 Found 350 hits with Last Name = 'moritani' and Initial = 'y'
Found 350 hits with Last Name = 'moritani' and Initial = 'y' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor X
(Homo sapiens (Human)) | BDBM50358251
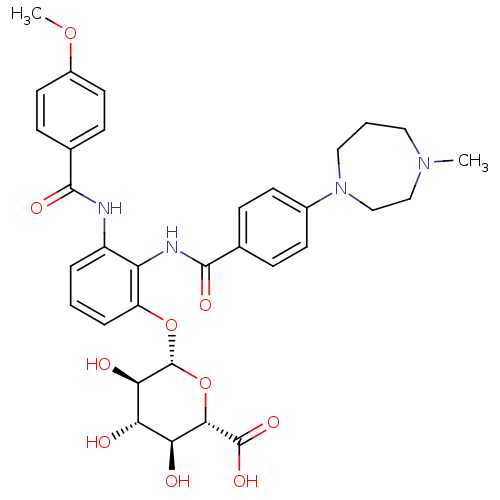
(CHEMBL1922344)Show SMILES COc1ccc(cc1)C(=O)Nc1cccc(O[C@@H]2O[C@@H]([C@@H](O)[C@H](O)[C@H]2O)C(O)=O)c1NC(=O)c1ccc(cc1)N1CCCN(C)CC1 |r| Show InChI InChI=1S/C33H38N4O10/c1-36-15-4-16-37(18-17-36)21-11-7-19(8-12-21)31(42)35-25-23(34-30(41)20-9-13-22(45-2)14-10-20)5-3-6-24(25)46-33-28(40)26(38)27(39)29(47-33)32(43)44/h3,5-14,26-29,33,38-40H,4,15-18H2,1-2H3,(H,34,41)(H,35,42)(H,43,44)/t26-,27-,28+,29-,33+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10A using chromogenic substrate S2222 by dixon plot analysis |
J Med Chem 54: 8051-65 (2011)
Article DOI: 10.1021/jm200868m
BindingDB Entry DOI: 10.7270/Q2D50ND9 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50358252
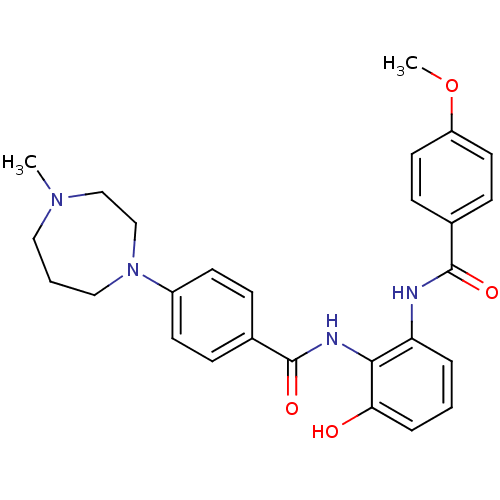
(CHEMBL1922235)Show SMILES COc1ccc(cc1)C(=O)Nc1cccc(O)c1NC(=O)c1ccc(cc1)N1CCCN(C)CC1 Show InChI InChI=1S/C27H30N4O4/c1-30-15-4-16-31(18-17-30)21-11-7-19(8-12-21)27(34)29-25-23(5-3-6-24(25)32)28-26(33)20-9-13-22(35-2)14-10-20/h3,5-14,32H,4,15-18H2,1-2H3,(H,28,33)(H,29,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10A using chromogenic substrate S2222 by dixon plot analysis |
J Med Chem 54: 8051-65 (2011)
Article DOI: 10.1021/jm200868m
BindingDB Entry DOI: 10.7270/Q2D50ND9 |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50358252

(CHEMBL1922235)Show SMILES COc1ccc(cc1)C(=O)Nc1cccc(O)c1NC(=O)c1ccc(cc1)N1CCCN(C)CC1 Show InChI InChI=1S/C27H30N4O4/c1-30-15-4-16-31(18-17-30)21-11-7-19(8-12-21)27(34)29-25-23(5-3-6-24(25)32)28-26(33)20-9-13-22(35-2)14-10-20/h3,5-14,32H,4,15-18H2,1-2H3,(H,28,33)(H,29,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of human plasma kallikrein using chromogenic substrate S2302 by dixon plot analysis |
J Med Chem 54: 8051-65 (2011)
Article DOI: 10.1021/jm200868m
BindingDB Entry DOI: 10.7270/Q2D50ND9 |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50358251

(CHEMBL1922344)Show SMILES COc1ccc(cc1)C(=O)Nc1cccc(O[C@@H]2O[C@@H]([C@@H](O)[C@H](O)[C@H]2O)C(O)=O)c1NC(=O)c1ccc(cc1)N1CCCN(C)CC1 |r| Show InChI InChI=1S/C33H38N4O10/c1-36-15-4-16-37(18-17-36)21-11-7-19(8-12-21)31(42)35-25-23(34-30(41)20-9-13-22(45-2)14-10-20)5-3-6-24(25)46-33-28(40)26(38)27(39)29(47-33)32(43)44/h3,5-14,26-29,33,38-40H,4,15-18H2,1-2H3,(H,34,41)(H,35,42)(H,43,44)/t26-,27-,28+,29-,33+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of human plasma kallikrein using chromogenic substrate S2302 by dixon plot analysis |
J Med Chem 54: 8051-65 (2011)
Article DOI: 10.1021/jm200868m
BindingDB Entry DOI: 10.7270/Q2D50ND9 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50358252

(CHEMBL1922235)Show SMILES COc1ccc(cc1)C(=O)Nc1cccc(O)c1NC(=O)c1ccc(cc1)N1CCCN(C)CC1 Show InChI InChI=1S/C27H30N4O4/c1-30-15-4-16-31(18-17-30)21-11-7-19(8-12-21)27(34)29-25-23(5-3-6-24(25)32)28-26(33)20-9-13-22(35-2)14-10-20/h3,5-14,32H,4,15-18H2,1-2H3,(H,28,33)(H,29,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin using chromogenic substrate S2238 by dixon plot analysis |
J Med Chem 54: 8051-65 (2011)
Article DOI: 10.1021/jm200868m
BindingDB Entry DOI: 10.7270/Q2D50ND9 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50358251

(CHEMBL1922344)Show SMILES COc1ccc(cc1)C(=O)Nc1cccc(O[C@@H]2O[C@@H]([C@@H](O)[C@H](O)[C@H]2O)C(O)=O)c1NC(=O)c1ccc(cc1)N1CCCN(C)CC1 |r| Show InChI InChI=1S/C33H38N4O10/c1-36-15-4-16-37(18-17-36)21-11-7-19(8-12-21)31(42)35-25-23(34-30(41)20-9-13-22(45-2)14-10-20)5-3-6-24(25)46-33-28(40)26(38)27(39)29(47-33)32(43)44/h3,5-14,26-29,33,38-40H,4,15-18H2,1-2H3,(H,34,41)(H,35,42)(H,43,44)/t26-,27-,28+,29-,33+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin using chromogenic substrate S2238 by dixon plot analysis |
J Med Chem 54: 8051-65 (2011)
Article DOI: 10.1021/jm200868m
BindingDB Entry DOI: 10.7270/Q2D50ND9 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Canis lupus familiaris) | BDBM50130021
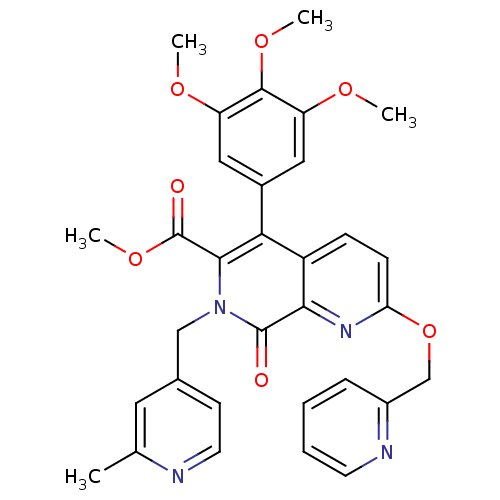
(7-(2-Methyl-pyridin-4-ylmethyl)-8-oxo-2-(pyridin-2...)Show SMILES COC(=O)c1c(-c2cc(OC)c(OC)c(OC)c2)c2ccc(OCc3ccccn3)nc2c(=O)n1Cc1ccnc(C)c1 Show InChI InChI=1S/C32H30N4O7/c1-19-14-20(11-13-33-19)17-36-29(32(38)42-5)27(21-15-24(39-2)30(41-4)25(16-21)40-3)23-9-10-26(35-28(23)31(36)37)43-18-22-8-6-7-12-34-22/h6-16H,17-18H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against phosphodiesterase 5 (PDE5) isolated from canine lung |
Bioorg Med Chem Lett 13: 2341-5 (2003)
BindingDB Entry DOI: 10.7270/Q2WQ04B2 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Canis lupus familiaris) | BDBM50100673

(CHEMBL538498 | methyl 2-(4-aminophenyl)-4-(4-bromo...)Show SMILES COC(=O)c1c(-c2cc(OC)c(Br)c(OC)c2)c2ccc(OCc3ccccn3)cc2c(=O)n1-c1ccc(N)cc1 Show InChI InChI=1S/C31H26BrN3O6/c1-38-25-14-18(15-26(39-2)28(25)32)27-23-12-11-22(41-17-20-6-4-5-13-34-20)16-24(23)30(36)35(29(27)31(37)40-3)21-9-7-19(33)8-10-21/h4-16H,17,33H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against canine lung Phosphodiesterase 5 (PDE5) |
J Med Chem 44: 2204-18 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4XT5 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Canis lupus familiaris) | BDBM50130017

(2-(2-Methyl-pyridin-4-ylmethyl)-1-oxo-8-(pyrimidin...)Show SMILES COC(=O)c1c(-c2cc(OC)c(OC)c(OC)c2)c2ccnc(OCc3ncccn3)c2c(=O)n1Cc1ccnc(C)c1 Show InChI InChI=1S/C31H29N5O7/c1-18-13-19(7-11-32-18)16-36-27(31(38)42-5)25(20-14-22(39-2)28(41-4)23(15-20)40-3)21-8-12-35-29(26(21)30(36)37)43-17-24-33-9-6-10-34-24/h6-15H,16-17H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against phosphodiesterase 5 (PDE5) isolated from canine lung |
Bioorg Med Chem Lett 13: 2341-5 (2003)
BindingDB Entry DOI: 10.7270/Q2WQ04B2 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Canis lupus familiaris) | BDBM50130025
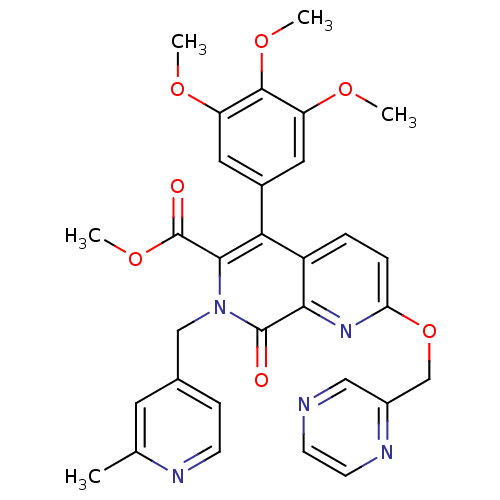
(7-(2-Methyl-pyridin-4-ylmethyl)-8-oxo-2-(pyrazin-2...)Show SMILES COC(=O)c1c(-c2cc(OC)c(OC)c(OC)c2)c2ccc(OCc3cnccn3)nc2c(=O)n1Cc1ccnc(C)c1 Show InChI InChI=1S/C31H29N5O7/c1-18-12-19(8-9-33-18)16-36-28(31(38)42-5)26(20-13-23(39-2)29(41-4)24(14-20)40-3)22-6-7-25(35-27(22)30(36)37)43-17-21-15-32-10-11-34-21/h6-15H,16-17H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against phosphodiesterase 5 (PDE5) isolated from canine lung |
Bioorg Med Chem Lett 13: 2341-5 (2003)
BindingDB Entry DOI: 10.7270/Q2WQ04B2 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Canis lupus familiaris) | BDBM50130020
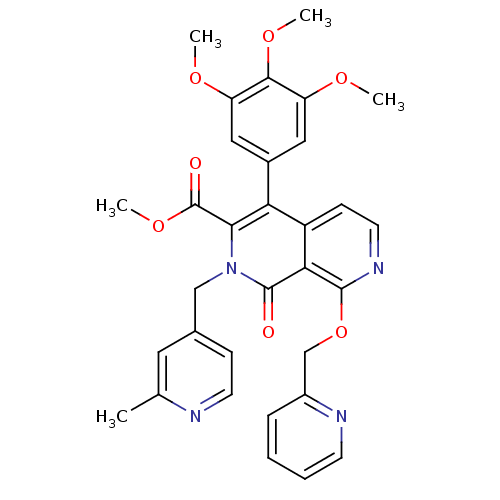
(2-(2-Methyl-pyridin-4-ylmethyl)-1-oxo-8-(pyridin-2...)Show SMILES COC(=O)c1c(-c2cc(OC)c(OC)c(OC)c2)c2ccnc(OCc3ccccn3)c2c(=O)n1Cc1ccnc(C)c1 Show InChI InChI=1S/C32H30N4O7/c1-19-14-20(9-12-33-19)17-36-28(32(38)42-5)26(21-15-24(39-2)29(41-4)25(16-21)40-3)23-10-13-35-30(27(23)31(36)37)43-18-22-8-6-7-11-34-22/h6-16H,17-18H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against phosphodiesterase 5 (PDE5) isolated from canine lung |
Bioorg Med Chem Lett 13: 2341-5 (2003)
BindingDB Entry DOI: 10.7270/Q2WQ04B2 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Canis lupus familiaris) | BDBM50130027
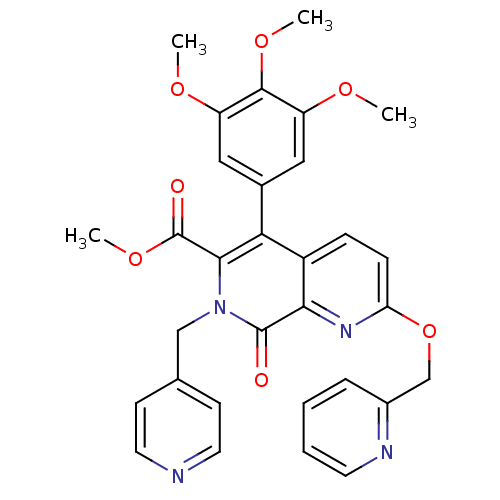
(8-Oxo-2-(pyridin-2-ylmethoxy)-7-pyridin-4-ylmethyl...)Show SMILES COC(=O)c1c(-c2cc(OC)c(OC)c(OC)c2)c2ccc(OCc3ccccn3)nc2c(=O)n1Cc1ccncc1 Show InChI InChI=1S/C31H28N4O7/c1-38-23-15-20(16-24(39-2)29(23)40-3)26-22-8-9-25(42-18-21-7-5-6-12-33-21)34-27(22)30(36)35(28(26)31(37)41-4)17-19-10-13-32-14-11-19/h5-16H,17-18H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against phosphodiesterase 5 (PDE5) isolated from canine lung |
Bioorg Med Chem Lett 13: 2341-5 (2003)
BindingDB Entry DOI: 10.7270/Q2WQ04B2 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Canis lupus familiaris) | BDBM50100674
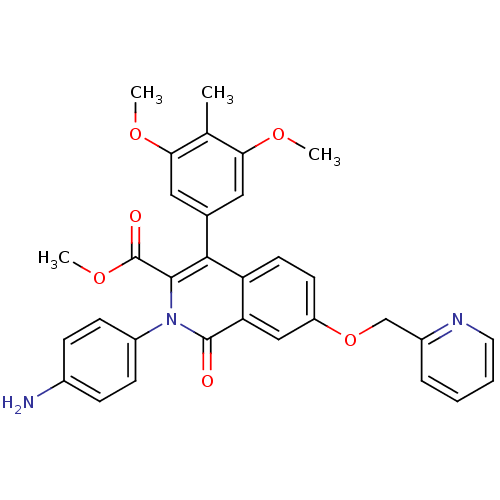
(CHEMBL541818 | methyl 2-(4-aminophenyl)-4-(3,5-dim...)Show SMILES COC(=O)c1c(-c2cc(OC)c(C)c(OC)c2)c2ccc(OCc3ccccn3)cc2c(=O)n1-c1ccc(N)cc1 Show InChI InChI=1S/C32H29N3O6/c1-19-27(38-2)15-20(16-28(19)39-3)29-25-13-12-24(41-18-22-7-5-6-14-34-22)17-26(25)31(36)35(30(29)32(37)40-4)23-10-8-21(33)9-11-23/h5-17H,18,33H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against canine lung Phosphodiesterase 5 (PDE5) |
J Med Chem 44: 2204-18 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4XT5 |
More data for this
Ligand-Target Pair | |
Extracellular calcium-sensing receptor
(Rattus norvegicus) | BDBM50272596
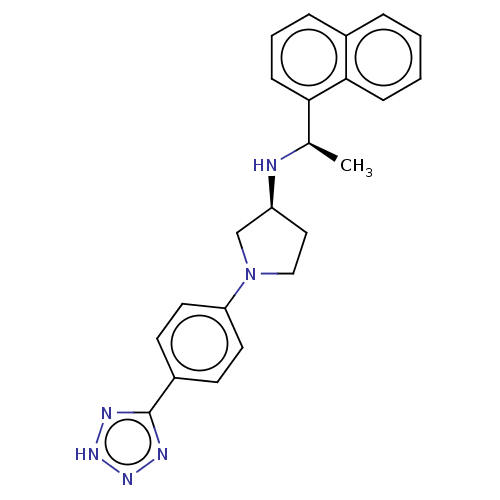
(CHEMBL4126450)Show SMILES Cl.C[C@@H](N[C@H]1CCN(C1)c1ccc(cc1)-c1nn[nH]n1)c1cccc2ccccc12 |r| Show InChI InChI=1S/C23H24N6.ClH/c1-16(21-8-4-6-17-5-2-3-7-22(17)21)24-19-13-14-29(15-19)20-11-9-18(10-12-20)23-25-27-28-26-23;/h2-12,16,19,24H,13-15H2,1H3,(H,25,26,27,28);1H/t16-,19+;/m1./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at CaSR in rat parathyroid cells assessed as inhibition of PTH (1 to 84 residues) production in presence of Cacl2 by ELISA |
Bioorg Med Chem Lett 28: 2055-2060 (2018)
Article DOI: 10.1016/j.bmcl.2018.04.055
BindingDB Entry DOI: 10.7270/Q2DF6TP0 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Canis lupus familiaris) | BDBM50130028
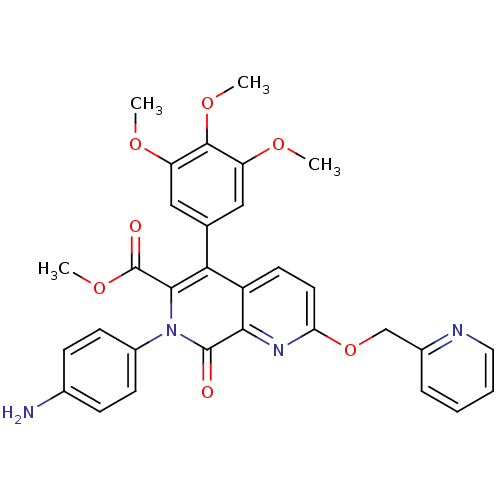
(7-(4-Amino-phenyl)-8-oxo-2-(pyridin-2-ylmethoxy)-5...)Show SMILES COC(=O)c1c(-c2cc(OC)c(OC)c(OC)c2)c2ccc(OCc3ccccn3)nc2c(=O)n1-c1ccc(N)cc1 Show InChI InChI=1S/C31H28N4O7/c1-38-23-15-18(16-24(39-2)29(23)40-3)26-22-12-13-25(42-17-20-7-5-6-14-33-20)34-27(22)30(36)35(28(26)31(37)41-4)21-10-8-19(32)9-11-21/h5-16H,17,32H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against phosphodiesterase 5 (PDE5) isolated from canine lung |
Bioorg Med Chem Lett 13: 2341-5 (2003)
BindingDB Entry DOI: 10.7270/Q2WQ04B2 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Canis lupus familiaris) | BDBM50130024

(2-(2-Methyl-pyridin-4-ylmethyl)-1-oxo-8-(2-pyridin...)Show SMILES COC(=O)c1c(-c2cc(OC)c(OC)c(OC)c2)c2ccnc(OCCc3ccccn3)c2c(=O)n1Cc1ccnc(C)c1 Show InChI InChI=1S/C33H32N4O7/c1-20-16-21(9-13-34-20)19-37-29(33(39)43-5)27(22-17-25(40-2)30(42-4)26(18-22)41-3)24-10-14-36-31(28(24)32(37)38)44-15-11-23-8-6-7-12-35-23/h6-10,12-14,16-18H,11,15,19H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against phosphodiesterase 5 (PDE5) isolated from canine lung |
Bioorg Med Chem Lett 13: 2341-5 (2003)
BindingDB Entry DOI: 10.7270/Q2WQ04B2 |
More data for this
Ligand-Target Pair | |
Extracellular calcium-sensing receptor
(Rattus norvegicus) | BDBM50272602
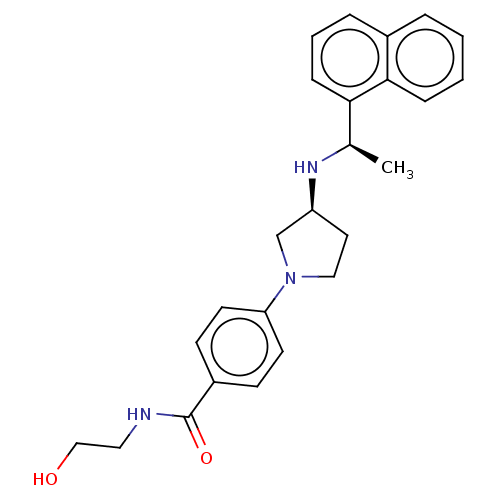
(CHEMBL4126877)Show SMILES Cl.Cl.C[C@@H](N[C@H]1CCN(C1)c1ccc(cc1)C(=O)NCCO)c1cccc2ccccc12 |r| Show InChI InChI=1S/C25H29N3O2.2ClH/c1-18(23-8-4-6-19-5-2-3-7-24(19)23)27-21-13-15-28(17-21)22-11-9-20(10-12-22)25(30)26-14-16-29;;/h2-12,18,21,27,29H,13-17H2,1H3,(H,26,30);2*1H/t18-,21+;;/m1../s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at CaSR in rat parathyroid cells assessed as inhibition of PTH (1 to 84 residues) production in presence of Cacl2 by ELISA |
Bioorg Med Chem Lett 28: 2055-2060 (2018)
Article DOI: 10.1016/j.bmcl.2018.04.055
BindingDB Entry DOI: 10.7270/Q2DF6TP0 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Canis lupus familiaris) | BDBM14390

(5-[2-ethoxy-5-(4-methyl-1-piperazinylsulfonyl)phen...)Show SMILES CCCc1nn(C)c2c1nc([nH]c2=O)-c1cc(ccc1OCC)S(=O)(=O)N1CCN(C)CC1 Show InChI InChI=1S/C22H30N6O4S/c1-5-7-17-19-20(27(4)25-17)22(29)24-21(23-19)16-14-15(8-9-18(16)32-6-2)33(30,31)28-12-10-26(3)11-13-28/h8-9,14H,5-7,10-13H2,1-4H3,(H,23,24,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against phosphodiesterase 5 (PDE5) isolated from canine lung |
Bioorg Med Chem Lett 13: 2341-5 (2003)
BindingDB Entry DOI: 10.7270/Q2WQ04B2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Canis lupus familiaris) | BDBM50100662

(CHEMBL553371 | CHEMBL77971 | methyl 2-(4-aminophen...)Show SMILES COC(=O)c1c(-c2cc(OC)c(OC)c(OC)c2)c2ccc(OCc3ccccn3)cc2c(=O)n1-c1ccc(N)cc1 Show InChI InChI=1S/C32H29N3O7/c1-38-26-15-19(16-27(39-2)30(26)40-3)28-24-13-12-23(42-18-21-7-5-6-14-34-21)17-25(24)31(36)35(29(28)32(37)41-4)22-10-8-20(33)9-11-22/h5-17H,18,33H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against canine lung Phosphodiesterase 5 (PDE5) |
J Med Chem 44: 2204-18 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4XT5 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Canis lupus familiaris) | BDBM50100662

(CHEMBL553371 | CHEMBL77971 | methyl 2-(4-aminophen...)Show SMILES COC(=O)c1c(-c2cc(OC)c(OC)c(OC)c2)c2ccc(OCc3ccccn3)cc2c(=O)n1-c1ccc(N)cc1 Show InChI InChI=1S/C32H29N3O7/c1-38-26-15-19(16-27(39-2)30(26)40-3)28-24-13-12-23(42-18-21-7-5-6-14-34-21)17-25(24)31(36)35(29(28)32(37)41-4)22-10-8-20(33)9-11-22/h5-17H,18,33H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against phosphodiesterase 5 (PDE5) isolated from canine lung |
Bioorg Med Chem Lett 13: 2341-5 (2003)
BindingDB Entry DOI: 10.7270/Q2WQ04B2 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Canis lupus familiaris) | BDBM50100659
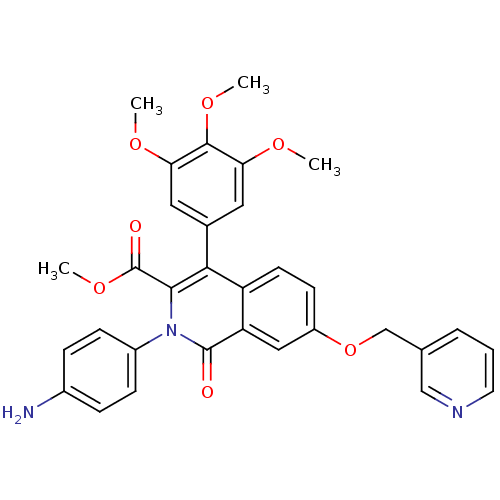
(CHEMBL544289 | methyl 2-(4-aminophenyl)-1-oxo-7-(3...)Show SMILES COC(=O)c1c(-c2cc(OC)c(OC)c(OC)c2)c2ccc(OCc3cccnc3)cc2c(=O)n1-c1ccc(N)cc1 Show InChI InChI=1S/C32H29N3O7/c1-38-26-14-20(15-27(39-2)30(26)40-3)28-24-12-11-23(42-18-19-6-5-13-34-17-19)16-25(24)31(36)35(29(28)32(37)41-4)22-9-7-21(33)8-10-22/h5-17H,18,33H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against canine lung Phosphodiesterase 5 (PDE5) |
J Med Chem 44: 2204-18 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4XT5 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Canis lupus familiaris) | BDBM50130029
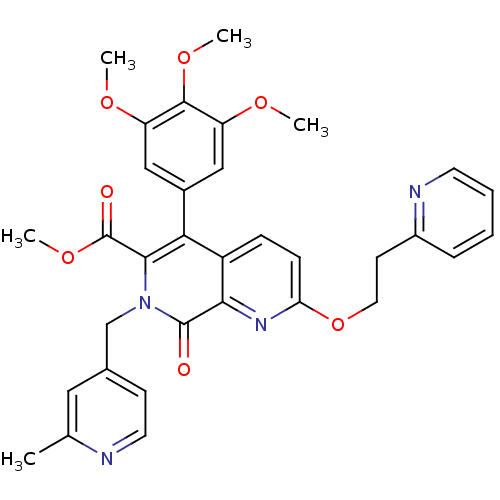
(7-(2-Methyl-pyridin-4-ylmethyl)-8-oxo-2-(2-pyridin...)Show SMILES COC(=O)c1c(-c2cc(OC)c(OC)c(OC)c2)c2ccc(OCCc3ccccn3)nc2c(=O)n1Cc1ccnc(C)c1 Show InChI InChI=1S/C33H32N4O7/c1-20-16-21(11-14-34-20)19-37-30(33(39)43-5)28(22-17-25(40-2)31(42-4)26(18-22)41-3)24-9-10-27(36-29(24)32(37)38)44-15-12-23-8-6-7-13-35-23/h6-11,13-14,16-18H,12,15,19H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against phosphodiesterase 5 (PDE5) isolated from canine lung |
Bioorg Med Chem Lett 13: 2341-5 (2003)
BindingDB Entry DOI: 10.7270/Q2WQ04B2 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Canis lupus familiaris) | BDBM50100656

(2-(4-Amino-phenyl)-7-benzyloxy-1-oxo-4-(3,4,5-trim...)Show SMILES COC(=O)c1c(-c2cc(OC)c(OC)c(OC)c2)c2ccc(OCc3ccccc3)cc2c(=O)n1-c1ccc(N)cc1 Show InChI InChI=1S/C33H30N2O7/c1-38-27-16-21(17-28(39-2)31(27)40-3)29-25-15-14-24(42-19-20-8-6-5-7-9-20)18-26(25)32(36)35(30(29)33(37)41-4)23-12-10-22(34)11-13-23/h5-18H,19,34H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against canine lung Phosphodiesterase 5 (PDE5) |
J Med Chem 44: 2204-18 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4XT5 |
More data for this
Ligand-Target Pair | |
Extracellular calcium-sensing receptor
(Rattus norvegicus) | BDBM50272605

(CHEMBL4128542)Show SMILES Cl.Cl.C[C@@H](N[C@@H]1CCCN(C1)c1cccc(OC(F)(F)F)c1)c1cccc2ccccc12 |r| Show InChI InChI=1S/C24H25F3N2O.2ClH/c1-17(22-13-4-8-18-7-2-3-12-23(18)22)28-19-9-6-14-29(16-19)20-10-5-11-21(15-20)30-24(25,26)27;;/h2-5,7-8,10-13,15,17,19,28H,6,9,14,16H2,1H3;2*1H/t17-,19-;;/m1../s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at CaSR in rat parathyroid cells assessed as inhibition of PTH (1 to 84 residues) production in presence of Cacl2 by ELISA |
Bioorg Med Chem Lett 28: 2055-2060 (2018)
Article DOI: 10.1016/j.bmcl.2018.04.055
BindingDB Entry DOI: 10.7270/Q2DF6TP0 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Canis lupus familiaris) | BDBM50366818

(CHEMBL1202833)Show SMILES COC(=O)c1c(-c2cc(OC)c(OC)c(OC)c2)c2ccc(NCc3ccccn3)nc2c(=O)n1Cc1ccnc(C)c1 Show InChI InChI=1S/C32H31N5O6/c1-19-14-20(11-13-33-19)18-37-29(32(39)43-5)27(21-15-24(40-2)30(42-4)25(16-21)41-3)23-9-10-26(36-28(23)31(37)38)35-17-22-8-6-7-12-34-22/h6-16H,17-18H2,1-5H3,(H,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against phosphodiesterase 5 (PDE5) isolated from canine lung |
Bioorg Med Chem Lett 13: 2341-5 (2003)
BindingDB Entry DOI: 10.7270/Q2WQ04B2 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Canis lupus familiaris) | BDBM50130026

(7-(2-Methyl-pyridin-4-ylmethyl)-8-oxo-2-(pyrimidin...)Show SMILES COC(=O)c1c(-c2cc(OC)c(OC)c(OC)c2)c2ccc(OCc3ncccn3)nc2c(=O)n1Cc1ccnc(C)c1 Show InChI InChI=1S/C31H29N5O7/c1-18-13-19(9-12-32-18)16-36-28(31(38)42-5)26(20-14-22(39-2)29(41-4)23(15-20)40-3)21-7-8-25(35-27(21)30(36)37)43-17-24-33-10-6-11-34-24/h6-15H,16-17H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against phosphodiesterase 5 (PDE5) isolated from canine lung |
Bioorg Med Chem Lett 13: 2341-5 (2003)
BindingDB Entry DOI: 10.7270/Q2WQ04B2 |
More data for this
Ligand-Target Pair | |
Extracellular calcium-sensing receptor
(Rattus norvegicus) | BDBM50272606

(CHEMBL4125917)Show SMILES Cl.Cl.C[C@@H](N[C@@H]1CCCN(C1)c1cccc(c1)C(F)(F)F)c1cccc2ccccc12 |r| Show InChI InChI=1S/C24H25F3N2.2ClH/c1-17(22-13-4-8-18-7-2-3-12-23(18)22)28-20-10-6-14-29(16-20)21-11-5-9-19(15-21)24(25,26)27;;/h2-5,7-9,11-13,15,17,20,28H,6,10,14,16H2,1H3;2*1H/t17-,20-;;/m1../s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at CaSR in rat parathyroid cells assessed as inhibition of PTH (1 to 84 residues) production in presence of Cacl2 by ELISA |
Bioorg Med Chem Lett 28: 2055-2060 (2018)
Article DOI: 10.1016/j.bmcl.2018.04.055
BindingDB Entry DOI: 10.7270/Q2DF6TP0 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Canis lupus familiaris) | BDBM50130019
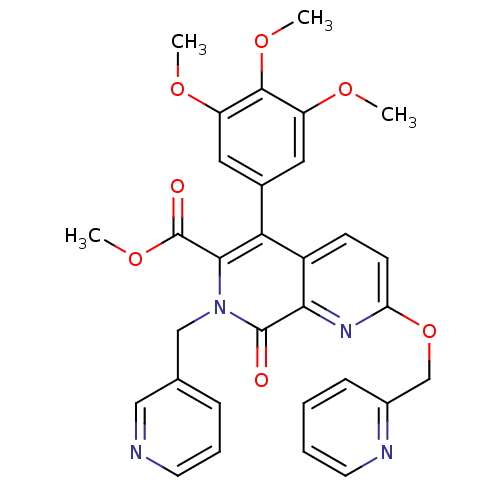
(8-Oxo-2-(pyridin-2-ylmethoxy)-7-pyridin-3-ylmethyl...)Show SMILES COC(=O)c1c(-c2cc(OC)c(OC)c(OC)c2)c2ccc(OCc3ccccn3)nc2c(=O)n1Cc1cccnc1 Show InChI InChI=1S/C31H28N4O7/c1-38-23-14-20(15-24(39-2)29(23)40-3)26-22-10-11-25(42-18-21-9-5-6-13-33-21)34-27(22)30(36)35(28(26)31(37)41-4)17-19-8-7-12-32-16-19/h5-16H,17-18H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against phosphodiesterase 5 (PDE5) isolated from canine lung |
Bioorg Med Chem Lett 13: 2341-5 (2003)
BindingDB Entry DOI: 10.7270/Q2WQ04B2 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Canis lupus familiaris) | BDBM50130016
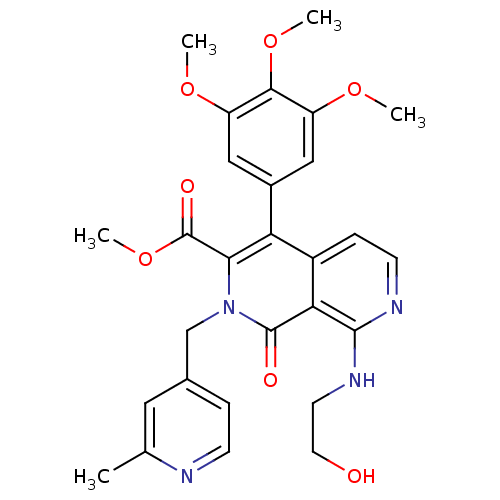
(8-(2-Hydroxy-ethylamino)-2-(2-methyl-pyridin-4-ylm...)Show SMILES COC(=O)c1c(-c2cc(OC)c(OC)c(OC)c2)c2ccnc(NCCO)c2c(=O)n1Cc1ccnc(C)c1 Show InChI InChI=1S/C28H30N4O7/c1-16-12-17(6-8-29-16)15-32-24(28(35)39-5)22(18-13-20(36-2)25(38-4)21(14-18)37-3)19-7-9-30-26(31-10-11-33)23(19)27(32)34/h6-9,12-14,33H,10-11,15H2,1-5H3,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against phosphodiesterase 5 (PDE5) isolated from canine lung |
Bioorg Med Chem Lett 13: 2341-5 (2003)
BindingDB Entry DOI: 10.7270/Q2WQ04B2 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Canis lupus familiaris) | BDBM50100670
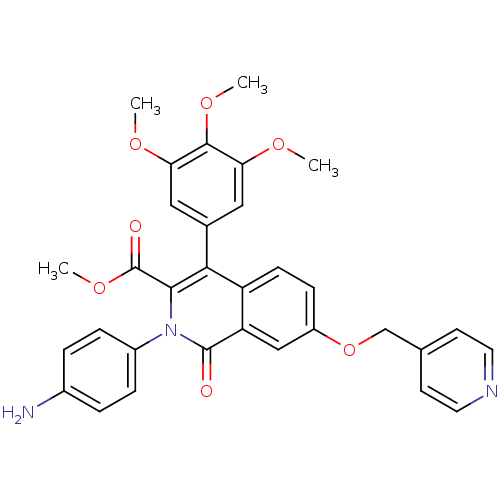
(CHEMBL553786 | methyl 2-(4-aminophenyl)-1-oxo-7-(4...)Show SMILES COC(=O)c1c(-c2cc(OC)c(OC)c(OC)c2)c2ccc(OCc3ccncc3)cc2c(=O)n1-c1ccc(N)cc1 Show InChI InChI=1S/C32H29N3O7/c1-38-26-15-20(16-27(39-2)30(26)40-3)28-24-10-9-23(42-18-19-11-13-34-14-12-19)17-25(24)31(36)35(29(28)32(37)41-4)22-7-5-21(33)6-8-22/h5-17H,18,33H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against canine lung Phosphodiesterase 5 (PDE5) |
J Med Chem 44: 2204-18 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4XT5 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Canis lupus familiaris) | BDBM14390

(5-[2-ethoxy-5-(4-methyl-1-piperazinylsulfonyl)phen...)Show SMILES CCCc1nn(C)c2c1nc([nH]c2=O)-c1cc(ccc1OCC)S(=O)(=O)N1CCN(C)CC1 Show InChI InChI=1S/C22H30N6O4S/c1-5-7-17-19-20(27(4)25-17)22(29)24-21(23-19)16-14-15(8-9-18(16)32-6-2)33(30,31)28-12-10-26(3)11-13-28/h8-9,14H,5-7,10-13H2,1-4H3,(H,23,24,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against canine lung Phosphodiesterase 5 (PDE5) |
J Med Chem 44: 2204-18 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4XT5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Extracellular calcium-sensing receptor
(Rattus norvegicus) | BDBM50272607
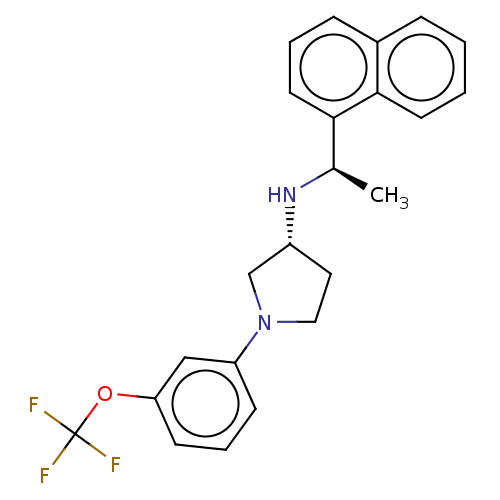
(CHEMBL4126057)Show SMILES Cl.Cl.C[C@@H](N[C@@H]1CCN(C1)c1cccc(OC(F)(F)F)c1)c1cccc2ccccc12 |r| Show InChI InChI=1S/C23H23F3N2O.2ClH/c1-16(21-11-4-7-17-6-2-3-10-22(17)21)27-18-12-13-28(15-18)19-8-5-9-20(14-19)29-23(24,25)26;;/h2-11,14,16,18,27H,12-13,15H2,1H3;2*1H/t16-,18-;;/m1../s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at CaSR in rat parathyroid cells assessed as inhibition of PTH (1 to 84 residues) production in presence of Cacl2 by ELISA |
Bioorg Med Chem Lett 28: 2055-2060 (2018)
Article DOI: 10.1016/j.bmcl.2018.04.055
BindingDB Entry DOI: 10.7270/Q2DF6TP0 |
More data for this
Ligand-Target Pair | |
P04972/P11541/P16586/P22571/P23439/Q95142
(Bos taurus-Bos taurus (Bovine)) | BDBM50130020

(2-(2-Methyl-pyridin-4-ylmethyl)-1-oxo-8-(pyridin-2...)Show SMILES COC(=O)c1c(-c2cc(OC)c(OC)c(OC)c2)c2ccnc(OCc3ccccn3)c2c(=O)n1Cc1ccnc(C)c1 Show InChI InChI=1S/C32H30N4O7/c1-19-14-20(9-12-33-19)17-36-28(32(38)42-5)26(21-15-24(39-2)29(41-4)25(16-21)40-3)23-10-13-35-30(27(23)31(36)37)43-18-22-8-6-7-11-34-22/h6-16H,17-18H2,1-5H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Phosphodiesterase 6 isolated from bovine retina |
Bioorg Med Chem Lett 13: 2341-5 (2003)
BindingDB Entry DOI: 10.7270/Q2WQ04B2 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Canis lupus familiaris) | BDBM50100664

(2-(4-Amino-phenyl)-1-oxo-7-(2-pyridin-2-yl-ethoxy)...)Show SMILES COC(=O)c1c(-c2cc(OC)c(OC)c(OC)c2)c2ccc(OCCc3ccccn3)cc2c(=O)n1-c1ccc(N)cc1 Show InChI InChI=1S/C33H31N3O7/c1-39-27-17-20(18-28(40-2)31(27)41-3)29-25-13-12-24(43-16-14-22-7-5-6-15-35-22)19-26(25)32(37)36(30(29)33(38)42-4)23-10-8-21(34)9-11-23/h5-13,15,17-19H,14,16,34H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against canine lung Phosphodiesterase 5 (PDE5) |
J Med Chem 44: 2204-18 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4XT5 |
More data for this
Ligand-Target Pair | |
P04972/P11541/P16586/P22571/P23439/Q95142
(Bos taurus-Bos taurus (Bovine)) | BDBM50130025

(7-(2-Methyl-pyridin-4-ylmethyl)-8-oxo-2-(pyrazin-2...)Show SMILES COC(=O)c1c(-c2cc(OC)c(OC)c(OC)c2)c2ccc(OCc3cnccn3)nc2c(=O)n1Cc1ccnc(C)c1 Show InChI InChI=1S/C31H29N5O7/c1-18-12-19(8-9-33-18)16-36-28(31(38)42-5)26(20-13-23(39-2)29(41-4)24(14-20)40-3)22-6-7-25(35-27(22)30(36)37)43-17-21-15-32-10-11-34-21/h6-15H,16-17H2,1-5H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Phosphodiesterase 6 isolated from bovine retina |
Bioorg Med Chem Lett 13: 2341-5 (2003)
BindingDB Entry DOI: 10.7270/Q2WQ04B2 |
More data for this
Ligand-Target Pair | |
Extracellular calcium-sensing receptor
(Rattus norvegicus) | BDBM50272604

(CHEMBL4129011)Show SMILES Cl.Cl.C[C@@H](N[C@H]1CCN(C1)c1ccc(OC(F)(F)F)cc1)c1cccc2ccccc12 |r| Show InChI InChI=1S/C23H23F3N2O.2ClH/c1-16(21-8-4-6-17-5-2-3-7-22(17)21)27-18-13-14-28(15-18)19-9-11-20(12-10-19)29-23(24,25)26;;/h2-12,16,18,27H,13-15H2,1H3;2*1H/t16-,18+;;/m1../s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at CaSR in rat parathyroid cells assessed as inhibition of PTH (1 to 84 residues) production in presence of Cacl2 by ELISA |
Bioorg Med Chem Lett 28: 2055-2060 (2018)
Article DOI: 10.1016/j.bmcl.2018.04.055
BindingDB Entry DOI: 10.7270/Q2DF6TP0 |
More data for this
Ligand-Target Pair | |
Extracellular calcium-sensing receptor
(Rattus norvegicus) | BDBM50261663

(CHEMBL4079960)Show SMILES C[C@@H](N[C@H]1CCN(C1)c1ccc(cc1)C(O)=O)c1cccc2ccccc12 |r| Show InChI InChI=1S/C23H24N2O2/c1-16(21-8-4-6-17-5-2-3-7-22(17)21)24-19-13-14-25(15-19)20-11-9-18(10-12-20)23(26)27/h2-12,16,19,24H,13-15H2,1H3,(H,26,27)/t16-,19+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at CaSR in rat parathyroid cells assessed as inhibition of PTH (1 to 84 residues) production in presence of Cacl2 by ELISA |
Bioorg Med Chem Lett 28: 2055-2060 (2018)
Article DOI: 10.1016/j.bmcl.2018.04.055
BindingDB Entry DOI: 10.7270/Q2DF6TP0 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50209900
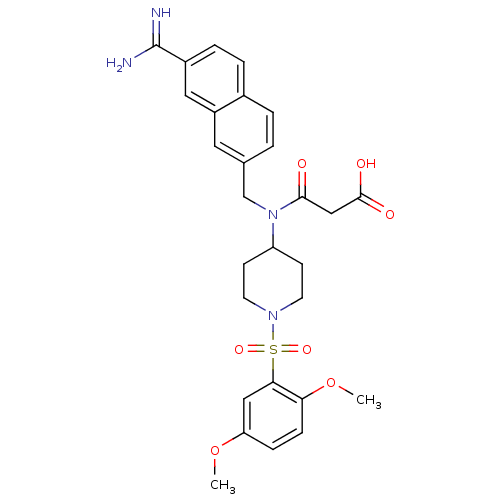
(3-(({7-[amino(imino)methyl]-2-naphthyl}methyl)-{1-...)Show SMILES COc1ccc(OC)c(c1)S(=O)(=O)N1CCC(CC1)N(Cc1ccc2ccc(cc2c1)C(N)=N)C(=O)CC(O)=O Show InChI InChI=1S/C28H32N4O7S/c1-38-23-7-8-24(39-2)25(15-23)40(36,37)31-11-9-22(10-12-31)32(26(33)16-27(34)35)17-18-3-4-19-5-6-20(28(29)30)14-21(19)13-18/h3-8,13-15,22H,9-12,16-17H2,1-2H3,(H3,29,30)(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem 15: 4175-92 (2007)
Article DOI: 10.1016/j.bmc.2007.03.066
BindingDB Entry DOI: 10.7270/Q27H1J90 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Canis lupus familiaris) | BDBM50100663
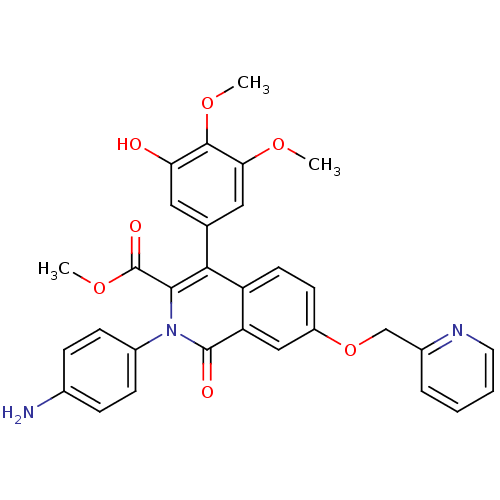
(CHEMBL542878 | methyl 2-(4-aminophenyl)-4-(3-hydro...)Show SMILES COC(=O)c1c(-c2cc(O)c(OC)c(OC)c2)c2ccc(OCc3ccccn3)cc2c(=O)n1-c1ccc(N)cc1 Show InChI InChI=1S/C31H27N3O7/c1-38-26-15-18(14-25(35)29(26)39-2)27-23-12-11-22(41-17-20-6-4-5-13-33-20)16-24(23)30(36)34(28(27)31(37)40-3)21-9-7-19(32)8-10-21/h4-16,35H,17,32H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against canine lung Phosphodiesterase 5 (PDE5) |
J Med Chem 44: 2204-18 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4XT5 |
More data for this
Ligand-Target Pair | |
P04972/P11541/P16586/P22571/P23439/Q95142
(Bos taurus-Bos taurus (Bovine)) | BDBM50130021

(7-(2-Methyl-pyridin-4-ylmethyl)-8-oxo-2-(pyridin-2...)Show SMILES COC(=O)c1c(-c2cc(OC)c(OC)c(OC)c2)c2ccc(OCc3ccccn3)nc2c(=O)n1Cc1ccnc(C)c1 Show InChI InChI=1S/C32H30N4O7/c1-19-14-20(11-13-33-19)17-36-29(32(38)42-5)27(21-15-24(39-2)30(41-4)25(16-21)40-3)23-9-10-26(35-28(23)31(36)37)43-18-22-8-6-7-12-34-22/h6-16H,17-18H2,1-5H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Phosphodiesterase 6 isolated from bovine retina |
Bioorg Med Chem Lett 13: 2341-5 (2003)
BindingDB Entry DOI: 10.7270/Q2WQ04B2 |
More data for this
Ligand-Target Pair | |
Extracellular calcium-sensing receptor
(Rattus norvegicus) | BDBM50272608

(CHEMBL4125688)Show SMILES Cl.Cl.C[C@@H](N[C@@H]1CCN(C1)c1cccc(c1)C(F)(F)F)c1cccc2ccccc12 |r| Show InChI InChI=1S/C23H23F3N2.2ClH/c1-16(21-11-4-7-17-6-2-3-10-22(17)21)27-19-12-13-28(15-19)20-9-5-8-18(14-20)23(24,25)26;;/h2-11,14,16,19,27H,12-13,15H2,1H3;2*1H/t16-,19-;;/m1../s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at CaSR in rat parathyroid cells assessed as inhibition of PTH (1 to 84 residues) production in presence of Cacl2 by ELISA |
Bioorg Med Chem Lett 28: 2055-2060 (2018)
Article DOI: 10.1016/j.bmcl.2018.04.055
BindingDB Entry DOI: 10.7270/Q2DF6TP0 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50209891

(CHEMBL390266 | [(((E)-3-{3-[amino(imino)methyl]phe...)Show SMILES COc1ccc(OC)c(c1)S(=O)(=O)N1CCC(CC1)N(C\C=C\c1cccc(c1)C(N)=N)S(=O)(=O)CC(O)=O Show InChI InChI=1S/C25H32N4O8S2/c1-36-21-8-9-22(37-2)23(16-21)39(34,35)28-13-10-20(11-14-28)29(38(32,33)17-24(30)31)12-4-6-18-5-3-7-19(15-18)25(26)27/h3-9,15-16,20H,10-14,17H2,1-2H3,(H3,26,27)(H,30,31)/b6-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem 15: 4175-92 (2007)
Article DOI: 10.1016/j.bmc.2007.03.066
BindingDB Entry DOI: 10.7270/Q27H1J90 |
More data for this
Ligand-Target Pair | |
P04972/P11541/P16586/P22571/P23439/Q95142
(Bos taurus-Bos taurus (Bovine)) | BDBM50130027

(8-Oxo-2-(pyridin-2-ylmethoxy)-7-pyridin-4-ylmethyl...)Show SMILES COC(=O)c1c(-c2cc(OC)c(OC)c(OC)c2)c2ccc(OCc3ccccn3)nc2c(=O)n1Cc1ccncc1 Show InChI InChI=1S/C31H28N4O7/c1-38-23-15-20(16-24(39-2)29(23)40-3)26-22-8-9-25(42-18-21-7-5-6-12-33-21)34-27(22)30(36)35(28(26)31(37)41-4)17-19-10-13-32-14-11-19/h5-16H,17-18H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Phosphodiesterase 6 isolated from bovine retina |
Bioorg Med Chem Lett 13: 2341-5 (2003)
BindingDB Entry DOI: 10.7270/Q2WQ04B2 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Canis lupus familiaris) | BDBM50130018
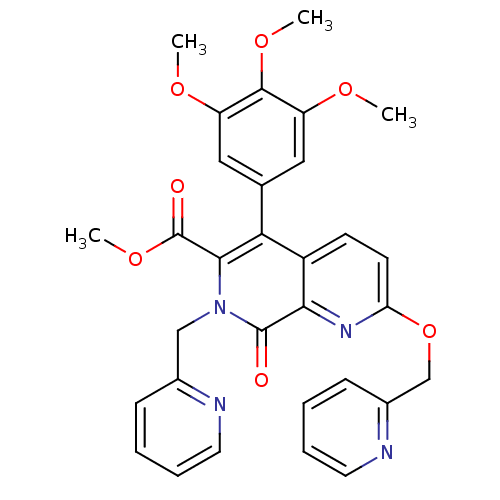
(8-Oxo-2-(pyridin-2-ylmethoxy)-7-pyridin-2-ylmethyl...)Show SMILES COC(=O)c1c(-c2cc(OC)c(OC)c(OC)c2)c2ccc(OCc3ccccn3)nc2c(=O)n1Cc1ccccn1 Show InChI InChI=1S/C31H28N4O7/c1-38-23-15-19(16-24(39-2)29(23)40-3)26-22-11-12-25(42-18-21-10-6-8-14-33-21)34-27(22)30(36)35(28(26)31(37)41-4)17-20-9-5-7-13-32-20/h5-16H,17-18H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against phosphodiesterase 5 (PDE5) isolated from canine lung |
Bioorg Med Chem Lett 13: 2341-5 (2003)
BindingDB Entry DOI: 10.7270/Q2WQ04B2 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50209893
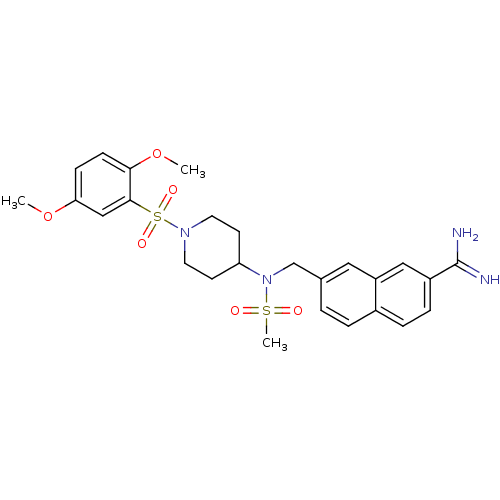
(7-({[1-(2,5-dimethoxy-benzenesulfonyl)-piperidin-4...)Show SMILES COc1ccc(OC)c(c1)S(=O)(=O)N1CCC(CC1)N(Cc1ccc2ccc(cc2c1)C(N)=N)S(C)(=O)=O Show InChI InChI=1S/C26H32N4O6S2/c1-35-23-8-9-24(36-2)25(16-23)38(33,34)29-12-10-22(11-13-29)30(37(3,31)32)17-18-4-5-19-6-7-20(26(27)28)15-21(19)14-18/h4-9,14-16,22H,10-13,17H2,1-3H3,(H3,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem 15: 4175-92 (2007)
Article DOI: 10.1016/j.bmc.2007.03.066
BindingDB Entry DOI: 10.7270/Q27H1J90 |
More data for this
Ligand-Target Pair | |
Extracellular calcium-sensing receptor
(Rattus norvegicus) | BDBM50272592
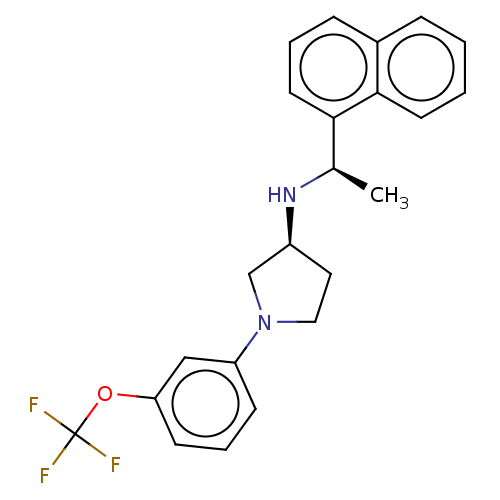
(CHEMBL4125724)Show SMILES Cl.Cl.C[C@@H](N[C@H]1CCN(C1)c1cccc(OC(F)(F)F)c1)c1cccc2ccccc12 |r| Show InChI InChI=1S/C23H23F3N2O.2ClH/c1-16(21-11-4-7-17-6-2-3-10-22(17)21)27-18-12-13-28(15-18)19-8-5-9-20(14-19)29-23(24,25)26;;/h2-11,14,16,18,27H,12-13,15H2,1H3;2*1H/t16-,18+;;/m1../s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at CaSR in rat parathyroid cells assessed as inhibition of PTH (1 to 84 residues) production in presence of Cacl2 by ELISA |
Bioorg Med Chem Lett 28: 2055-2060 (2018)
Article DOI: 10.1016/j.bmcl.2018.04.055
BindingDB Entry DOI: 10.7270/Q2DF6TP0 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50209895
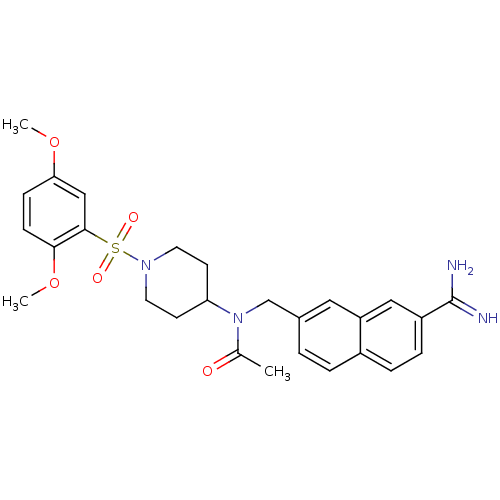
(CHEMBL244221 | N-({7-[amino(imino)methyl]-2-naphth...)Show SMILES COc1ccc(OC)c(c1)S(=O)(=O)N1CCC(CC1)N(Cc1ccc2ccc(cc2c1)C(N)=N)C(C)=O Show InChI InChI=1S/C27H32N4O5S/c1-18(32)31(17-19-4-5-20-6-7-21(27(28)29)15-22(20)14-19)23-10-12-30(13-11-23)37(33,34)26-16-24(35-2)8-9-25(26)36-3/h4-9,14-16,23H,10-13,17H2,1-3H3,(H3,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem 15: 4175-92 (2007)
Article DOI: 10.1016/j.bmc.2007.03.066
BindingDB Entry DOI: 10.7270/Q27H1J90 |
More data for this
Ligand-Target Pair | |
Extracellular calcium-sensing receptor
(Rattus norvegicus) | BDBM50272595

(CHEMBL4127153)Show SMILES Cl.Cl.C[C@@H](N[C@H]1CCN(C1)c1cccc(c1)C(F)(F)F)c1cccc2ccccc12 |r| Show InChI InChI=1S/C23H23F3N2.2ClH/c1-16(21-11-4-7-17-6-2-3-10-22(17)21)27-19-12-13-28(15-19)20-9-5-8-18(14-20)23(24,25)26;;/h2-11,14,16,19,27H,12-13,15H2,1H3;2*1H/t16-,19+;;/m1../s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at CaSR in rat parathyroid cells assessed as inhibition of PTH (1 to 84 residues) production in presence of Cacl2 by ELISA |
Bioorg Med Chem Lett 28: 2055-2060 (2018)
Article DOI: 10.1016/j.bmcl.2018.04.055
BindingDB Entry DOI: 10.7270/Q2DF6TP0 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50209899
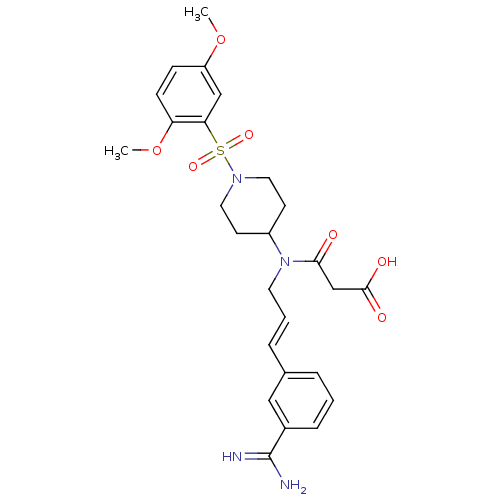
(3-(((E)-3-{3-[amino(imino)methyl]phenyl}prop-2-en-...)Show SMILES COc1ccc(OC)c(c1)S(=O)(=O)N1CCC(CC1)N(C\C=C\c1cccc(c1)C(N)=N)C(=O)CC(O)=O Show InChI InChI=1S/C26H32N4O7S/c1-36-21-8-9-22(37-2)23(16-21)38(34,35)29-13-10-20(11-14-29)30(24(31)17-25(32)33)12-4-6-18-5-3-7-19(15-18)26(27)28/h3-9,15-16,20H,10-14,17H2,1-2H3,(H3,27,28)(H,32,33)/b6-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem 15: 4175-92 (2007)
Article DOI: 10.1016/j.bmc.2007.03.066
BindingDB Entry DOI: 10.7270/Q27H1J90 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50209904
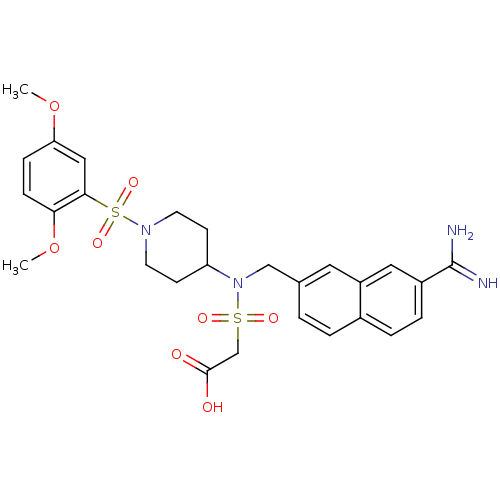
(CHEMBL244436 | [(({7-[amino(imino)methyl]-2-naphth...)Show SMILES COc1ccc(OC)c(c1)S(=O)(=O)N1CCC(CC1)N(Cc1ccc2ccc(cc2c1)C(N)=N)S(=O)(=O)CC(O)=O Show InChI InChI=1S/C27H32N4O8S2/c1-38-23-7-8-24(39-2)25(15-23)41(36,37)30-11-9-22(10-12-30)31(40(34,35)17-26(32)33)16-18-3-4-19-5-6-20(27(28)29)14-21(19)13-18/h3-8,13-15,22H,9-12,16-17H2,1-2H3,(H3,28,29)(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem 15: 4175-92 (2007)
Article DOI: 10.1016/j.bmc.2007.03.066
BindingDB Entry DOI: 10.7270/Q27H1J90 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data