 Found 350 hits with Last Name = 'moy' and Initial = 'ly'
Found 350 hits with Last Name = 'moy' and Initial = 'ly' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50114806
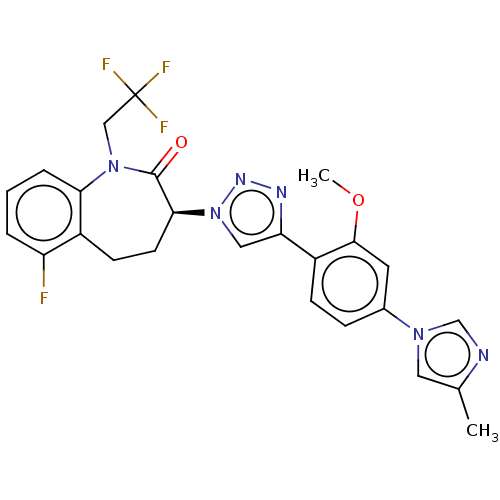
(CHEMBL3609752)Show SMILES COc1cc(ccc1-c1cn(nn1)[C@H]1CCc2c(F)cccc2N(CC(F)(F)F)C1=O)-n1cnc(C)c1 |r| Show InChI InChI=1S/C25H22F4N6O2/c1-15-11-33(14-30-15)16-6-7-18(23(10-16)37-2)20-12-35(32-31-20)22-9-8-17-19(26)4-3-5-21(17)34(24(22)36)13-25(27,28)29/h3-7,10-12,14,22H,8-9,13H2,1-2H3/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG expressed in HEK293 cells by radioligand displacement assay |
Bioorg Med Chem Lett 25: 3495-500 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.032
BindingDB Entry DOI: 10.7270/Q2125VFP |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50114807
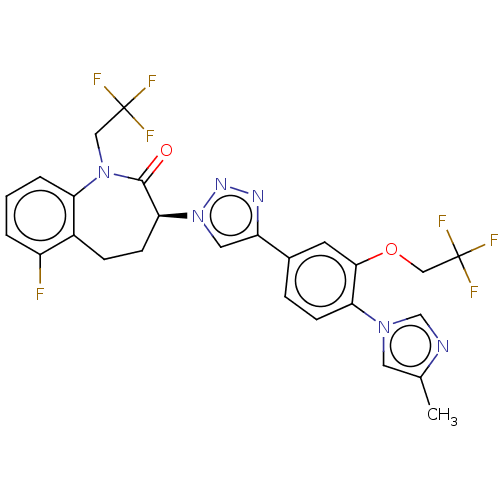
(CHEMBL3608385)Show SMILES Cc1cn(cn1)-c1ccc(cc1OCC(F)(F)F)-c1cn(nn1)[C@H]1CCc2c(F)cccc2N(CC(F)(F)F)C1=O |r| Show InChI InChI=1S/C26H21F7N6O2/c1-15-10-37(14-34-15)21-7-5-16(9-23(21)41-13-26(31,32)33)19-11-39(36-35-19)22-8-6-17-18(27)3-2-4-20(17)38(24(22)40)12-25(28,29)30/h2-5,7,9-11,14,22H,6,8,12-13H2,1H3/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG expressed in HEK293 cells by radioligand displacement assay |
Bioorg Med Chem Lett 25: 3495-500 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.032
BindingDB Entry DOI: 10.7270/Q2125VFP |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50114800

(CHEMBL3609749)Show SMILES COc1cc(ccc1-n1cnc(C)c1)-c1cn(nn1)[C@H]1CCc2c(F)cccc2N(CC(F)(F)F)C1=O |r| Show InChI InChI=1S/C25H22F4N6O2/c1-15-11-33(14-30-15)21-8-6-16(10-23(21)37-2)19-12-35(32-31-19)22-9-7-17-18(26)4-3-5-20(17)34(24(22)36)13-25(27,28)29/h3-6,8,10-12,14,22H,7,9,13H2,1-2H3/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG expressed in HEK293 cells by radioligand displacement assay |
Bioorg Med Chem Lett 25: 3495-500 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.032
BindingDB Entry DOI: 10.7270/Q2125VFP |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50114836
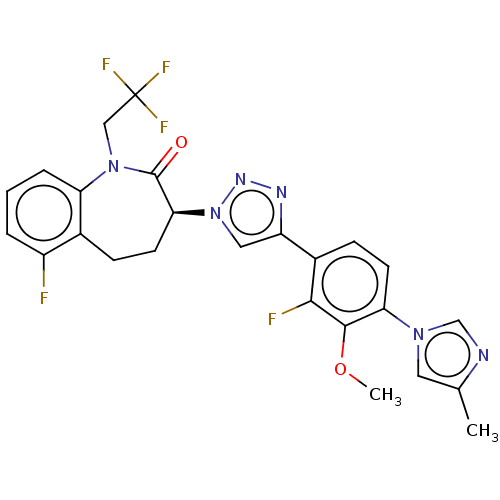
(CHEMBL3608387)Show SMILES COc1c(F)c(ccc1-n1cnc(C)c1)-c1cn(nn1)[C@H]1CCc2c(F)cccc2N(CC(F)(F)F)C1=O |r| Show InChI InChI=1S/C25H21F5N6O2/c1-14-10-34(13-31-14)20-8-7-16(22(27)23(20)38-2)18-11-36(33-32-18)21-9-6-15-17(26)4-3-5-19(15)35(24(21)37)12-25(28,29)30/h3-5,7-8,10-11,13,21H,6,9,12H2,1-2H3/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG expressed in HEK293 cells by radioligand displacement assay |
Bioorg Med Chem Lett 25: 3495-500 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.032
BindingDB Entry DOI: 10.7270/Q2125VFP |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50114805
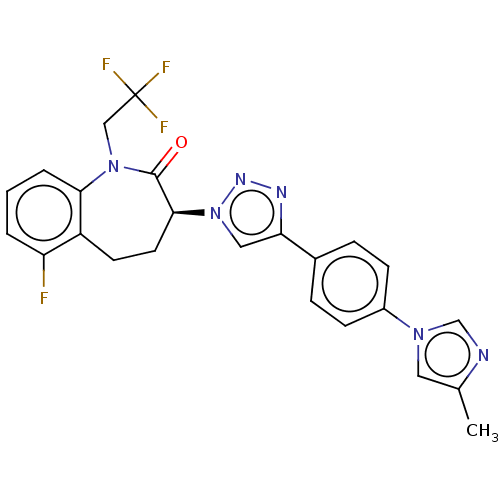
(CHEMBL3608384)Show SMILES Cc1cn(cn1)-c1ccc(cc1)-c1cn(nn1)[C@H]1CCc2c(F)cccc2N(CC(F)(F)F)C1=O |r| Show InChI InChI=1S/C24H20F4N6O/c1-15-11-32(14-29-15)17-7-5-16(6-8-17)20-12-34(31-30-20)22-10-9-18-19(25)3-2-4-21(18)33(23(22)35)13-24(26,27)28/h2-8,11-12,14,22H,9-10,13H2,1H3/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG expressed in HEK293 cells by radioligand displacement assay |
Bioorg Med Chem Lett 25: 3495-500 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.032
BindingDB Entry DOI: 10.7270/Q2125VFP |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50114837
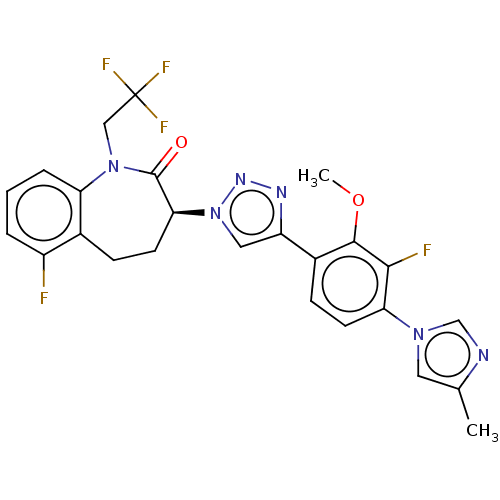
(CHEMBL3609755)Show SMILES COc1c(F)c(ccc1-c1cn(nn1)[C@H]1CCc2c(F)cccc2N(CC(F)(F)F)C1=O)-n1cnc(C)c1 |r| Show InChI InChI=1S/C25H21F5N6O2/c1-14-10-34(13-31-14)20-8-7-16(23(38-2)22(20)27)18-11-36(33-32-18)21-9-6-15-17(26)4-3-5-19(15)35(24(21)37)12-25(28,29)30/h3-5,7-8,10-11,13,21H,6,9,12H2,1-2H3/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG expressed in HEK293 cells by radioligand displacement assay |
Bioorg Med Chem Lett 25: 3495-500 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.032
BindingDB Entry DOI: 10.7270/Q2125VFP |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50114810
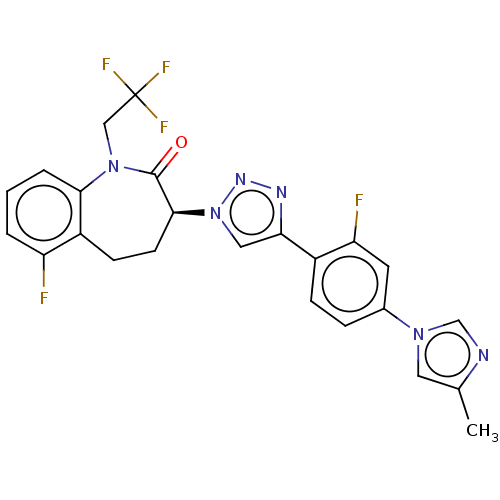
(CHEMBL3609754)Show SMILES Cc1cn(cn1)-c1ccc(-c2cn(nn2)[C@H]2CCc3c(F)cccc3N(CC(F)(F)F)C2=O)c(F)c1 |r| Show InChI InChI=1S/C24H19F5N6O/c1-14-10-33(13-30-14)15-5-6-16(19(26)9-15)20-11-35(32-31-20)22-8-7-17-18(25)3-2-4-21(17)34(23(22)36)12-24(27,28)29/h2-6,9-11,13,22H,7-8,12H2,1H3/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG expressed in HEK293 cells by radioligand displacement assay |
Bioorg Med Chem Lett 25: 3495-500 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.032
BindingDB Entry DOI: 10.7270/Q2125VFP |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50114845
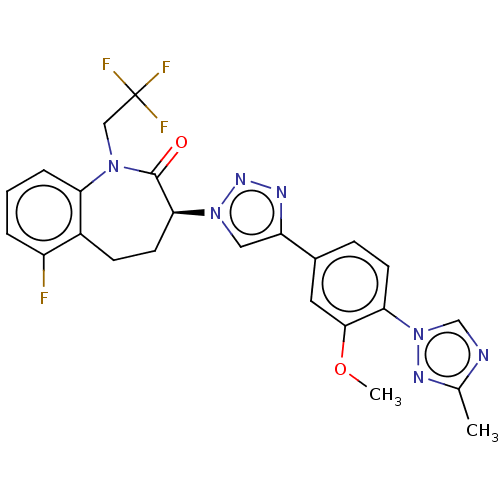
(CHEMBL3609759)Show SMILES COc1cc(ccc1-n1cnc(C)n1)-c1cn(nn1)[C@H]1CCc2c(F)cccc2N(CC(F)(F)F)C1=O |r| Show InChI InChI=1S/C24H21F4N7O2/c1-14-29-13-35(31-14)20-8-6-15(10-22(20)37-2)18-11-34(32-30-18)21-9-7-16-17(25)4-3-5-19(16)33(23(21)36)12-24(26,27)28/h3-6,8,10-11,13,21H,7,9,12H2,1-2H3/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG expressed in HEK293 cells by radioligand displacement assay |
Bioorg Med Chem Lett 25: 3495-500 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.032
BindingDB Entry DOI: 10.7270/Q2125VFP |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50075813
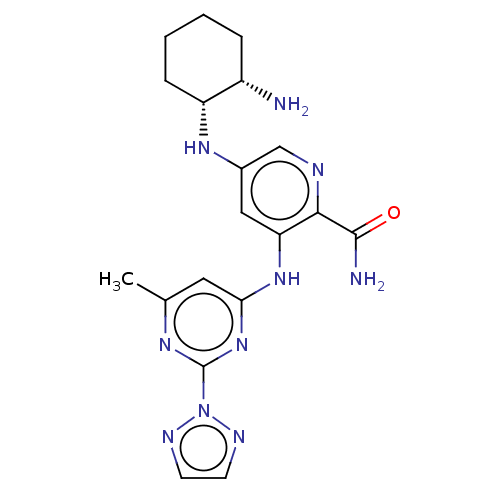
(CHEMBL3415598)Show SMILES Cc1cc(Nc2cc(N[C@@H]3CCCC[C@@H]3N)cnc2C(N)=O)nc(n1)-n1nccn1 |r| Show InChI InChI=1S/C19H24N10O/c1-11-8-16(28-19(25-11)29-23-6-7-24-29)27-15-9-12(10-22-17(15)18(21)30)26-14-5-3-2-4-13(14)20/h6-10,13-14,26H,2-5,20H2,1H3,(H2,21,30)(H,25,27,28)/t13-,14+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length GST-tagged human Syk preincubated for 10 mins followed by peptide substrate/ATP addition measured after 45 mins by TR-FRET ... |
J Med Chem 58: 1929-39 (2015)
Article DOI: 10.1021/jm5018169
BindingDB Entry DOI: 10.7270/Q2028T7D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50075735
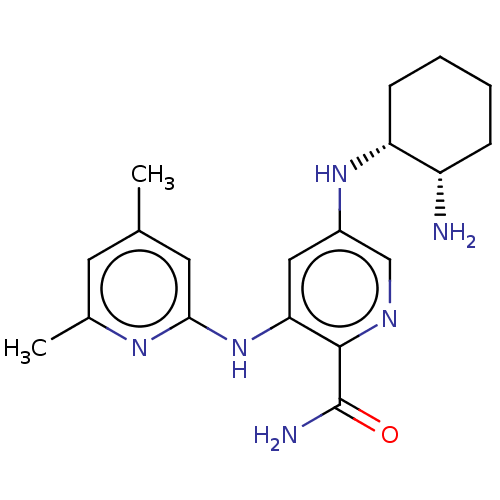
(CHEMBL3415583)Show SMILES Cc1cc(C)nc(Nc2cc(N[C@@H]3CCCC[C@@H]3N)cnc2C(N)=O)c1 |r| Show InChI InChI=1S/C19H26N6O/c1-11-7-12(2)23-17(8-11)25-16-9-13(10-22-18(16)19(21)26)24-15-6-4-3-5-14(15)20/h7-10,14-15,24H,3-6,20H2,1-2H3,(H2,21,26)(H,23,25)/t14-,15+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length GST-tagged human Syk preincubated for 10 mins followed by peptide substrate/ATP addition measured after 45 mins by TR-FRET ... |
J Med Chem 58: 1929-39 (2015)
Article DOI: 10.1021/jm5018169
BindingDB Entry DOI: 10.7270/Q2028T7D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50075740

(CHEMBL3415589)Show SMILES Cc1cc(C)nc(Nc2cc(N[C@@H]3CS(=O)(=O)CC[C@@H]3N)cnc2C(N)=O)c1 |r| Show InChI InChI=1S/C18H24N6O3S/c1-10-5-11(2)22-16(6-10)24-14-7-12(8-21-17(14)18(20)25)23-15-9-28(26,27)4-3-13(15)19/h5-8,13,15,23H,3-4,9,19H2,1-2H3,(H2,20,25)(H,22,24)/t13-,15+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length GST-tagged human Syk preincubated for 10 mins followed by peptide substrate/ATP addition measured after 45 mins by TR-FRET ... |
J Med Chem 58: 1929-39 (2015)
Article DOI: 10.1021/jm5018169
BindingDB Entry DOI: 10.7270/Q2028T7D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50075922
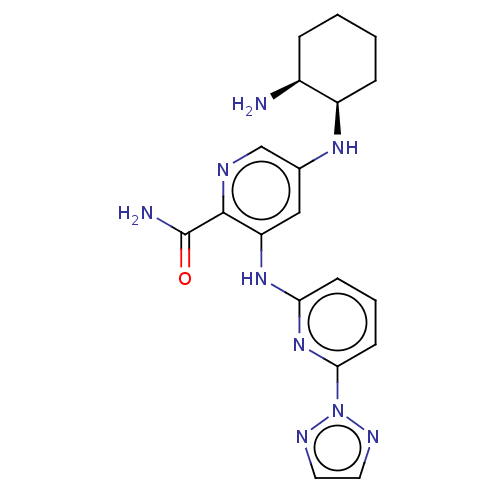
(CHEMBL3415606)Show SMILES N[C@H]1CCCC[C@H]1Nc1cnc(C(N)=O)c(Nc2cccc(n2)-n2nccn2)c1 |r| Show InChI InChI=1S/C19H23N9O/c20-13-4-1-2-5-14(13)25-12-10-15(18(19(21)29)22-11-12)26-16-6-3-7-17(27-16)28-23-8-9-24-28/h3,6-11,13-14,25H,1-2,4-5,20H2,(H2,21,29)(H,26,27)/t13-,14+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length GST-tagged human Syk preincubated for 10 mins followed by peptide substrate/ATP addition measured after 45 mins by TR-FRET ... |
J Med Chem 58: 1929-39 (2015)
Article DOI: 10.1021/jm5018169
BindingDB Entry DOI: 10.7270/Q2028T7D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50075744
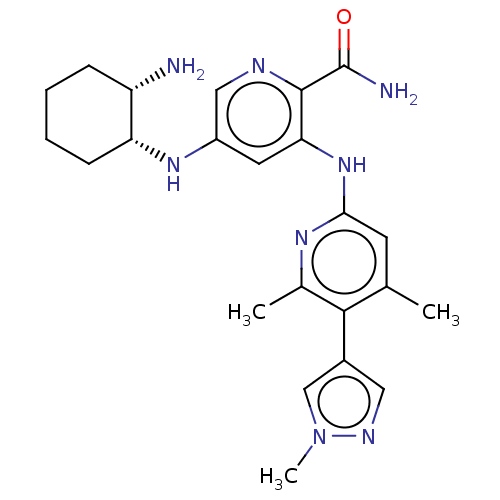
(CHEMBL3415594)Show SMILES Cc1cc(Nc2cc(N[C@@H]3CCCC[C@@H]3N)cnc2C(N)=O)nc(C)c1-c1cnn(C)c1 |r| Show InChI InChI=1S/C23H30N8O/c1-13-8-20(28-14(2)21(13)15-10-27-31(3)12-15)30-19-9-16(11-26-22(19)23(25)32)29-18-7-5-4-6-17(18)24/h8-12,17-18,29H,4-7,24H2,1-3H3,(H2,25,32)(H,28,30)/t17-,18+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length GST-tagged human Syk preincubated for 10 mins followed by peptide substrate/ATP addition measured after 45 mins by TR-FRET ... |
J Med Chem 58: 1929-39 (2015)
Article DOI: 10.1021/jm5018169
BindingDB Entry DOI: 10.7270/Q2028T7D |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50095475
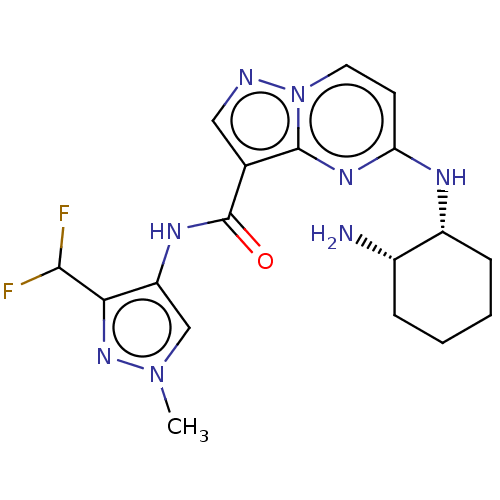
(CHEMBL3590479)Show SMILES Cn1cc(NC(=O)c2cnn3ccc(N[C@@H]4CCCC[C@@H]4N)nc23)c(n1)C(F)F |r| Show InChI InChI=1S/C23H28N4O3/c1-26(2)14-7-15-30-22-16-21(27(25-22)17-18-8-5-4-6-9-18)23(28)24-19-10-12-20(29-3)13-11-19/h4-6,8-13,16H,7,14-15,17H2,1-3H3,(H,24,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of human IRAK4 assessed as phosphorylation of fluorescent peptide substrate after 30 mins by fluorescent polarization reader |
ACS Med Chem Lett 6: 683-8 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00107
BindingDB Entry DOI: 10.7270/Q2QF8VNH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50075747

(CHEMBL3415597)Show SMILES Cc1nc(Nc2cc(N[C@@H]3CCCC[C@@H]3N)cnc2C(N)=O)cc(OCC(C)(C)O)n1 |r| Show InChI InChI=1S/C21H31N7O3/c1-12-25-17(9-18(26-12)31-11-21(2,3)30)28-16-8-13(10-24-19(16)20(23)29)27-15-7-5-4-6-14(15)22/h8-10,14-15,27,30H,4-7,11,22H2,1-3H3,(H2,23,29)(H,25,26,28)/t14-,15+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length GST-tagged human Syk preincubated for 10 mins followed by peptide substrate/ATP addition measured after 45 mins by TR-FRET ... |
J Med Chem 58: 1929-39 (2015)
Article DOI: 10.1021/jm5018169
BindingDB Entry DOI: 10.7270/Q2028T7D |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | CHEMBL5267350

| PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | CHEMBL5286106
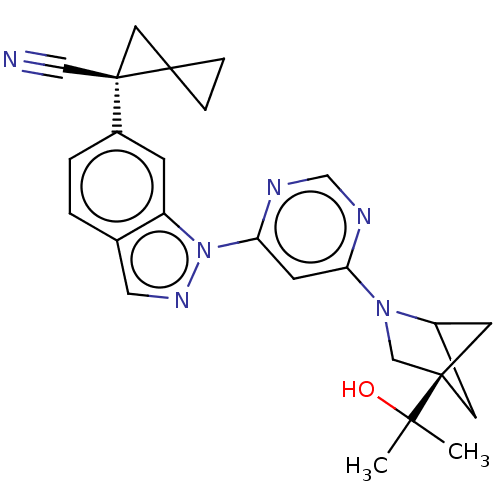
| PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | CHEMBL5286106

| PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50095474
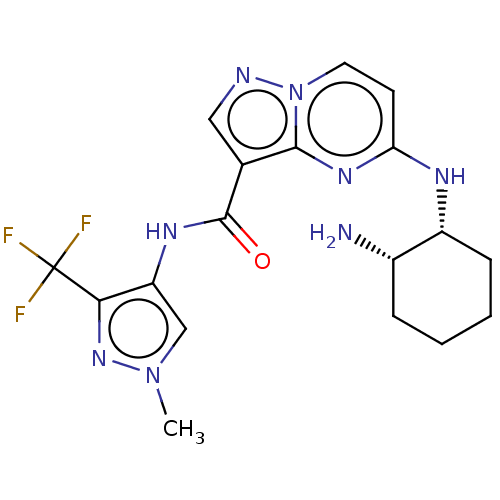
(CHEMBL3590478 | US10329294, Example 2)Show SMILES Cn1cc(NC(=O)c2cnn3ccc(N[C@@H]4CCCC[C@@H]4N)nc23)c(n1)C(F)(F)F |r| Show InChI InChI=1S/C20H23N3O/c1-22(2)14-9-15-24-20-16-19(17-10-5-3-6-11-17)23(21-20)18-12-7-4-8-13-18/h3-8,10-13,16H,9,14-15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of human IRAK4 assessed as phosphorylation of fluorescent peptide substrate after 30 mins by fluorescent polarization reader |
ACS Med Chem Lett 6: 683-8 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00107
BindingDB Entry DOI: 10.7270/Q2QF8VNH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50095470
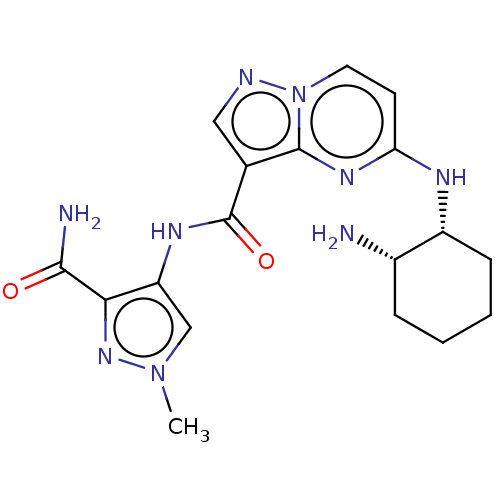
(CHEMBL3590474 | US10155765, Example 9)Show SMILES Cn1cc(NC(=O)c2cnn3ccc(N[C@@H]4CCCC[C@@H]4N)nc23)c(n1)C(N)=O |r| Show InChI InChI=1S/C21H25N3O/c1-23(2)14-9-15-25-21-16-20(19-12-7-4-8-13-19)24(22-21)17-18-10-5-3-6-11-18/h3-8,10-13,16H,9,14-15,17H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of human IRAK4 assessed as phosphorylation of fluorescent peptide substrate after 30 mins by fluorescent polarization reader |
ACS Med Chem Lett 6: 683-8 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00107
BindingDB Entry DOI: 10.7270/Q2QF8VNH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50075819

(CHEMBL3415604)Show SMILES Cc1cc(CCC(C)(C)O)cc(Nc2cc(N[C@@H]3CCCC[C@@H]3N)cnc2C(N)=O)n1 |r| Show InChI InChI=1S/C23H34N6O2/c1-14-10-15(8-9-23(2,3)31)11-20(27-14)29-19-12-16(13-26-21(19)22(25)30)28-18-7-5-4-6-17(18)24/h10-13,17-18,28,31H,4-9,24H2,1-3H3,(H2,25,30)(H,27,29)/t17-,18+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length GST-tagged human Syk preincubated for 10 mins followed by peptide substrate/ATP addition measured after 45 mins by TR-FRET ... |
J Med Chem 58: 1929-39 (2015)
Article DOI: 10.1021/jm5018169
BindingDB Entry DOI: 10.7270/Q2028T7D |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | CHEMBL5284341

| PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50095534

(CHEMBL3590516 | US10329294, Example 252)Show SMILES Cn1cc(NC(=O)c2cnn3ccc(nc23)N2C[C@H](N)C[C@@H](F)C2)c(n1)C(F)(F)F |r| Show InChI InChI=1S/C9H14N2O2/c1-6-8(13-11-9(6)12)7-2-4-10-5-3-7/h7,10H,2-5H2,1H3,(H,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of human IRAK4 assessed as phosphorylation of fluorescent peptide substrate after 30 mins by fluorescent polarization reader |
ACS Med Chem Lett 6: 683-8 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00107
BindingDB Entry DOI: 10.7270/Q2QF8VNH |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50095532
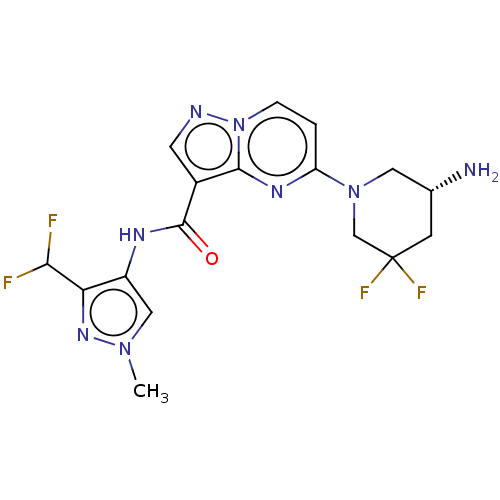
(CHEMBL3590515 | US10329294, Example 173)Show SMILES Cn1cc(NC(=O)c2cnn3ccc(nc23)N2C[C@H](N)CC(F)(F)C2)c(n1)C(F)F |r| Show InChI InChI=1S/C15H18N2O2/c18-15-13(10-11-4-2-1-3-5-11)14(19-17-15)12-6-8-16-9-7-12/h1-5,12,16H,6-10H2,(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of human IRAK4 assessed as phosphorylation of fluorescent peptide substrate after 30 mins by fluorescent polarization reader |
ACS Med Chem Lett 6: 683-8 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00107
BindingDB Entry DOI: 10.7270/Q2QF8VNH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50075742
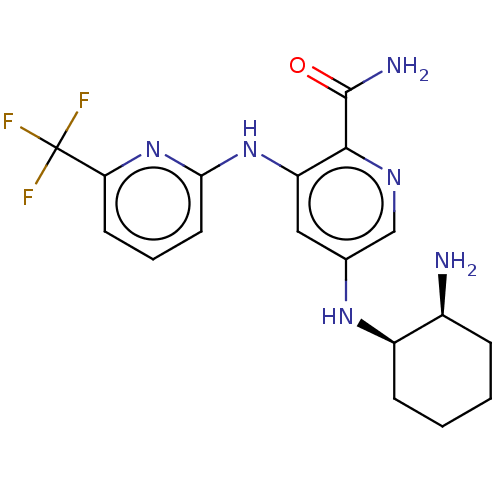
(CHEMBL3415592)Show SMILES N[C@H]1CCCC[C@H]1Nc1cnc(C(N)=O)c(Nc2cccc(n2)C(F)(F)F)c1 |r| Show InChI InChI=1S/C18H21F3N6O/c19-18(20,21)14-6-3-7-15(27-14)26-13-8-10(9-24-16(13)17(23)28)25-12-5-2-1-4-11(12)22/h3,6-9,11-12,25H,1-2,4-5,22H2,(H2,23,28)(H,26,27)/t11-,12+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length GST-tagged human Syk preincubated for 10 mins followed by peptide substrate/ATP addition measured after 45 mins by TR-FRET ... |
J Med Chem 58: 1929-39 (2015)
Article DOI: 10.1021/jm5018169
BindingDB Entry DOI: 10.7270/Q2028T7D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50075738
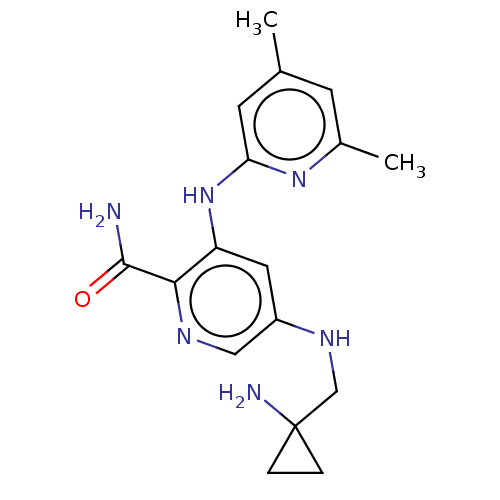
(CHEMBL3415587)Show InChI InChI=1S/C17H22N6O/c1-10-5-11(2)22-14(6-10)23-13-7-12(8-20-15(13)16(18)24)21-9-17(19)3-4-17/h5-8,21H,3-4,9,19H2,1-2H3,(H2,18,24)(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length GST-tagged human Syk preincubated for 10 mins followed by peptide substrate/ATP addition measured after 45 mins by TR-FRET ... |
J Med Chem 58: 1929-39 (2015)
Article DOI: 10.1021/jm5018169
BindingDB Entry DOI: 10.7270/Q2028T7D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50075732
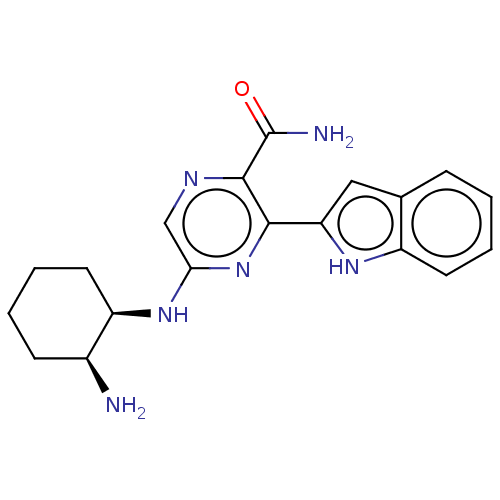
(CHEMBL3414584 | US9775839, 2.1)Show SMILES N[C@H]1CCCC[C@H]1Nc1cnc(C(N)=O)c(n1)-c1cc2ccccc2[nH]1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length GST-tagged human Syk preincubated for 10 mins followed by peptide substrate/ATP addition measured after 45 mins by TR-FRET ... |
J Med Chem 58: 1929-39 (2015)
Article DOI: 10.1021/jm5018169
BindingDB Entry DOI: 10.7270/Q2028T7D |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM257207
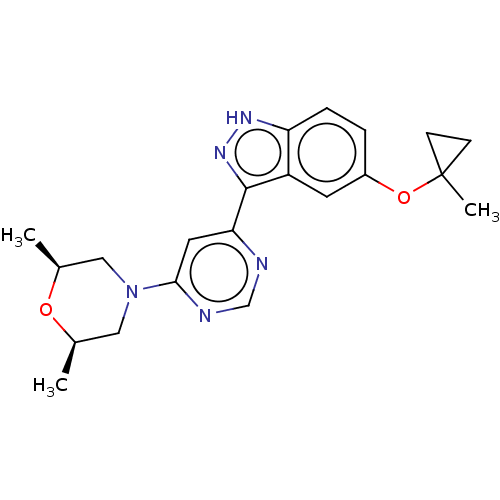
(US9493440, 51)Show SMILES C[C@H]1CN(C[C@@H](C)O1)c1cc(ncn1)-c1n[nH]c2ccc(OC3(C)CC3)cc12 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50075730
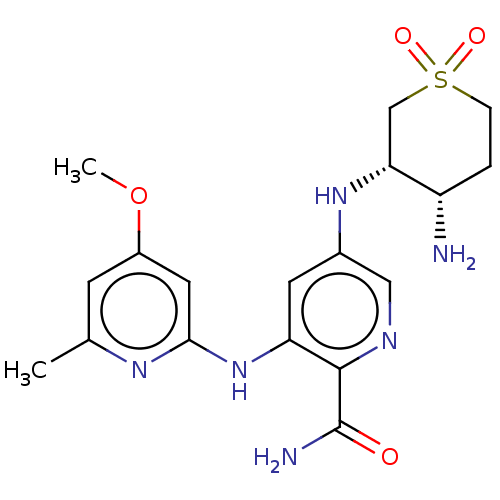
(CHEMBL3415599)Show SMILES COc1cc(C)nc(Nc2cc(N[C@@H]3CS(=O)(=O)CC[C@@H]3N)cnc2C(N)=O)c1 |r| Show InChI InChI=1S/C18H24N6O4S/c1-10-5-12(28-2)7-16(22-10)24-14-6-11(8-21-17(14)18(20)25)23-15-9-29(26,27)4-3-13(15)19/h5-8,13,15,23H,3-4,9,19H2,1-2H3,(H2,20,25)(H,22,24)/t13-,15+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length GST-tagged human Syk preincubated for 10 mins followed by peptide substrate/ATP addition measured after 45 mins by TR-FRET ... |
J Med Chem 58: 1929-39 (2015)
Article DOI: 10.1021/jm5018169
BindingDB Entry DOI: 10.7270/Q2028T7D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50075733
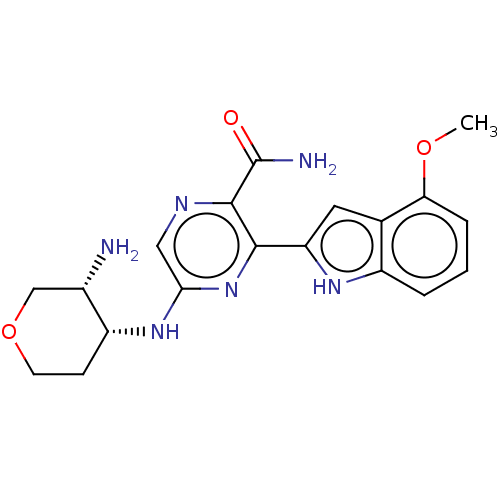
(CHEMBL3415610 | US9775839, 8.5)Show SMILES COc1cccc2[nH]c(cc12)-c1nc(N[C@@H]2CCOC[C@@H]2N)cnc1C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length GST-tagged human Syk preincubated for 10 mins followed by peptide substrate/ATP addition measured after 45 mins by TR-FRET ... |
J Med Chem 58: 1929-39 (2015)
Article DOI: 10.1021/jm5018169
BindingDB Entry DOI: 10.7270/Q2028T7D |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | CHEMBL5284341

| PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50075743
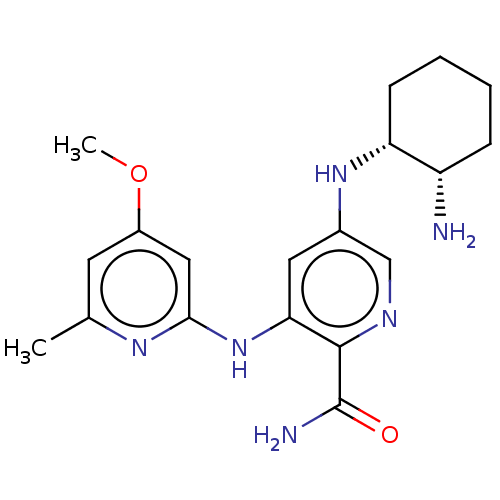
(CHEMBL3415593)Show SMILES COc1cc(C)nc(Nc2cc(N[C@@H]3CCCC[C@@H]3N)cnc2C(N)=O)c1 |r| Show InChI InChI=1S/C19H26N6O2/c1-11-7-13(27-2)9-17(23-11)25-16-8-12(10-22-18(16)19(21)26)24-15-6-4-3-5-14(15)20/h7-10,14-15,24H,3-6,20H2,1-2H3,(H2,21,26)(H,23,25)/t14-,15+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length GST-tagged human Syk preincubated for 10 mins followed by peptide substrate/ATP addition measured after 45 mins by TR-FRET ... |
J Med Chem 58: 1929-39 (2015)
Article DOI: 10.1021/jm5018169
BindingDB Entry DOI: 10.7270/Q2028T7D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50075739
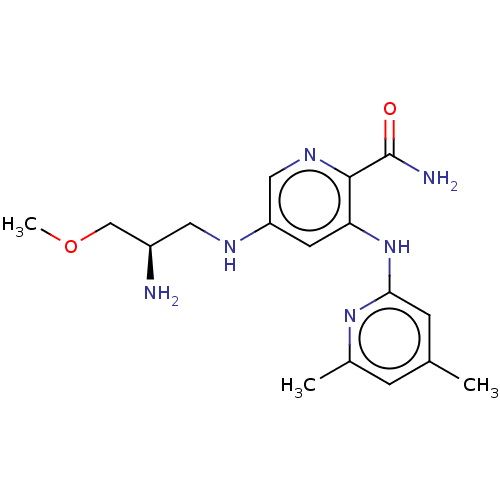
(CHEMBL3415588)Show SMILES COC[C@H](N)CNc1cnc(C(N)=O)c(Nc2cc(C)cc(C)n2)c1 |r| Show InChI InChI=1S/C17H24N6O2/c1-10-4-11(2)22-15(5-10)23-14-6-13(8-21-16(14)17(19)24)20-7-12(18)9-25-3/h4-6,8,12,20H,7,9,18H2,1-3H3,(H2,19,24)(H,22,23)/t12-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length GST-tagged human Syk preincubated for 10 mins followed by peptide substrate/ATP addition measured after 45 mins by TR-FRET ... |
J Med Chem 58: 1929-39 (2015)
Article DOI: 10.1021/jm5018169
BindingDB Entry DOI: 10.7270/Q2028T7D |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50095473

(CHEMBL3590477 | US10329294, Example 282)Show SMILES Cn1cc(NC(=O)c2cnn3ccc(N[C@@H]4CCCC[C@@H]4N)nc23)c(n1)C#N |r| Show InChI InChI=1S/C18H21N9O/c1-26-10-15(14(8-19)25-26)23-18(28)11-9-21-27-7-6-16(24-17(11)27)22-13-5-3-2-4-12(13)20/h6-7,9-10,12-13H,2-5,20H2,1H3,(H,22,24)(H,23,28)/t12-,13+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of human IRAK4 assessed as phosphorylation of fluorescent peptide substrate after 30 mins by fluorescent polarization reader |
ACS Med Chem Lett 6: 683-8 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00107
BindingDB Entry DOI: 10.7270/Q2QF8VNH |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | CHEMBL5286106

| PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50075745

(CHEMBL3415595)Show SMILES Cc1cc(CC(C)(C)O)cc(Nc2cc(N[C@@H]3CCCC[C@@H]3N)cnc2C(N)=O)n1 |r| Show InChI InChI=1S/C22H32N6O2/c1-13-8-14(11-22(2,3)30)9-19(26-13)28-18-10-15(12-25-20(18)21(24)29)27-17-7-5-4-6-16(17)23/h8-10,12,16-17,27,30H,4-7,11,23H2,1-3H3,(H2,24,29)(H,26,28)/t16-,17+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length GST-tagged human Syk preincubated for 10 mins followed by peptide substrate/ATP addition measured after 45 mins by TR-FRET ... |
J Med Chem 58: 1929-39 (2015)
Article DOI: 10.1021/jm5018169
BindingDB Entry DOI: 10.7270/Q2028T7D |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM329724
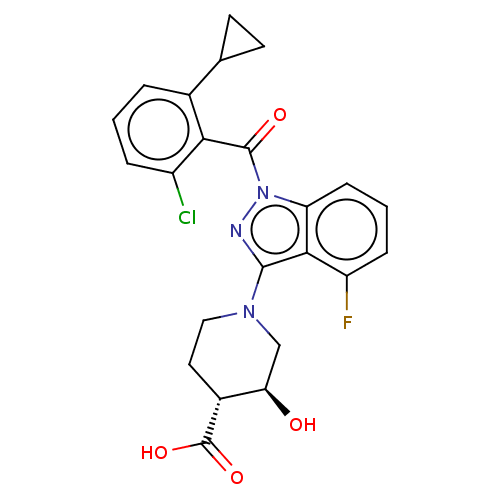
((3S,4R)-1-(1-(2-chloro-6-cyclopropylbenzoyl)-4-flu...)Show SMILES O[C@@H]1CN(CC[C@H]1C(O)=O)c1nn(C(=O)c2c(Cl)cccc2C2CC2)c2cccc(F)c12 Show InChI InChI=1S/C23H21ClFN3O4/c24-15-4-1-3-13(12-7-8-12)19(15)22(30)28-17-6-2-5-16(25)20(17)21(26-28)27-10-9-14(23(31)32)18(29)11-27/h1-6,12,14,18,29H,7-11H2,(H,31,32)/t14-,18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Allosteric inhibition of recombinant His6-tagged RORgammat LBD (unknown origin) expressed in Escherichia coli BL21(DE3) assessed as inhibition of bio... |
ACS Med Chem Lett 11: 114-119 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00431
BindingDB Entry DOI: 10.7270/Q24Q7Z9C |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50075821
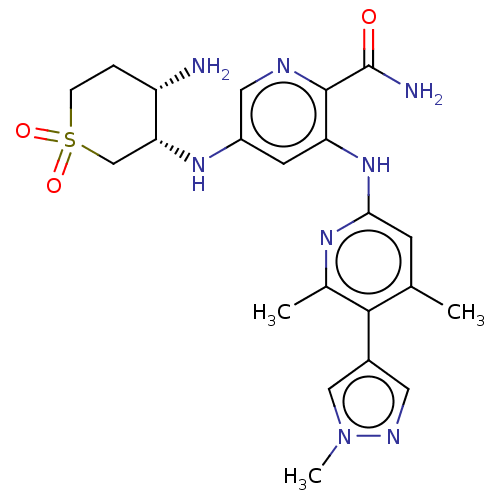
(CHEMBL3415605)Show SMILES Cc1cc(Nc2cc(N[C@@H]3CS(=O)(=O)CC[C@@H]3N)cnc2C(N)=O)nc(C)c1-c1cnn(C)c1 |r| Show InChI InChI=1S/C22H28N8O3S/c1-12-6-19(27-13(2)20(12)14-8-26-30(3)10-14)29-17-7-15(9-25-21(17)22(24)31)28-18-11-34(32,33)5-4-16(18)23/h6-10,16,18,28H,4-5,11,23H2,1-3H3,(H2,24,31)(H,27,29)/t16-,18+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length GST-tagged human Syk preincubated for 10 mins followed by peptide substrate/ATP addition measured after 45 mins by TR-FRET ... |
J Med Chem 58: 1929-39 (2015)
Article DOI: 10.1021/jm5018169
BindingDB Entry DOI: 10.7270/Q2028T7D |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50095505

(CHEMBL3590488 | US10329294, Example 88)Show SMILES Cn1cc(NC(=O)c2cnn3ccc(nc23)N2CCNCC2)c(n1)C(F)F Show InChI InChI=1S/C17H13ClO4/c18-14-7-2-1-5-12(14)8-11-4-3-6-13(9-11)15(19)10-16(20)17(21)22/h1-7,9H,8,10H2,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of human IRAK4 assessed as phosphorylation of fluorescent peptide substrate after 30 mins by fluorescent polarization reader |
ACS Med Chem Lett 6: 683-8 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00107
BindingDB Entry DOI: 10.7270/Q2QF8VNH |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50095531
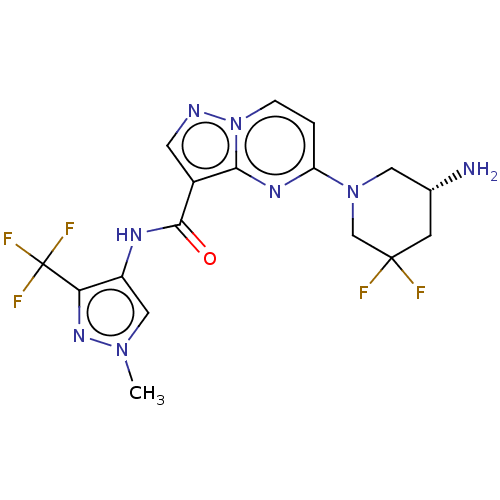
(CHEMBL3590493 | US10329294, Example 60)Show SMILES Cn1cc(NC(=O)c2cnn3ccc(nc23)N2C[C@H](N)CC(F)(F)C2)c(n1)C(F)(F)F |r| Show InChI InChI=1S/C5H8N2O2/c1-3-4(2-6)9-7-5(3)8/h2,6H2,1H3,(H,7,8) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of human IRAK4 assessed as phosphorylation of fluorescent peptide substrate after 30 mins by fluorescent polarization reader |
ACS Med Chem Lett 6: 683-8 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00107
BindingDB Entry DOI: 10.7270/Q2QF8VNH |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM257207

(US9493440, 51)Show SMILES C[C@H]1CN(C[C@@H](C)O1)c1cc(ncn1)-c1n[nH]c2ccc(OC3(C)CC3)cc12 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50095476
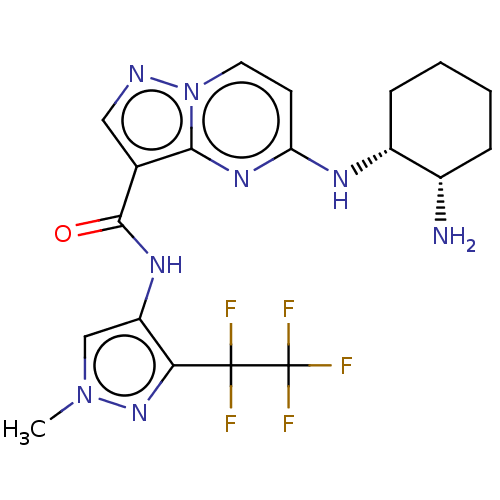
(CHEMBL3590480 | US10329294, Example 297)Show SMILES Cn1cc(NC(=O)c2cnn3ccc(N[C@@H]4CCCC[C@@H]4N)nc23)c(n1)C(F)(F)C(F)(F)F |r| Show InChI InChI=1S/C19H21F5N8O/c1-31-9-13(15(30-31)18(20,21)19(22,23)24)28-17(33)10-8-26-32-7-6-14(29-16(10)32)27-12-5-3-2-4-11(12)25/h6-9,11-12H,2-5,25H2,1H3,(H,27,29)(H,28,33)/t11-,12+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of human IRAK4 assessed as phosphorylation of fluorescent peptide substrate after 30 mins by fluorescent polarization reader |
ACS Med Chem Lett 6: 683-8 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00107
BindingDB Entry DOI: 10.7270/Q2QF8VNH |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM329726
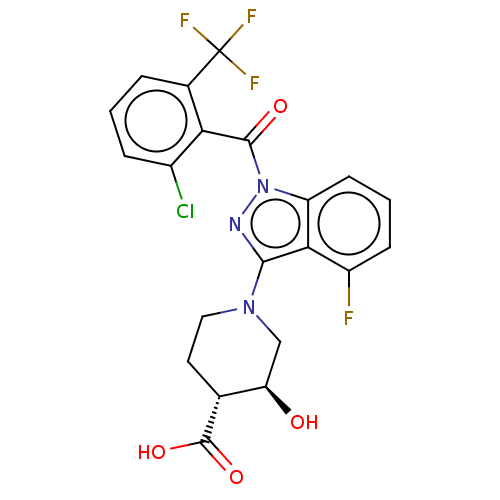
((3S,4R)-1-(1-(2- chloro-6- (trifluoromethyl)benzoy...)Show SMILES O[C@@H]1CN(CC[C@H]1C(O)=O)c1nn(C(=O)c2c(Cl)cccc2C(F)(F)F)c2cccc(F)c12 Show InChI InChI=1S/C21H16ClF4N3O4/c22-12-4-1-3-11(21(24,25)26)16(12)19(31)29-14-6-2-5-13(23)17(14)18(27-29)28-8-7-10(20(32)33)15(30)9-28/h1-6,10,15,30H,7-9H2,(H,32,33)/t10-,15-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Allosteric inhibition of recombinant His6-tagged RORgammat LBD (unknown origin) expressed in Escherichia coli BL21(DE3) assessed as inhibition of bio... |
ACS Med Chem Lett 11: 114-119 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00431
BindingDB Entry DOI: 10.7270/Q24Q7Z9C |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50075943
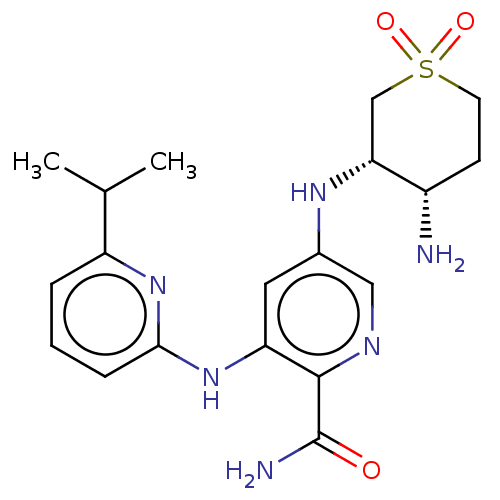
(CHEMBL3415607)Show SMILES CC(C)c1cccc(Nc2cc(N[C@@H]3CS(=O)(=O)CC[C@@H]3N)cnc2C(N)=O)n1 |r| Show InChI InChI=1S/C19H26N6O3S/c1-11(2)14-4-3-5-17(24-14)25-15-8-12(9-22-18(15)19(21)26)23-16-10-29(27,28)7-6-13(16)20/h3-5,8-9,11,13,16,23H,6-7,10,20H2,1-2H3,(H2,21,26)(H,24,25)/t13-,16+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length GST-tagged human Syk preincubated for 10 mins followed by peptide substrate/ATP addition measured after 45 mins by TR-FRET ... |
J Med Chem 58: 1929-39 (2015)
Article DOI: 10.1021/jm5018169
BindingDB Entry DOI: 10.7270/Q2028T7D |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50095465
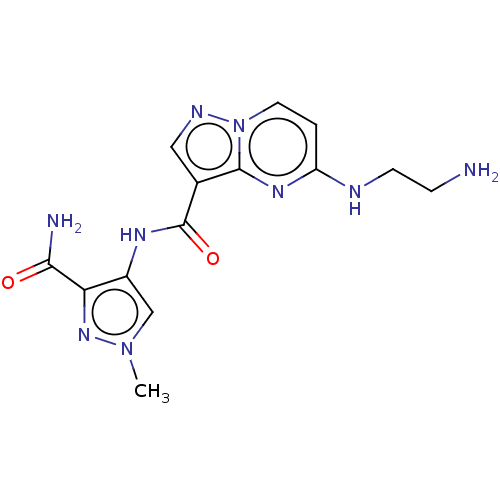
(CHEMBL3590438 | US10155765, Example 28)Show SMILES Cn1cc(NC(=O)c2cnn3ccc(NCCN)nc23)c(n1)C(N)=O Show InChI InChI=1S/C14H17N9O2/c1-22-7-9(11(21-22)12(16)24)19-14(25)8-6-18-23-5-2-10(17-4-3-15)20-13(8)23/h2,5-7H,3-4,15H2,1H3,(H2,16,24)(H,17,20)(H,19,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of human IRAK4 assessed as phosphorylation of fluorescent peptide substrate after 30 mins by fluorescent polarization reader |
ACS Med Chem Lett 6: 683-8 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00107
BindingDB Entry DOI: 10.7270/Q2QF8VNH |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | CHEMBL5267690
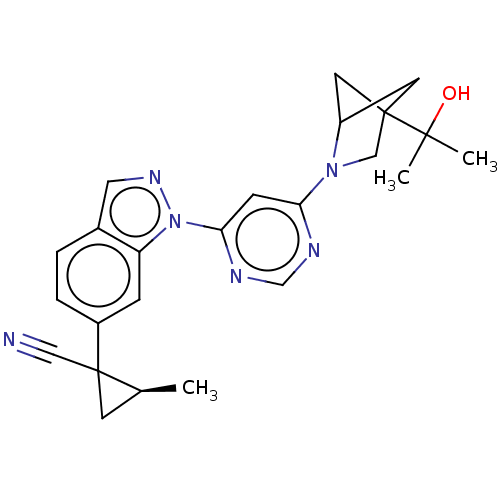
| PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | CHEMBL5267690

| PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50106301
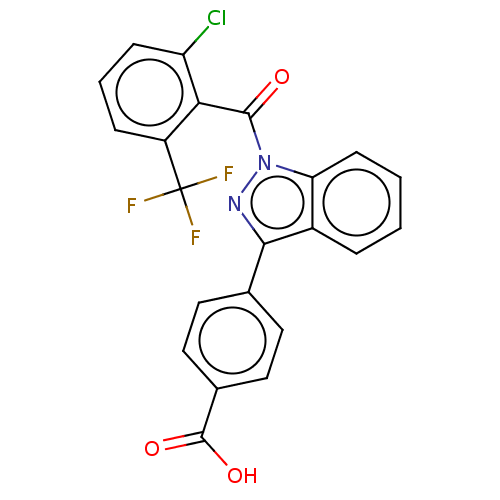
(CHEMBL3598140)Show SMILES OC(=O)c1ccc(cc1)-c1nn(C(=O)c2c(Cl)cccc2C(F)(F)F)c2ccccc12 Show InChI InChI=1S/C22H12ClF3N2O3/c23-16-6-3-5-15(22(24,25)26)18(16)20(29)28-17-7-2-1-4-14(17)19(27-28)12-8-10-13(11-9-12)21(30)31/h1-11H,(H,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Allosteric inhibition of recombinant His6-tagged RORgammat LBD (unknown origin) expressed in Escherichia coli BL21(DE3) assessed as inhibition of bio... |
ACS Med Chem Lett 11: 114-119 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00431
BindingDB Entry DOI: 10.7270/Q24Q7Z9C |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50075944
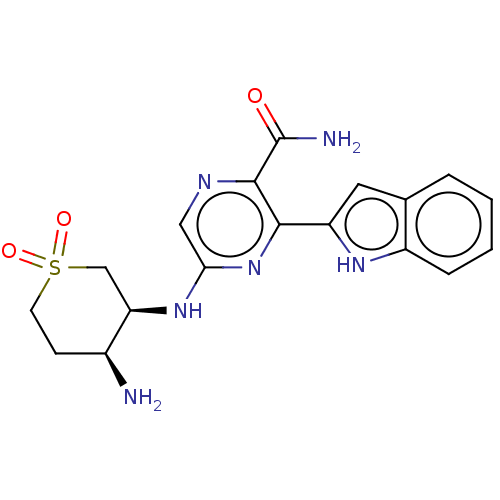
(CHEMBL3415609 | US9775839, 3.1)Show SMILES N[C@H]1CCS(=O)(=O)C[C@H]1Nc1cnc(C(N)=O)c(n1)-c1cc2ccccc2[nH]1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length GST-tagged human Syk preincubated for 10 mins followed by peptide substrate/ATP addition measured after 45 mins by TR-FRET ... |
J Med Chem 58: 1929-39 (2015)
Article DOI: 10.1021/jm5018169
BindingDB Entry DOI: 10.7270/Q2028T7D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50011182
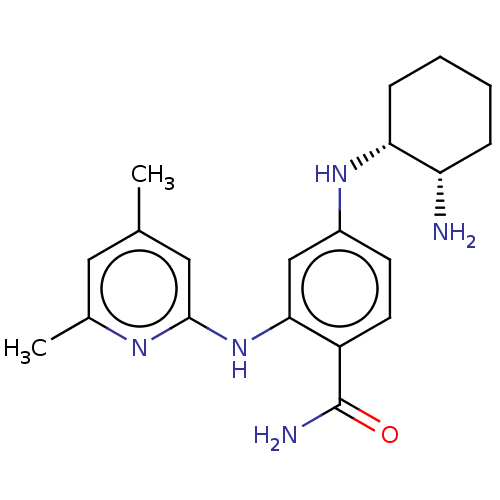
(CHEMBL3260318)Show SMILES Cc1cc(C)nc(Nc2cc(N[C@@H]3CCCC[C@@H]3N)ccc2C(N)=O)c1 |r| Show InChI InChI=1S/C20H27N5O/c1-12-9-13(2)23-19(10-12)25-18-11-14(7-8-15(18)20(22)26)24-17-6-4-3-5-16(17)21/h7-11,16-17,24H,3-6,21H2,1-2H3,(H2,22,26)(H,23,25)/t16-,17+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length GST-tagged human Syk preincubated for 10 mins followed by peptide substrate/ATP addition measured after 45 mins by TR-FRET ... |
J Med Chem 58: 1929-39 (2015)
Article DOI: 10.1021/jm5018169
BindingDB Entry DOI: 10.7270/Q2028T7D |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data