 Found 384 hits with Last Name = 'moxham' and Initial = 'c'
Found 384 hits with Last Name = 'moxham' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50114806
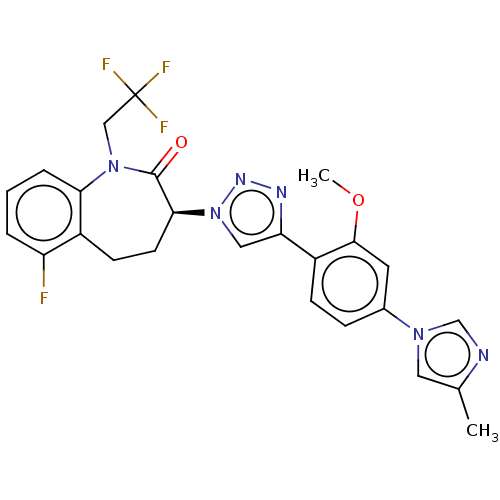
(CHEMBL3609752)Show SMILES COc1cc(ccc1-c1cn(nn1)[C@H]1CCc2c(F)cccc2N(CC(F)(F)F)C1=O)-n1cnc(C)c1 |r| Show InChI InChI=1S/C25H22F4N6O2/c1-15-11-33(14-30-15)16-6-7-18(23(10-16)37-2)20-12-35(32-31-20)22-9-8-17-19(26)4-3-5-21(17)34(24(22)36)13-25(27,28)29/h3-7,10-12,14,22H,8-9,13H2,1-2H3/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG expressed in HEK293 cells by radioligand displacement assay |
Bioorg Med Chem Lett 25: 3495-500 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.032
BindingDB Entry DOI: 10.7270/Q2125VFP |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50114807
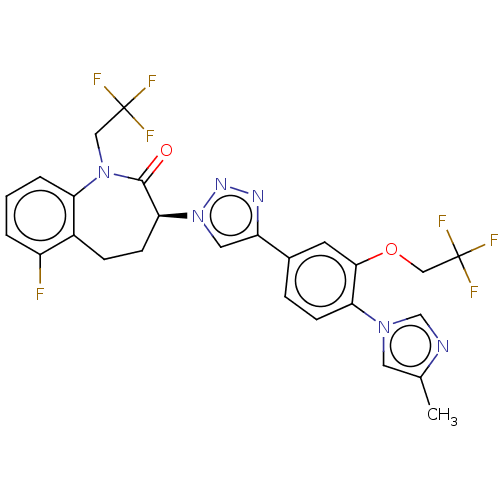
(CHEMBL3608385)Show SMILES Cc1cn(cn1)-c1ccc(cc1OCC(F)(F)F)-c1cn(nn1)[C@H]1CCc2c(F)cccc2N(CC(F)(F)F)C1=O |r| Show InChI InChI=1S/C26H21F7N6O2/c1-15-10-37(14-34-15)21-7-5-16(9-23(21)41-13-26(31,32)33)19-11-39(36-35-19)22-8-6-17-18(27)3-2-4-20(17)38(24(22)40)12-25(28,29)30/h2-5,7,9-11,14,22H,6,8,12-13H2,1H3/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG expressed in HEK293 cells by radioligand displacement assay |
Bioorg Med Chem Lett 25: 3495-500 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.032
BindingDB Entry DOI: 10.7270/Q2125VFP |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50114800

(CHEMBL3609749)Show SMILES COc1cc(ccc1-n1cnc(C)c1)-c1cn(nn1)[C@H]1CCc2c(F)cccc2N(CC(F)(F)F)C1=O |r| Show InChI InChI=1S/C25H22F4N6O2/c1-15-11-33(14-30-15)21-8-6-16(10-23(21)37-2)19-12-35(32-31-19)22-9-7-17-18(26)4-3-5-20(17)34(24(22)36)13-25(27,28)29/h3-6,8,10-12,14,22H,7,9,13H2,1-2H3/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG expressed in HEK293 cells by radioligand displacement assay |
Bioorg Med Chem Lett 25: 3495-500 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.032
BindingDB Entry DOI: 10.7270/Q2125VFP |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50114836
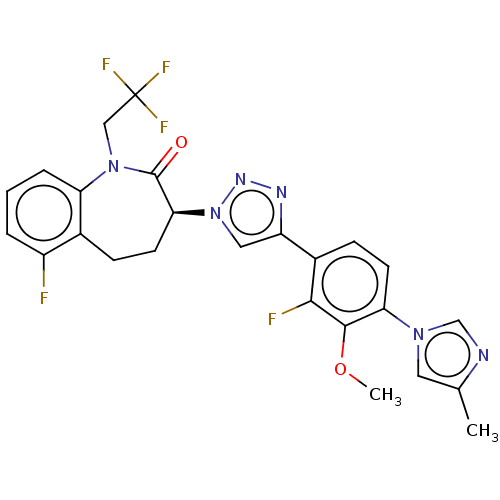
(CHEMBL3608387)Show SMILES COc1c(F)c(ccc1-n1cnc(C)c1)-c1cn(nn1)[C@H]1CCc2c(F)cccc2N(CC(F)(F)F)C1=O |r| Show InChI InChI=1S/C25H21F5N6O2/c1-14-10-34(13-31-14)20-8-7-16(22(27)23(20)38-2)18-11-36(33-32-18)21-9-6-15-17(26)4-3-5-19(15)35(24(21)37)12-25(28,29)30/h3-5,7-8,10-11,13,21H,6,9,12H2,1-2H3/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG expressed in HEK293 cells by radioligand displacement assay |
Bioorg Med Chem Lett 25: 3495-500 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.032
BindingDB Entry DOI: 10.7270/Q2125VFP |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50114805
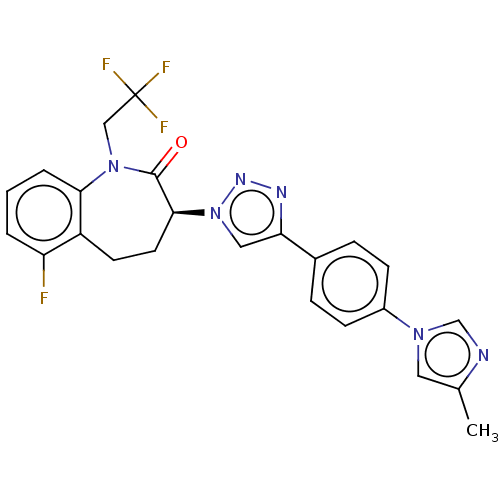
(CHEMBL3608384)Show SMILES Cc1cn(cn1)-c1ccc(cc1)-c1cn(nn1)[C@H]1CCc2c(F)cccc2N(CC(F)(F)F)C1=O |r| Show InChI InChI=1S/C24H20F4N6O/c1-15-11-32(14-29-15)17-7-5-16(6-8-17)20-12-34(31-30-20)22-10-9-18-19(25)3-2-4-21(18)33(23(22)35)13-24(26,27)28/h2-8,11-12,14,22H,9-10,13H2,1H3/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG expressed in HEK293 cells by radioligand displacement assay |
Bioorg Med Chem Lett 25: 3495-500 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.032
BindingDB Entry DOI: 10.7270/Q2125VFP |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50114837
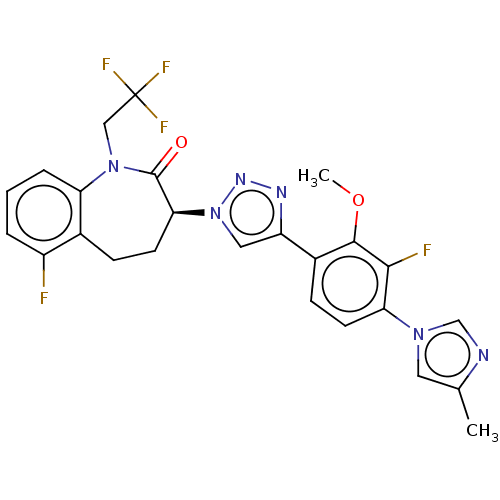
(CHEMBL3609755)Show SMILES COc1c(F)c(ccc1-c1cn(nn1)[C@H]1CCc2c(F)cccc2N(CC(F)(F)F)C1=O)-n1cnc(C)c1 |r| Show InChI InChI=1S/C25H21F5N6O2/c1-14-10-34(13-31-14)20-8-7-16(23(38-2)22(20)27)18-11-36(33-32-18)21-9-6-15-17(26)4-3-5-19(15)35(24(21)37)12-25(28,29)30/h3-5,7-8,10-11,13,21H,6,9,12H2,1-2H3/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG expressed in HEK293 cells by radioligand displacement assay |
Bioorg Med Chem Lett 25: 3495-500 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.032
BindingDB Entry DOI: 10.7270/Q2125VFP |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50114810
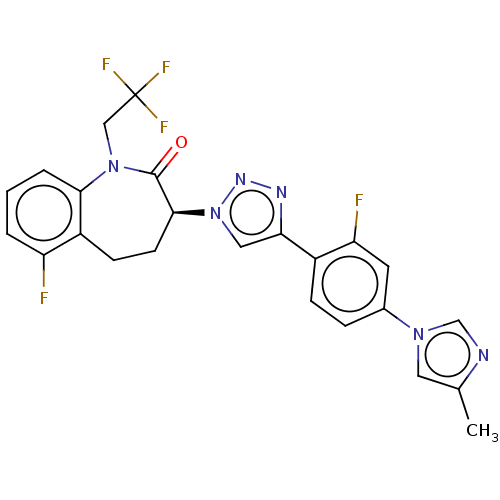
(CHEMBL3609754)Show SMILES Cc1cn(cn1)-c1ccc(-c2cn(nn2)[C@H]2CCc3c(F)cccc3N(CC(F)(F)F)C2=O)c(F)c1 |r| Show InChI InChI=1S/C24H19F5N6O/c1-14-10-33(13-30-14)15-5-6-16(19(26)9-15)20-11-35(32-31-20)22-8-7-17-18(25)3-2-4-21(17)34(23(22)36)12-24(27,28)29/h2-6,9-11,13,22H,7-8,12H2,1H3/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG expressed in HEK293 cells by radioligand displacement assay |
Bioorg Med Chem Lett 25: 3495-500 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.032
BindingDB Entry DOI: 10.7270/Q2125VFP |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50114845
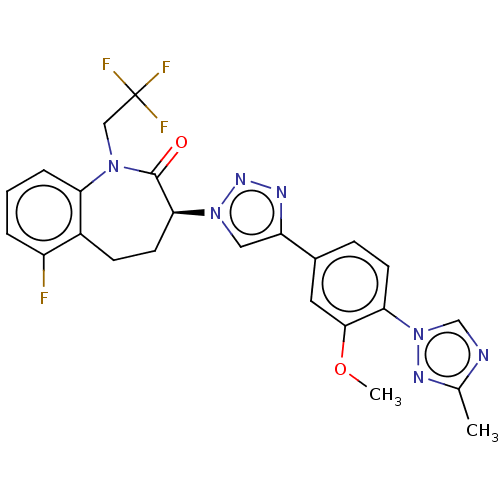
(CHEMBL3609759)Show SMILES COc1cc(ccc1-n1cnc(C)n1)-c1cn(nn1)[C@H]1CCc2c(F)cccc2N(CC(F)(F)F)C1=O |r| Show InChI InChI=1S/C24H21F4N7O2/c1-14-29-13-35(31-14)20-8-6-15(10-22(20)37-2)18-11-34(32-30-18)21-9-7-16-17(25)4-3-5-19(16)33(23(21)36)12-24(26,27)28/h3-6,8,10-11,13,21H,7,9,12H2,1-2H3/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG expressed in HEK293 cells by radioligand displacement assay |
Bioorg Med Chem Lett 25: 3495-500 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.032
BindingDB Entry DOI: 10.7270/Q2125VFP |
More data for this
Ligand-Target Pair | |
Gamma-secretase subunit APH-1A/Gamma-secretase subunit APH-1B/Gamma-secretase subunit PEN-2/Nicastrin/Presenilin-1/Presenilin-2
(Homo sapiens (Human)) | BDBM50114798
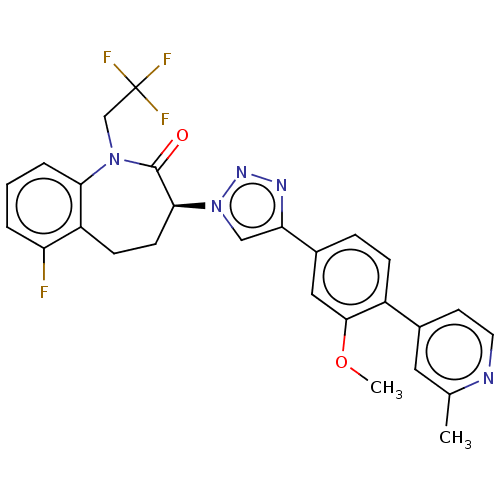
(CHEMBL3608381)Show SMILES COc1cc(ccc1-c1ccnc(C)c1)-c1cn(nn1)[C@H]1CCc2c(F)cccc2N(CC(F)(F)F)C1=O |r| Show InChI InChI=1S/C27H23F4N5O2/c1-16-12-17(10-11-32-16)19-7-6-18(13-25(19)38-2)22-14-36(34-33-22)24-9-8-20-21(28)4-3-5-23(20)35(26(24)37)15-27(29,30)31/h3-7,10-14,24H,8-9,15H2,1-2H3/t24-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Modulation of gamma-secretase in human SH-SY5Y cells overexpressing APP C-terminal fragment SPA4CT assessed as inhibition of intracellular amyloid be... |
Bioorg Med Chem Lett 25: 3495-500 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.032
BindingDB Entry DOI: 10.7270/Q2125VFP |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50484994
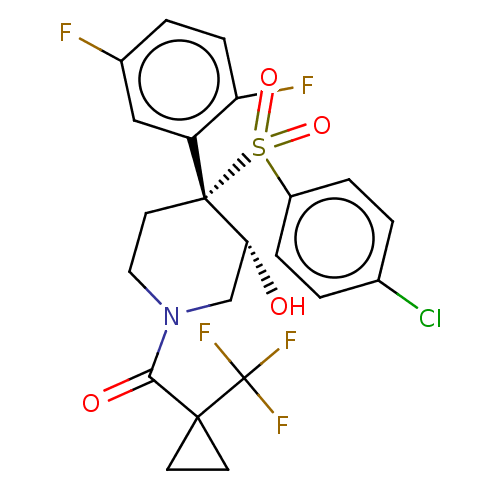
(CHEMBL2019019)Show SMILES O[C@@H]1CN(CC[C@]1(c1cc(F)ccc1F)S(=O)(=O)c1ccc(Cl)cc1)C(=O)C1(CC1)C(F)(F)F |r| Show InChI InChI=1S/C22H19ClF5NO4S/c23-13-1-4-15(5-2-13)34(32,33)21(16-11-14(24)3-6-17(16)25)9-10-29(12-18(21)30)19(31)20(7-8-20)22(26,27)28/h1-6,11,18,30H,7-10,12H2/t18-,21-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of gamma-secretase in human SH-SY5Y cells co-expressing APP C-terminal fragment SPA4CT assessed as inhibition of amyloid beta 40 productio... |
Bioorg Med Chem Lett 22: 3203-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.038
BindingDB Entry DOI: 10.7270/Q2D221GG |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50498583

(CHEMBL3609639)Show SMILES COc1cc(ccc1-n1cnc(C)c1)-c1cn(nn1)C1CCc2c(F)c(Br)ccc2N(CC(F)(F)F)C1=O Show InChI InChI=1S/C25H21BrF4N6O2/c1-14-10-34(13-31-14)20-6-3-15(9-22(20)38-2)18-11-36(33-32-18)21-7-4-16-19(8-5-17(26)23(16)27)35(24(21)37)12-25(28,29)30/h3,5-6,8-11,13,21H,4,7,12H2,1-2H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Boston
Curated by ChEMBL
| Assay Description
Inhibition of gamma-secretase in human SH-SY5Y cells expressing beta-APP C-terminal fragment SPA4CT assessed as decrease of amyloid beta-42 level by ... |
Bioorg Med Chem Lett 25: 3488-94 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.003
BindingDB Entry DOI: 10.7270/Q2MG7SHX |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50484994

(CHEMBL2019019)Show SMILES O[C@@H]1CN(CC[C@]1(c1cc(F)ccc1F)S(=O)(=O)c1ccc(Cl)cc1)C(=O)C1(CC1)C(F)(F)F |r| Show InChI InChI=1S/C22H19ClF5NO4S/c23-13-1-4-15(5-2-13)34(32,33)21(16-11-14(24)3-6-17(16)25)9-10-29(12-18(21)30)19(31)20(7-8-20)22(26,27)28/h1-6,11,18,30H,7-10,12H2/t18-,21-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of gamma-secretase in human SH-SY5Y cells co-expressing APP C-terminal fragment SPA4CT assessed as inhibition of amyloid beta 40 productio... |
Bioorg Med Chem Lett 22: 3203-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.038
BindingDB Entry DOI: 10.7270/Q2D221GG |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50484994

(CHEMBL2019019)Show SMILES O[C@@H]1CN(CC[C@]1(c1cc(F)ccc1F)S(=O)(=O)c1ccc(Cl)cc1)C(=O)C1(CC1)C(F)(F)F |r| Show InChI InChI=1S/C22H19ClF5NO4S/c23-13-1-4-15(5-2-13)34(32,33)21(16-11-14(24)3-6-17(16)25)9-10-29(12-18(21)30)19(31)20(7-8-20)22(26,27)28/h1-6,11,18,30H,7-10,12H2/t18-,21-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of gamma-secretase in human SH-SY5Y cells co-expressing APP C-terminal fragment SPA4CT assessed as inhibition of amyloid beta 42 productio... |
Bioorg Med Chem Lett 22: 3203-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.038
BindingDB Entry DOI: 10.7270/Q2D221GG |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50484994

(CHEMBL2019019)Show SMILES O[C@@H]1CN(CC[C@]1(c1cc(F)ccc1F)S(=O)(=O)c1ccc(Cl)cc1)C(=O)C1(CC1)C(F)(F)F |r| Show InChI InChI=1S/C22H19ClF5NO4S/c23-13-1-4-15(5-2-13)34(32,33)21(16-11-14(24)3-6-17(16)25)9-10-29(12-18(21)30)19(31)20(7-8-20)22(26,27)28/h1-6,11,18,30H,7-10,12H2/t18-,21-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of gamma-secretase in human SH-SY5Y cells co-expressing APP C-terminal fragment SPA4CT assessed as inhibition of amyloid beta 42 productio... |
Bioorg Med Chem Lett 22: 3203-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.038
BindingDB Entry DOI: 10.7270/Q2D221GG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50079859

((R)-2-[2,6-Dibromo-4-(6-bromo-benzo[b]naphtho[2,3-...)Show SMILES OC(=O)[C@@H](CCN1C(=O)c2ccccc2C1=O)Oc1c(Br)cc(cc1Br)-c1c2c3ccccc3sc2c(Br)c2ccccc12 Show InChI InChI=1S/C34H20Br3NO5S/c35-23-15-17(27-18-7-1-2-8-19(18)29(37)31-28(27)22-11-5-6-12-26(22)44-31)16-24(36)30(23)43-25(34(41)42)13-14-38-32(39)20-9-3-4-10-21(20)33(38)40/h1-12,15-16,25H,13-14H2,(H,41,42)/t25-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
The compound was tested in vitro for the inhibitory activity against Protein-tyrosine phosphatase 1B (human PTPases.) |
J Med Chem 42: 3199-202 (1999)
Article DOI: 10.1021/jm990260v
BindingDB Entry DOI: 10.7270/Q2DF6QCQ |
More data for this
Ligand-Target Pair | |
Gamma-secretase subunit APH-1A/Gamma-secretase subunit APH-1B/Gamma-secretase subunit PEN-2/Nicastrin/Presenilin-1/Presenilin-2
(Homo sapiens (Human)) | BDBM50114806

(CHEMBL3609752)Show SMILES COc1cc(ccc1-c1cn(nn1)[C@H]1CCc2c(F)cccc2N(CC(F)(F)F)C1=O)-n1cnc(C)c1 |r| Show InChI InChI=1S/C25H22F4N6O2/c1-15-11-33(14-30-15)16-6-7-18(23(10-16)37-2)20-12-35(32-31-20)22-9-8-17-19(26)4-3-5-21(17)34(24(22)36)13-25(27,28)29/h3-7,10-12,14,22H,8-9,13H2,1-2H3/t22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Modulation of gamma-secretase in human SH-SY5Y cells overexpressing APP C-terminal fragment SPA4CT assessed as inhibition of intracellular amyloid be... |
Bioorg Med Chem Lett 25: 3495-500 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.032
BindingDB Entry DOI: 10.7270/Q2125VFP |
More data for this
Ligand-Target Pair | |
Gamma-secretase subunit APH-1A/Gamma-secretase subunit APH-1B/Gamma-secretase subunit PEN-2/Nicastrin/Presenilin-1/Presenilin-2
(Homo sapiens (Human)) | BDBM50114837

(CHEMBL3609755)Show SMILES COc1c(F)c(ccc1-c1cn(nn1)[C@H]1CCc2c(F)cccc2N(CC(F)(F)F)C1=O)-n1cnc(C)c1 |r| Show InChI InChI=1S/C25H21F5N6O2/c1-14-10-34(13-31-14)20-8-7-16(23(38-2)22(20)27)18-11-36(33-32-18)21-9-6-15-17(26)4-3-5-19(15)35(24(21)37)12-25(28,29)30/h3-5,7-8,10-11,13,21H,6,9,12H2,1-2H3/t21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Modulation of gamma-secretase in human SH-SY5Y cells overexpressing APP C-terminal fragment SPA4CT assessed as inhibition of intracellular amyloid be... |
Bioorg Med Chem Lett 25: 3495-500 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.032
BindingDB Entry DOI: 10.7270/Q2125VFP |
More data for this
Ligand-Target Pair | |
Gamma-secretase subunit APH-1A/Gamma-secretase subunit APH-1B/Gamma-secretase subunit PEN-2/Nicastrin/Presenilin-1/Presenilin-2
(Homo sapiens (Human)) | BDBM50114798

(CHEMBL3608381)Show SMILES COc1cc(ccc1-c1ccnc(C)c1)-c1cn(nn1)[C@H]1CCc2c(F)cccc2N(CC(F)(F)F)C1=O |r| Show InChI InChI=1S/C27H23F4N5O2/c1-16-12-17(10-11-32-16)19-7-6-18(13-25(19)38-2)22-14-36(34-33-22)24-9-8-20-21(28)4-3-5-23(20)35(26(24)37)15-27(29,30)31/h3-7,10-14,24H,8-9,15H2,1-2H3/t24-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Modulation of gamma-secretase in human SH-SY5Y cells overexpressing APP C-terminal fragment SPA4CT assessed as inhibition of intracellular amyloid be... |
Bioorg Med Chem Lett 25: 3495-500 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.032
BindingDB Entry DOI: 10.7270/Q2125VFP |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50086986
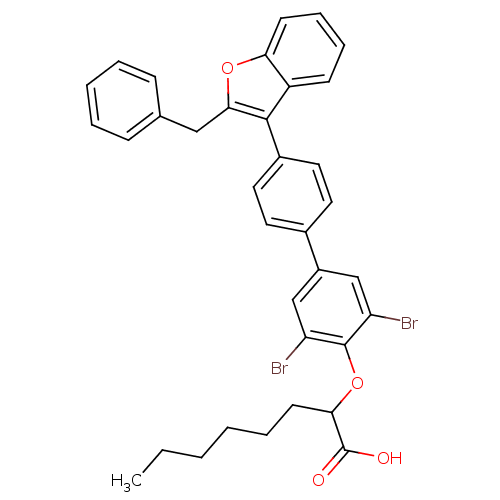
(2-[4'-(2-Benzyl-benzofuran-3-yl)-3,5-dibromo-biphe...)Show SMILES CCCCCCC(Oc1c(Br)cc(cc1Br)-c1ccc(cc1)-c1c(Cc2ccccc2)oc2ccccc12)C(O)=O Show InChI InChI=1S/C35H32Br2O4/c1-2-3-4-8-15-31(35(38)39)41-34-28(36)21-26(22-29(34)37)24-16-18-25(19-17-24)33-27-13-9-10-14-30(27)40-32(33)20-23-11-6-5-7-12-23/h5-7,9-14,16-19,21-22,31H,2-4,8,15,20H2,1H3,(H,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) |
J Med Chem 43: 1293-310 (2001)
BindingDB Entry DOI: 10.7270/Q2W958FT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50086896

(4-[4''-(2-Benzyl-benzo[b]thiophen-3-yl)-3-bromo-bi...)Show SMILES OC(=O)c1ccc(cc1O)S(=O)(=O)Oc1ccc(cc1Br)-c1ccc(cc1)-c1c(Cc2ccccc2)sc2ccccc12 Show InChI InChI=1S/C34H23BrO6S2/c35-28-19-24(14-17-30(28)41-43(39,40)25-15-16-26(34(37)38)29(36)20-25)22-10-12-23(13-11-22)33-27-8-4-5-9-31(27)42-32(33)18-21-6-2-1-3-7-21/h1-17,19-20,36H,18H2,(H,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) |
J Med Chem 43: 1293-310 (2001)
BindingDB Entry DOI: 10.7270/Q2W958FT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50086947

(2-[4'-(2-Benzyl-benzo[b]thiophen-3-yl)-3,5-dibromo...)Show SMILES OC(=O)C(Cc1ccccc1)Oc1c(Br)cc(cc1Br)-c1ccc(cc1)-c1c(Cc2ccccc2)sc2ccccc12 Show InChI InChI=1S/C36H26Br2O3S/c37-29-21-27(22-30(38)35(29)41-31(36(39)40)19-23-9-3-1-4-10-23)25-15-17-26(18-16-25)34-28-13-7-8-14-32(28)42-33(34)20-24-11-5-2-6-12-24/h1-18,21-22,31H,19-20H2,(H,39,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) |
J Med Chem 43: 1293-310 (2001)
BindingDB Entry DOI: 10.7270/Q2W958FT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50086972

(2-{4-[4-(2-benzyl-1-benzothiophen-3-yl)phenyl]-2,6...)Show SMILES COc1cccc(c1)-c1cc(cc(-c2cccc(OC)c2)c1OCC(O)=O)-c1ccc(cc1)-c1c(Cc2ccccc2)sc2ccccc12 Show InChI InChI=1S/C43H34O5S/c1-46-34-14-8-12-31(23-34)37-25-33(26-38(43(37)48-27-41(44)45)32-13-9-15-35(24-32)47-2)29-18-20-30(21-19-29)42-36-16-6-7-17-39(36)49-40(42)22-28-10-4-3-5-11-28/h3-21,23-26H,22,27H2,1-2H3,(H,44,45) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) |
J Med Chem 43: 1293-310 (2001)
BindingDB Entry DOI: 10.7270/Q2W958FT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50086950
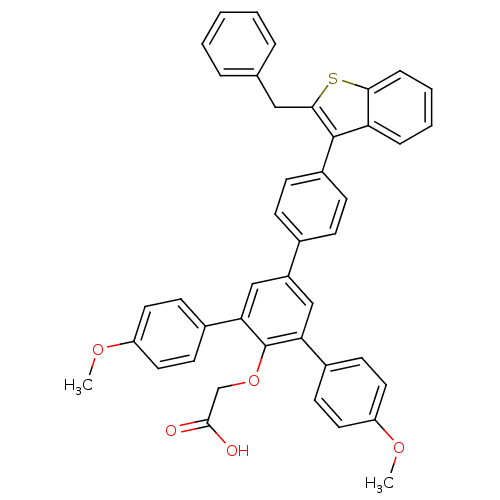
(2-[4-[4-(2-benzylbenzo[b]thiophen-3-yl)phenyl]-2,6...)Show SMILES COc1ccc(cc1)-c1cc(cc(-c2ccc(OC)cc2)c1OCC(O)=O)-c1ccc(cc1)-c1c(Cc2ccccc2)sc2ccccc12 Show InChI InChI=1S/C43H34O5S/c1-46-34-20-16-30(17-21-34)37-25-33(26-38(43(37)48-27-41(44)45)31-18-22-35(47-2)23-19-31)29-12-14-32(15-13-29)42-36-10-6-7-11-39(36)49-40(42)24-28-8-4-3-5-9-28/h3-23,25-26H,24,27H2,1-2H3,(H,44,45) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) |
J Med Chem 43: 1293-310 (2001)
BindingDB Entry DOI: 10.7270/Q2W958FT |
More data for this
Ligand-Target Pair | |
Gamma-secretase subunit APH-1A/Gamma-secretase subunit APH-1B/Gamma-secretase subunit PEN-2/Nicastrin/Presenilin-1/Presenilin-2
(Homo sapiens (Human)) | BDBM50114793
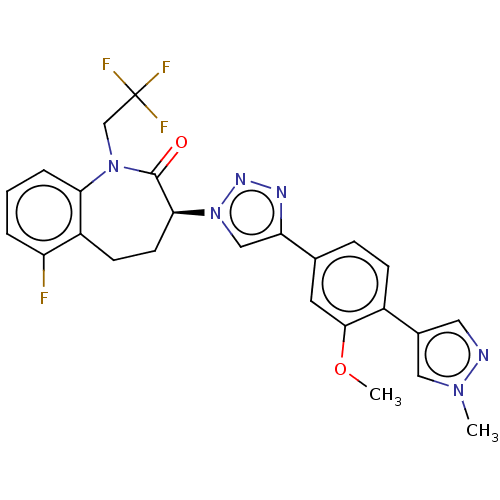
(CHEMBL3608392)Show SMILES COc1cc(ccc1-c1cnn(C)c1)-c1cn(nn1)[C@H]1CCc2c(F)cccc2N(CC(F)(F)F)C1=O |r| Show InChI InChI=1S/C25H22F4N6O2/c1-33-12-16(11-30-33)17-7-6-15(10-23(17)37-2)20-13-35(32-31-20)22-9-8-18-19(26)4-3-5-21(18)34(24(22)36)14-25(27,28)29/h3-7,10-13,22H,8-9,14H2,1-2H3/t22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Modulation of gamma-secretase in human SH-SY5Y cells overexpressing APP C-terminal fragment SPA4CT assessed as inhibition of intracellular amyloid be... |
Bioorg Med Chem Lett 25: 3495-500 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.032
BindingDB Entry DOI: 10.7270/Q2125VFP |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50086911

(5-[4''-(2-Benzyl-benzofuran-3-yl)-biphenyl-4-yloxy...)Show SMILES OC(=O)c1cc(ccc1O)S(=O)(=O)Oc1ccc(cc1)-c1ccc(cc1)-c1c(Cc2ccccc2)oc2ccccc12 Show InChI InChI=1S/C34H24O7S/c35-30-19-18-27(21-29(30)34(36)37)42(38,39)41-26-16-14-24(15-17-26)23-10-12-25(13-11-23)33-28-8-4-5-9-31(28)40-32(33)20-22-6-2-1-3-7-22/h1-19,21,35H,20H2,(H,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) |
J Med Chem 43: 1293-310 (2001)
BindingDB Entry DOI: 10.7270/Q2W958FT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50086923

(4-[4''-(2-Benzyl-benzofuran-3-yl)-3-cyclopentyl-bi...)Show SMILES OC(=O)c1ccc(cc1O)S(=O)(=O)Oc1ccc(cc1C1CCCC1)-c1ccc(cc1)-c1c(Cc2ccccc2)oc2ccccc12 Show InChI InChI=1S/C39H32O7S/c40-34-24-30(19-20-31(34)39(41)42)47(43,44)46-36-21-18-29(23-33(36)27-10-4-5-11-27)26-14-16-28(17-15-26)38-32-12-6-7-13-35(32)45-37(38)22-25-8-2-1-3-9-25/h1-3,6-9,12-21,23-24,27,40H,4-5,10-11,22H2,(H,41,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) |
J Med Chem 43: 1293-310 (2001)
BindingDB Entry DOI: 10.7270/Q2W958FT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50086975

(Benzothiophene derivative | CHEMBL25628 | [4-(2-Be...)Show SMILES COc1cccc(c1)-c1cc(cc(Br)c1OCC(O)=O)-c1ccc(cc1)-c1c(Cc2ccccc2)sc2ccccc12 Show InChI InChI=1S/C36H27BrO4S/c1-40-28-11-7-10-26(19-28)30-20-27(21-31(37)36(30)41-22-34(38)39)24-14-16-25(17-15-24)35-29-12-5-6-13-32(29)42-33(35)18-23-8-3-2-4-9-23/h2-17,19-21H,18,22H2,1H3,(H,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) |
J Med Chem 43: 1293-310 (2001)
BindingDB Entry DOI: 10.7270/Q2W958FT |
More data for this
Ligand-Target Pair | |
Gamma-secretase subunit APH-1A/Gamma-secretase subunit APH-1B/Gamma-secretase subunit PEN-2/Nicastrin/Presenilin-1/Presenilin-2
(Homo sapiens (Human)) | BDBM50114796
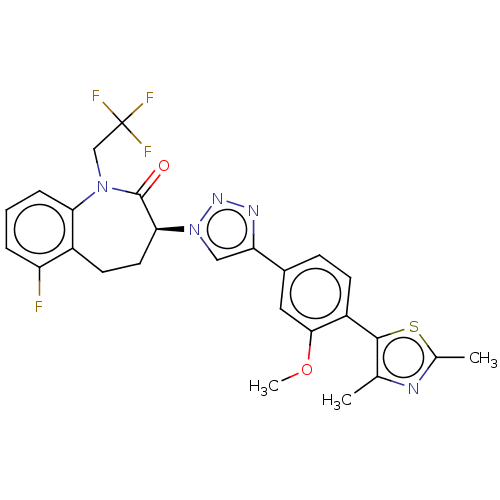
(CHEMBL3608380)Show SMILES COc1cc(ccc1-c1sc(C)nc1C)-c1cn(nn1)[C@H]1CCc2c(F)cccc2N(CC(F)(F)F)C1=O |r| Show InChI InChI=1S/C26H23F4N5O2S/c1-14-24(38-15(2)31-14)18-8-7-16(11-23(18)37-3)20-12-35(33-32-20)22-10-9-17-19(27)5-4-6-21(17)34(25(22)36)13-26(28,29)30/h4-8,11-12,22H,9-10,13H2,1-3H3/t22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Modulation of gamma-secretase in human SH-SY5Y cells overexpressing APP C-terminal fragment SPA4CT assessed as inhibition of intracellular amyloid be... |
Bioorg Med Chem Lett 25: 3495-500 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.032
BindingDB Entry DOI: 10.7270/Q2125VFP |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50086970

(4-[4'-(2-Benzyl-benzo[b]thiophen-3-yl)-biphenyl-4-...)Show SMILES OC(=O)c1ccc(cc1O)S(=O)(=O)Oc1ccc(cc1)-c1ccc(cc1)-c1c(Cc2ccccc2)sc2ccccc12 Show InChI InChI=1S/C34H24O6S2/c35-30-21-27(18-19-28(30)34(36)37)42(38,39)40-26-16-14-24(15-17-26)23-10-12-25(13-11-23)33-29-8-4-5-9-31(29)41-32(33)20-22-6-2-1-3-7-22/h1-19,21,35H,20H2,(H,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) |
J Med Chem 43: 1293-310 (2001)
BindingDB Entry DOI: 10.7270/Q2W958FT |
More data for this
Ligand-Target Pair | |
Gamma-secretase subunit APH-1A/Gamma-secretase subunit APH-1B/Gamma-secretase subunit PEN-2/Nicastrin/Presenilin-1/Presenilin-2
(Homo sapiens (Human)) | BDBM50114803
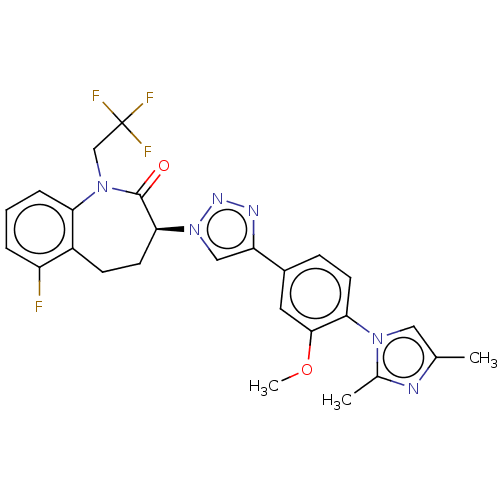
(CHEMBL3608383)Show SMILES COc1cc(ccc1-n1cc(C)nc1C)-c1cn(nn1)[C@H]1CCc2c(F)cccc2N(CC(F)(F)F)C1=O |r| Show InChI InChI=1S/C26H24F4N6O2/c1-15-12-34(16(2)31-15)22-9-7-17(11-24(22)38-3)20-13-36(33-32-20)23-10-8-18-19(27)5-4-6-21(18)35(25(23)37)14-26(28,29)30/h4-7,9,11-13,23H,8,10,14H2,1-3H3/t23-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Modulation of gamma-secretase in human SH-SY5Y cells overexpressing APP C-terminal fragment SPA4CT assessed as inhibition of intracellular amyloid be... |
Bioorg Med Chem Lett 25: 3495-500 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.032
BindingDB Entry DOI: 10.7270/Q2125VFP |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50086981
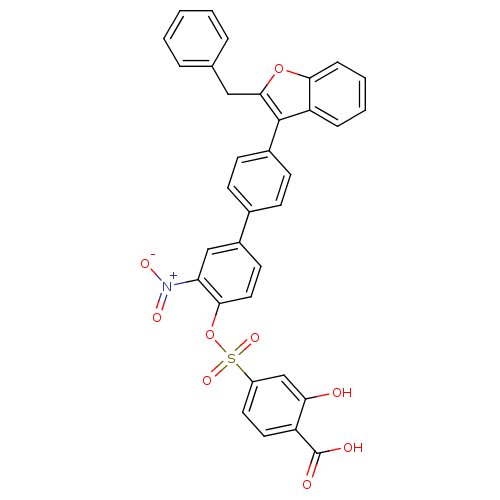
(4-[4''-(2-Benzyl-benzofuran-3-yl)-3-nitro-biphenyl...)Show SMILES OC(=O)c1ccc(cc1O)S(=O)(=O)Oc1ccc(cc1[N+]([O-])=O)-c1ccc(cc1)-c1c(Cc2ccccc2)oc2ccccc12 Show InChI InChI=1S/C34H23NO9S/c36-29-20-25(15-16-26(29)34(37)38)45(41,42)44-31-17-14-24(19-28(31)35(39)40)22-10-12-23(13-11-22)33-27-8-4-5-9-30(27)43-32(33)18-21-6-2-1-3-7-21/h1-17,19-20,36H,18H2,(H,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) |
J Med Chem 43: 1293-310 (2001)
BindingDB Entry DOI: 10.7270/Q2W958FT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50086954

(CHEMBL278092 | [4-(2-Benzyl-benzo[b]thiophen-3-yl)...)Show SMILES COc1ccc(cc1)-c1cc(cc(Br)c1OCC(O)=O)-c1ccc(cc1)-c1c(Cc2ccccc2)sc2ccccc12 Show InChI InChI=1S/C36H27BrO4S/c1-40-28-17-15-25(16-18-28)30-20-27(21-31(37)36(30)41-22-34(38)39)24-11-13-26(14-12-24)35-29-9-5-6-10-32(29)42-33(35)19-23-7-3-2-4-8-23/h2-18,20-21H,19,22H2,1H3,(H,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) |
J Med Chem 43: 1293-310 (2001)
BindingDB Entry DOI: 10.7270/Q2W958FT |
More data for this
Ligand-Target Pair | |
Gamma-secretase subunit APH-1A/Gamma-secretase subunit APH-1B/Gamma-secretase subunit PEN-2/Nicastrin/Presenilin-1/Presenilin-2
(Homo sapiens (Human)) | BDBM50114842
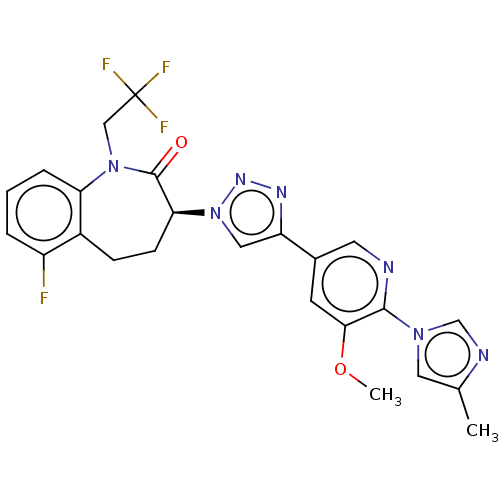
(CHEMBL3608390)Show SMILES COc1cc(cnc1-n1cnc(C)c1)-c1cn(nn1)[C@H]1CCc2c(F)cccc2N(CC(F)(F)F)C1=O |r| Show InChI InChI=1S/C24H21F4N7O2/c1-14-10-33(13-30-14)22-21(37-2)8-15(9-29-22)18-11-35(32-31-18)20-7-6-16-17(25)4-3-5-19(16)34(23(20)36)12-24(26,27)28/h3-5,8-11,13,20H,6-7,12H2,1-2H3/t20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Modulation of gamma-secretase in human SH-SY5Y cells overexpressing APP C-terminal fragment SPA4CT assessed as inhibition of intracellular amyloid be... |
Bioorg Med Chem Lett 25: 3495-500 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.032
BindingDB Entry DOI: 10.7270/Q2125VFP |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50086955
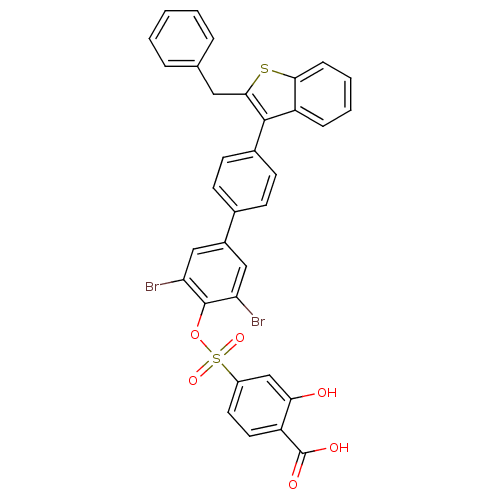
(4-[4''-(2-Benzyl-benzo[b]thiophen-3-yl)-3,5-dibrom...)Show SMILES OC(=O)c1ccc(cc1O)S(=O)(=O)Oc1c(Br)cc(cc1Br)-c1ccc(cc1)-c1c(Cc2ccccc2)sc2ccccc12 Show InChI InChI=1S/C34H22Br2O6S2/c35-27-17-23(18-28(36)33(27)42-44(40,41)24-14-15-25(34(38)39)29(37)19-24)21-10-12-22(13-11-21)32-26-8-4-5-9-30(26)43-31(32)16-20-6-2-1-3-7-20/h1-15,17-19,37H,16H2,(H,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) |
J Med Chem 43: 1293-310 (2001)
BindingDB Entry DOI: 10.7270/Q2W958FT |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50484998

(CHEMBL2019013)Show SMILES Fc1ccc(F)c(c1)C1(CCN(CC1)C(=O)C1(CC1)C(F)(F)F)S(=O)(=O)c1ccc(Cl)cc1 Show InChI InChI=1S/C22H19ClF5NO3S/c23-14-1-4-16(5-2-14)33(31,32)21(17-13-15(24)3-6-18(17)25)9-11-29(12-10-21)19(30)20(7-8-20)22(26,27)28/h1-6,13H,7-12H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of gamma-secretase in human SH-SY5Y cells co-expressing APP C-terminal fragment SPA4CT assessed as inhibition of amyloid beta 40 productio... |
Bioorg Med Chem Lett 22: 3203-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.038
BindingDB Entry DOI: 10.7270/Q2D221GG |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50484998

(CHEMBL2019013)Show SMILES Fc1ccc(F)c(c1)C1(CCN(CC1)C(=O)C1(CC1)C(F)(F)F)S(=O)(=O)c1ccc(Cl)cc1 Show InChI InChI=1S/C22H19ClF5NO3S/c23-14-1-4-16(5-2-14)33(31,32)21(17-13-15(24)3-6-18(17)25)9-11-29(12-10-21)19(30)20(7-8-20)22(26,27)28/h1-6,13H,7-12H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of gamma-secretase in human SH-SY5Y cells co-expressing APP C-terminal fragment SPA4CT assessed as inhibition of amyloid beta 42 productio... |
Bioorg Med Chem Lett 22: 3203-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.038
BindingDB Entry DOI: 10.7270/Q2D221GG |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50498590

(CHEMBL3609637)Show SMILES COc1cc(ccc1-n1cnc(C)c1)-c1cn(nn1)C1CCc2c(F)cccc2N(CC(F)(F)F)C1=O Show InChI InChI=1S/C25H22F4N6O2/c1-15-11-33(14-30-15)21-8-6-16(10-23(21)37-2)19-12-35(32-31-19)22-9-7-17-18(26)4-3-5-20(17)34(24(22)36)13-25(27,28)29/h3-6,8,10-12,14,22H,7,9,13H2,1-2H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Boston
Curated by ChEMBL
| Assay Description
Inhibition of gamma-secretase in human SH-SY5Y cells expressing beta-APP C-terminal fragment SPA4CT assessed as decrease of amyloid beta-42 level by ... |
Bioorg Med Chem Lett 25: 3488-94 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.003
BindingDB Entry DOI: 10.7270/Q2MG7SHX |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50498578

(CHEMBL3609635)Show SMILES COc1cc(ccc1-n1cnc(C)c1)-c1cn(nn1)C1CCc2c(ccc3ccccc23)N(CC(F)(F)F)C1=O Show InChI InChI=1S/C29H25F3N6O2/c1-18-14-36(17-33-18)25-11-8-20(13-27(25)40-2)23-15-38(35-34-23)26-12-9-22-21-6-4-3-5-19(21)7-10-24(22)37(28(26)39)16-29(30,31)32/h3-8,10-11,13-15,17,26H,9,12,16H2,1-2H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Boston
Curated by ChEMBL
| Assay Description
Inhibition of gamma-secretase in human SH-SY5Y cells expressing beta-APP C-terminal fragment SPA4CT assessed as decrease of amyloid beta-42 level by ... |
Bioorg Med Chem Lett 25: 3488-94 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.003
BindingDB Entry DOI: 10.7270/Q2MG7SHX |
More data for this
Ligand-Target Pair | |
Gamma-secretase subunit APH-1A/Gamma-secretase subunit APH-1B/Gamma-secretase subunit PEN-2/Nicastrin/Presenilin-1/Presenilin-2
(Homo sapiens (Human)) | BDBM50114800

(CHEMBL3609749)Show SMILES COc1cc(ccc1-n1cnc(C)c1)-c1cn(nn1)[C@H]1CCc2c(F)cccc2N(CC(F)(F)F)C1=O |r| Show InChI InChI=1S/C25H22F4N6O2/c1-15-11-33(14-30-15)21-8-6-16(10-23(21)37-2)19-12-35(32-31-19)22-9-7-17-18(26)4-3-5-20(17)34(24(22)36)13-25(27,28)29/h3-6,8,10-12,14,22H,7,9,13H2,1-2H3/t22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Modulation of gamma-secretase in human SH-SY5Y cells overexpressing APP C-terminal fragment SPA4CT assessed as inhibition of intracellular amyloid be... |
Bioorg Med Chem Lett 25: 3495-500 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.032
BindingDB Entry DOI: 10.7270/Q2125VFP |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50498590

(CHEMBL3609637)Show SMILES COc1cc(ccc1-n1cnc(C)c1)-c1cn(nn1)C1CCc2c(F)cccc2N(CC(F)(F)F)C1=O Show InChI InChI=1S/C25H22F4N6O2/c1-15-11-33(14-30-15)21-8-6-16(10-23(21)37-2)19-12-35(32-31-19)22-9-7-17-18(26)4-3-5-20(17)34(24(22)36)13-25(27,28)29/h3-6,8,10-12,14,22H,7,9,13H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Boston
Curated by ChEMBL
| Assay Description
Inhibition of SERT (unknown origin) |
Bioorg Med Chem Lett 25: 3488-94 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.003
BindingDB Entry DOI: 10.7270/Q2MG7SHX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50086951

(4-[4'-(2-Benzyl-4,5-dimethyl-thiophen-3-yl)-biphen...)Show SMILES Cc1sc(Cc2ccccc2)c(c1C)-c1ccc(cc1)-c1ccc(OS(=O)(=O)c2ccc(C(O)=O)c(O)c2)cc1 Show InChI InChI=1S/C32H26O6S2/c1-20-21(2)39-30(18-22-6-4-3-5-7-22)31(20)25-10-8-23(9-11-25)24-12-14-26(15-13-24)38-40(36,37)27-16-17-28(32(34)35)29(33)19-27/h3-17,19,33H,18H2,1-2H3,(H,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) |
J Med Chem 43: 1293-310 (2001)
BindingDB Entry DOI: 10.7270/Q2W958FT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50086902

(4-[4''-(2-Benzyl-benzofuran-3-yl)-3,5-dimethyl-bip...)Show SMILES Cc1cc(cc(C)c1OS(=O)(=O)c1ccc(C(O)=O)c(O)c1)-c1ccc(cc1)-c1c(Cc2ccccc2)oc2ccccc12 Show InChI InChI=1S/C36H28O7S/c1-22-18-27(19-23(2)35(22)43-44(40,41)28-16-17-29(36(38)39)31(37)21-28)25-12-14-26(15-13-25)34-30-10-6-7-11-32(30)42-33(34)20-24-8-4-3-5-9-24/h3-19,21,37H,20H2,1-2H3,(H,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) |
J Med Chem 43: 1293-310 (2001)
BindingDB Entry DOI: 10.7270/Q2W958FT |
More data for this
Ligand-Target Pair | |
Gamma-secretase subunit APH-1A/Gamma-secretase subunit APH-1B/Gamma-secretase subunit PEN-2/Nicastrin/Presenilin-1/Presenilin-2
(Homo sapiens (Human)) | BDBM50114836

(CHEMBL3608387)Show SMILES COc1c(F)c(ccc1-n1cnc(C)c1)-c1cn(nn1)[C@H]1CCc2c(F)cccc2N(CC(F)(F)F)C1=O |r| Show InChI InChI=1S/C25H21F5N6O2/c1-14-10-34(13-31-14)20-8-7-16(22(27)23(20)38-2)18-11-36(33-32-18)21-9-6-15-17(26)4-3-5-19(15)35(24(21)37)12-25(28,29)30/h3-5,7-8,10-11,13,21H,6,9,12H2,1-2H3/t21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Modulation of gamma-secretase in human SH-SY5Y cells overexpressing APP C-terminal fragment SPA4CT assessed as inhibition of intracellular amyloid be... |
Bioorg Med Chem Lett 25: 3495-500 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.032
BindingDB Entry DOI: 10.7270/Q2125VFP |
More data for this
Ligand-Target Pair | |
Gamma-secretase subunit APH-1A/Gamma-secretase subunit APH-1B/Gamma-secretase subunit PEN-2/Nicastrin/Presenilin-1/Presenilin-2
(Homo sapiens (Human)) | BDBM50114802

(CHEMBL3609750)Show SMILES COc1cc(ccc1-n1ccnc1C)-c1cn(nn1)[C@H]1CCc2c(F)cccc2N(CC(F)(F)F)C1=O |r| Show InChI InChI=1S/C25H22F4N6O2/c1-15-30-10-11-33(15)21-8-6-16(12-23(21)37-2)19-13-35(32-31-19)22-9-7-17-18(26)4-3-5-20(17)34(24(22)36)14-25(27,28)29/h3-6,8,10-13,22H,7,9,14H2,1-2H3/t22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Modulation of gamma-secretase in human SH-SY5Y cells overexpressing APP C-terminal fragment SPA4CT assessed as inhibition of intracellular amyloid be... |
Bioorg Med Chem Lett 25: 3495-500 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.032
BindingDB Entry DOI: 10.7270/Q2125VFP |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50086892

(2-[4'-(2-Benzyl-benzofuran-3-yl)-3,5-dibromo-biphe...)Show SMILES OC(=O)C(Cc1ccccc1)Oc1c(Br)cc(cc1Br)-c1ccc(cc1)-c1c(Cc2ccccc2)oc2ccccc12 Show InChI InChI=1S/C36H26Br2O4/c37-29-21-27(22-30(38)35(29)42-33(36(39)40)20-24-11-5-2-6-12-24)25-15-17-26(18-16-25)34-28-13-7-8-14-31(28)41-32(34)19-23-9-3-1-4-10-23/h1-18,21-22,33H,19-20H2,(H,39,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) |
J Med Chem 43: 1293-310 (2001)
BindingDB Entry DOI: 10.7270/Q2W958FT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50086963

(4-[4'-(2-Benzyl-benzofuran-3-yl)-biphenyl-4-yloxys...)Show SMILES OC(=O)c1ccc(cc1O)S(=O)(=O)Oc1ccc(cc1)-c1ccc(cc1)-c1c(Cc2ccccc2)oc2ccccc12 Show InChI InChI=1S/C34H24O7S/c35-30-21-27(18-19-28(30)34(36)37)42(38,39)41-26-16-14-24(15-17-26)23-10-12-25(13-11-23)33-29-8-4-5-9-31(29)40-32(33)20-22-6-2-1-3-7-22/h1-19,21,35H,20H2,(H,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) |
J Med Chem 43: 1293-310 (2001)
BindingDB Entry DOI: 10.7270/Q2W958FT |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50498588

(CHEMBL3609633)Show SMILES COc1cc(ccc1-n1cnc(C)c1)-c1cn(nn1)[C@H]1C[C@H](C)c2ccccc2N(CC(F)(F)F)C1=O |r| Show InChI InChI=1S/C26H25F3N6O2/c1-16-10-23(25(36)34(14-26(27,28)29)21-7-5-4-6-19(16)21)35-13-20(31-32-35)18-8-9-22(24(11-18)37-3)33-12-17(2)30-15-33/h4-9,11-13,15-16,23H,10,14H2,1-3H3/t16-,23-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Boston
Curated by ChEMBL
| Assay Description
Inhibition of gamma-secretase in human SH-SY5Y cells expressing beta-APP C-terminal fragment SPA4CT assessed as decrease of amyloid beta-42 level by ... |
Bioorg Med Chem Lett 25: 3488-94 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.003
BindingDB Entry DOI: 10.7270/Q2MG7SHX |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50498580
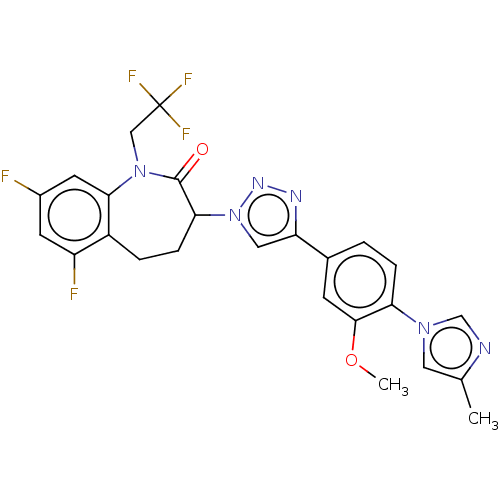
(CHEMBL3609638)Show SMILES COc1cc(ccc1-n1cnc(C)c1)-c1cn(nn1)C1CCc2c(F)cc(F)cc2N(CC(F)(F)F)C1=O Show InChI InChI=1S/C25H21F5N6O2/c1-14-10-34(13-31-14)20-5-3-15(7-23(20)38-2)19-11-36(33-32-19)21-6-4-17-18(27)8-16(26)9-22(17)35(24(21)37)12-25(28,29)30/h3,5,7-11,13,21H,4,6,12H2,1-2H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Boston
Curated by ChEMBL
| Assay Description
Inhibition of gamma-secretase in human SH-SY5Y cells expressing beta-APP C-terminal fragment SPA4CT assessed as decrease of amyloid beta-42 level by ... |
Bioorg Med Chem Lett 25: 3488-94 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.003
BindingDB Entry DOI: 10.7270/Q2MG7SHX |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50498582
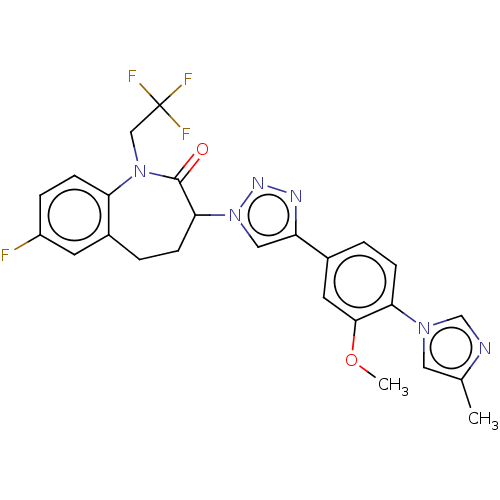
(CHEMBL3609636)Show SMILES COc1cc(ccc1-n1cnc(C)c1)-c1cn(nn1)C1CCc2cc(F)ccc2N(CC(F)(F)F)C1=O Show InChI InChI=1S/C25H22F4N6O2/c1-15-11-33(14-30-15)21-6-3-16(10-23(21)37-2)19-12-35(32-31-19)22-7-4-17-9-18(26)5-8-20(17)34(24(22)36)13-25(27,28)29/h3,5-6,8-12,14,22H,4,7,13H2,1-2H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Boston
Curated by ChEMBL
| Assay Description
Inhibition of gamma-secretase in human SH-SY5Y cells expressing beta-APP C-terminal fragment SPA4CT assessed as decrease of amyloid beta-42 level by ... |
Bioorg Med Chem Lett 25: 3488-94 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.003
BindingDB Entry DOI: 10.7270/Q2MG7SHX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50086973

(4-[4'-(2-Benzoyl-benzofuran-3-yl)-3-cyclopentyl-bi...)Show SMILES OC(=O)c1ccc(cc1O)S(=O)(=O)Oc1ccc(cc1C1CCCC1)-c1ccc(cc1)-c1c(oc2ccccc12)C(=O)c1ccccc1 Show InChI InChI=1S/C39H30O8S/c40-33-23-29(19-20-30(33)39(42)43)48(44,45)47-35-21-18-28(22-32(35)25-8-4-5-9-25)24-14-16-26(17-15-24)36-31-12-6-7-13-34(31)46-38(36)37(41)27-10-2-1-3-11-27/h1-3,6-7,10-23,25,40H,4-5,8-9H2,(H,42,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B) |
J Med Chem 43: 1293-310 (2001)
BindingDB Entry DOI: 10.7270/Q2W958FT |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data