

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SRSF protein kinase 1 (Homo sapiens (Human)) | BDBM448544 (N-(2-(4-(furan-2-ylmethyl)piperazin-1-yl)-5- (trif...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
Exonate Limited US Patent | Assay Description Screening of a series of molecules identified compounds (Compounds 5, 8, 16 to 18 and 43 of Table 3) that had improved potency against SRPK1 against ... | US Patent US10696661 (2020) BindingDB Entry DOI: 10.7270/Q23B635R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| SRSF protein kinase 1 (Homo sapiens (Human)) | BDBM448683 (N-(2-(4-(thiazol-5-ylmethyl)piperazin-1-yl)-5- (tr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Exonate Limited US Patent | Assay Description Screening of a series of molecules identified compounds (Compounds 5, 8, 16 to 18 and 43 of Table 3) that had improved potency against SRPK1 against ... | US Patent US10696661 (2020) BindingDB Entry DOI: 10.7270/Q23B635R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| SRSF protein kinase 1 (Homo sapiens (Human)) | BDBM448727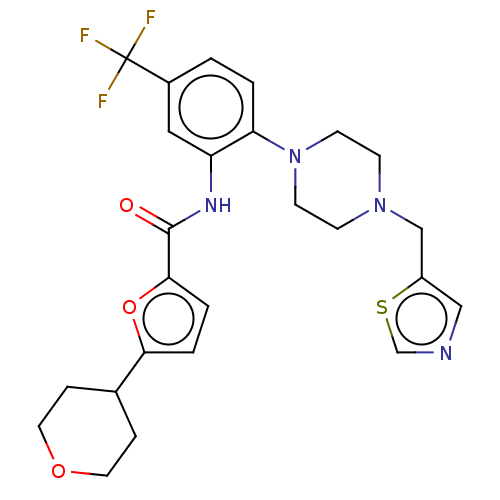 (5-(tetrahydro-2H-pyran-4-yl)-N-(2-(4-(thiazol-5- y...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Exonate Limited US Patent | Assay Description Screening of a series of molecules identified compounds (Compounds 5, 8, 16 to 18 and 43 of Table 3) that had improved potency against SRPK1 against ... | US Patent US10696661 (2020) BindingDB Entry DOI: 10.7270/Q23B635R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| SRSF protein kinase 1 (Homo sapiens (Human)) | BDBM448695 (N-(2-(4-(furan-3-ylmethyl)piperazin-1-yl)-5- (trif...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Exonate Limited US Patent | Assay Description Screening of a series of molecules identified compounds (Compounds 5, 8, 16 to 18 and 43 of Table 3) that had improved potency against SRPK1 against ... | US Patent US10696661 (2020) BindingDB Entry DOI: 10.7270/Q23B635R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| SRSF protein kinase 1 (Homo sapiens (Human)) | BDBM448663 (N-(2-(4-(oxazol-5-ylmethyl)piperazin-1-yl)-5- (tri...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
Exonate Limited US Patent | Assay Description Screening of a series of molecules identified compounds (Compounds 5, 8, 16 to 18 and 43 of Table 3) that had improved potency against SRPK1 against ... | US Patent US10696661 (2020) BindingDB Entry DOI: 10.7270/Q23B635R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Canis familiaris) | BDBM50395256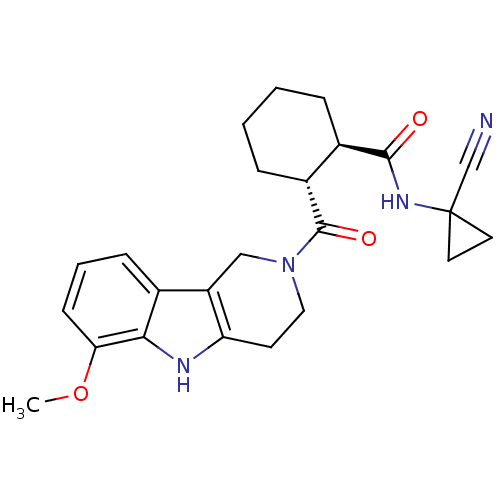 (CHEMBL2163587) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of dog recombinant CatK assessed as suppression of enzyme-mediated Z-Phe-Arg-AMC cleavage by QFRET assay | J Med Chem 55: 6363-74 (2012) Article DOI: 10.1021/jm3007257 BindingDB Entry DOI: 10.7270/Q2833T5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50395254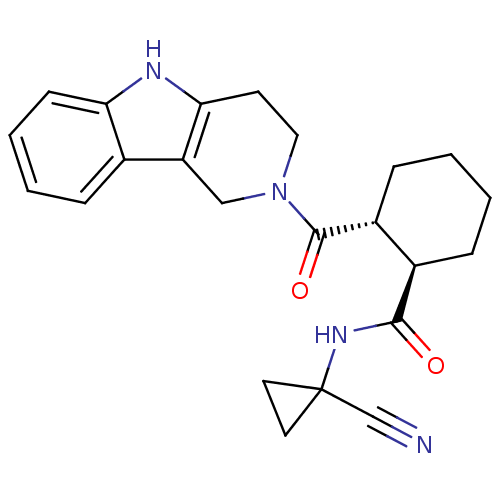 (CHEMBL2164682) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant CatK assessed as suppression of enzyme-mediated Z-Phe-Arg-AMC cleavage incubated for 1 hrs by QFRET assay | J Med Chem 55: 6363-74 (2012) Article DOI: 10.1021/jm3007257 BindingDB Entry DOI: 10.7270/Q2833T5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50395235 (CHEMBL2164670) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant CatK assessed as suppression of enzyme-mediated Z-Phe-Arg-AMC cleavage incubated for 1 hrs by QFRET assay | J Med Chem 55: 6363-74 (2012) Article DOI: 10.1021/jm3007257 BindingDB Entry DOI: 10.7270/Q2833T5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50395236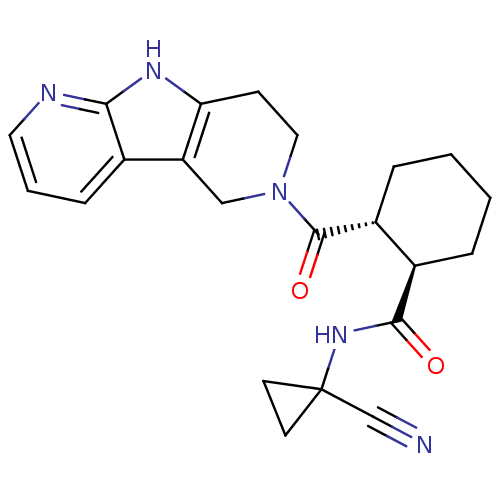 (CHEMBL2163360) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant CatK assessed as suppression of enzyme-mediated Z-Phe-Arg-AMC cleavage incubated for 1 hrs by QFRET assay | J Med Chem 55: 6363-74 (2012) Article DOI: 10.1021/jm3007257 BindingDB Entry DOI: 10.7270/Q2833T5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| SRSF protein kinase 1 (Homo sapiens (Human)) | BDBM448673 (N-(5-chloro-2-(4-(furan-2-ylmethyl)piperazin-1-yl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Exonate Limited US Patent | Assay Description Screening of a series of molecules identified compounds (Compounds 5, 8, 16 to 18 and 43 of Table 3) that had improved potency against SRPK1 against ... | US Patent US10696661 (2020) BindingDB Entry DOI: 10.7270/Q23B635R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50414644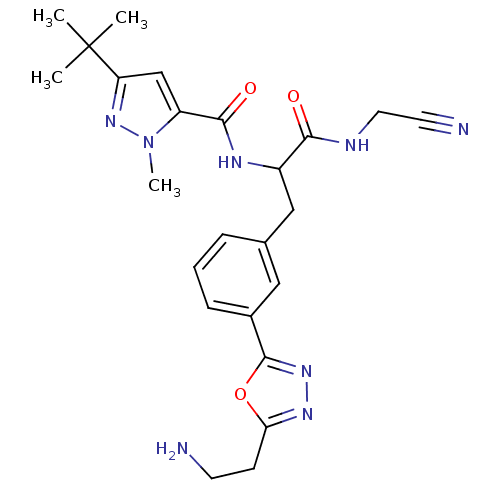 (CHEMBL555122) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.26 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of cathepsin L assessed as inhibition of fluorogenic substrate cleavage | Bioorg Med Chem Lett 19: 4622-5 (2009) Article DOI: 10.1016/j.bmcl.2009.06.090 BindingDB Entry DOI: 10.7270/Q2N017S3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| SRSF protein kinase 1 (Homo sapiens (Human)) | BDBM448662 (N-(2-(4-(oxazol-4-ylmethyl)piperazin-1-yl)-5- (tri...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Exonate Limited US Patent | Assay Description Screening of a series of molecules identified compounds (Compounds 5, 8, 16 to 18 and 43 of Table 3) that had improved potency against SRPK1 against ... | US Patent US10696661 (2020) BindingDB Entry DOI: 10.7270/Q23B635R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| SRSF protein kinase 1 (Homo sapiens (Human)) | BDBM448675 (N-(2-(4-(furan-2-ylmethyl)piperazin-1-yl)-5- (trif...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Exonate Limited US Patent | Assay Description Screening of a series of molecules identified compounds (Compounds 5, 8, 16 to 18 and 43 of Table 3) that had improved potency against SRPK1 against ... | US Patent US10696661 (2020) BindingDB Entry DOI: 10.7270/Q23B635R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| SRSF protein kinase 1 (Homo sapiens (Human)) | BDBM448664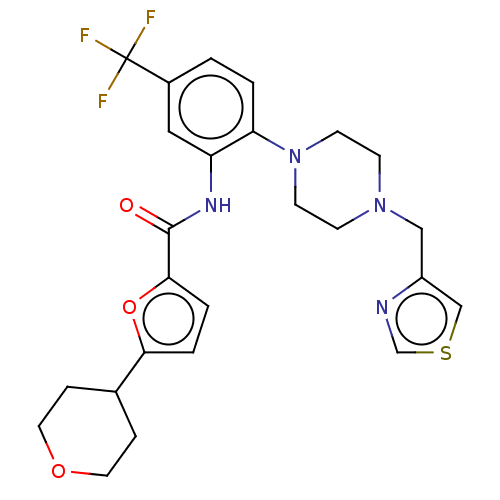 (5-(tetrahydro-2H-pyran-4-yl)-N-(2-(4-(thiazol-4- y...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Exonate Limited US Patent | Assay Description Screening of a series of molecules identified compounds (Compounds 5, 8, 16 to 18 and 43 of Table 3) that had improved potency against SRPK1 against ... | US Patent US10696661 (2020) BindingDB Entry DOI: 10.7270/Q23B635R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| SRSF protein kinase 1 (Homo sapiens (Human)) | BDBM448697 (N-(2-(4-(furan-3-ylmethyl)piperazin-1-yl)-5- (trif...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Exonate Limited US Patent | Assay Description Screening of a series of molecules identified compounds (Compounds 5, 8, 16 to 18 and 43 of Table 3) that had improved potency against SRPK1 against ... | US Patent US10696661 (2020) BindingDB Entry DOI: 10.7270/Q23B635R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| SRSF protein kinase 1 (Homo sapiens (Human)) | BDBM448726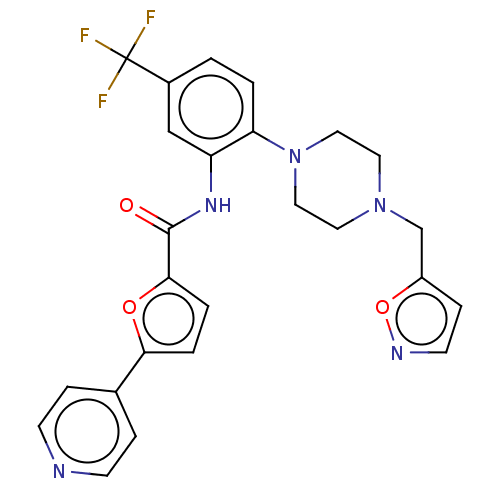 (N-(2-(4-(isoxazol-5-ylmethyl)piperazin-1-yl)-5-(tr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Exonate Limited US Patent | Assay Description Screening of a series of molecules identified compounds (Compounds 5, 8, 16 to 18 and 43 of Table 3) that had improved potency against SRPK1 against ... | US Patent US10696661 (2020) BindingDB Entry DOI: 10.7270/Q23B635R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin L2 (Homo sapiens (Human)) | BDBM50414641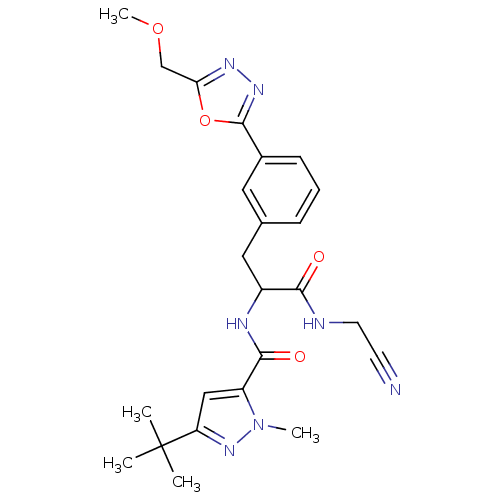 (CHEMBL554065) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of cathepsin L2 assessed as inhibition of fluorogenic substrate cleavage | Bioorg Med Chem Lett 19: 4622-5 (2009) Article DOI: 10.1016/j.bmcl.2009.06.090 BindingDB Entry DOI: 10.7270/Q2N017S3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50414643 (CHEMBL557455) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of cathepsin L assessed as inhibition of fluorogenic substrate cleavage | Bioorg Med Chem Lett 19: 4622-5 (2009) Article DOI: 10.1016/j.bmcl.2009.06.090 BindingDB Entry DOI: 10.7270/Q2N017S3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50395238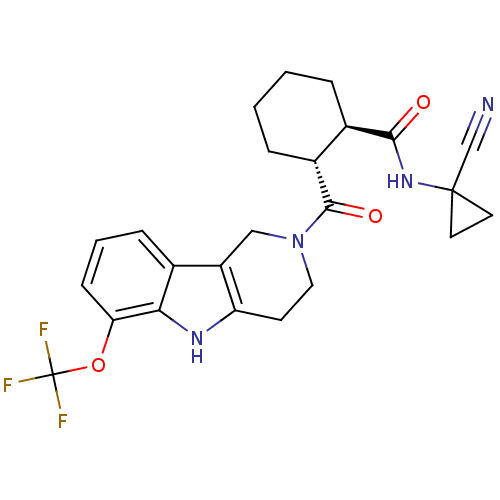 (CHEMBL2163589) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant CatK assessed as suppression of enzyme-mediated Z-Phe-Arg-AMC cleavage incubated for 1 hrs by QFRET assay | J Med Chem 55: 6363-74 (2012) Article DOI: 10.1021/jm3007257 BindingDB Entry DOI: 10.7270/Q2833T5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50395228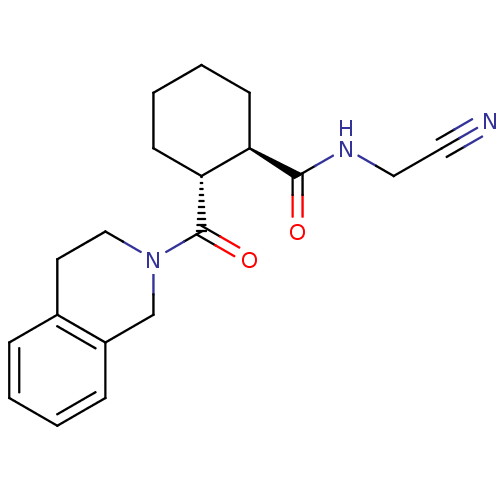 (CHEMBL2164674) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant CatK assessed as suppression of enzyme-mediated Z-Phe-Arg-AMC cleavage incubated for 1 hrs by QFRET assay | J Med Chem 55: 6363-74 (2012) Article DOI: 10.1021/jm3007257 BindingDB Entry DOI: 10.7270/Q2833T5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50249413 (5-amino-4-(bicyclo[2.2.1]heptan-2-ylamino)-6-morph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of cathepsin K (unknown origin) | Bioorg Med Chem Lett 19: 1658-61 (2009) Article DOI: 10.1016/j.bmcl.2009.01.110 BindingDB Entry DOI: 10.7270/Q2571BWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50395241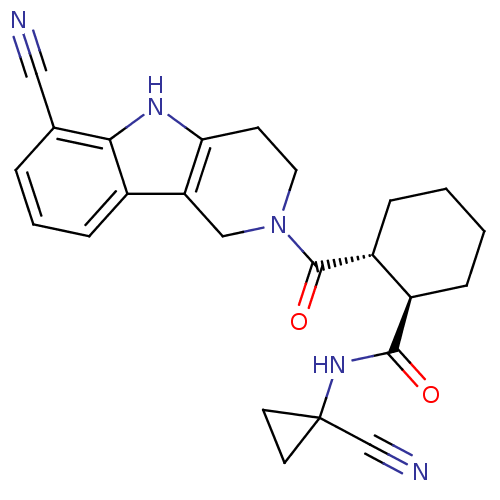 (CHEMBL2163585) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant CatK assessed as suppression of enzyme-mediated Z-Phe-Arg-AMC cleavage incubated for 1 hrs by QFRET assay | J Med Chem 55: 6363-74 (2012) Article DOI: 10.1021/jm3007257 BindingDB Entry DOI: 10.7270/Q2833T5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50395244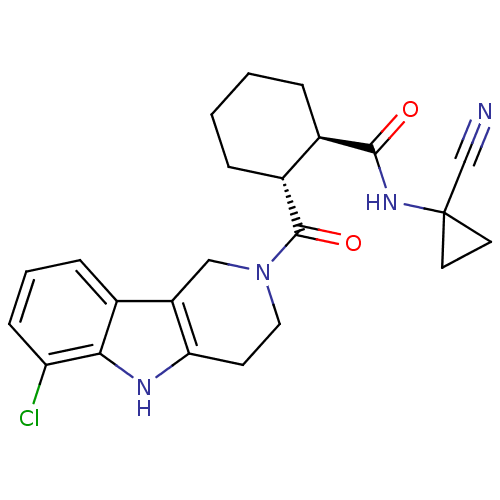 (CHEMBL2163582) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant CatK assessed as suppression of enzyme-mediated Z-Phe-Arg-AMC cleavage incubated for 1 hrs by QFRET assay | J Med Chem 55: 6363-74 (2012) Article DOI: 10.1021/jm3007257 BindingDB Entry DOI: 10.7270/Q2833T5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50414636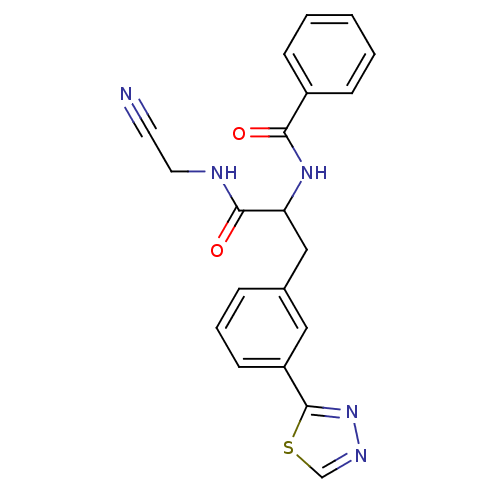 (CHEMBL559880) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.51 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of cathepsin S assessed as inhibition of fluorogenic substrate cleavage | Bioorg Med Chem Lett 19: 4622-5 (2009) Article DOI: 10.1016/j.bmcl.2009.06.090 BindingDB Entry DOI: 10.7270/Q2N017S3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| SRSF protein kinase 1 (Homo sapiens (Human)) | BDBM448715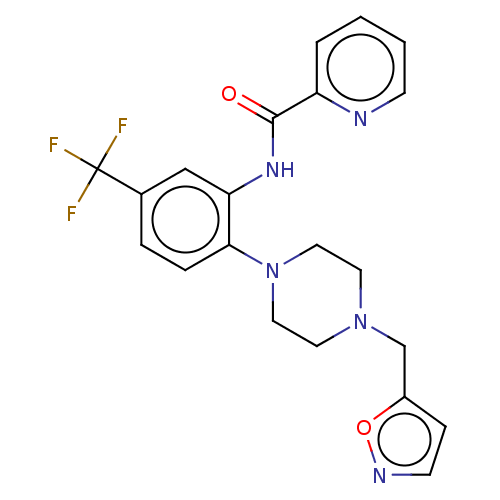 (N-(2-(4-(isoxazol-5-ylmethyl)piperazin-1-yl)-5-(tr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Exonate Limited US Patent | Assay Description Screening of a series of molecules identified compounds (Compounds 5, 8, 16 to 18 and 43 of Table 3) that had improved potency against SRPK1 against ... | US Patent US10696661 (2020) BindingDB Entry DOI: 10.7270/Q23B635R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50395243 (CHEMBL2163583) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant CatK assessed as suppression of enzyme-mediated Z-Phe-Arg-AMC cleavage incubated for 1 hrs by QFRET assay | J Med Chem 55: 6363-74 (2012) Article DOI: 10.1021/jm3007257 BindingDB Entry DOI: 10.7270/Q2833T5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50397138 (CHEMBL2172003) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin-k using Z-Phe-Arg-AMC as substrate preincubated for 15 mins measured after 1 hr by QFRET assay | J Med Chem 55: 8827-37 (2012) Article DOI: 10.1021/jm301119s BindingDB Entry DOI: 10.7270/Q2HX1DS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50395251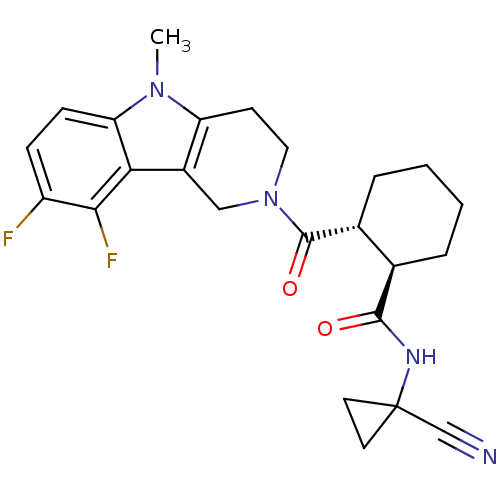 (CHEMBL2164686) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant CatK assessed as suppression of enzyme-mediated Z-Phe-Arg-AMC cleavage incubated for 1 hrs by QFRET assay | J Med Chem 55: 6363-74 (2012) Article DOI: 10.1021/jm3007257 BindingDB Entry DOI: 10.7270/Q2833T5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50395250 (CHEMBL2164687) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant CatK assessed as suppression of enzyme-mediated Z-Phe-Arg-AMC cleavage incubated for 1 hrs by QFRET assay | J Med Chem 55: 6363-74 (2012) Article DOI: 10.1021/jm3007257 BindingDB Entry DOI: 10.7270/Q2833T5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50395234 (CHEMBL2164669) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant CatK assessed as suppression of enzyme-mediated Z-Phe-Arg-AMC cleavage incubated for 1 hrs by QFRET assay | J Med Chem 55: 6363-74 (2012) Article DOI: 10.1021/jm3007257 BindingDB Entry DOI: 10.7270/Q2833T5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin L2 (Homo sapiens (Human)) | BDBM50414640 (CHEMBL562844) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.16 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of cathepsin L2 assessed as inhibition of fluorogenic substrate cleavage | Bioorg Med Chem Lett 19: 4622-5 (2009) Article DOI: 10.1016/j.bmcl.2009.06.090 BindingDB Entry DOI: 10.7270/Q2N017S3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| SRSF protein kinase 1 (Homo sapiens (Human)) | BDBM448647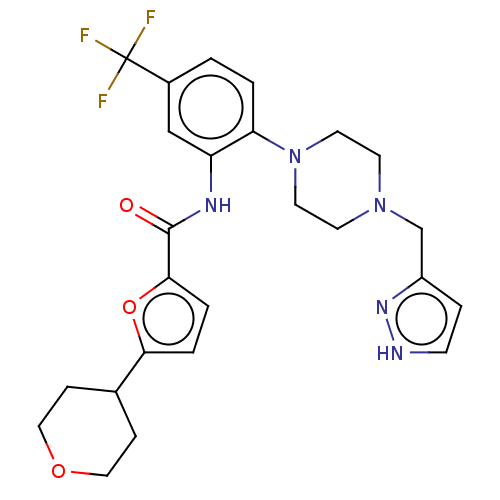 (N-(2-(4-((1H-pyrazol-3-yl)methyl)piperazin-1-yl)-5...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Exonate Limited US Patent | Assay Description Screening of a series of molecules identified compounds (Compounds 5, 8, 16 to 18 and 43 of Table 3) that had improved potency against SRPK1 against ... | US Patent US10696661 (2020) BindingDB Entry DOI: 10.7270/Q23B635R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| SRSF protein kinase 1 (Homo sapiens (Human)) | BDBM448696 (N-(2-(4-((5-methylfuran-2-yl)methyl)piperazin-1-yl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Exonate Limited US Patent | Assay Description Screening of a series of molecules identified compounds (Compounds 5, 8, 16 to 18 and 43 of Table 3) that had improved potency against SRPK1 against ... | US Patent US10696661 (2020) BindingDB Entry DOI: 10.7270/Q23B635R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50414641 (CHEMBL554065) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.98 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of cathepsin L assessed as inhibition of fluorogenic substrate cleavage | Bioorg Med Chem Lett 19: 4622-5 (2009) Article DOI: 10.1016/j.bmcl.2009.06.090 BindingDB Entry DOI: 10.7270/Q2N017S3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50395242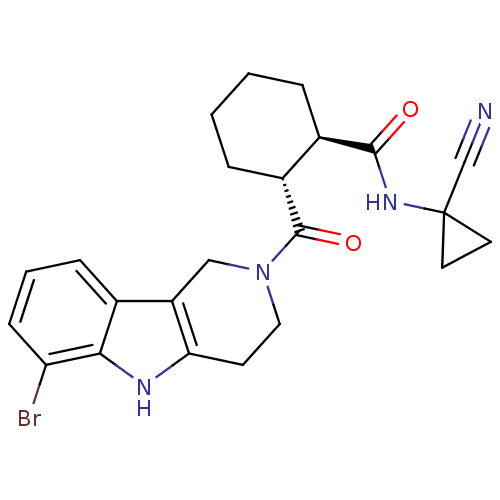 (CHEMBL2163584) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant CatK assessed as suppression of enzyme-mediated Z-Phe-Arg-AMC cleavage incubated for 1 hrs by QFRET assay | J Med Chem 55: 6363-74 (2012) Article DOI: 10.1021/jm3007257 BindingDB Entry DOI: 10.7270/Q2833T5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50395239 (CHEMBL2163588) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant CatK assessed as suppression of enzyme-mediated Z-Phe-Arg-AMC cleavage incubated for 1 hrs by QFRET assay | J Med Chem 55: 6363-74 (2012) Article DOI: 10.1021/jm3007257 BindingDB Entry DOI: 10.7270/Q2833T5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50419562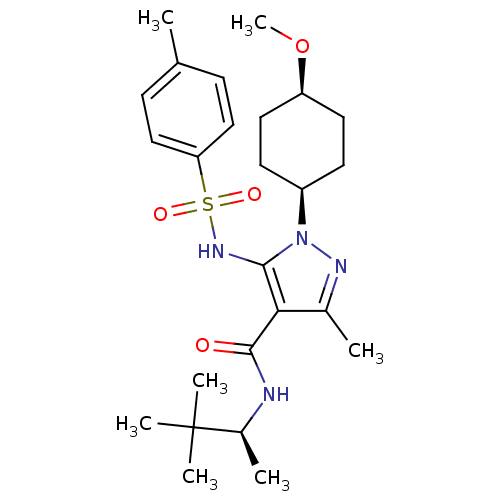 (CHEMBL1934424) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at human recombinant FPR1 in expressed in HEK293 cells assessed as inhibition of FMLP-stimulated intracellular calcium mobilisati... | Bioorg Med Chem Lett 22: 532-6 (2011) Article DOI: 10.1016/j.bmcl.2011.10.090 BindingDB Entry DOI: 10.7270/Q27P90NM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50395253 (CHEMBL2164684) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant CatK assessed as suppression of enzyme-mediated Z-Phe-Arg-AMC cleavage incubated for 1 hrs by QFRET assay | J Med Chem 55: 6363-74 (2012) Article DOI: 10.1021/jm3007257 BindingDB Entry DOI: 10.7270/Q2833T5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50397142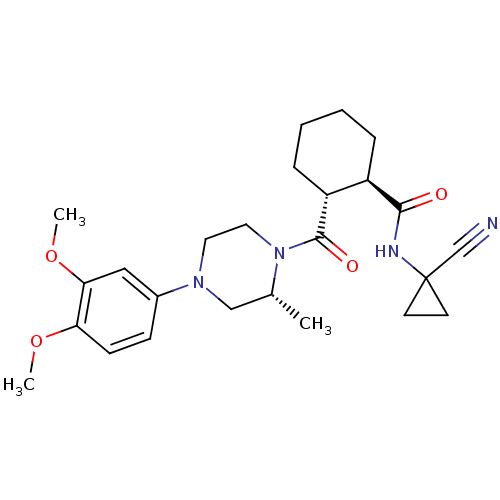 (CHEMBL2172005) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin-k using Z-Phe-Arg-AMC as substrate preincubated for 15 mins measured after 1 hr by QFRET assay | J Med Chem 55: 8827-37 (2012) Article DOI: 10.1021/jm301119s BindingDB Entry DOI: 10.7270/Q2HX1DS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| SRSF protein kinase 1 (Homo sapiens (Human)) | BDBM448661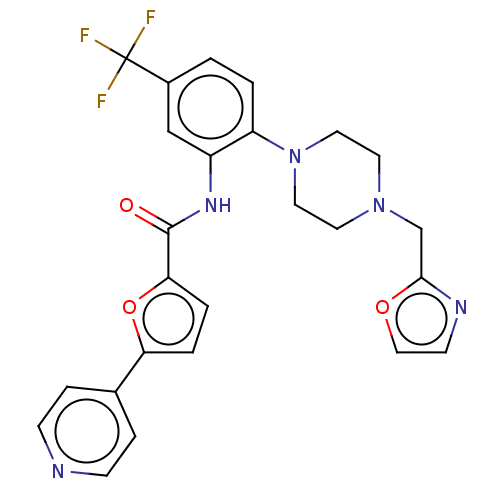 (N-(2-(4-(oxazol-2-ylmethyl)piperazin-1-yl)-5- (tri...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Exonate Limited US Patent | Assay Description Screening of a series of molecules identified compounds (Compounds 5, 8, 16 to 18 and 43 of Table 3) that had improved potency against SRPK1 against ... | US Patent US10696661 (2020) BindingDB Entry DOI: 10.7270/Q23B635R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19855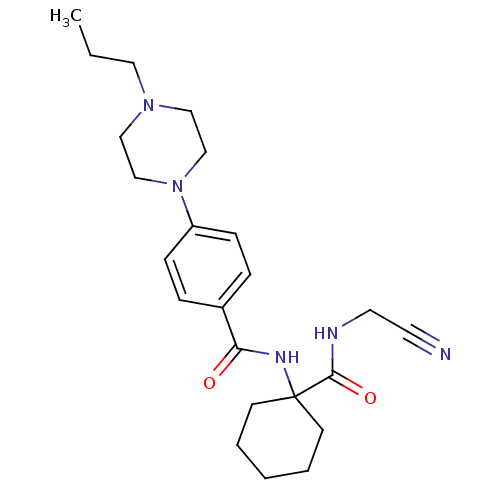 (Balicatib | CHEMBL371064 | N-[1-(cyanomethylcarbam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant CatK assessed as suppression of enzyme-mediated Z-Phe-Arg-AMC cleavage incubated for 1 hrs by QFRET assay | J Med Chem 55: 6363-74 (2012) Article DOI: 10.1021/jm3007257 BindingDB Entry DOI: 10.7270/Q2833T5F | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Glycogen phosphorylase, liver form (Homo sapiens (Human)) | BDBM50194411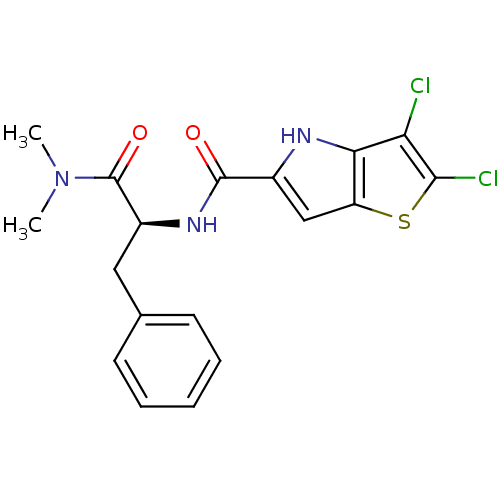 ((S)-2,3-dichloro-N-(1-(dimethylamino)-1-oxo-3-phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human liver GPa by multienzyme coupled assay | Bioorg Med Chem Lett 16: 5567-71 (2006) Article DOI: 10.1016/j.bmcl.2006.08.047 BindingDB Entry DOI: 10.7270/Q2KW5FP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50397137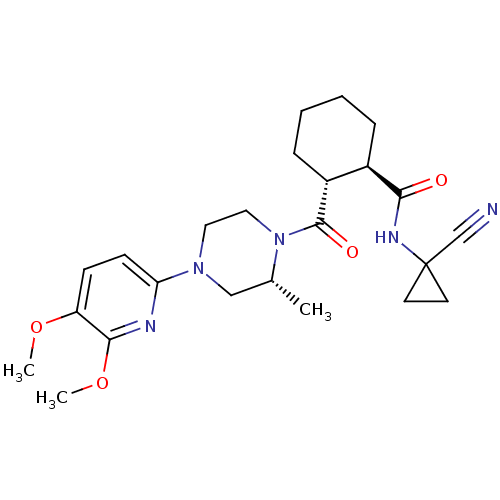 (CHEMBL2172002) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin-k using Z-Phe-Arg-AMC as substrate preincubated for 15 mins measured after 1 hr by QFRET assay | J Med Chem 55: 8827-37 (2012) Article DOI: 10.1021/jm301119s BindingDB Entry DOI: 10.7270/Q2HX1DS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19855 (Balicatib | CHEMBL371064 | N-[1-(cyanomethylcarbam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of cathepsin-k | J Med Chem 55: 8827-37 (2012) Article DOI: 10.1021/jm301119s BindingDB Entry DOI: 10.7270/Q2HX1DS7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50085853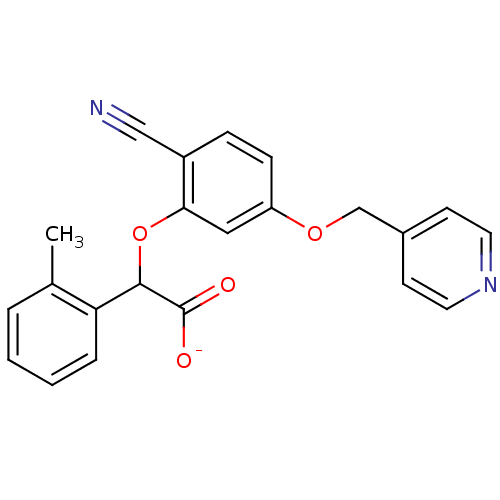 (CHEMBL173251 | Sodium; [2-cyano-5-(pyridin-4-ylmet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description In vitro ability to antagonise the binding of [125I]-ET-1 to the rat aortic A10 cell membrane endothelin A receptor. | J Med Chem 43: 900-10 (2000) BindingDB Entry DOI: 10.7270/Q2154G73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50395240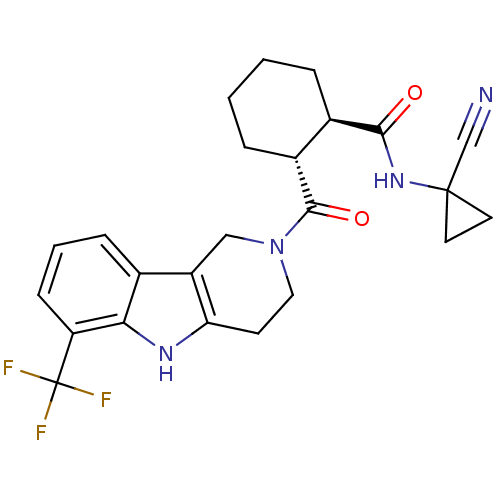 (CHEMBL2163586) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant CatK assessed as suppression of enzyme-mediated Z-Phe-Arg-AMC cleavage incubated for 1 hrs by QFRET assay | J Med Chem 55: 6363-74 (2012) Article DOI: 10.1021/jm3007257 BindingDB Entry DOI: 10.7270/Q2833T5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50414638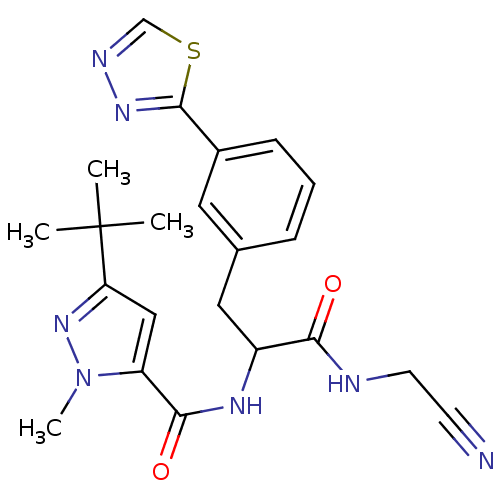 (CHEMBL549378) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.01 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of cathepsin L assessed as inhibition of fluorogenic substrate cleavage | Bioorg Med Chem Lett 19: 4622-5 (2009) Article DOI: 10.1016/j.bmcl.2009.06.090 BindingDB Entry DOI: 10.7270/Q2N017S3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50414639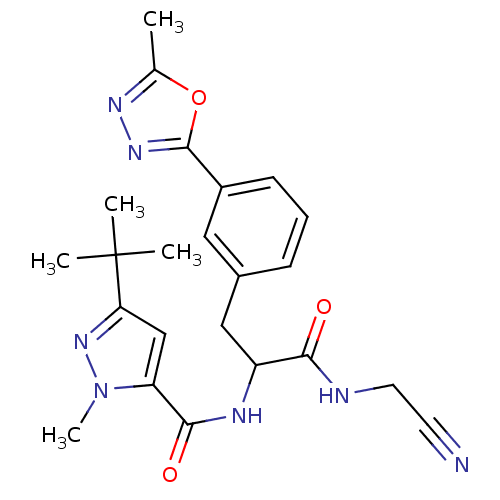 (CHEMBL550872) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.01 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of cathepsin L assessed as inhibition of fluorogenic substrate cleavage | Bioorg Med Chem Lett 19: 4622-5 (2009) Article DOI: 10.1016/j.bmcl.2009.06.090 BindingDB Entry DOI: 10.7270/Q2N017S3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| SRSF protein kinase 1 (Homo sapiens (Human)) | BDBM448729 (N-(2-(4-(furan-3-ylmethyl)piperazin-1-yl)-5- (trif...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Exonate Limited US Patent | Assay Description Screening of a series of molecules identified compounds (Compounds 5, 8, 16 to 18 and 43 of Table 3) that had improved potency against SRPK1 against ... | US Patent US10696661 (2020) BindingDB Entry DOI: 10.7270/Q23B635R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| SRSF protein kinase 1 (Homo sapiens (Human)) | BDBM448717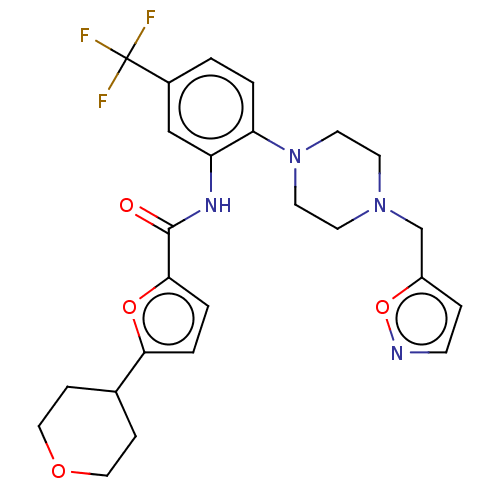 (N-(2-(4-(isoxazol-5-ylmethyl)piperazin-1-yl)-5-(tr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Exonate Limited US Patent | Assay Description Screening of a series of molecules identified compounds (Compounds 5, 8, 16 to 18 and 43 of Table 3) that had improved potency against SRPK1 against ... | US Patent US10696661 (2020) BindingDB Entry DOI: 10.7270/Q23B635R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1290 total ) | Next | Last >> |