 Found 35246 hits with Last Name = 'mu' and Initial = 'c'
Found 35246 hits with Last Name = 'mu' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50140757
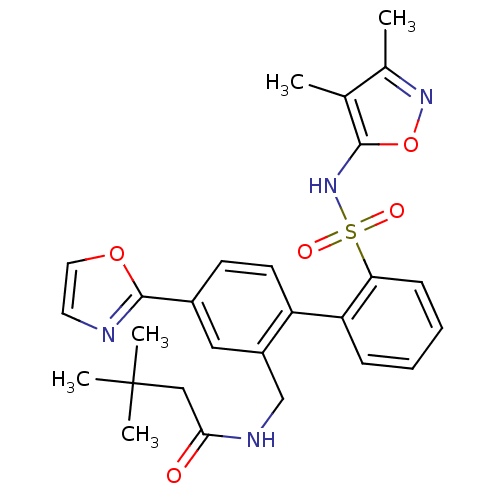
(BMS-207940 | CHEMBL277447 | N-[2'-(3,4-Dimethyl-is...)Show SMILES Cc1noc(NS(=O)(=O)c2ccccc2-c2ccc(cc2CNC(=O)CC(C)(C)C)-c2ncco2)c1C Show InChI InChI=1S/C27H30N4O5S/c1-17-18(2)30-36-25(17)31-37(33,34)23-9-7-6-8-22(23)21-11-10-19(26-28-12-13-35-26)14-20(21)16-29-24(32)15-27(3,4)5/h6-14,31H,15-16H2,1-5H3,(H,29,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Prestwick Chemical Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Endothelin A receptor |
J Med Chem 47: 1303-14 (2004)
Article DOI: 10.1021/jm030480f
BindingDB Entry DOI: 10.7270/Q2FN16X5 |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor
(RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50085683
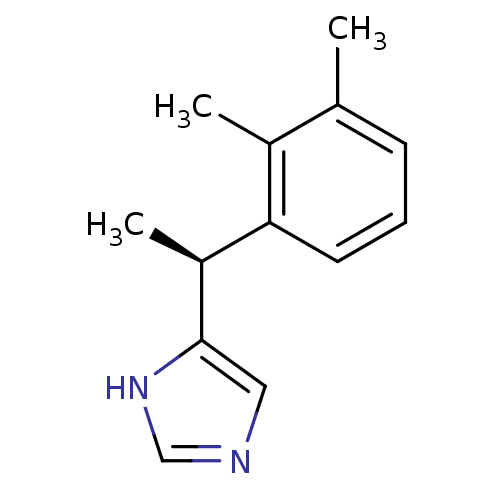
((+)-4-((S)-alpha,2,3-trimethylbenzyl)imidazole | 4...)Show InChI InChI=1S/C13H16N2/c1-9-5-4-6-12(10(9)2)11(3)13-7-14-8-15-13/h4-8,11H,1-3H3,(H,14,15)/t11-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
In vitro binding affinity against alpha-2 adrenergic receptor in rat |
J Med Chem 42: 5064-71 (1999)
Article DOI: 10.1021/jm990005a
BindingDB Entry DOI: 10.7270/Q2F76G95 |
More data for this
Ligand-Target Pair | |
Alpha-1D adrenergic receptor
(Homo sapiens (Human)) | BDBM50085683

((+)-4-((S)-alpha,2,3-trimethylbenzyl)imidazole | 4...)Show InChI InChI=1S/C13H16N2/c1-9-5-4-6-12(10(9)2)11(3)13-7-14-8-15-13/h4-8,11H,1-3H3,(H,14,15)/t11-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
In vitro binding affinity against alpha-2D adrenergic receptor of male Wistar rat |
J Med Chem 44: 863-72 (2001)
BindingDB Entry DOI: 10.7270/Q23R0TKP |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM50216315
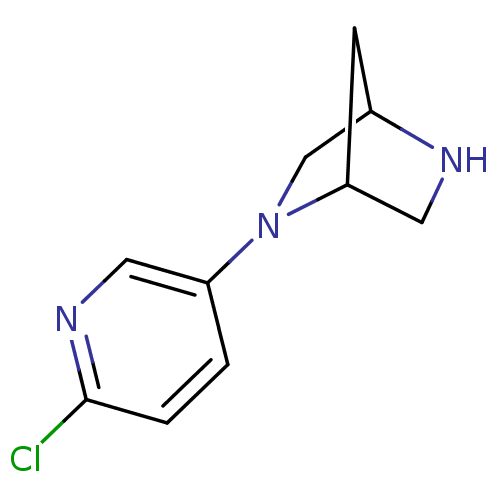
((1R,4R)-2-(6-chloro-3-pyridinyl)-2,5-diazabicyclo[...)Show InChI InChI=1S/C10H12ClN3/c11-10-2-1-8(4-13-10)14-6-7-3-9(14)5-12-7/h1-2,4,7,9,12H,3,5-6H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Sassari
Curated by ChEMBL
| Assay Description
Displacement of [3H]cytosine form alpha4beta2 nAChR in rat striatum |
Bioorg Med Chem Lett 18: 6147-50 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.002
BindingDB Entry DOI: 10.7270/Q29G5MPJ |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM50275878
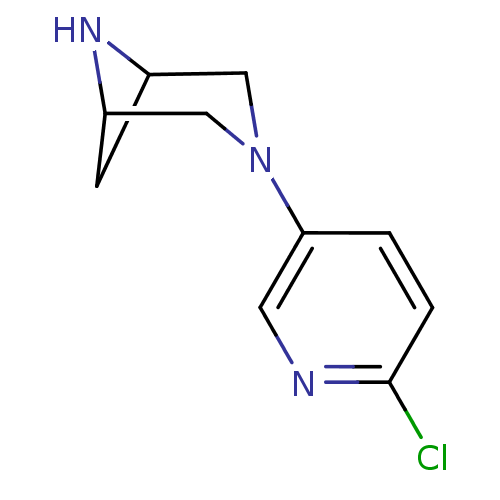
(3-(6-chloropyridin-3-yl)-3,6-diazabicyclo[3.1.1]he...)Show InChI InChI=1S/C10H12ClN3/c11-10-2-1-9(4-12-10)14-5-7-3-8(6-14)13-7/h1-2,4,7-8,13H,3,5-6H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Sassari
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine form alpha4beta2 nAChR in rat cortex |
Bioorg Med Chem Lett 18: 6147-50 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.002
BindingDB Entry DOI: 10.7270/Q29G5MPJ |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM50275880
(3-(6-bromopyridin-3-yl)-3,6-diazabicyclo[3.1.1]hep...)Show InChI InChI=1S/C10H12BrN3/c11-10-2-1-9(4-12-10)14-5-7-3-8(6-14)13-7/h1-2,4,7-8,13H,3,5-6H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Sassari
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine form alpha4beta2 nAChR in rat cortex |
Bioorg Med Chem Lett 18: 6147-50 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.002
BindingDB Entry DOI: 10.7270/Q29G5MPJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50200169
(6-methyl-1-(2',4'-dichlorophenyl)-N-piperidin-1-yl...)Show SMILES Cc1ccc-2c(Cc3c(nn(c-23)-c2ccc(Cl)cc2Cl)C(=O)NN2CCCCC2)c1 Show InChI InChI=1S/C23H22Cl2N4O/c1-14-5-7-17-15(11-14)12-18-21(23(30)27-28-9-3-2-4-10-28)26-29(22(17)18)20-8-6-16(24)13-19(20)25/h5-8,11,13H,2-4,9-10,12H2,1H3,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP 55940 from human CB2 receptor in cell free system |
Eur J Med Chem 46: 547-55 (2011)
Article DOI: 10.1016/j.ejmech.2010.11.034
BindingDB Entry DOI: 10.7270/Q2CF9QCJ |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens) | BDBM50514737
(CHEMBL4482861)Show SMILES CCOP(=O)(OCC)C1(N=CC2C1C(=O)N(C2=O)c1ccccc1)c1ccccc1 |c:9| Show InChI InChI=1S/C22H23N2O5P/c1-3-28-30(27,29-4-2)22(16-11-7-5-8-12-16)19-18(15-23-22)20(25)24(21(19)26)17-13-9-6-10-14-17/h5-15,18-19H,3-4H2,1-2H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0417 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona
Curated by ChEMBL
| Assay Description
Displacement of [3H]RX821002 from alpha2-AR in human brain frontal cortex incubated for 30 mins by liquid scintillation spectrometry |
J Med Chem 63: 3610-3633 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02080
BindingDB Entry DOI: 10.7270/Q2FB569H |
More data for this
Ligand-Target Pair | |
Protein O-GlcNAcase
(Homo sapiens (Human)) | BDBM139926

(US8901087, 2)Show SMILES CCCNC1=N[C@H]2[C@H](O[C@H]([C@H](C)O)[C@@H](O)[C@@H]2O)S1 |t:4| Show InChI InChI=1S/C11H20N2O4S/c1-3-4-12-11-13-6-7(15)8(16)9(5(2)14)17-10(6)18-11/h5-10,14-16H,3-4H2,1-2H3,(H,12,13)/t5-,6+,7+,8-,9+,10+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0500 | -58.8 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Enzymatic reactions are carried out in a reaction containing 50 mM NaH2PO4, 100 mM NaCl and 0.1% BSA (pH 7.0) using 2 mM 4-Methylumbelliferyl N-acety... |
US Patent US8901087 (2014)
BindingDB Entry DOI: 10.7270/Q24B301W |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50580083
(CHEMBL5094268)Show SMILES Cc1cc(F)c(NC(=O)NCCC(C)(C)C)cc1NC(=O)c1cccc2c(N)ncnc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CRAF Y340D/Y341D mutant (unknown origin) using inactive MAP2K1 as substrate preincubated for 30 mins measured after 90 mins by DELFIA a... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02085
BindingDB Entry DOI: 10.7270/Q2GT5S13 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50557775
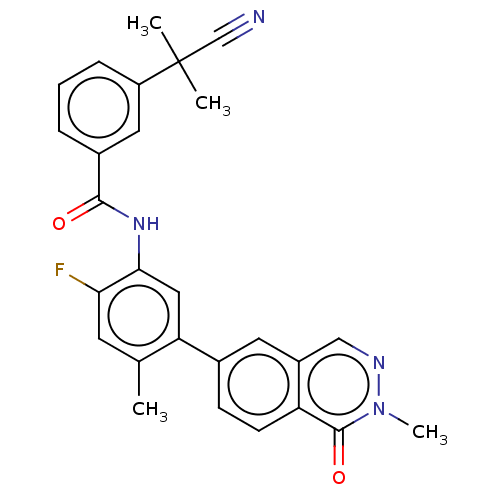
(CHEMBL4758903)Show SMILES Cc1cc(F)c(NC(=O)c2cccc(c2)C(C)(C)C#N)cc1-c1ccc2c(cnn(C)c2=O)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CRAF Y340D/Y341D mutant (unknown origin) assessed as using inactive phosphorylated MAP2K1 substrate preincubated for 30 mins measured a... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00063
BindingDB Entry DOI: 10.7270/Q2DZ0D0Z |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50580080
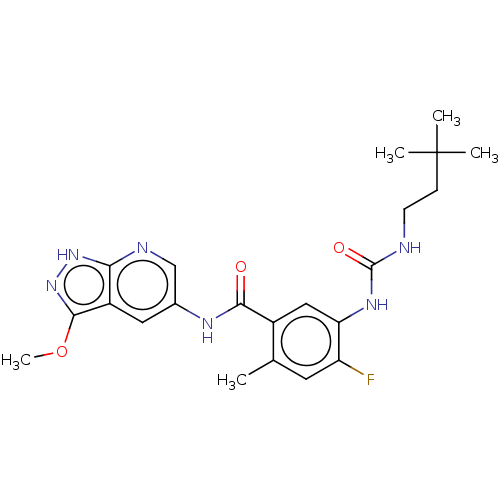
(CHEMBL5090624)Show SMILES COc1n[nH]c2ncc(NC(=O)c3cc(NC(=O)NCCC(C)(C)C)c(F)cc3C)cc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CRAF Y340D/Y341D mutant (unknown origin) using inactive MAP2K1 as substrate preincubated for 30 mins measured after 90 mins by DELFIA a... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02085
BindingDB Entry DOI: 10.7270/Q2GT5S13 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50580082
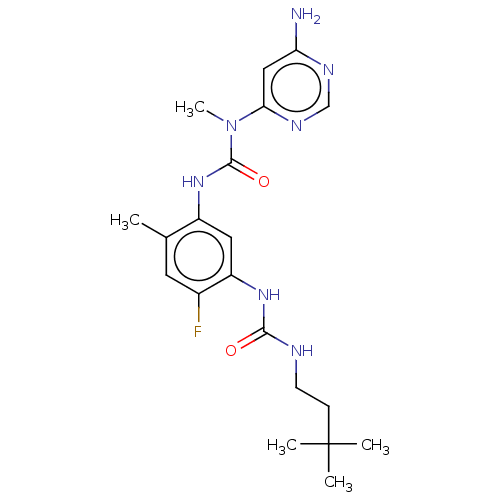
(CHEMBL5079215)Show SMILES CN(C(=O)Nc1cc(NC(=O)NCCC(C)(C)C)c(F)cc1C)c1cc(N)ncn1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CRAF Y340D/Y341D mutant (unknown origin) using inactive MAP2K1 as substrate preincubated for 30 mins measured after 90 mins by DELFIA a... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02085
BindingDB Entry DOI: 10.7270/Q2GT5S13 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50580084
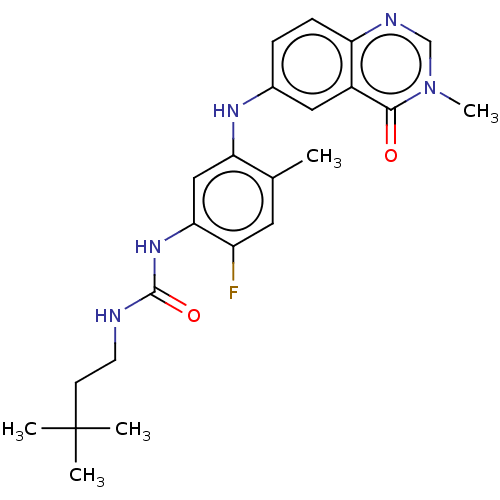
(CHEMBL5075174)Show SMILES Cc1cc(F)c(NC(=O)NCCC(C)(C)C)cc1Nc1ccc2ncn(C)c(=O)c2c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CRAF Y340D/Y341D mutant (unknown origin) using inactive MAP2K1 as substrate preincubated for 30 mins measured after 90 mins by DELFIA a... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02085
BindingDB Entry DOI: 10.7270/Q2GT5S13 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50557772
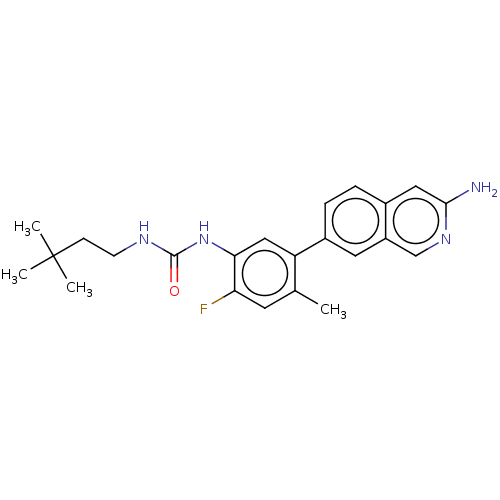
(CHEMBL4775998)Show SMILES Cc1cc(F)c(NC(=O)NCCC(C)(C)C)cc1-c1ccc2cc(N)ncc2c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CRAF Y340D/Y341D mutant (unknown origin) assessed as using inactive phosphorylated MAP2K1 substrate preincubated for 30 mins measured a... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00063
BindingDB Entry DOI: 10.7270/Q2DZ0D0Z |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50557773
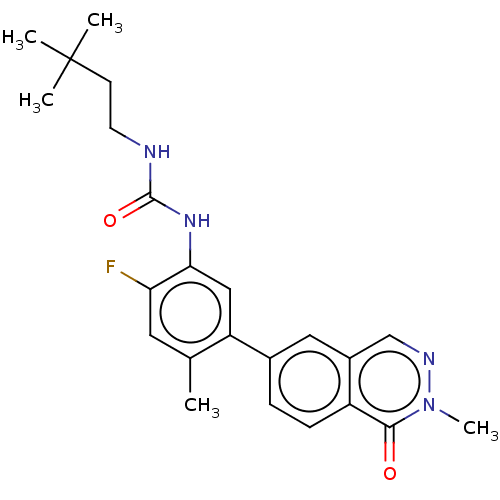
(CHEMBL4778772)Show SMILES Cc1cc(F)c(NC(=O)NCCC(C)(C)C)cc1-c1ccc2c(cnn(C)c2=O)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CRAF Y340D/Y341D mutant (unknown origin) assessed as using inactive phosphorylated MAP2K1 substrate preincubated for 30 mins measured a... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00063
BindingDB Entry DOI: 10.7270/Q2DZ0D0Z |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens) | BDBM50514738
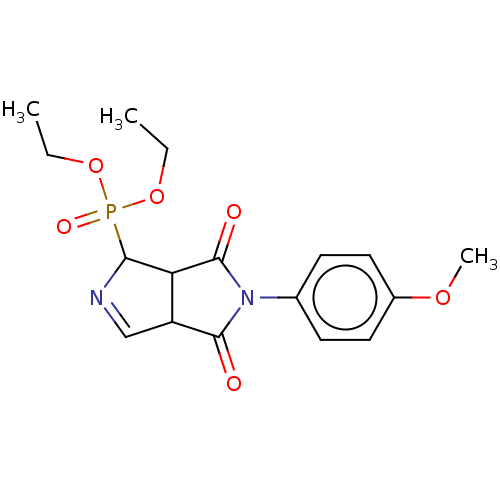
(CHEMBL4536304)Show SMILES CCOP(=O)(OCC)C1N=CC2C1C(=O)N(C2=O)c1ccc(OC)cc1 |c:9| Show InChI InChI=1S/C17H21N2O6P/c1-4-24-26(22,25-5-2)15-14-13(10-18-15)16(20)19(17(14)21)11-6-8-12(23-3)9-7-11/h6-10,13-15H,4-5H2,1-3H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0537 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona
Curated by ChEMBL
| Assay Description
Displacement of [3H]RX821002 from alpha2-AR in human brain frontal cortex incubated for 30 mins by liquid scintillation spectrometry |
J Med Chem 63: 3610-3633 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02080
BindingDB Entry DOI: 10.7270/Q2FB569H |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM50275879
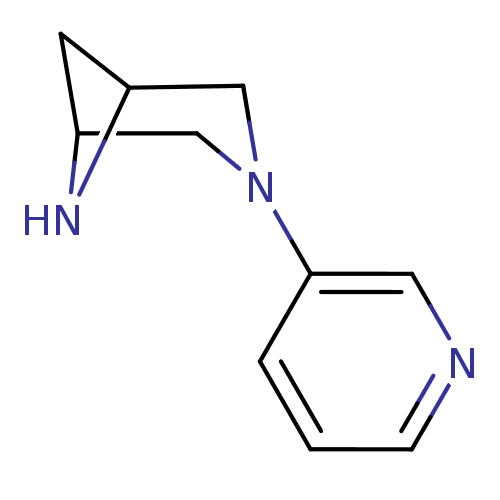
(3-(pyridin-3-yl)-3,6-diazabicyclo[3.1.1]heptane | ...)Show InChI InChI=1S/C10H13N3/c1-2-10(5-11-3-1)13-6-8-4-9(7-13)12-8/h1-3,5,8-9,12H,4,6-7H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Sassari
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine form alpha4beta2 nAChR in rat cortex |
Bioorg Med Chem Lett 18: 6147-50 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.002
BindingDB Entry DOI: 10.7270/Q29G5MPJ |
More data for this
Ligand-Target Pair | |
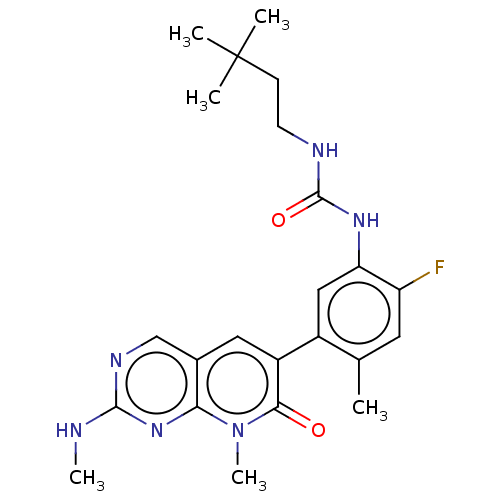
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50580081
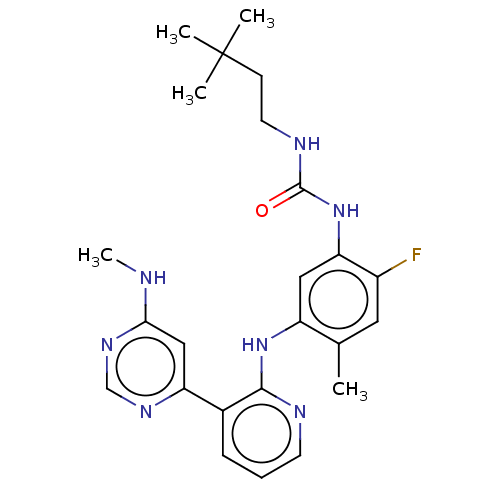
(CHEMBL5094514)Show SMILES CNc1cc(ncn1)-c1cccnc1Nc1cc(NC(=O)NCCC(C)(C)C)c(F)cc1C | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CRAF Y340D/Y341D mutant (unknown origin) using inactive MAP2K1 as substrate preincubated for 30 mins measured after 90 mins by DELFIA a... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02085
BindingDB Entry DOI: 10.7270/Q2GT5S13 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50557774
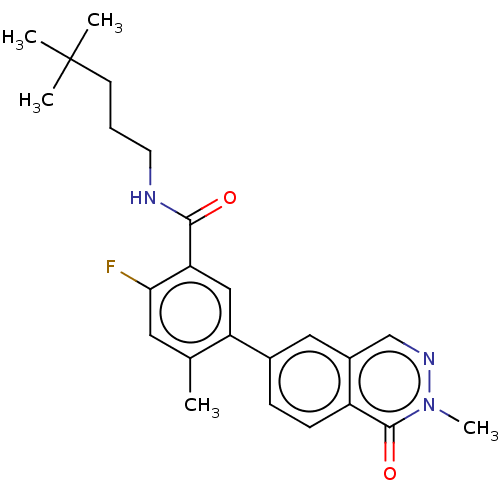
(CHEMBL4776565)Show SMILES Cc1cc(F)c(cc1-c1ccc2c(cnn(C)c2=O)c1)C(=O)NCCCC(C)(C)C | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CRAF Y340D/Y341D mutant (unknown origin) assessed as using inactive phosphorylated MAP2K1 substrate preincubated for 30 mins measured a... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00063
BindingDB Entry DOI: 10.7270/Q2DZ0D0Z |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50096279
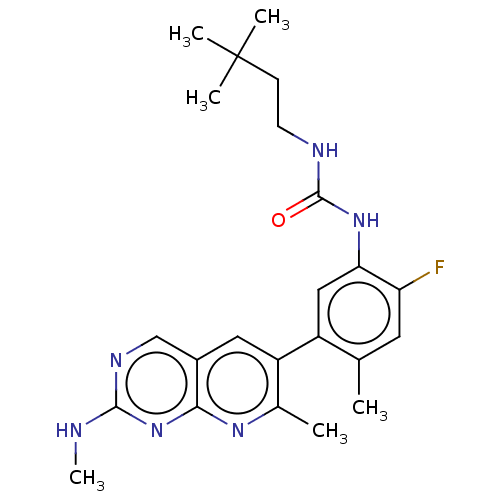
(CHEMBL3577124)Show SMILES CNc1ncc2cc(c(C)nc2n1)-c1cc(NC(=O)NCCC(C)(C)C)c(F)cc1C Show InChI InChI=1S/C18H13N3O5/c22-14(23)5-4-10-8-21-13-7-12-9(6-15(24)25)2-1-3-11(12)16(13)20-18(26)17(21)19-10/h1-5,8H,6-7H2,(H,20,26)(H,22,23)(H,24,25)/b5-4+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.0610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CRAF Y340D/Y341D mutant (unknown origin) assessed as using inactive phosphorylated MAP2K1 substrate preincubated for 30 mins measured a... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00063
BindingDB Entry DOI: 10.7270/Q2DZ0D0Z |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50557770

(CHEMBL4780060)Show SMILES Cc1cc(F)c(NC(=O)NCCC(C)(C)C)cc1Nc1ccc2ncn(C)c(=O)c2c1F | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.0610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CRAF Y340D/Y341D mutant (unknown origin) using inactive MAP2K1 as substrate preincubated for 30 mins measured after 90 mins by DELFIA a... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02085
BindingDB Entry DOI: 10.7270/Q2GT5S13 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50096279

(CHEMBL3577124)Show SMILES CNc1ncc2cc(c(C)nc2n1)-c1cc(NC(=O)NCCC(C)(C)C)c(F)cc1C Show InChI InChI=1S/C18H13N3O5/c22-14(23)5-4-10-8-21-13-7-12-9(6-15(24)25)2-1-3-11(12)16(13)20-18(26)17(21)19-10/h1-5,8H,6-7H2,(H,20,26)(H,22,23)(H,24,25)/b5-4+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.0610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CRAF Y340D/Y341D mutant (unknown origin) using inactive MAP2K1 as substrate preincubated for 30 mins measured after 90 mins by DELFIA a... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02085
BindingDB Entry DOI: 10.7270/Q2GT5S13 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50557770

(CHEMBL4780060)Show SMILES Cc1cc(F)c(NC(=O)NCCC(C)(C)C)cc1Nc1ccc2ncn(C)c(=O)c2c1F | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.0610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CRAF Y340D/Y341D mutant (unknown origin) assessed as using inactive phosphorylated MAP2K1 substrate preincubated for 30 mins measured a... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00063
BindingDB Entry DOI: 10.7270/Q2DZ0D0Z |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50557776
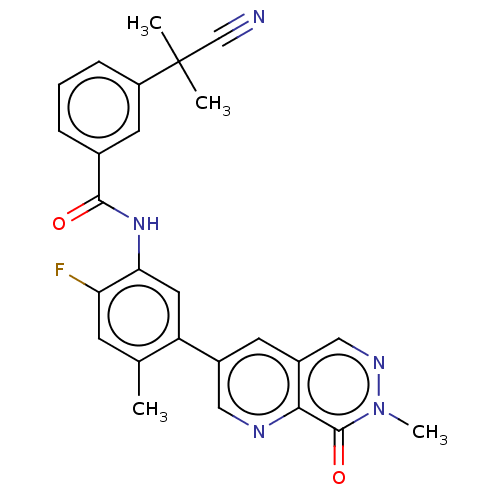
(CHEMBL4778419)Show SMILES Cc1cc(F)c(NC(=O)c2cccc(c2)C(C)(C)C#N)cc1-c1cnc2c(cnn(C)c2=O)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CRAF Y340D/Y341D mutant (unknown origin) assessed as using inactive phosphorylated MAP2K1 substrate preincubated for 30 mins measured a... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00063
BindingDB Entry DOI: 10.7270/Q2DZ0D0Z |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens) | BDBM50514722
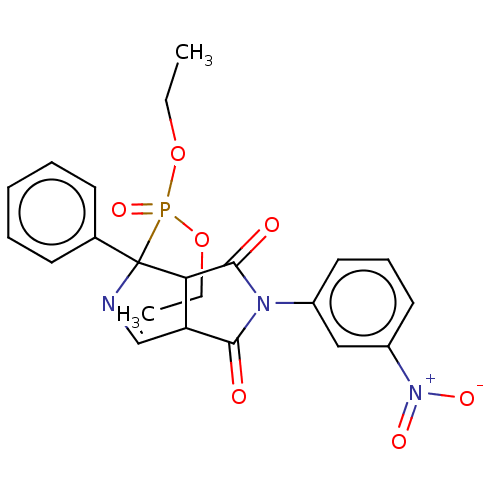
(CHEMBL4438801)Show SMILES CCOP(=O)(OCC)C1(N=CC2C1C(=O)N(C2=O)c1cccc(c1)[N+]([O-])=O)c1ccccc1 |c:9| Show InChI InChI=1S/C22H22N3O7P/c1-3-31-33(30,32-4-2)22(15-9-6-5-7-10-15)19-18(14-23-22)20(26)24(21(19)27)16-11-8-12-17(13-16)25(28)29/h5-14,18-19H,3-4H2,1-2H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0661 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona
Curated by ChEMBL
| Assay Description
Displacement of [3H]RX821002 from alpha2-AR in human brain frontal cortex incubated for 30 mins by liquid scintillation spectrometry |
J Med Chem 63: 3610-3633 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02080
BindingDB Entry DOI: 10.7270/Q2FB569H |
More data for this
Ligand-Target Pair | |
Alpha-1D adrenergic receptor
(Homo sapiens (Human)) | BDBM50370037
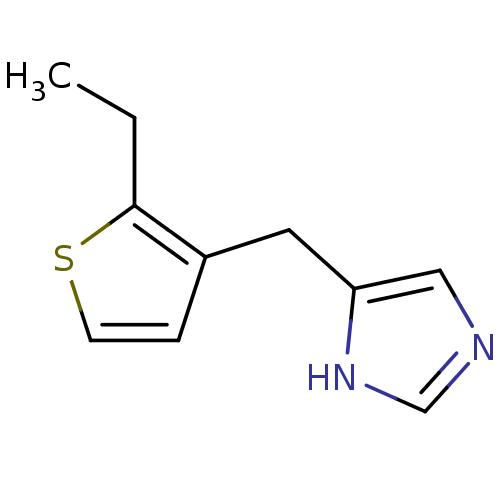
(CHEMBL1744288)Show InChI InChI=1S/C10H12N2S/c1-2-10-8(3-4-13-10)5-9-6-11-7-12-9/h3-4,6-7H,2,5H2,1H3,(H,11,12) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
In vitro binding affinity against alpha-2D adrenergic receptor of male Wistar rat |
J Med Chem 44: 863-72 (2001)
BindingDB Entry DOI: 10.7270/Q23R0TKP |
More data for this
Ligand-Target Pair | |
Alpha-1D adrenergic receptor
(Homo sapiens (Human)) | BDBM50370036
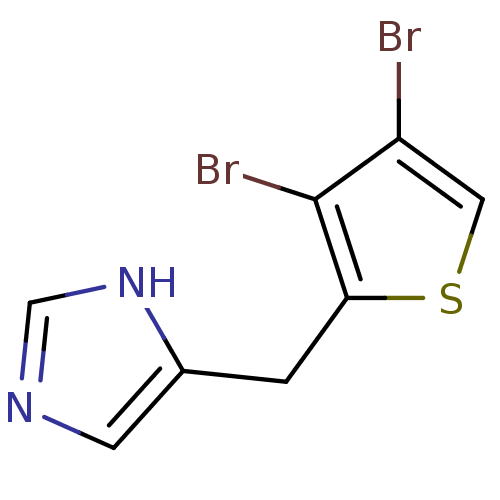
(CHEMBL1203855)Show InChI InChI=1S/C8H6Br2N2S/c9-6-3-13-7(8(6)10)1-5-2-11-4-12-5/h2-4H,1H2,(H,11,12) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
In vitro binding affinity against alpha-2D adrenergic receptor of male Wistar rat |
J Med Chem 44: 863-72 (2001)
BindingDB Entry DOI: 10.7270/Q23R0TKP |
More data for this
Ligand-Target Pair | |
Polyamine oxidase 1
(Zea mays) | BDBM50294105
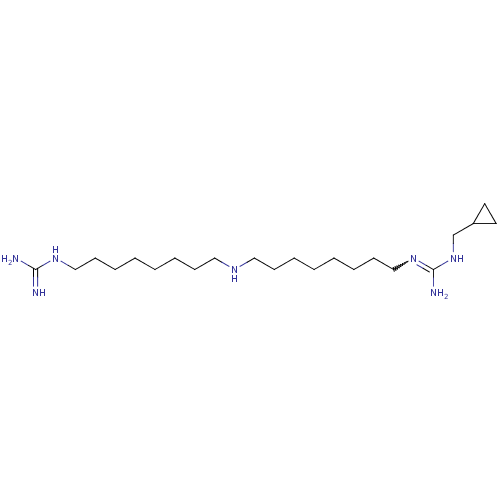
(1-(Guanidino)-17-(N1-(methylcyclopropyl)guanidino)...)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#7]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#7]=[#6](-[#7])-[#7]-[#6]-[#6]-1-[#6]-[#6]-1 |w:21.20| Show InChI InChI=1S/C22H47N7/c23-21(24)27-17-11-7-3-1-5-9-15-26-16-10-6-2-4-8-12-18-28-22(25)29-19-20-13-14-20/h20,26H,1-19H2,(H4,23,24,27)(H3,25,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | 6.5 | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Inhibition of maize PAO at pH 6.5 by spectrophotometry-based Dixon plot method |
J Med Chem 52: 4774-85 (2009)
Article DOI: 10.1021/jm900371z
BindingDB Entry DOI: 10.7270/Q2TB16W1 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Alpha-1D adrenergic receptor
(Homo sapiens (Human)) | BDBM50370035
(CHEMBL1788145)Show InChI InChI=1S/C9H9BrN2S/c1-6(9-2-11-5-12-9)7-3-13-4-8(7)10/h2-6H,1H3,(H,11,12) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
In vitro binding affinity against alpha-2D adrenergic receptor of male Wistar rat |
J Med Chem 44: 863-72 (2001)
BindingDB Entry DOI: 10.7270/Q23R0TKP |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50580084

(CHEMBL5075174)Show SMILES Cc1cc(F)c(NC(=O)NCCC(C)(C)C)cc1Nc1ccc2ncn(C)c(=O)c2c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BRAF (unknown origin) (416 to 766) inactive MAP2K1 as substrate preincubated for 30 mins measured after 90 mins by DELFIA assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02085
BindingDB Entry DOI: 10.7270/Q2GT5S13 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50557775

(CHEMBL4758903)Show SMILES Cc1cc(F)c(NC(=O)c2cccc(c2)C(C)(C)C#N)cc1-c1ccc2c(cnn(C)c2=O)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BRAF (unknown origin) (416 to 766) assessed as using inactive phosphorylated MAP2K1 substrate preincubated for 30 mins measured after 9... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00063
BindingDB Entry DOI: 10.7270/Q2DZ0D0Z |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50580081

(CHEMBL5094514)Show SMILES CNc1cc(ncn1)-c1cccnc1Nc1cc(NC(=O)NCCC(C)(C)C)c(F)cc1C | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BRAF (unknown origin) (416 to 766) inactive MAP2K1 as substrate preincubated for 30 mins measured after 90 mins by DELFIA assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02085
BindingDB Entry DOI: 10.7270/Q2GT5S13 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50580082

(CHEMBL5079215)Show SMILES CN(C(=O)Nc1cc(NC(=O)NCCC(C)(C)C)c(F)cc1C)c1cc(N)ncn1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BRAF (unknown origin) (416 to 766) inactive MAP2K1 as substrate preincubated for 30 mins measured after 90 mins by DELFIA assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02085
BindingDB Entry DOI: 10.7270/Q2GT5S13 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50580083

(CHEMBL5094268)Show SMILES Cc1cc(F)c(NC(=O)NCCC(C)(C)C)cc1NC(=O)c1cccc2c(N)ncnc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BRAF (unknown origin) (416 to 766) inactive MAP2K1 as substrate preincubated for 30 mins measured after 90 mins by DELFIA assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02085
BindingDB Entry DOI: 10.7270/Q2GT5S13 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50557770

(CHEMBL4780060)Show SMILES Cc1cc(F)c(NC(=O)NCCC(C)(C)C)cc1Nc1ccc2ncn(C)c(=O)c2c1F | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| <0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BRAF (unknown origin) (416 to 766) inactive MAP2K1 as substrate preincubated for 30 mins measured after 90 mins by DELFIA assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02085
BindingDB Entry DOI: 10.7270/Q2GT5S13 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50557770

(CHEMBL4780060)Show SMILES Cc1cc(F)c(NC(=O)NCCC(C)(C)C)cc1Nc1ccc2ncn(C)c(=O)c2c1F | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| <0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BRAF (unknown origin) (416 to 766) assessed as using inactive phosphorylated MAP2K1 substrate preincubated for 30 mins measured after 9... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00063
BindingDB Entry DOI: 10.7270/Q2DZ0D0Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50557772

(CHEMBL4775998)Show SMILES Cc1cc(F)c(NC(=O)NCCC(C)(C)C)cc1-c1ccc2cc(N)ncc2c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BRAF (unknown origin) (416 to 766) assessed as using inactive phosphorylated MAP2K1 substrate preincubated for 30 mins measured after 9... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00063
BindingDB Entry DOI: 10.7270/Q2DZ0D0Z |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50557773

(CHEMBL4778772)Show SMILES Cc1cc(F)c(NC(=O)NCCC(C)(C)C)cc1-c1ccc2c(cnn(C)c2=O)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BRAF (unknown origin) (416 to 766) assessed as using inactive phosphorylated MAP2K1 substrate preincubated for 30 mins measured after 9... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00063
BindingDB Entry DOI: 10.7270/Q2DZ0D0Z |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50557771
(CHEMBL4740241)Show SMILES CNc1ncc2cc(-c3cc(NC(=O)NCCC(C)(C)C)c(F)cc3C)c(=O)n(C)c2n1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CRAF Y340D/Y341D mutant (unknown origin) assessed as using inactive phosphorylated MAP2K1 substrate preincubated for 30 mins measured a... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00063
BindingDB Entry DOI: 10.7270/Q2DZ0D0Z |
More data for this
Ligand-Target Pair | |
Alpha-1D adrenergic receptor
(Homo sapiens (Human)) | BDBM50370026
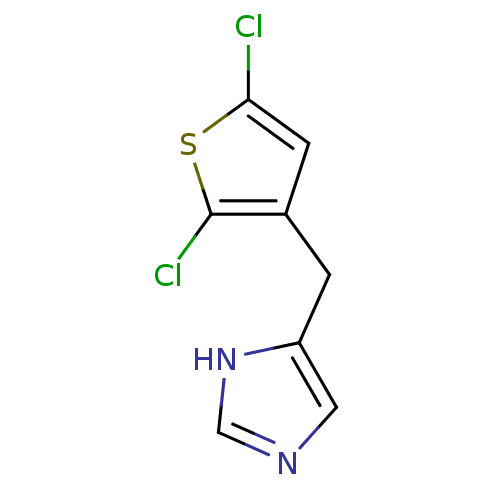
(CHEMBL1744273)Show InChI InChI=1S/C8H6Cl2N2S/c9-7-2-5(8(10)13-7)1-6-3-11-4-12-6/h2-4H,1H2,(H,11,12) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
In vitro binding affinity against alpha-2D adrenergic receptor of male Wistar rat |
J Med Chem 44: 863-72 (2001)
BindingDB Entry DOI: 10.7270/Q23R0TKP |
More data for this
Ligand-Target Pair | |
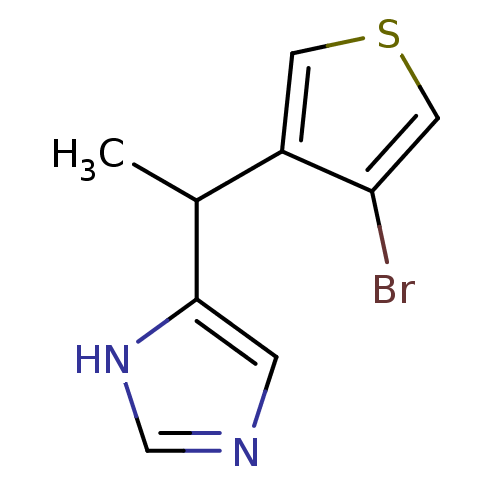
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50392561
(CHEMBL2152813)Show SMILES CCCCCn1cc(C(=O)N[C@@H]2C(C)(C)C3CC[C@]2(C)C3)c(=O)c2ccc(Sc3ccccc3)cc12 |r| Show InChI InChI=1S/C31H38N2O2S/c1-5-6-10-17-33-20-25(28(35)32-29-30(2,3)21-15-16-31(29,4)19-21)27(34)24-14-13-23(18-26(24)33)36-22-11-8-7-9-12-22/h7-9,11-14,18,20-21,29H,5-6,10,15-17,19H2,1-4H3,(H,32,35)/t21?,29-,31-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human recombinant CB2 receptor transfected in HEK cell membranes |
Eur J Med Chem 58: 30-43 (2012)
Article DOI: 10.1016/j.ejmech.2012.09.035
BindingDB Entry DOI: 10.7270/Q2JD4XW2 |
More data for this
Ligand-Target Pair | |
Alpha-1D adrenergic receptor
(Homo sapiens (Human)) | BDBM50370020
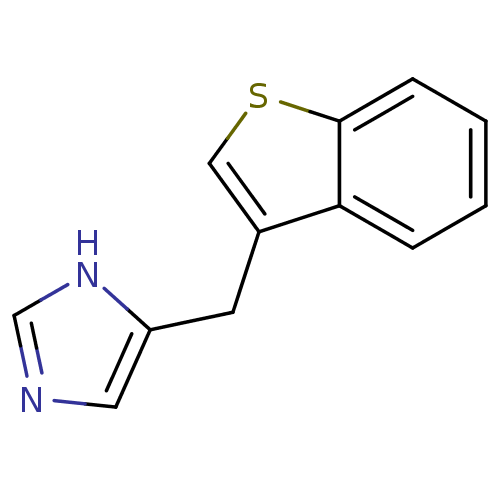
(CHEMBL1744270)Show InChI InChI=1S/C12H10N2S/c1-2-4-12-11(3-1)9(7-15-12)5-10-6-13-8-14-10/h1-4,6-8H,5H2,(H,13,14) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
In vitro binding affinity against alpha-2D adrenergic receptor of male Wistar rat |
J Med Chem 44: 863-72 (2001)
BindingDB Entry DOI: 10.7270/Q23R0TKP |
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 1
(Homo sapiens (Human)) | BDBM22000
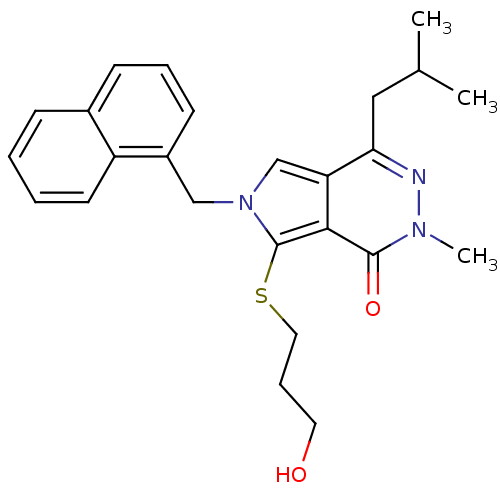
(7-[(3-hydroxypropyl)sulfanyl]-2-methyl-4-(2-methyl...)Show SMILES CC(C)Cc1nn(C)c(=O)c2c(SCCCO)n(Cc3cccc4ccccc34)cc12 Show InChI InChI=1S/C25H29N3O2S/c1-17(2)14-22-21-16-28(15-19-10-6-9-18-8-4-5-11-20(18)19)25(31-13-7-12-29)23(21)24(30)27(3)26-22/h4-6,8-11,16-17,29H,7,12-15H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0955 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood
Curated by ChEMBL
| Assay Description
Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-... |
Nat Chem Biol 1: 371-6 (2005)
BindingDB Entry DOI: 10.7270/Q2MC9063 |
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 1
(Homo sapiens (Human)) | BDBM22000

(7-[(3-hydroxypropyl)sulfanyl]-2-methyl-4-(2-methyl...)Show SMILES CC(C)Cc1nn(C)c(=O)c2c(SCCCO)n(Cc3cccc4ccccc34)cc12 Show InChI InChI=1S/C25H29N3O2S/c1-17(2)14-22-21-16-28(15-19-10-6-9-18-8-4-5-11-20(18)19)25(31-13-7-12-29)23(21)24(30)27(3)26-22/h4-6,8-11,16-17,29H,7,12-15H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | -56.5 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
AstraZeneca
| Assay Description
Jurkat cell membranes were incubated with [3H]-labeled ligand in the absence or presence of increasing concentrations of test compound. The reagents ... |
Bioorg Med Chem Lett 16: 2260-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.024
BindingDB Entry DOI: 10.7270/Q2C24TQK |
More data for this
Ligand-Target Pair | |
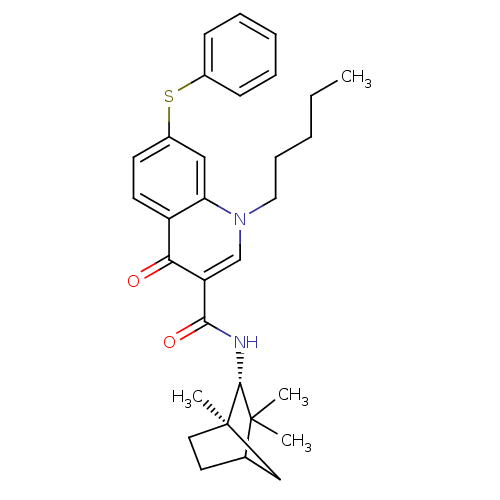
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM164488
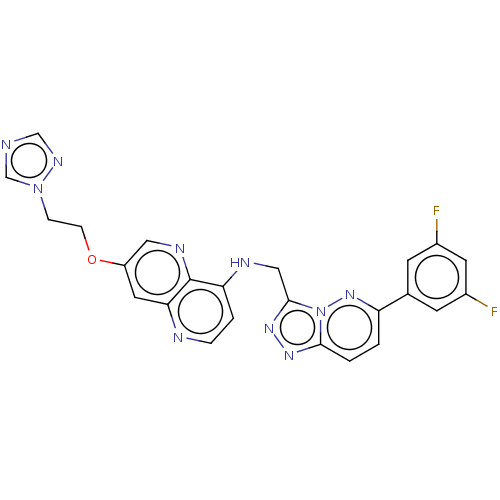
(US9066954, 373)Show SMILES Fc1cc(F)cc(c1)-c1ccc2nnc(CNc3ccnc4cc(OCCn5cncn5)cnc34)n2n1 Show InChI InChI=1S/C24H18F2N10O/c25-16-7-15(8-17(26)9-16)19-1-2-22-32-33-23(36(22)34-19)12-29-20-3-4-28-21-10-18(11-30-24(20)21)37-6-5-35-14-27-13-31-35/h1-4,7-11,13-14H,5-6,12H2,(H,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.100 | -57.5 | n/a | n/a | n/a | n/a | n/a | 8.0 | 27 |
Amgen Inc.
US Patent
| Assay Description
A PCR product covering residues 1058-1365 of c-Met (c-Met kinase domain) is generated from Human Liver QuickClone cDNA (Invitrogen) using forward pri... |
US Patent US9066954 (2015)
BindingDB Entry DOI: 10.7270/Q2N878JD |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50392560
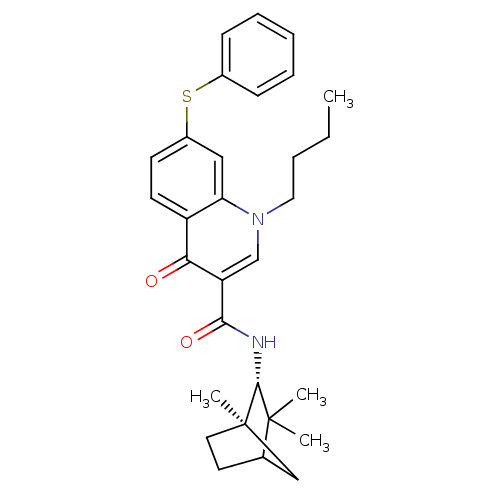
(CHEMBL2152812)Show SMILES CCCCn1cc(C(=O)N[C@@H]2C(C)(C)C3CC[C@]2(C)C3)c(=O)c2ccc(Sc3ccccc3)cc12 |r| Show InChI InChI=1S/C30H36N2O2S/c1-5-6-16-32-19-24(27(34)31-28-29(2,3)20-14-15-30(28,4)18-20)26(33)23-13-12-22(17-25(23)32)35-21-10-8-7-9-11-21/h7-13,17,19-20,28H,5-6,14-16,18H2,1-4H3,(H,31,34)/t20?,28-,30-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human recombinant CB2 receptor transfected in HEK cell membranes |
Eur J Med Chem 58: 30-43 (2012)
Article DOI: 10.1016/j.ejmech.2012.09.035
BindingDB Entry DOI: 10.7270/Q2JD4XW2 |
More data for this
Ligand-Target Pair | |
Alpha-1D adrenergic receptor
(Homo sapiens (Human)) | BDBM50370021
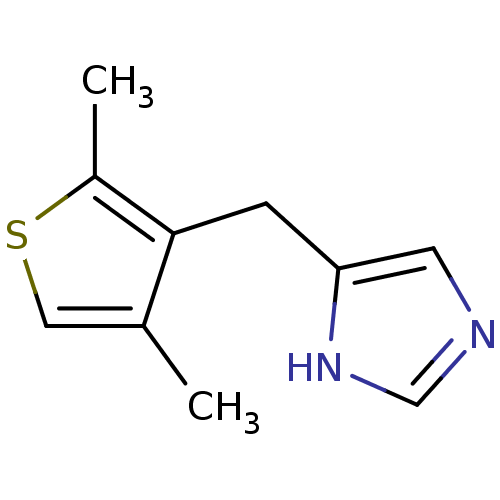
(CHEMBL1744272)Show InChI InChI=1S/C10H12N2S/c1-7-5-13-8(2)10(7)3-9-4-11-6-12-9/h4-6H,3H2,1-2H3,(H,11,12) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
In vitro binding affinity against alpha-2D adrenergic receptor of male Wistar rat |
J Med Chem 44: 863-72 (2001)
BindingDB Entry DOI: 10.7270/Q23R0TKP |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM21301
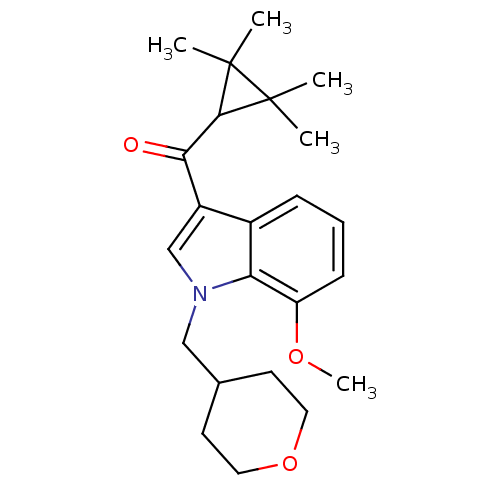
(7-methoxy-1-(oxan-4-ylmethyl)-3-[(2,2,3,3-tetramet...)Show SMILES COc1cccc2c(cn(CC3CCOCC3)c12)C(=O)C1C(C)(C)C1(C)C Show InChI InChI=1S/C23H31NO3/c1-22(2)21(23(22,3)4)20(25)17-14-24(13-15-9-11-27-12-10-15)19-16(17)7-6-8-18(19)26-5/h6-8,14-15,21H,9-13H2,1-5H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP 55940 from human CB2 receptor in cell free system |
Eur J Med Chem 46: 547-55 (2011)
Article DOI: 10.1016/j.ejmech.2010.11.034
BindingDB Entry DOI: 10.7270/Q2CF9QCJ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50557771

(CHEMBL4740241)Show SMILES CNc1ncc2cc(-c3cc(NC(=O)NCCC(C)(C)C)c(F)cc3C)c(=O)n(C)c2n1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.102 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BRAF (unknown origin) (416 to 766) assessed as using inactive phosphorylated MAP2K1 substrate preincubated for 30 mins measured after 9... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00063
BindingDB Entry DOI: 10.7270/Q2DZ0D0Z |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data