiens (Human)) | BDBM50339730 ((5R)-N-(1-Ethyl-1-phenylpropyl)-2,7,7-trimethyl-5-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Antagonist activity at CaSR expressed in CHO cells assessed as inhibition of [35S]GTPgammaS binding after 10 mins by scintillation counting | Bioorg Med Chem 19: 1881-94 (2011) Article DOI: 10.1016/j.bmc.2011.02.001 BindingDB Entry DOI: 10.7270/Q2MW2HFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Homo sapiens (Human)) | BDBM50339731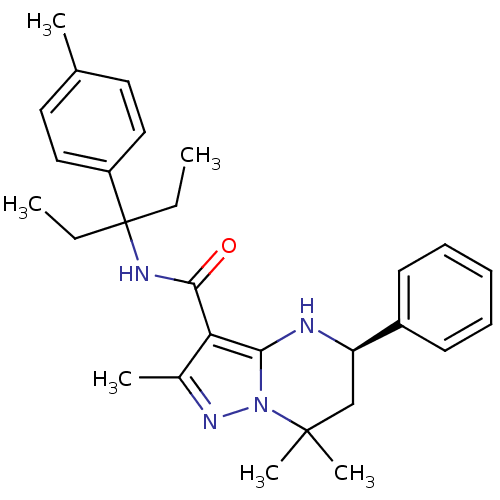 ((5R)-N-[1-Ethyl-1-(4-methylphenyl)propyl]-2,7,7-tr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Antagonist activity at CaSR expressed in CHO cells assessed as inhibition of [35S]GTPgammaS binding after 10 mins by scintillation counting | Bioorg Med Chem 19: 1881-94 (2011) Article DOI: 10.1016/j.bmc.2011.02.001 BindingDB Entry DOI: 10.7270/Q2MW2HFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase (Homo sapiens (Human)) | BDBM50090514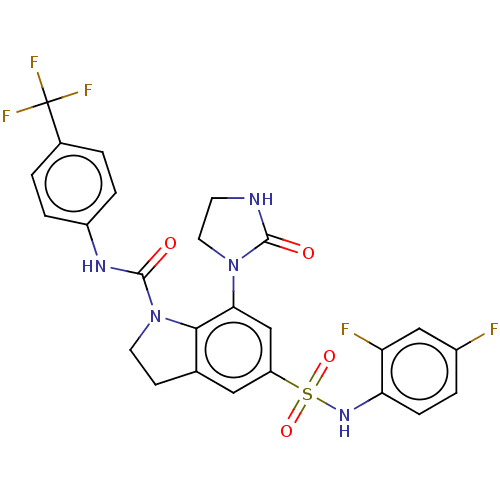 (CHEMBL3581736) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of full length human MGAT2 transfected in FreeStyle293 cells assessed as dioleoylglycerol by RapidFire/MS assay | J Med Chem 58: 3892-909 (2015) Article DOI: 10.1021/acs.jmedchem.5b00178 BindingDB Entry DOI: 10.7270/Q2XK8H94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50364210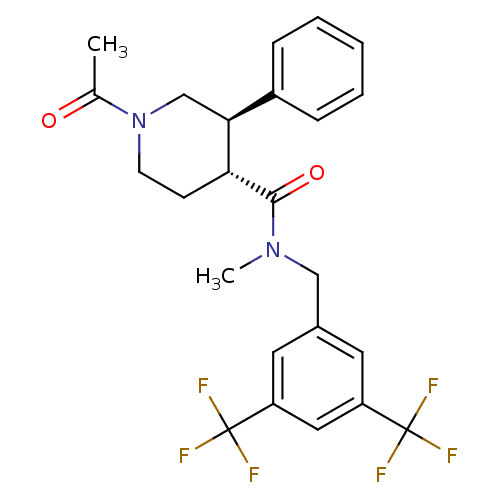 (CHEMBL1951618) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]BH-SP from tachykinin NK1 receptor in human IM9 cells after 30 mins by scintillation counting | Bioorg Med Chem 20: 962-77 (2012) Article DOI: 10.1016/j.bmc.2011.11.048 BindingDB Entry DOI: 10.7270/Q2NP24WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50364212 (CHEMBL1951616) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]BH-SP from tachykinin NK1 receptor in human IM9 cells after 30 mins by scintillation counting | Bioorg Med Chem 20: 962-77 (2012) Article DOI: 10.1016/j.bmc.2011.11.048 BindingDB Entry DOI: 10.7270/Q2NP24WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM50268214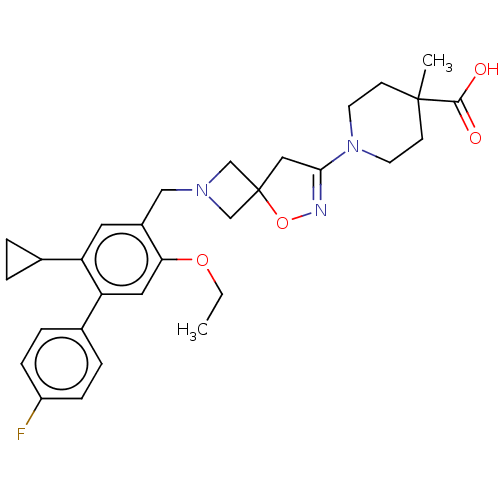 (CHEMBL4070852) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Division, Takeda Pharmaceutical Co., Ltd., 26-1, Muraoka-Higashi 2-Chome, Fujisawa, Kanagawa 251-8555, Japan. Electronic address: takeshi.yamasaki@takeda.com. Curated by ChEMBL | Assay Description Antagonist activity at human SSTR5 expressed in CHO cells assessed as inhibition of SST14-induced forskolin-stimulated intracellular cAMP level incub... | Bioorg Med Chem 25: 4153-4162 (2017) Article DOI: 10.1016/j.bmc.2017.06.003 BindingDB Entry DOI: 10.7270/Q28G8P5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50364206 (CHEMBL1951622) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]BH-SP from tachykinin NK1 receptor in human IM9 cells after 30 mins by scintillation counting | Bioorg Med Chem 20: 962-77 (2012) Article DOI: 10.1016/j.bmc.2011.11.048 BindingDB Entry DOI: 10.7270/Q2NP24WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Homo sapiens (Human)) | BDBM50339732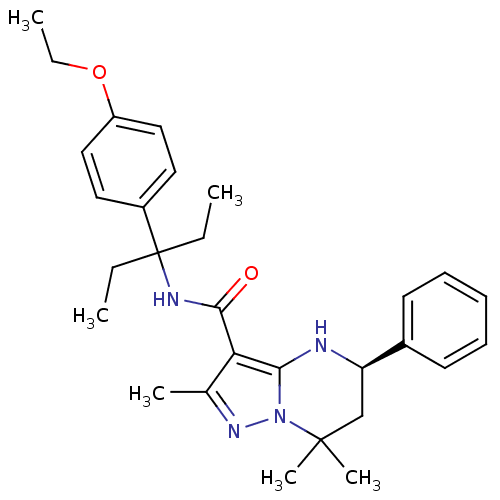 ((5R)-N-[1-(4-Ethoxyphenyl)-1-ethylpropyl]-2,7,7-tr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Antagonist activity at CaSR expressed in CHO cells assessed as inhibition of [35S]GTPgammaS binding after 10 mins by scintillation counting | Bioorg Med Chem 19: 1881-94 (2011) Article DOI: 10.1016/j.bmc.2011.02.001 BindingDB Entry DOI: 10.7270/Q2MW2HFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase (Homo sapiens (Human)) | BDBM50090617 (CHEMBL3581734) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of full length human MGAT2 transfected in FreeStyle293 cells assessed as dioleoylglycerol by RapidFire/MS assay | J Med Chem 58: 3892-909 (2015) Article DOI: 10.1021/acs.jmedchem.5b00178 BindingDB Entry DOI: 10.7270/Q2XK8H94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50364209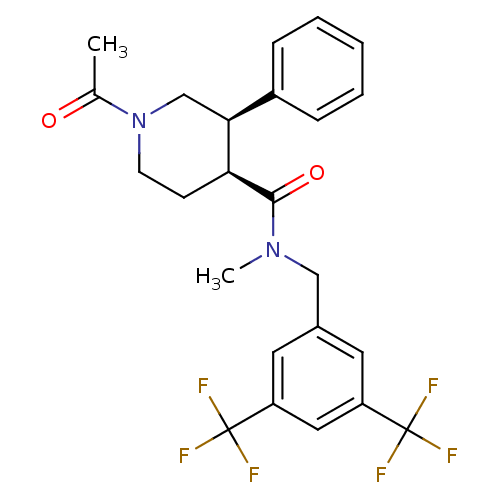 (CHEMBL1951619) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]BH-SP from tachykinin NK1 receptor in human IM9 cells after 30 mins by scintillation counting | Bioorg Med Chem 20: 962-77 (2012) Article DOI: 10.1016/j.bmc.2011.11.048 BindingDB Entry DOI: 10.7270/Q2NP24WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase (Homo sapiens (Human)) | BDBM50090650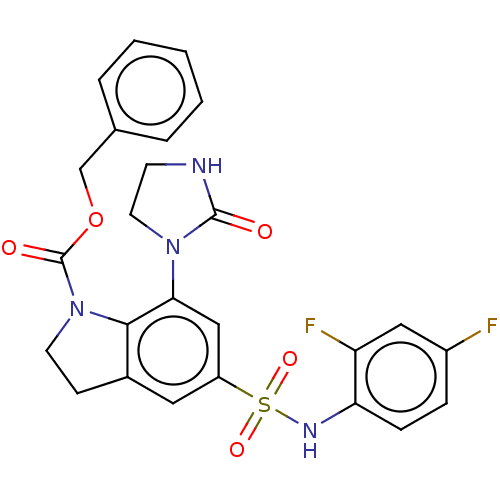 (CHEMBL3581730) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of full length human MGAT2 transfected in FreeStyle293 cells assessed as dioleoylglycerol by RapidFire/MS assay | J Med Chem 58: 3892-909 (2015) Article DOI: 10.1021/acs.jmedchem.5b00178 BindingDB Entry DOI: 10.7270/Q2XK8H94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase (Homo sapiens (Human)) | BDBM50090616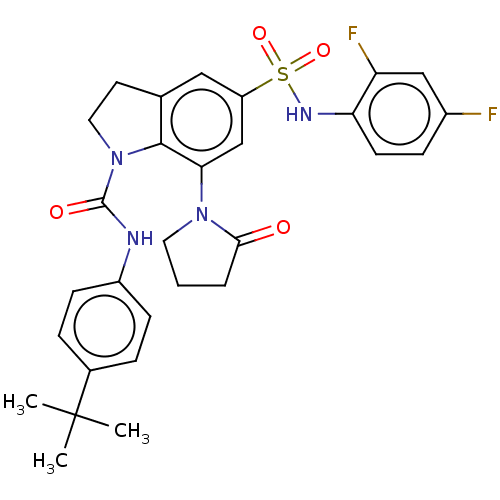 (CHEMBL3581735) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of full length human MGAT2 transfected in FreeStyle293 cells assessed as dioleoylglycerol by RapidFire/MS assay | J Med Chem 58: 3892-909 (2015) Article DOI: 10.1021/acs.jmedchem.5b00178 BindingDB Entry DOI: 10.7270/Q2XK8H94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase (Homo sapiens (Human)) | BDBM50090656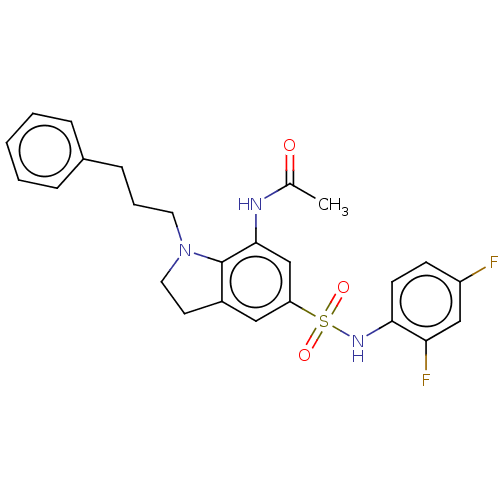 (CHEMBL3581724) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of full length human MGAT2 transfected in FreeStyle293 cells assessed as dioleoylglycerol by RapidFire/MS assay | J Med Chem 58: 3892-909 (2015) Article DOI: 10.1021/acs.jmedchem.5b00178 BindingDB Entry DOI: 10.7270/Q2XK8H94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM50268237 (CHEMBL4060964) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Division, Takeda Pharmaceutical Co., Ltd., 26-1, Muraoka-Higashi 2-Chome, Fujisawa, Kanagawa 251-8555, Japan. Electronic address: takeshi.yamasaki@takeda.com. Curated by ChEMBL | Assay Description Antagonist activity at human SSTR5 expressed in CHO cells assessed as inhibition of SST14-induced forskolin-stimulated intracellular cAMP level incub... | Bioorg Med Chem 25: 4153-4162 (2017) Article DOI: 10.1016/j.bmc.2017.06.003 BindingDB Entry DOI: 10.7270/Q28G8P5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Mus musculus) | BDBM50268236 (CHEMBL4101718) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Division, Takeda Pharmaceutical Co., Ltd., 26-1, Muraoka-Higashi 2-Chome, Fujisawa, Kanagawa 251-8555, Japan. Electronic address: takeshi.yamasaki@takeda.com. Curated by ChEMBL | Assay Description Antagonist activity at mouse SSTR5 expressed in CHO cells assessed as inhibition of SST14-induced forskolin-stimulated intracellular cAMP level incub... | Bioorg Med Chem 25: 4153-4162 (2017) Article DOI: 10.1016/j.bmc.2017.06.003 BindingDB Entry DOI: 10.7270/Q28G8P5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Homo sapiens (Human)) | BDBM50339731 ((5R)-N-[1-Ethyl-1-(4-methylphenyl)propyl]-2,7,7-tr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Antagonist activity at CaSR expressed in CHO cells assessed as inhibition of [35S]GTPgammaS binding after 10 mins by scintillation counting | Bioorg Med Chem 19: 1881-94 (2011) Article DOI: 10.1016/j.bmc.2011.02.001 BindingDB Entry DOI: 10.7270/Q2MW2HFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Homo sapiens (Human)) | BDBM50339725 ((5R)-N-[1-Ethyl-1-(4-ethylphenyl)propyl]-2,7,7-tri...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Antagonist activity at CaSR expressed in CHO cells assessed as inhibition of [35S]GTPgammaS binding after 10 mins by scintillation counting | Bioorg Med Chem 19: 1881-94 (2011) Article DOI: 10.1016/j.bmc.2011.02.001 BindingDB Entry DOI: 10.7270/Q2MW2HFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Mus musculus) | BDBM50268214 (CHEMBL4070852) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Division, Takeda Pharmaceutical Co., Ltd., 26-1, Muraoka-Higashi 2-Chome, Fujisawa, Kanagawa 251-8555, Japan. Electronic address: takeshi.yamasaki@takeda.com. Curated by ChEMBL | Assay Description Antagonist activity at mouse SSTR5 expressed in CHO cells assessed as inhibition of SST14-induced forskolin-stimulated intracellular cAMP level incub... | Bioorg Med Chem 25: 4153-4162 (2017) Article DOI: 10.1016/j.bmc.2017.06.003 BindingDB Entry DOI: 10.7270/Q28G8P5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50460165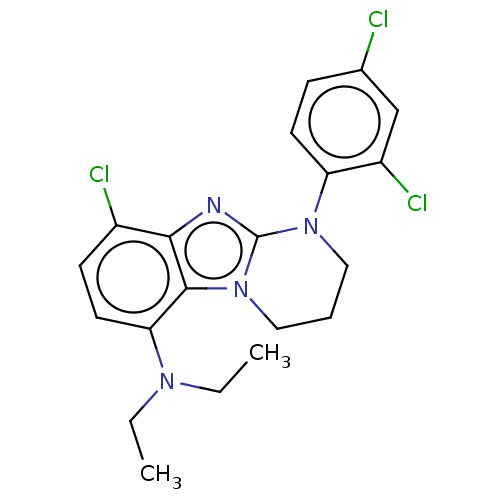 (CHEMBL4225254) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Antagonist activity at human CRF1 receptor expressed in CHO cells assessed as inhibition of human CRF-stimulated cAMP accumulation after 4 hrs by luc... | Bioorg Med Chem 26: 2229-2250 (2018) Article DOI: 10.1016/j.bmc.2018.01.020 BindingDB Entry DOI: 10.7270/Q2474DG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 3607 total ) | Next | Last >> |