

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peptide deformylase (Escherichia coli) | BDBM50153088 (CHEMBL365416 | N-((3S,6R)-3-tert-Butyl-2,5-dioxo-1...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Inhibitory effect against E. coli peptide deformylase (PDF) by AAP assay | J Med Chem 47: 4941-9 (2004) Article DOI: 10.1021/jm049592c BindingDB Entry DOI: 10.7270/Q2WD401S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Escherichia coli) | BDBM50153085 (CHEMBL364836 | N-((3S,6R)-3-tert-Butyl-2,5-dioxo-1...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Inhibitory effect against E. coli peptide deformylase (PDF) by AAP assay | J Med Chem 47: 4941-9 (2004) Article DOI: 10.1021/jm049592c BindingDB Entry DOI: 10.7270/Q2WD401S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Escherichia coli) | BDBM50153083 (CHEMBL441502 | N-((3S,6R)-3-tert-Butyl-2,5-dioxo-1...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Inhibitory effect against E. coli peptide deformylase (PDF) by AAP assay | J Med Chem 47: 4941-9 (2004) Article DOI: 10.1021/jm049592c BindingDB Entry DOI: 10.7270/Q2WD401S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM25771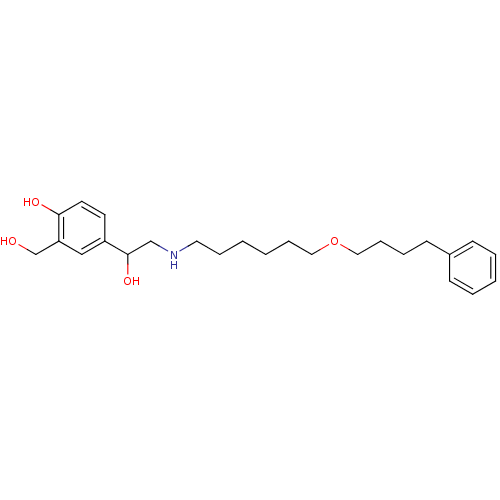 (1-hydroxy-2-naphthoic acid;4-[1-hydroxy-2-[6-(4-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [125I]cyanopindolol from human recombinant beta2 adrenergic receptor expressed in CHO cells by filtration assay | J Med Chem 53: 3675-84 (2010) Article DOI: 10.1021/jm100068m BindingDB Entry DOI: 10.7270/Q27H1JRT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peptide deformylase (Escherichia coli) | BDBM50153086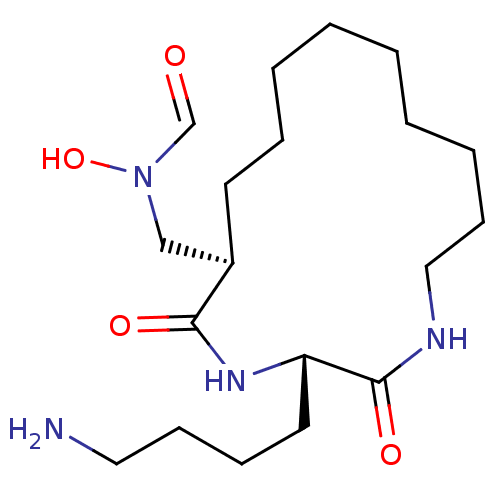 (CHEMBL188671 | N-[(1R,2S)-3-(4-Amino-butyl)-2,5-di...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Inhibitory effect against E. coli peptide deformylase (PDF) by AAP assay | J Med Chem 47: 4941-9 (2004) Article DOI: 10.1021/jm049592c BindingDB Entry DOI: 10.7270/Q2WD401S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50318156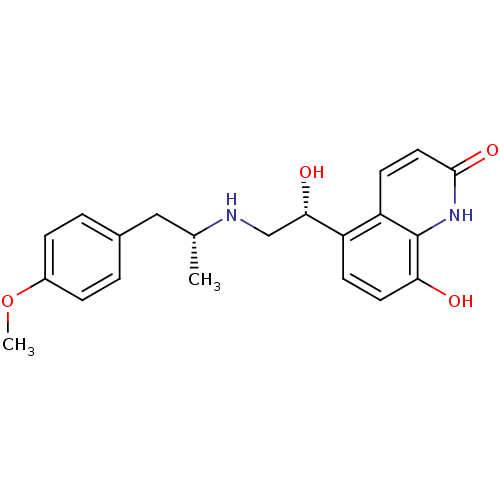 (CHEMBL1094785 | carmoterol) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [125I]cyanopindolol from human recombinant beta2 adrenergic receptor expressed in CHO cells by filtration assay | J Med Chem 53: 3675-84 (2010) Article DOI: 10.1021/jm100068m BindingDB Entry DOI: 10.7270/Q27H1JRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Escherichia coli) | BDBM50104501 ((R)-2-[(Formyl-hydroxy-amino)-methyl]-hexanoic aci...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Inhibitory effect against E. coli peptide deformylase (PDF) by DPPI assay | J Med Chem 47: 4941-9 (2004) Article DOI: 10.1021/jm049592c BindingDB Entry DOI: 10.7270/Q2WD401S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peptide deformylase (Escherichia coli) | BDBM50153084 (CHEMBL361449 | N-(4-{(3R,14S)-14-[(Formyl-hydroxy-...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Inhibitory effect against E. coli peptide deformylase (PDF) by AAP assay | J Med Chem 47: 4941-9 (2004) Article DOI: 10.1021/jm049592c BindingDB Entry DOI: 10.7270/Q2WD401S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Escherichia coli) | BDBM50153087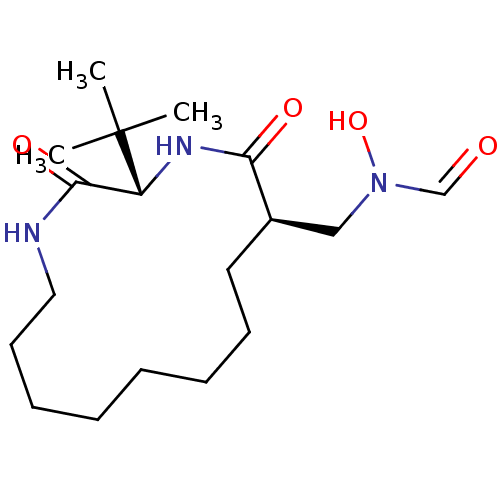 (CHEMBL188894 | N-((3S,6R)-3-tert-Butyl-2,5-dioxo-1...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Inhibitory effect against E. coli peptide deformylase (PDF) by AAP assay | J Med Chem 47: 4941-9 (2004) Article DOI: 10.1021/jm049592c BindingDB Entry DOI: 10.7270/Q2WD401S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50151720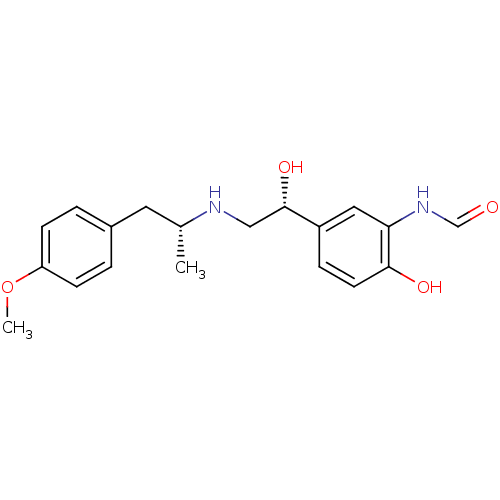 (ARFORMOTEROL TARTRATE | CHEMBL1363 | CHEMBL605993 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [125I]cyanopindolol from human recombinant beta2 adrenergic receptor expressed in CHO cells by filtration assay | J Med Chem 53: 3675-84 (2010) Article DOI: 10.1021/jm100068m BindingDB Entry DOI: 10.7270/Q27H1JRT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peptide deformylase (Escherichia coli) | BDBM50153080 ((R)-2-[(Formyl-hydroxy-amino)-methyl]-hexanoic aci...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Inhibitory effect against E. coli peptide deformylase (PDF) by DPPI assay | J Med Chem 47: 4941-9 (2004) Article DOI: 10.1021/jm049592c BindingDB Entry DOI: 10.7270/Q2WD401S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Escherichia coli) | BDBM50153081 (CHEMBL365910 | N-((3S,6R)-3-tert-Butyl-2,5-dioxo-1...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Inhibitory effect against E. coli peptide deformylase (PDF) by AAP assay | J Med Chem 47: 4941-9 (2004) Article DOI: 10.1021/jm049592c BindingDB Entry DOI: 10.7270/Q2WD401S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Escherichia coli) | BDBM50153079 (CHEMBL364979 | N-[(3S,6R)-3-(4-Amino-butyl)-2,5-di...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Inhibitory effect against E. coli peptide deformylase (PDF) by AAP assay | J Med Chem 47: 4941-9 (2004) Article DOI: 10.1021/jm049592c BindingDB Entry DOI: 10.7270/Q2WD401S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Escherichia coli) | BDBM50153087 (CHEMBL188894 | N-((3S,6R)-3-tert-Butyl-2,5-dioxo-1...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Inhibitory effect against E. coli peptide deformylase (PDF) by DPPI assay | J Med Chem 47: 4941-9 (2004) Article DOI: 10.1021/jm049592c BindingDB Entry DOI: 10.7270/Q2WD401S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Escherichia coli) | BDBM50153082 ((R)-2-[(Formyl-hydroxy-amino)-methyl]-heptanoic ac...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Inhibitory effect against E. coli peptide deformylase (PDF) by DPPI assay | J Med Chem 47: 4941-9 (2004) Article DOI: 10.1021/jm049592c BindingDB Entry DOI: 10.7270/Q2WD401S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50318159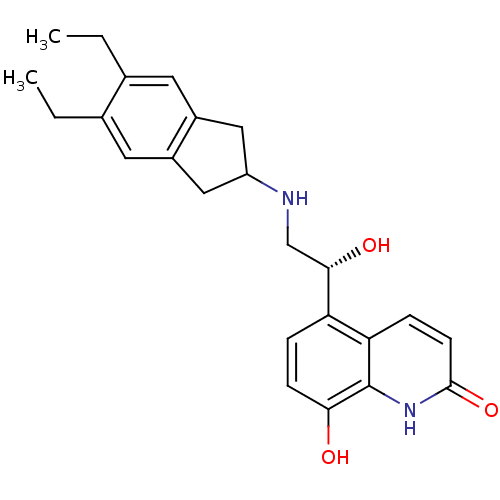 (8-Hydroxy-5-[(R)-1-hydroxy-2-(5,6-diethylindan-2-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [125I]cyanopindolol from human recombinant beta2 adrenergic receptor expressed in CHO cells by filtration assay | J Med Chem 53: 3675-84 (2010) Article DOI: 10.1021/jm100068m BindingDB Entry DOI: 10.7270/Q27H1JRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Escherichia coli) | BDBM50153088 (CHEMBL365416 | N-((3S,6R)-3-tert-Butyl-2,5-dioxo-1...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 77 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Inhibitory effect against E. coli peptide deformylase (PDF) by DPPI assay | J Med Chem 47: 4941-9 (2004) Article DOI: 10.1021/jm049592c BindingDB Entry DOI: 10.7270/Q2WD401S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Escherichia coli) | BDBM50153086 (CHEMBL188671 | N-[(1R,2S)-3-(4-Amino-butyl)-2,5-di...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 92 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Inhibitory effect against E. coli peptide deformylase (PDF) by DPPI assay | J Med Chem 47: 4941-9 (2004) Article DOI: 10.1021/jm049592c BindingDB Entry DOI: 10.7270/Q2WD401S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Escherichia coli) | BDBM50153085 (CHEMBL364836 | N-((3S,6R)-3-tert-Butyl-2,5-dioxo-1...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 96 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Inhibitory effect against E. coli peptide deformylase (PDF) by DPPI assay | J Med Chem 47: 4941-9 (2004) Article DOI: 10.1021/jm049592c BindingDB Entry DOI: 10.7270/Q2WD401S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Escherichia coli) | BDBM50153083 (CHEMBL441502 | N-((3S,6R)-3-tert-Butyl-2,5-dioxo-1...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 109 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Inhibitory effect against E. coli peptide deformylase (PDF) by DPPI assay | J Med Chem 47: 4941-9 (2004) Article DOI: 10.1021/jm049592c BindingDB Entry DOI: 10.7270/Q2WD401S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50318157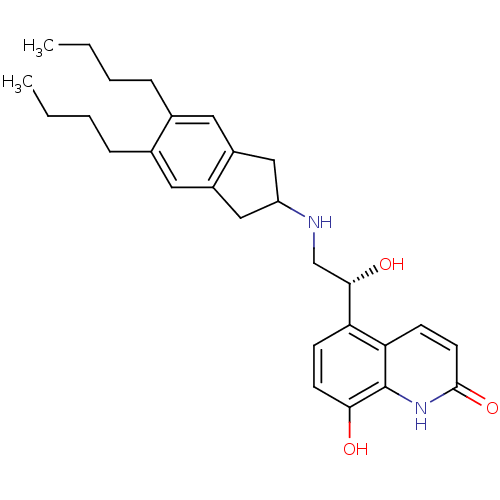 (8-Hydroxy-5-[(R)-1-hydroxy-2-(5,6-di-n-butylindan-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 112 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [125I]cyanopindolol from human recombinant beta2 adrenergic receptor expressed in CHO cells by filtration assay | J Med Chem 53: 3675-84 (2010) Article DOI: 10.1021/jm100068m BindingDB Entry DOI: 10.7270/Q27H1JRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50318158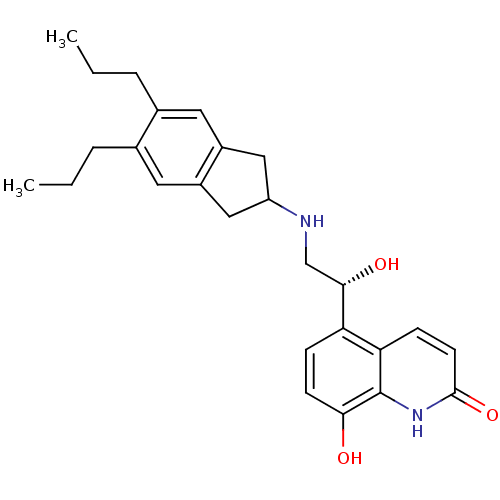 (8-Hydroxy-5-[(R)-1-hydroxy-2-(5,6-di-n-propylindan...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 119 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [125I]cyanopindolol from human recombinant beta2 adrenergic receptor expressed in CHO cells by filtration assay | J Med Chem 53: 3675-84 (2010) Article DOI: 10.1021/jm100068m BindingDB Entry DOI: 10.7270/Q27H1JRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50318161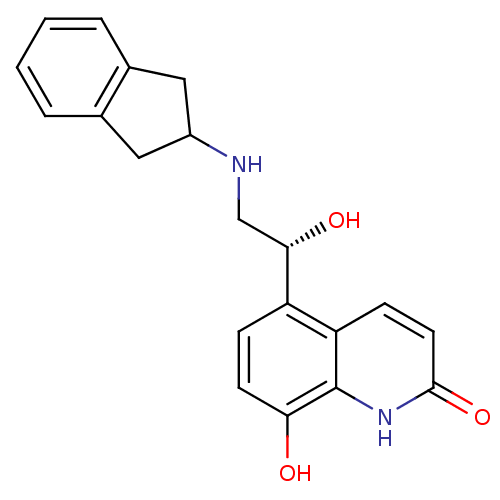 (8-Hydroxy-5-[(R)-1-hydroxy-2-(indan-2-ylamino)-eth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 218 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [125I]cyanopindolol from human recombinant beta2 adrenergic receptor expressed in CHO cells by filtration assay | J Med Chem 53: 3675-84 (2010) Article DOI: 10.1021/jm100068m BindingDB Entry DOI: 10.7270/Q27H1JRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Escherichia coli) | BDBM50153079 (CHEMBL364979 | N-[(3S,6R)-3-(4-Amino-butyl)-2,5-di...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 258 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Inhibitory effect against E. coli peptide deformylase (PDF) by DPPI assay | J Med Chem 47: 4941-9 (2004) Article DOI: 10.1021/jm049592c BindingDB Entry DOI: 10.7270/Q2WD401S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50318155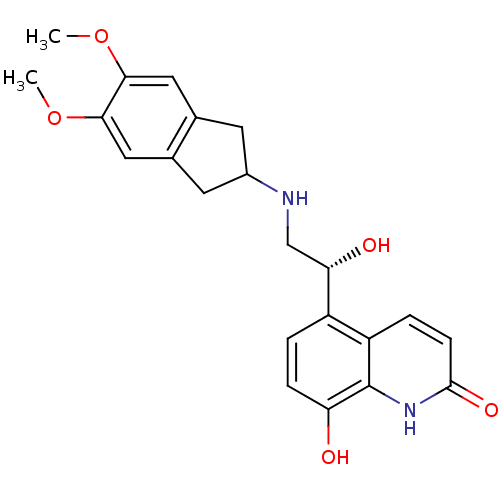 (8-Hydroxy-5-[(R)-1-hydroxy-2-(5,6-dimethoxyindan-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 342 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [125I]cyanopindolol from human recombinant beta2 adrenergic receptor expressed in CHO cells by filtration assay | J Med Chem 53: 3675-84 (2010) Article DOI: 10.1021/jm100068m BindingDB Entry DOI: 10.7270/Q27H1JRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50318160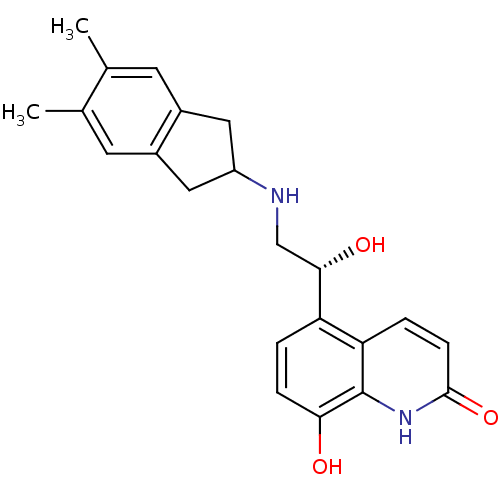 (8-Hydroxy-5-[(R)-1-hydroxy-2-(5,6-dimethylindan-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 522 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [125I]cyanopindolol from human recombinant beta2 adrenergic receptor expressed in CHO cells by filtration assay | J Med Chem 53: 3675-84 (2010) Article DOI: 10.1021/jm100068m BindingDB Entry DOI: 10.7270/Q27H1JRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50318154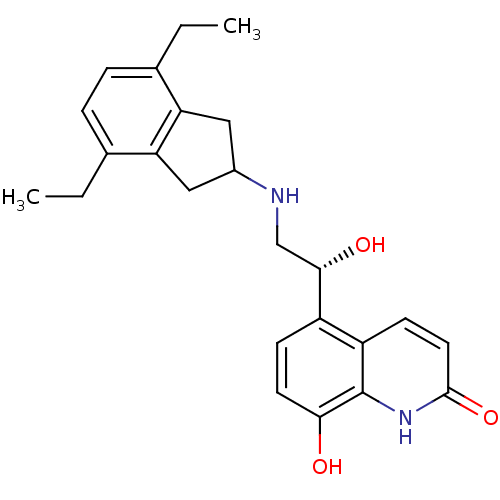 (8-Hydroxy-5-[(R)-1-hydroxy-2-(4,7-diethylindan-2-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 692 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [125I]cyanopindolol from human recombinant beta2 adrenergic receptor expressed in CHO cells by filtration assay | J Med Chem 53: 3675-84 (2010) Article DOI: 10.1021/jm100068m BindingDB Entry DOI: 10.7270/Q27H1JRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Escherichia coli) | BDBM50153084 (CHEMBL361449 | N-(4-{(3R,14S)-14-[(Formyl-hydroxy-...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Inhibitory effect against E. coli peptide deformylase (PDF) by DPPI assay | J Med Chem 47: 4941-9 (2004) Article DOI: 10.1021/jm049592c BindingDB Entry DOI: 10.7270/Q2WD401S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM25769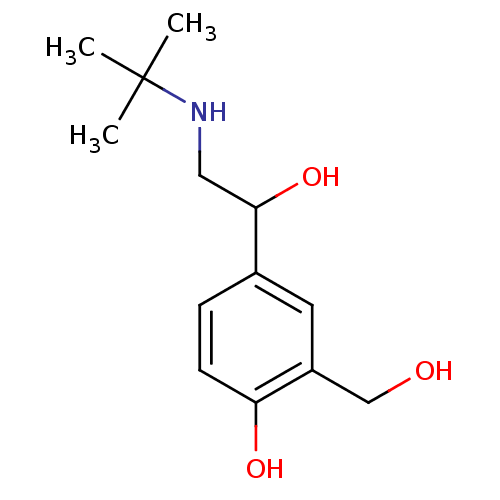 (4-[2-(tert-butylamino)-1-hydroxyethyl]-2-(hydroxym...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 1.83E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [125I]cyanopindolol from human recombinant beta2 adrenergic receptor expressed in CHO cells by filtration assay | J Med Chem 53: 3675-84 (2010) Article DOI: 10.1021/jm100068m BindingDB Entry DOI: 10.7270/Q27H1JRT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peptide deformylase (Escherichia coli) | BDBM50153081 (CHEMBL365910 | N-((3S,6R)-3-tert-Butyl-2,5-dioxo-1...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Inhibitory effect against E. coli peptide deformylase (PDF) by DPPI assay | J Med Chem 47: 4941-9 (2004) Article DOI: 10.1021/jm049592c BindingDB Entry DOI: 10.7270/Q2WD401S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50207120 (3-isobutyl-8-((6-methoxyisoquinolin-4-yl)methyl)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research Curated by ChEMBL | Assay Description Inhibition of adenosine A1 receptor | Bioorg Med Chem Lett 17: 2376-9 (2007) Article DOI: 10.1016/j.bmcl.2006.11.019 BindingDB Entry DOI: 10.7270/Q2X63NSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50318153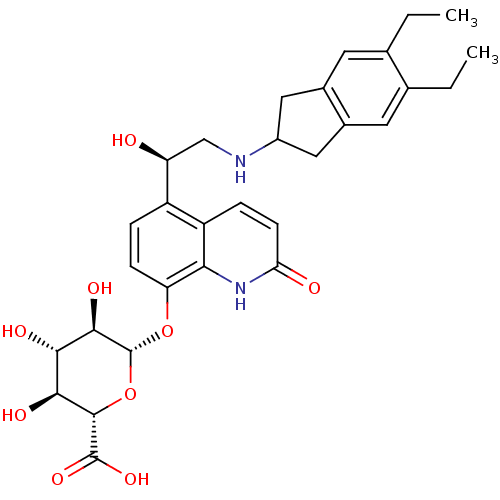 ((2S,3S,4S,5R,6S)-6-(5-((R)-2-(5,6-diethyl-2,3-dihy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [125I]cyanopindolol from human recombinant beta2 adrenergic receptor expressed in CHO cells by filtration assay | J Med Chem 53: 3675-84 (2010) Article DOI: 10.1021/jm100068m BindingDB Entry DOI: 10.7270/Q27H1JRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (GUINEA PIG) | BDBM50318156 (CHEMBL1094785 | carmoterol) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Agonist activity at beta2 adrenergic receptor in guinea pig tracheal strip assessed as inhibition of electrically-induced bronchocontractile response... | J Med Chem 53: 3675-84 (2010) Article DOI: 10.1021/jm100068m BindingDB Entry DOI: 10.7270/Q27H1JRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (GUINEA PIG) | BDBM50151720 (ARFORMOTEROL TARTRATE | CHEMBL1363 | CHEMBL605993 ...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Agonist activity at beta2 adrenergic receptor in guinea pig tracheal strip assessed as inhibition of electrically-induced bronchocontractile response... | J Med Chem 53: 3675-84 (2010) Article DOI: 10.1021/jm100068m BindingDB Entry DOI: 10.7270/Q27H1JRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (GUINEA PIG) | BDBM50318161 (8-Hydroxy-5-[(R)-1-hydroxy-2-(indan-2-ylamino)-eth...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Agonist activity at beta2 adrenergic receptor in guinea pig tracheal strip assessed as inhibition of electrically-induced bronchocontractile response... | J Med Chem 53: 3675-84 (2010) Article DOI: 10.1021/jm100068m BindingDB Entry DOI: 10.7270/Q27H1JRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50207130 (8-((6,7-dimethoxy-1-methylisoquinolin-4-yl)methyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human platelet PDE5 by [3H]cGMP scintillation proximity assay | Bioorg Med Chem Lett 17: 2376-9 (2007) Article DOI: 10.1016/j.bmcl.2006.11.019 BindingDB Entry DOI: 10.7270/Q2X63NSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50207120 (3-isobutyl-8-((6-methoxyisoquinolin-4-yl)methyl)-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human platelet PDE5 by [3H]cGMP scintillation proximity assay | Bioorg Med Chem Lett 17: 2376-9 (2007) Article DOI: 10.1016/j.bmcl.2006.11.019 BindingDB Entry DOI: 10.7270/Q2X63NSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (GUINEA PIG) | BDBM50318155 (8-Hydroxy-5-[(R)-1-hydroxy-2-(5,6-dimethoxyindan-2...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Agonist activity at beta2 adrenergic receptor in guinea pig tracheal strip assessed as inhibition of electrically-induced bronchocontractile response... | J Med Chem 53: 3675-84 (2010) Article DOI: 10.1021/jm100068m BindingDB Entry DOI: 10.7270/Q27H1JRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50117734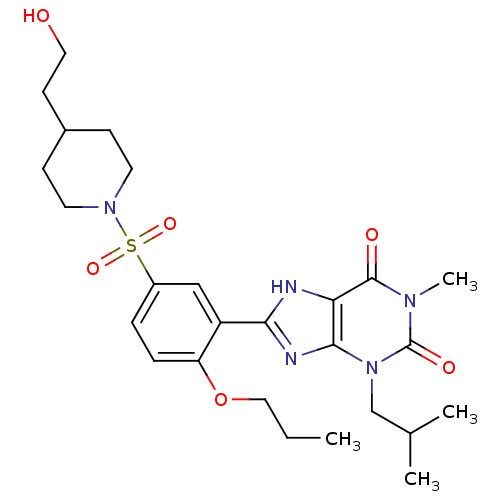 (8-{5-[4-(2-Hydroxy-ethyl)-piperidine-1-sulfonyl]-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Inhibitory activity against human platelet Phosphodiesterase 5 (PDE5) | Bioorg Med Chem Lett 12: 2587-90 (2002) BindingDB Entry DOI: 10.7270/Q2KW5FC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P04972/P11541/P16586/P22571/P23439/Q95142 (Bos taurus-Bos taurus (Bovine)) | BDBM50117721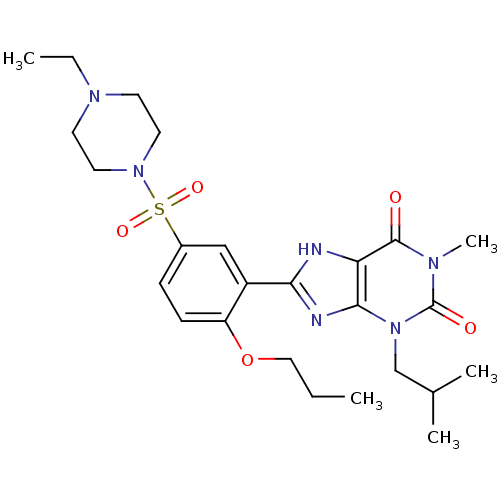 (3-Isobutyl-1-methyl-8-[5-(piperazin-1-ylmethanesul...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Inhibitory concentration against bovine retina phosphodiesterase 6 activity | Bioorg Med Chem Lett 12: 2587-90 (2002) BindingDB Entry DOI: 10.7270/Q2KW5FC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50117711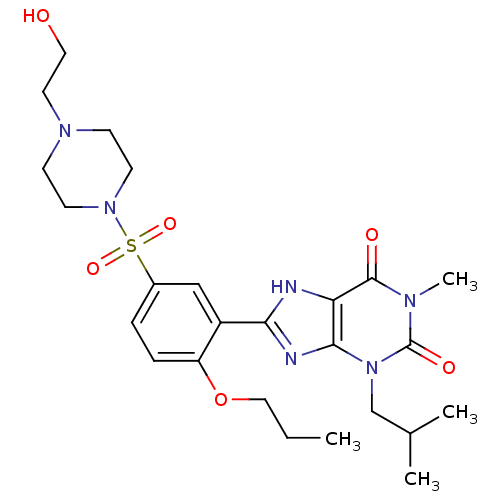 (8-{5-[4-(2-Hydroxy-ethyl)-piperazine-1-sulfonyl]-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Inhibitory activity against human platelet Phosphodiesterase 5 (PDE5) | Bioorg Med Chem Lett 12: 2587-90 (2002) BindingDB Entry DOI: 10.7270/Q2KW5FC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50117719 (4-[3-(3-Isobutyl-1-methyl-2,6-dioxo-2,3,6,7-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Inhibitory activity against human platelet Phosphodiesterase 5 (PDE5) | Bioorg Med Chem Lett 12: 2587-90 (2002) BindingDB Entry DOI: 10.7270/Q2KW5FC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (GUINEA PIG) | BDBM25771 (1-hydroxy-2-naphthoic acid;4-[1-hydroxy-2-[6-(4-ph...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Agonist activity at beta2 adrenergic receptor in guinea pig tracheal strip assessed as inhibition of electrically-induced bronchocontractile response... | J Med Chem 53: 3675-84 (2010) Article DOI: 10.1021/jm100068m BindingDB Entry DOI: 10.7270/Q27H1JRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50117724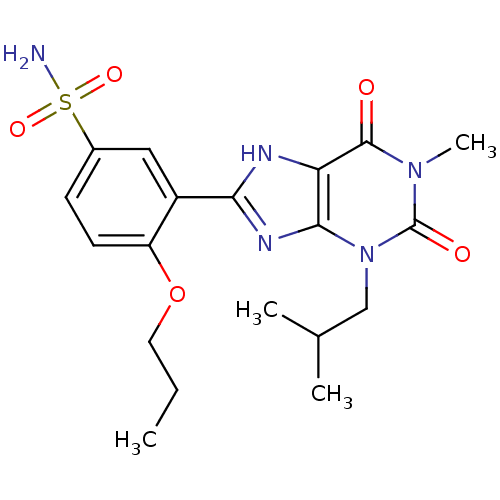 (3-(3-Isobutyl-1-methyl-2,6-dioxo-2,3,6,7-tetrahydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Inhibitory activity against human platelet Phosphodiesterase 5 (PDE5) | Bioorg Med Chem Lett 12: 2587-90 (2002) BindingDB Entry DOI: 10.7270/Q2KW5FC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50207121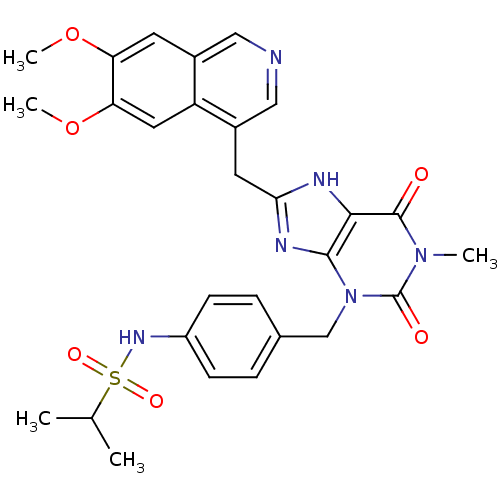 (CHEMBL245648 | N-(4-((8-((6,7-dimethoxyisoquinolin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human platelet PDE5 by [3H]cGMP scintillation proximity assay | Bioorg Med Chem Lett 17: 2376-9 (2007) Article DOI: 10.1016/j.bmcl.2006.11.019 BindingDB Entry DOI: 10.7270/Q2X63NSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50117721 (3-Isobutyl-1-methyl-8-[5-(piperazin-1-ylmethanesul...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Inhibitory activity against human platelet Phosphodiesterase 5 (PDE5) | Bioorg Med Chem Lett 12: 2587-90 (2002) BindingDB Entry DOI: 10.7270/Q2KW5FC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50207123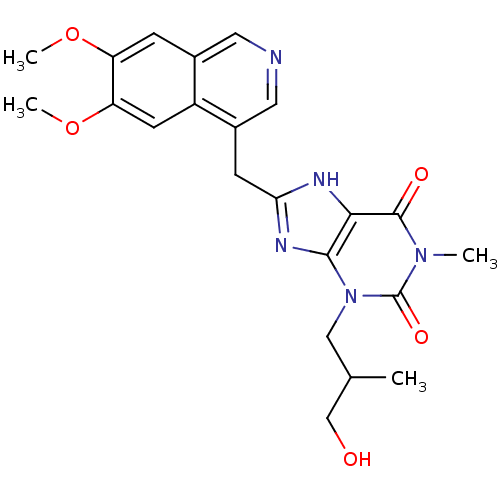 (8-((6,7-dimethoxyisoquinolin-4-yl)methyl)-3-(3-hyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human platelet PDE5 by [3H]cGMP scintillation proximity assay | Bioorg Med Chem Lett 17: 2376-9 (2007) Article DOI: 10.1016/j.bmcl.2006.11.019 BindingDB Entry DOI: 10.7270/Q2X63NSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P04972/P11541/P16586/P22571/P23439/Q95142 (Bos taurus-Bos taurus (Bovine)) | BDBM50117715 (3-Isobutyl-1-methyl-8-[5-(4-methyl-piperazine-1-su...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Inhibitory concentration against bovine retina phosphodiesterase 6 activity | Bioorg Med Chem Lett 12: 2587-90 (2002) BindingDB Entry DOI: 10.7270/Q2KW5FC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (GUINEA PIG) | BDBM50318159 (8-Hydroxy-5-[(R)-1-hydroxy-2-(5,6-diethylindan-2-y...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Agonist activity at beta2 adrenergic receptor in guinea pig tracheal strip assessed as inhibition of electrically-induced bronchocontractile response... | J Med Chem 53: 3675-84 (2010) Article DOI: 10.1021/jm100068m BindingDB Entry DOI: 10.7270/Q27H1JRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50207127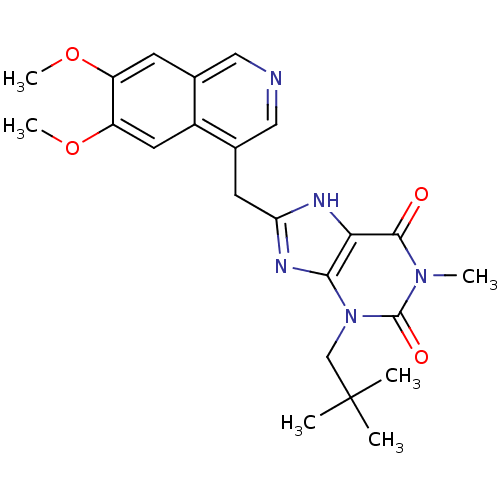 (8-((6,7-dimethoxyisoquinolin-4-yl)methyl)-1-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human platelet PDE5 by [3H]cGMP scintillation proximity assay | Bioorg Med Chem Lett 17: 2376-9 (2007) Article DOI: 10.1016/j.bmcl.2006.11.019 BindingDB Entry DOI: 10.7270/Q2X63NSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 224 total ) | Next | Last >> |