 Found 464 hits with Last Name = 'mukherjee' and Initial = 'a'
Found 464 hits with Last Name = 'mukherjee' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Protein arginine N-methyltransferase 5
(Homo sapiens (Human)) | BDBM50597886

(CHEMBL5180177)Show SMILES [H][C@]1(O[C@@H]([C@@H](O)[C@H]1O)n1ccc2c(N)ncnc12)[C@H](O)c1ccc(Cl)c(F)c1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01208
BindingDB Entry DOI: 10.7270/Q25X2DZR |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50597868

(CHEMBL5202438)Show SMILES CC(C)N(C[C@@H]1O[C@@H]([C@@H](O)[C@H]1O)n1cnc2c(N)ncnc12)C1CC(CCc2nc3cc(ccc3[nH]2)C(C)(C)C)C1 |r,wU:5.4,7.12,wD:8.8,10.11,(-4.59,1.12,;-3.05,1.12,;-2.28,2.45,;-2.28,-.21,;-3.05,-1.55,;-4.59,-1.55,;-5.62,-.4,;-7.02,-1.03,;-6.86,-2.56,;-7.95,-3.65,;-5.35,-2.88,;-4.59,-4.21,;-8.29,-.15,;-8.4,1.38,;-9.89,1.75,;-10.71,.44,;-12.23,.17,;-13.22,1.35,;-12.75,-1.29,;-11.75,-2.46,;-10.24,-2.19,;-9.71,-.73,;-.74,-.21,;.37,.89,;1.46,-.2,;3,-.2,;3.77,1.14,;5.31,1.14,;6.22,2.38,;7.68,1.91,;9.01,2.67,;10.34,1.9,;10.35,.37,;9.02,-.4,;7.68,.37,;6.22,-.11,;11.68,2.67,;11.68,4.21,;12.45,1.34,;13.22,2.67,;.35,-1.3,)| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01208
BindingDB Entry DOI: 10.7270/Q25X2DZR |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50597877
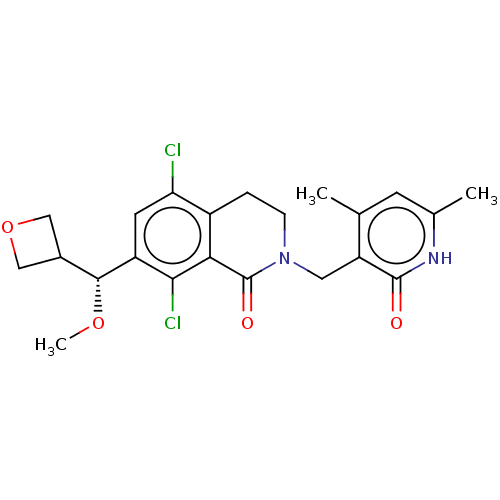
(CHEMBL5191399)Show SMILES CO[C@H](C1COC1)c1cc(Cl)c2CCN(Cc3c(C)cc(C)[nH]c3=O)C(=O)c2c1Cl |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01208
BindingDB Entry DOI: 10.7270/Q25X2DZR |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM18161

((1S,2S,7S,10R,11S,14S,15S)-14-hydroxy-2,15-dimethy...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC[C@@]2([H])CC(=O)CC[C@]12C |r| Show InChI InChI=1S/C19H30O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h12,14-17,21H,3-11H2,1-2H3/t12-,14-,15-,16-,17-,18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.280 | -50.7 | n/a | n/a | 1 | n/a | n/a | 7.4 | 4 |
University of Tennessee at Memphis
| Assay Description
The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... |
Biochem Biophys Res Commun 244: 1-4 (1998)
Article DOI: 10.1006/bbrc.1998.8209
BindingDB Entry DOI: 10.7270/Q2930RFC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Homo sapiens (Human)) | BDBM18699
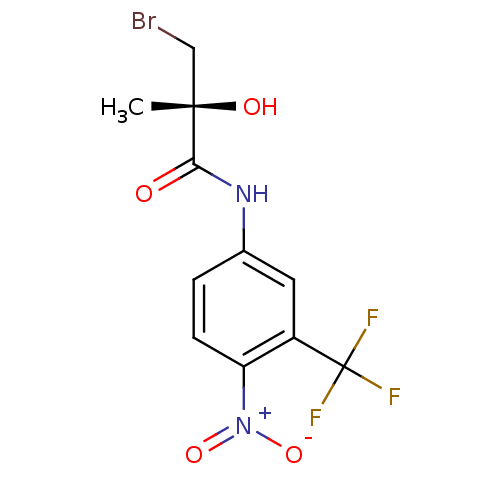
((2R)-3-bromo-2-hydroxy-2-methyl-N-[4-nitro-3-(trif...)Show SMILES C[C@](O)(CBr)C(=O)Nc1ccc(c(c1)C(F)(F)F)[N+]([O-])=O |r| Show InChI InChI=1S/C11H10BrF3N2O4/c1-10(19,5-12)9(18)16-6-2-3-8(17(20)21)7(4-6)11(13,14)15/h2-4,19H,5H2,1H3,(H,16,18)/t10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tennessee-Health Science Center
Curated by ChEMBL
| Assay Description
Binding affinity against human androgen receptor (hAR) in competitive binding assay |
J Med Chem 44: 1729-40 (2001)
BindingDB Entry DOI: 10.7270/Q25M650Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM18699

((2R)-3-bromo-2-hydroxy-2-methyl-N-[4-nitro-3-(trif...)Show SMILES C[C@](O)(CBr)C(=O)Nc1ccc(c(c1)C(F)(F)F)[N+]([O-])=O |r| Show InChI InChI=1S/C11H10BrF3N2O4/c1-10(19,5-12)9(18)16-6-2-3-8(17(20)21)7(4-6)11(13,14)15/h2-4,19H,5H2,1H3,(H,16,18)/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.300 | -50.5 | n/a | n/a | 500 | n/a | n/a | 7.4 | 4 |
University of Tennessee at Memphis
| Assay Description
The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... |
Biochem Biophys Res Commun 244: 1-4 (1998)
Article DOI: 10.1006/bbrc.1998.8209
BindingDB Entry DOI: 10.7270/Q2930RFC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM18681

((2R)-N-[4-cyano-3-(trifluoromethyl)phenyl]-2-hydro...)Show SMILES C[C@](O)(CSc1ccc(cc1)N=C=S)C(=O)Nc1ccc(C#N)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C19H14F3N3O2S2/c1-18(27,10-29-15-6-4-13(5-7-15)24-11-28)17(26)25-14-3-2-12(9-23)16(8-14)19(20,21)22/h2-8,27H,10H2,1H3,(H,25,26)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | -48.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
University of Tennessee at Memphis
| Assay Description
The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... |
J Med Chem 43: 581-90 (2000)
Article DOI: 10.1021/jm990027x
BindingDB Entry DOI: 10.7270/Q2DR2SR2 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50193709
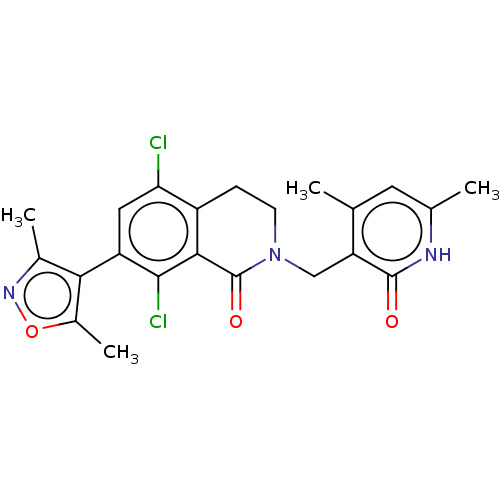
(CHEMBL3911017)Show SMILES Cc1noc(C)c1-c1cc(Cl)c2CCN(Cc3c(C)cc(C)[nH]c3=O)C(=O)c2c1Cl |(59.06,-26.01,;58.74,-27.52,;59.77,-28.66,;59,-30,;57.5,-29.68,;56.35,-30.71,;57.33,-28.15,;55.99,-27.38,;54.66,-28.14,;53.34,-27.37,;52.01,-28.14,;53.35,-25.85,;52.02,-25.09,;52.02,-23.55,;53.35,-22.77,;53.35,-21.23,;52.02,-20.46,;52.02,-18.92,;53.36,-18.16,;50.7,-18.15,;49.36,-18.92,;48.03,-18.14,;49.35,-20.46,;50.69,-21.24,;50.69,-22.78,;54.68,-23.55,;56.01,-22.79,;54.68,-25.09,;55.99,-25.86,;57.33,-25.09,)| Show InChI InChI=1S/C22H21Cl2N3O3/c1-10-7-11(2)25-21(28)16(10)9-27-6-5-14-17(23)8-15(20(24)19(14)22(27)29)18-12(3)26-30-13(18)4/h7-8H,5-6,9H2,1-4H3,(H,25,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01208
BindingDB Entry DOI: 10.7270/Q25X2DZR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM18701
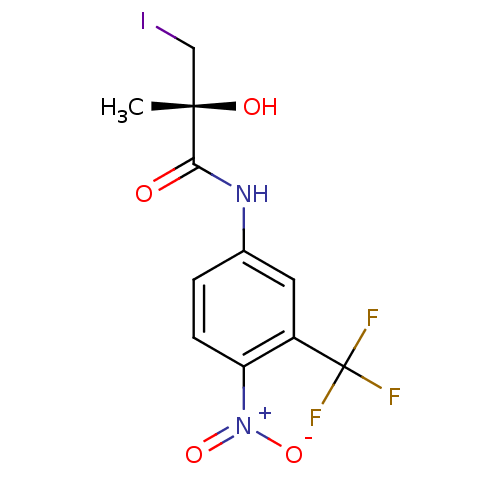
((2R)-2-hydroxy-3-iodo-2-methyl-N-[4-nitro-3-(trifl...)Show SMILES C[C@](O)(CI)C(=O)Nc1ccc(c(c1)C(F)(F)F)[N+]([O-])=O |r| Show InChI InChI=1S/C11H10F3IN2O4/c1-10(19,5-15)9(18)16-6-2-3-8(17(20)21)7(4-6)11(12,13)14/h2-4,19H,5H2,1H3,(H,16,18)/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.860 | -48.1 | n/a | n/a | 500 | n/a | n/a | 7.4 | 4 |
University of Tennessee at Memphis
| Assay Description
The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... |
Biochem Biophys Res Commun 244: 1-4 (1998)
Article DOI: 10.1006/bbrc.1998.8209
BindingDB Entry DOI: 10.7270/Q2930RFC |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM18685
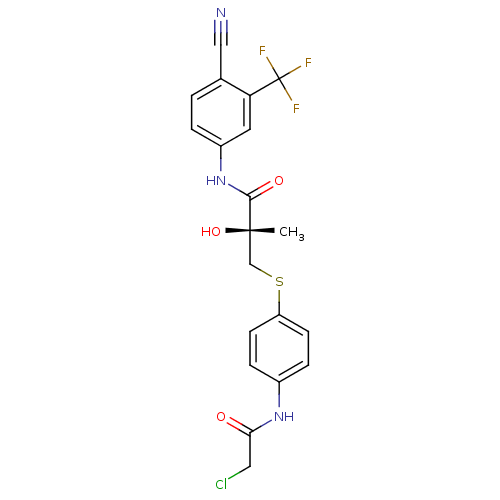
((2R)-3-{[4-(2-chloroacetamido)phenyl]sulfanyl}-N-[...)Show SMILES C[C@](O)(CSc1ccc(NC(=O)CCl)cc1)C(=O)Nc1ccc(C#N)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C20H17ClF3N3O3S/c1-19(30,11-31-15-6-4-13(5-7-15)26-17(28)9-21)18(29)27-14-3-2-12(10-25)16(8-14)20(22,23)24/h2-8,30H,9,11H2,1H3,(H,26,28)(H,27,29)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.65 | -46.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
University of Tennessee at Memphis
| Assay Description
The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... |
J Med Chem 43: 581-90 (2000)
Article DOI: 10.1021/jm990027x
BindingDB Entry DOI: 10.7270/Q2DR2SR2 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM18685

((2R)-3-{[4-(2-chloroacetamido)phenyl]sulfanyl}-N-[...)Show SMILES C[C@](O)(CSc1ccc(NC(=O)CCl)cc1)C(=O)Nc1ccc(C#N)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C20H17ClF3N3O3S/c1-19(30,11-31-15-6-4-13(5-7-15)26-17(28)9-21)18(29)27-14-3-2-12(10-25)16(8-14)20(22,23)24/h2-8,30H,9,11H2,1H3,(H,26,28)(H,27,29)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.65 | -46.6 | n/a | n/a | 100 | n/a | n/a | 7.4 | 4 |
University of Tennessee at Memphis
| Assay Description
The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... |
Biochem Biophys Res Commun 244: 1-4 (1998)
Article DOI: 10.1006/bbrc.1998.8209
BindingDB Entry DOI: 10.7270/Q2930RFC |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM18700
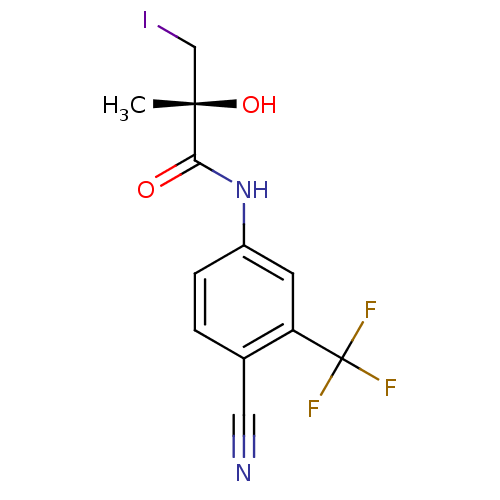
((2R)-N-[4-cyano-3-(trifluoromethyl)phenyl]-2-hydro...)Show SMILES C[C@](O)(CI)C(=O)Nc1ccc(C#N)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C12H10F3IN2O2/c1-11(20,6-16)10(19)18-8-3-2-7(5-17)9(4-8)12(13,14)15/h2-4,20H,6H2,1H3,(H,18,19)/t11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.79 | -45.4 | n/a | n/a | 500 | n/a | n/a | 7.4 | 4 |
University of Tennessee at Memphis
| Assay Description
The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... |
Biochem Biophys Res Commun 244: 1-4 (1998)
Article DOI: 10.1006/bbrc.1998.8209
BindingDB Entry DOI: 10.7270/Q2930RFC |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM18698
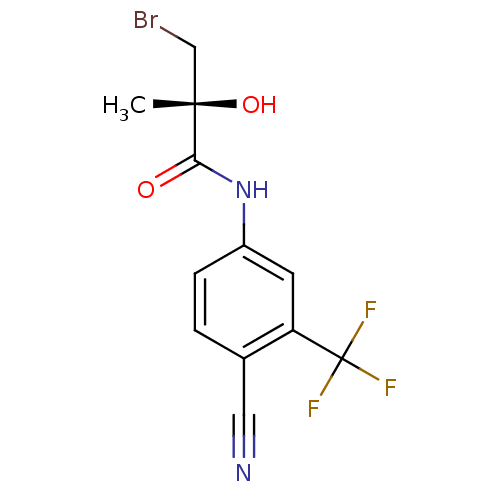
((2R)-3-bromo-N-[4-cyano-3-(trifluoromethyl)phenyl]...)Show SMILES C[C@](O)(CBr)C(=O)Nc1ccc(C#N)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C12H10BrF3N2O2/c1-11(20,6-13)10(19)18-8-3-2-7(5-17)9(4-8)12(14,15)16/h2-4,20H,6H2,1H3,(H,18,19)/t11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.98 | -43.0 | n/a | n/a | 500 | n/a | n/a | 7.4 | 4 |
University of Tennessee at Memphis
| Assay Description
The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... |
Biochem Biophys Res Commun 244: 1-4 (1998)
Article DOI: 10.1006/bbrc.1998.8209
BindingDB Entry DOI: 10.7270/Q2930RFC |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM18689
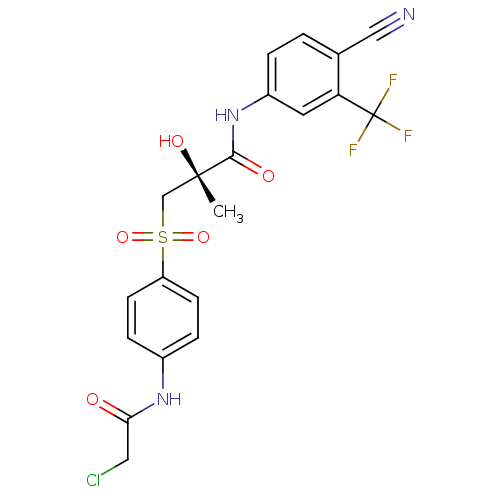
((2R)-3-{[4-(2-chloroacetamido)benzene]sulfonyl}-N-...)Show SMILES C[C@](O)(CS(=O)(=O)c1ccc(NC(=O)CCl)cc1)C(=O)Nc1ccc(C#N)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C20H17ClF3N3O5S/c1-19(30,11-33(31,32)15-6-4-13(5-7-15)26-17(28)9-21)18(29)27-14-3-2-12(10-25)16(8-14)20(22,23)24/h2-8,30H,9,11H2,1H3,(H,26,28)(H,27,29)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10.7 | -42.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
University of Tennessee at Memphis
| Assay Description
The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... |
J Med Chem 43: 581-90 (2000)
Article DOI: 10.1021/jm990027x
BindingDB Entry DOI: 10.7270/Q2DR2SR2 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM18678
((2R)-N-[4-cyano-3-(trifluoromethyl)phenyl]-3-[(4-f...)Show SMILES C[C@](O)(CS(=O)(=O)c1ccc(F)cc1)C(=O)Nc1ccc(C#N)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C18H14F4N2O4S/c1-17(26,10-29(27,28)14-6-3-12(19)4-7-14)16(25)24-13-5-2-11(9-23)15(8-13)18(20,21)22/h2-8,26H,10H2,1H3,(H,24,25)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 11 | -42.2 | n/a | n/a | 1.00E+3 | n/a | n/a | 7.4 | 4 |
University of Tennessee at Memphis
| Assay Description
The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... |
Biochem Biophys Res Commun 244: 1-4 (1998)
Article DOI: 10.1006/bbrc.1998.8209
BindingDB Entry DOI: 10.7270/Q2930RFC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM18678

((2R)-N-[4-cyano-3-(trifluoromethyl)phenyl]-3-[(4-f...)Show SMILES C[C@](O)(CS(=O)(=O)c1ccc(F)cc1)C(=O)Nc1ccc(C#N)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C18H14F4N2O4S/c1-17(26,10-29(27,28)14-6-3-12(19)4-7-14)16(25)24-13-5-2-11(9-23)15(8-13)18(20,21)22/h2-8,26H,10H2,1H3,(H,24,25)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 11 | -42.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
University of Tennessee at Memphis
| Assay Description
The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... |
J Med Chem 43: 581-90 (2000)
Article DOI: 10.1021/jm990027x
BindingDB Entry DOI: 10.7270/Q2DR2SR2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Homo sapiens (Human)) | BDBM50099679
(3-Bromo-2-hydroxy-2-methyl-N-(4-nitro-3-trifluorom...)Show SMILES C[C@@](O)(CBr)C(=O)Nc1ccc(c(c1)C(F)(F)F)[N+]([O-])=O Show InChI InChI=1S/C11H10BrF3N2O4/c1-10(19,5-12)9(18)16-6-2-3-8(17(20)21)7(4-6)11(13,14)15/h2-4,19H,5H2,1H3,(H,16,18)/t10-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 16.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tennessee-Health Science Center
Curated by ChEMBL
| Assay Description
Binding affinity against human androgen receptor (hAR) in competitive binding assay |
J Med Chem 44: 1729-40 (2001)
BindingDB Entry DOI: 10.7270/Q25M650Q |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM18683
((2R)-N-[4-cyano-3-(trifluoromethyl)phenyl]-2-hydro...)Show SMILES C[C@](O)(CS(=O)(=O)c1ccc(cc1)N=C=S)C(=O)Nc1ccc(C#N)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C19H14F3N3O4S2/c1-18(27,10-31(28,29)15-6-4-13(5-7-15)24-11-30)17(26)25-14-3-2-12(9-23)16(8-14)19(20,21)22/h2-8,27H,10H2,1H3,(H,25,26)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 41 | -39.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
University of Tennessee at Memphis
| Assay Description
The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... |
J Med Chem 43: 581-90 (2000)
Article DOI: 10.1021/jm990027x
BindingDB Entry DOI: 10.7270/Q2DR2SR2 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM18680

((2R)-3-[(3-aminophenyl)sulfanyl]-N-[4-cyano-3-(tri...)Show SMILES C[C@](O)(CSc1cccc(N)c1)C(=O)Nc1ccc(C#N)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C18H16F3N3O2S/c1-17(26,10-27-14-4-2-3-12(23)7-14)16(25)24-13-6-5-11(9-22)15(8-13)18(19,20)21/h2-8,26H,10,23H2,1H3,(H,24,25)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 65 | -38.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
University of Tennessee at Memphis
| Assay Description
The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... |
J Med Chem 43: 581-90 (2000)
Article DOI: 10.1021/jm990027x
BindingDB Entry DOI: 10.7270/Q2DR2SR2 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50099680
((R)-N-(4-Amino-3-trifluoromethyl-phenyl)-3-bromo-2...)Show InChI InChI=1S/C11H12BrF3N2O2/c1-10(19,5-12)9(18)17-6-2-3-8(16)7(4-6)11(13,14)15/h2-4,19H,5,16H2,1H3,(H,17,18)/t10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 80.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tennessee-Health Science Center
Curated by ChEMBL
| Assay Description
Binding affinity against human androgen receptor (hAR) in competitive binding assay |
J Med Chem 44: 1729-40 (2001)
BindingDB Entry DOI: 10.7270/Q25M650Q |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM18679
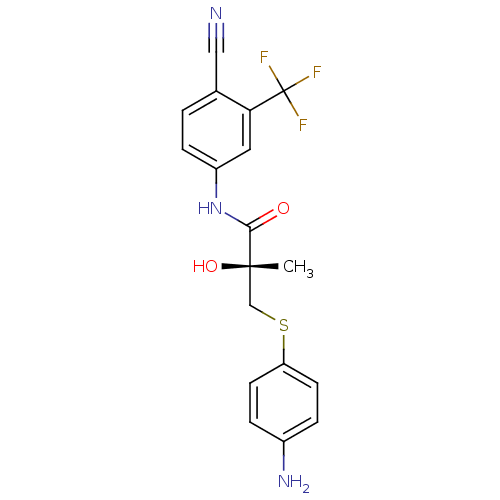
((2R)-3-[(4-aminophenyl)sulfanyl]-N-[4-cyano-3-(tri...)Show SMILES C[C@](O)(CSc1ccc(N)cc1)C(=O)Nc1ccc(C#N)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C18H16F3N3O2S/c1-17(26,10-27-14-6-3-12(23)4-7-14)16(25)24-13-5-2-11(9-22)15(8-13)18(19,20)21/h2-8,26H,10,23H2,1H3,(H,24,25)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 90 | -37.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
University of Tennessee at Memphis
| Assay Description
The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... |
J Med Chem 43: 581-90 (2000)
Article DOI: 10.1021/jm990027x
BindingDB Entry DOI: 10.7270/Q2DR2SR2 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM18687

((2R)-3-{[4-(2-bromoacetamido)phenyl]sulfanyl}-N-[4...)Show SMILES C[C@](O)(CSc1ccc(NC(=O)CBr)cc1)C(=O)Nc1ccc(C#N)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C20H17BrF3N3O3S/c1-19(30,11-31-15-6-4-13(5-7-15)26-17(28)9-21)18(29)27-14-3-2-12(10-25)16(8-14)20(22,23)24/h2-8,30H,9,11H2,1H3,(H,26,28)(H,27,29)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | -37.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
University of Tennessee at Memphis
| Assay Description
The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... |
J Med Chem 43: 581-90 (2000)
Article DOI: 10.1021/jm990027x
BindingDB Entry DOI: 10.7270/Q2DR2SR2 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM18690
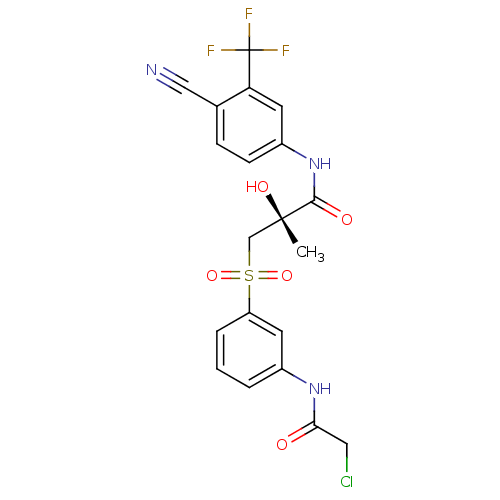
((2R)-3-{[3-(2-chloroacetamido)benzene]sulfonyl}-N-...)Show SMILES C[C@](O)(CS(=O)(=O)c1cccc(NC(=O)CCl)c1)C(=O)Nc1ccc(C#N)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C20H17ClF3N3O5S/c1-19(30,11-33(31,32)15-4-2-3-13(7-15)26-17(28)9-21)18(29)27-14-6-5-12(10-25)16(8-14)20(22,23)24/h2-8,30H,9,11H2,1H3,(H,26,28)(H,27,29)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 130 | -36.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
University of Tennessee at Memphis
| Assay Description
The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... |
J Med Chem 43: 581-90 (2000)
Article DOI: 10.1021/jm990027x
BindingDB Entry DOI: 10.7270/Q2DR2SR2 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM18696
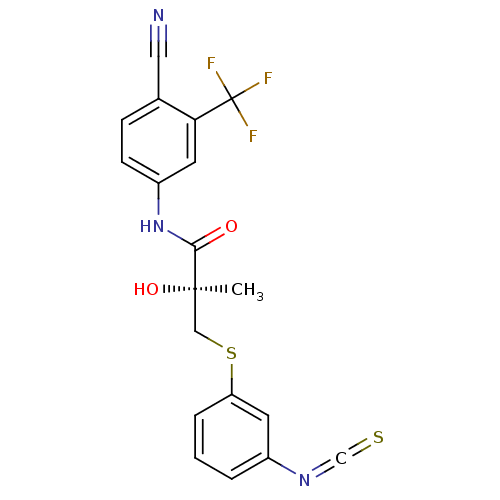
((2S)-N-[4-cyano-3-(trifluoromethyl)phenyl]-2-hydro...)Show SMILES C[C@@](O)(CSc1cccc(c1)N=C=S)C(=O)Nc1ccc(C#N)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C19H14F3N3O2S2/c1-18(27,10-29-15-4-2-3-13(7-15)24-11-28)17(26)25-14-6-5-12(9-23)16(8-14)19(20,21)22/h2-8,27H,10H2,1H3,(H,25,26)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 130 | -36.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
University of Tennessee at Memphis
| Assay Description
The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... |
J Med Chem 43: 581-90 (2000)
Article DOI: 10.1021/jm990027x
BindingDB Entry DOI: 10.7270/Q2DR2SR2 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM18684

((2R)-N-[4-cyano-3-(trifluoromethyl)phenyl]-2-hydro...)Show SMILES C[C@](O)(CS(=O)(=O)c1cccc(c1)N=C=S)C(=O)Nc1ccc(C#N)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C19H14F3N3O4S2/c1-18(27,10-31(28,29)15-4-2-3-13(7-15)24-11-30)17(26)25-14-6-5-12(9-23)16(8-14)19(20,21)22/h2-8,27H,10H2,1H3,(H,25,26)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 140 | -36.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
University of Tennessee at Memphis
| Assay Description
The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... |
J Med Chem 43: 581-90 (2000)
Article DOI: 10.1021/jm990027x
BindingDB Entry DOI: 10.7270/Q2DR2SR2 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50597860

(CHEMBL5172325)Show SMILES N[C@@H](CCSC[C@@H]1O[C@@H]([C@@H](O)[C@H]1O)n1cnc2c(N)ncnc12)C(O)=O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01208
BindingDB Entry DOI: 10.7270/Q25X2DZR |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50017292
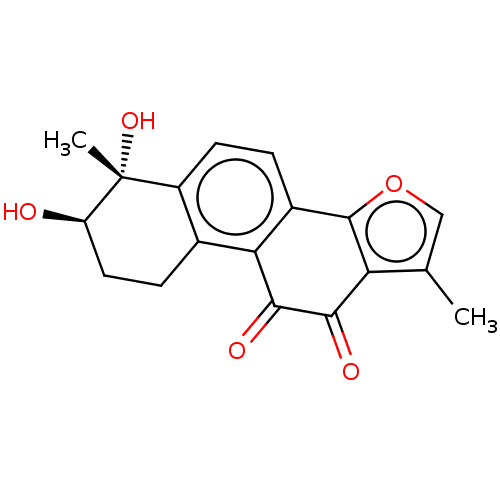
(CHEMBL3287734)Show SMILES Cc1coc-2c1C(=O)C(=O)c1c-2ccc2c1CC[C@@H](O)[C@]2(C)O |r| Show InChI InChI=1S/C18H16O5/c1-8-7-23-17-10-3-5-11-9(4-6-12(19)18(11,2)22)14(10)16(21)15(20)13(8)17/h3,5,7,12,19,22H,4,6H2,1-2H3/t12-,18-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 194 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01208
BindingDB Entry DOI: 10.7270/Q25X2DZR |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM18697
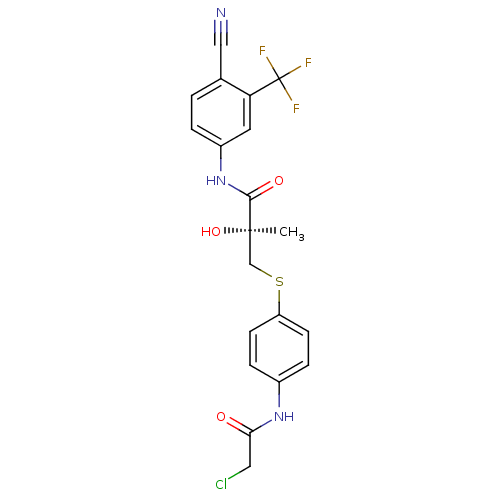
((2S)-3-{[4-(2-chloroacetamido)phenyl]sulfanyl}-N-[...)Show SMILES C[C@@](O)(CSc1ccc(NC(=O)CCl)cc1)C(=O)Nc1ccc(C#N)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C20H17ClF3N3O3S/c1-19(30,11-31-15-6-4-13(5-7-15)26-17(28)9-21)18(29)27-14-3-2-12(10-25)16(8-14)20(22,23)24/h2-8,30H,9,11H2,1H3,(H,26,28)(H,27,29)/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | -35.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
University of Tennessee at Memphis
| Assay Description
The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... |
J Med Chem 43: 581-90 (2000)
Article DOI: 10.1021/jm990027x
BindingDB Entry DOI: 10.7270/Q2DR2SR2 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM18682

((2R)-N-[4-cyano-3-(trifluoromethyl)phenyl]-2-hydro...)Show SMILES C[C@](O)(CSc1cccc(c1)N=C=S)C(=O)Nc1ccc(C#N)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C19H14F3N3O2S2/c1-18(27,10-29-15-4-2-3-13(7-15)24-11-28)17(26)25-14-6-5-12(9-23)16(8-14)19(20,21)22/h2-8,27H,10H2,1H3,(H,25,26)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 230 | -35.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
University of Tennessee at Memphis
| Assay Description
The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... |
J Med Chem 43: 581-90 (2000)
Article DOI: 10.1021/jm990027x
BindingDB Entry DOI: 10.7270/Q2DR2SR2 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50218197
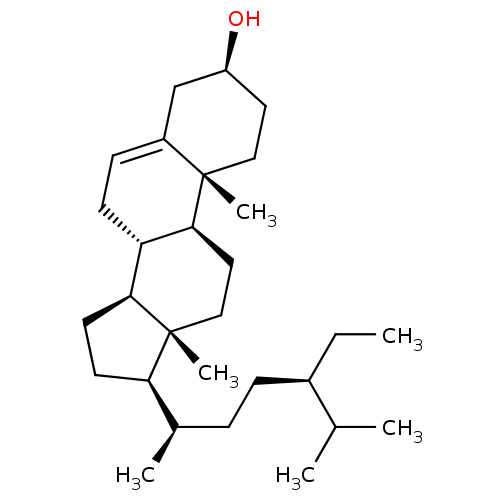
((-)-beta-Sitosterol | (24R)-Ethylcholest-5-en-3bet...)Show SMILES CC[C@H](CC[C@@H](C)[C@H]1CC[C@H]2[C@@H]3CC=C4C[C@@H](O)CC[C@]4(C)[C@H]3CC[C@]12C)C(C)C |r,t:13| Show InChI InChI=1S/C29H50O/c1-7-21(19(2)3)9-8-20(4)25-12-13-26-24-11-10-22-18-23(30)14-16-28(22,5)27(24)15-17-29(25,26)6/h10,19-21,23-27,30H,7-9,11-18H2,1-6H3/t20-,21-,23+,24+,25-,26+,27+,28+,29-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 267 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Medicinal and Aromatic Plants
Curated by ChEMBL
| Assay Description
Uncompetitive inhibition of human thrombin using T1637 as substrate by Michaelis-Menten plot analysis |
J Nat Prod 81: 2521-2530 (2018)
Article DOI: 10.1021/acs.jnatprod.8b00574
BindingDB Entry DOI: 10.7270/Q2BZ68RT |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50597869

(CHEMBL5194696)Show SMILES CNc1ncnc2n(cnc12)[C@H]1O[C@@H](CSCC[C@H](N)C(O)=O)[C@H](O)[C@@H]1O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01208
BindingDB Entry DOI: 10.7270/Q25X2DZR |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM18691

((2R)-3-{[4-(2-bromoacetamido)benzene]sulfonyl}-N-[...)Show SMILES C[C@](O)(CS(=O)(=O)c1ccc(NC(=O)CBr)cc1)C(=O)Nc1ccc(C#N)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C20H17BrF3N3O5S/c1-19(30,11-33(31,32)15-6-4-13(5-7-15)26-17(28)9-21)18(29)27-14-3-2-12(10-25)16(8-14)20(22,23)24/h2-8,30H,9,11H2,1H3,(H,26,28)(H,27,29)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 360 | -34.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
University of Tennessee at Memphis
| Assay Description
The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... |
J Med Chem 43: 581-90 (2000)
Article DOI: 10.1021/jm990027x
BindingDB Entry DOI: 10.7270/Q2DR2SR2 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM18692
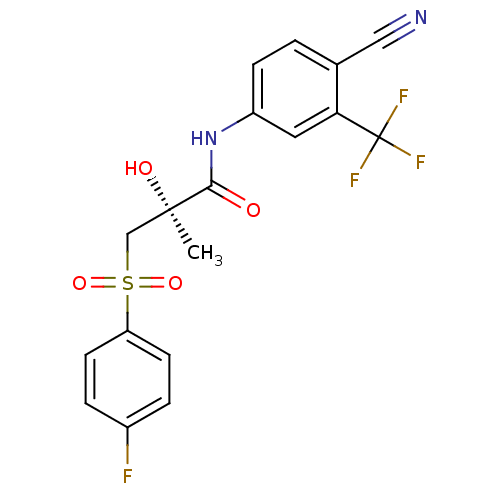
((2S)-N-[4-cyano-3-(trifluoromethyl)phenyl]-3-[(4-f...)Show SMILES C[C@@](O)(CS(=O)(=O)c1ccc(F)cc1)C(=O)Nc1ccc(C#N)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C18H14F4N2O4S/c1-17(26,10-29(27,28)14-6-3-12(19)4-7-14)16(25)24-13-5-2-11(9-23)15(8-13)18(20,21)22/h2-8,26H,10H2,1H3,(H,24,25)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 365 | -34.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
University of Tennessee at Memphis
| Assay Description
The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... |
J Med Chem 43: 581-90 (2000)
Article DOI: 10.1021/jm990027x
BindingDB Entry DOI: 10.7270/Q2DR2SR2 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM18695

((2S)-N-[4-cyano-3-(trifluoromethyl)phenyl]-2-hydro...)Show SMILES C[C@@](O)(CSc1ccc(cc1)N=C=S)C(=O)Nc1ccc(C#N)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C19H14F3N3O2S2/c1-18(27,10-29-15-6-4-13(5-7-15)24-11-28)17(26)25-14-3-2-12(9-23)16(8-14)19(20,21)22/h2-8,27H,10H2,1H3,(H,25,26)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 430 | -33.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
University of Tennessee at Memphis
| Assay Description
The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... |
J Med Chem 43: 581-90 (2000)
Article DOI: 10.1021/jm990027x
BindingDB Entry DOI: 10.7270/Q2DR2SR2 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM18693

((2S)-3-[(4-aminophenyl)sulfanyl]-N-[4-cyano-3-(tri...)Show SMILES C[C@@](O)(CSc1ccc(N)cc1)C(=O)Nc1ccc(C#N)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C18H16F3N3O2S/c1-17(26,10-27-14-6-3-12(23)4-7-14)16(25)24-13-5-2-11(9-22)15(8-13)18(19,20)21/h2-8,26H,10,23H2,1H3,(H,24,25)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 800 | -32.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
University of Tennessee at Memphis
| Assay Description
The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... |
J Med Chem 43: 581-90 (2000)
Article DOI: 10.1021/jm990027x
BindingDB Entry DOI: 10.7270/Q2DR2SR2 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM18686
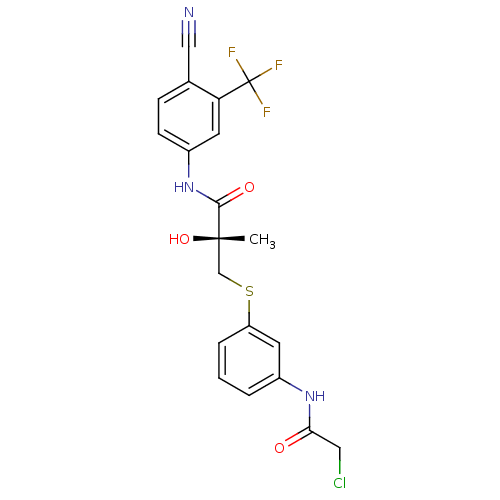
((2R)-3-{[3-(2-chloroacetamido)phenyl]sulfanyl}-N-[...)Show SMILES C[C@](O)(CSc1cccc(NC(=O)CCl)c1)C(=O)Nc1ccc(C#N)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C20H17ClF3N3O3S/c1-19(30,11-31-15-4-2-3-13(7-15)26-17(28)9-21)18(29)27-14-6-5-12(10-25)16(8-14)20(22,23)24/h2-8,30H,9,11H2,1H3,(H,26,28)(H,27,29)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | >-31.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
University of Tennessee at Memphis
| Assay Description
The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... |
J Med Chem 43: 581-90 (2000)
Article DOI: 10.1021/jm990027x
BindingDB Entry DOI: 10.7270/Q2DR2SR2 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM18694

((2S)-3-[(3-aminophenyl)sulfanyl]-N-[4-cyano-3-(tri...)Show SMILES C[C@@](O)(CSc1cccc(N)c1)C(=O)Nc1ccc(C#N)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C18H16F3N3O2S/c1-17(26,10-27-14-4-2-3-12(23)7-14)16(25)24-13-6-5-11(9-22)15(8-13)18(19,20)21/h2-8,26H,10,23H2,1H3,(H,24,25)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | >-31.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
University of Tennessee at Memphis
| Assay Description
The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... |
J Med Chem 43: 581-90 (2000)
Article DOI: 10.1021/jm990027x
BindingDB Entry DOI: 10.7270/Q2DR2SR2 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM18688

((2R)-3-{[3-(2-bromoacetamido)phenyl]sulfanyl}-N-[4...)Show SMILES C[C@](O)(CSc1cccc(NC(=O)CBr)c1)C(=O)Nc1ccc(C#N)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C20H17BrF3N3O3S/c1-19(30,11-31-15-4-2-3-13(7-15)26-17(28)9-21)18(29)27-14-6-5-12(10-25)16(8-14)20(22,23)24/h2-8,30H,9,11H2,1H3,(H,26,28)(H,27,29)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | >-31.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
University of Tennessee at Memphis
| Assay Description
The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... |
J Med Chem 43: 581-90 (2000)
Article DOI: 10.1021/jm990027x
BindingDB Entry DOI: 10.7270/Q2DR2SR2 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50010823
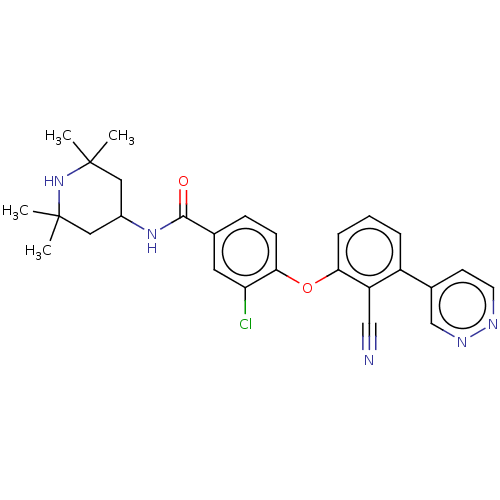
(CHEMBL3264787)Show SMILES CC1(C)CC(CC(C)(C)N1)NC(=O)c1ccc(Oc2cccc(-c3ccnnc3)c2C#N)c(Cl)c1 Show InChI InChI=1S/C27H28ClN5O2/c1-26(2)13-19(14-27(3,4)33-26)32-25(34)17-8-9-24(22(28)12-17)35-23-7-5-6-20(21(23)15-29)18-10-11-30-31-16-18/h5-12,16,19,33H,13-14H2,1-4H3,(H,32,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0320 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01208
BindingDB Entry DOI: 10.7270/Q25X2DZR |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50597874
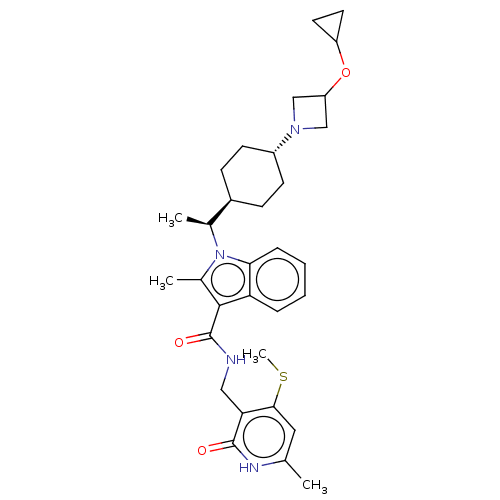
(CHEMBL5188476)Show SMILES [H][C@@]1(CC[C@@H](CC1)N1CC(C1)OC1CC1)[C@H](C)n1c(C)c(C(=O)NCc2c(SC)cc(C)[nH]c2=O)c2ccccc12 |r,wU:15.18,wD:4.7,1.0,(.87,-.92,;-.67,-.92,;.1,-2.26,;-.67,-3.59,;-2.21,-3.59,;-2.98,-2.26,;-2.21,-.92,;-2.98,-4.92,;-2.58,-6.44,;-4.07,-6.83,;-4.47,-5.32,;-4.84,-8.17,;-4.07,-9.5,;-4.07,-11.05,;-2.73,-10.27,;.1,.41,;1.64,.41,;-.67,1.75,;-.2,3.21,;1.29,3.61,;-1.44,4.11,;-1.44,5.65,;-2.78,6.43,;-.11,6.43,;-.11,7.97,;1.22,8.74,;2.56,7.97,;2.56,6.43,;3.89,5.66,;3.89,8.74,;3.89,10.28,;5.22,11.05,;2.56,11.05,;1.22,10.28,;-.11,11.05,;-2.69,3.21,;-4.2,3.53,;-5.22,2.38,;-4.75,.93,;-3.24,.61,;-2.21,1.75,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0570 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01208
BindingDB Entry DOI: 10.7270/Q25X2DZR |
More data for this
Ligand-Target Pair | |
Protein arginine N-methyltransferase 5
(Homo sapiens (Human)) | BDBM50597880
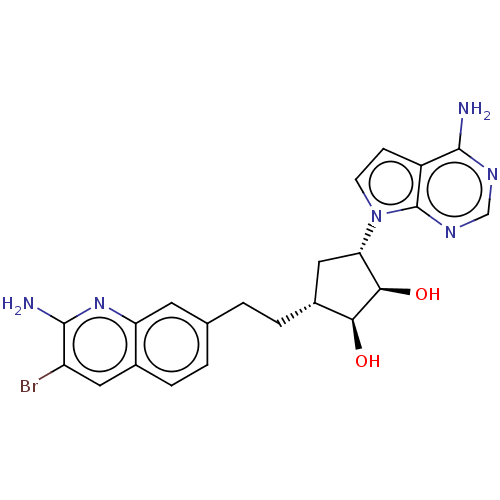
(CHEMBL5203719)Show SMILES Nc1nc2cc(CC[C@@H]3C[C@@H]([C@@H](O)[C@H]3O)n3ccc4c(N)ncnc34)ccc2cc1Br |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01208
BindingDB Entry DOI: 10.7270/Q25X2DZR |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50597863
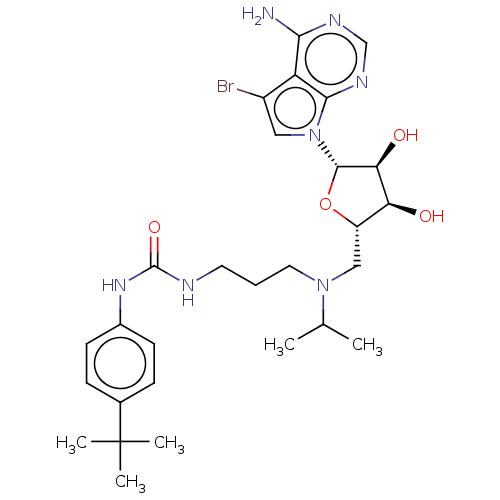
(CHEMBL5188291)Show SMILES CC(C)N(CCCNC(=O)Nc1ccc(cc1)C(C)(C)C)C[C@@H]1O[C@@H]([C@@H](O)[C@H]1O)n1cc(Br)c2c(N)ncnc12 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01208
BindingDB Entry DOI: 10.7270/Q25X2DZR |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50597863

(CHEMBL5188291)Show SMILES CC(C)N(CCCNC(=O)Nc1ccc(cc1)C(C)(C)C)C[C@@H]1O[C@@H]([C@@H](O)[C@H]1O)n1cc(Br)c2c(N)ncnc12 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01208
BindingDB Entry DOI: 10.7270/Q25X2DZR |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50597867
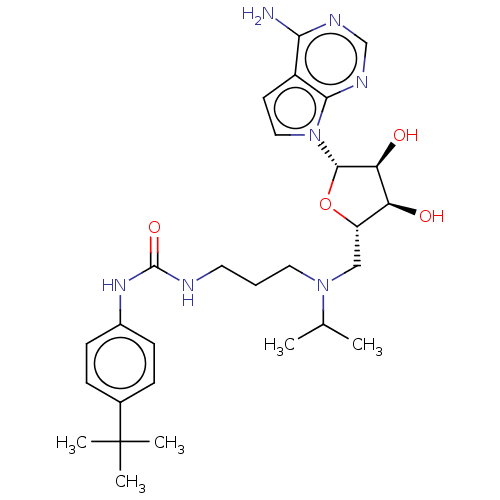
(CHEMBL5176983)Show SMILES CC(C)N(CCCNC(=O)Nc1ccc(cc1)C(C)(C)C)C[C@@H]1O[C@@H]([C@@H](O)[C@H]1O)n1ccc2c(N)ncnc12 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01208
BindingDB Entry DOI: 10.7270/Q25X2DZR |
More data for this
Ligand-Target Pair | |
Protein arginine N-methyltransferase 5
(Homo sapiens (Human)) | BDBM50238541
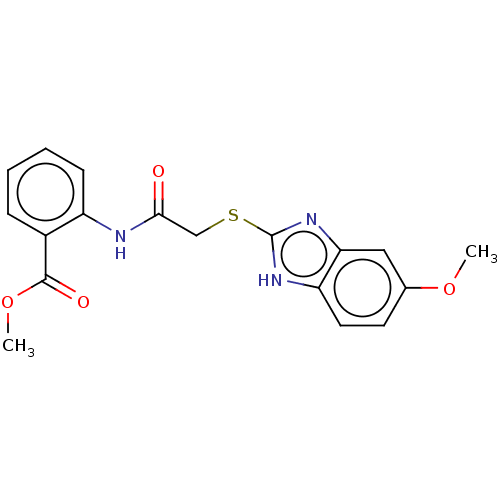
(CHEMBL4092336)Show SMILES COC(=O)c1ccccc1NC(=O)CSc1nc2cc(OC)ccc2[nH]1 Show InChI InChI=1S/C18H17N3O4S/c1-24-11-7-8-14-15(9-11)21-18(20-14)26-10-16(22)19-13-6-4-3-5-12(13)17(23)25-2/h3-9H,10H2,1-2H3,(H,19,22)(H,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01208
BindingDB Entry DOI: 10.7270/Q25X2DZR |
More data for this
Ligand-Target Pair | |
Protein arginine N-methyltransferase 5
(Homo sapiens (Human)) | BDBM50597881
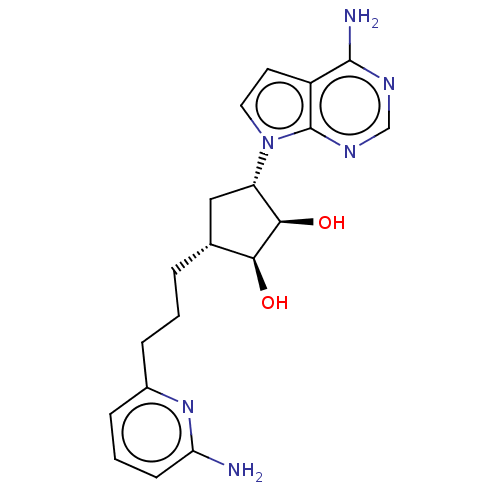
(CHEMBL5187591)Show SMILES Nc1cccc(CCC[C@@H]2C[C@@H]([C@@H](O)[C@H]2O)n2ccc3c(N)ncnc23)n1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01208
BindingDB Entry DOI: 10.7270/Q25X2DZR |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50536826

(CHEMBL4590355)Show SMILES CNc1ccnc(Nc2ccc3cc(C)n(-c4ccccc4Oc4cnc5n(C)cnc5c4)c3c2)n1 |(29.26,-10.25,;27.93,-11.02,;27.94,-12.56,;29.28,-13.33,;29.28,-14.87,;27.95,-15.64,;26.62,-14.88,;25.29,-15.64,;23.96,-14.88,;23.95,-13.33,;22.62,-12.56,;21.29,-13.34,;19.82,-12.85,;18.9,-14.1,;17.36,-14.08,;19.8,-15.36,;19.31,-16.81,;20.33,-17.95,;19.85,-19.4,;18.34,-19.72,;17.32,-18.56,;17.81,-17.11,;16.79,-15.96,;15.29,-16.26,;14.81,-17.71,;13.31,-18.01,;12.29,-16.86,;10.74,-16.84,;9.83,-18.07,;10.28,-15.37,;11.54,-14.47,;12.78,-15.39,;14.28,-15.1,;21.28,-14.89,;22.62,-15.65,;26.61,-13.34,)| Show InChI InChI=1S/C27H24N8O/c1-17-12-18-8-9-19(32-27-29-11-10-25(28-2)33-27)13-23(18)35(17)22-6-4-5-7-24(22)36-20-14-21-26(30-15-20)34(3)16-31-21/h4-16H,1-3H3,(H2,28,29,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01208
BindingDB Entry DOI: 10.7270/Q25X2DZR |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
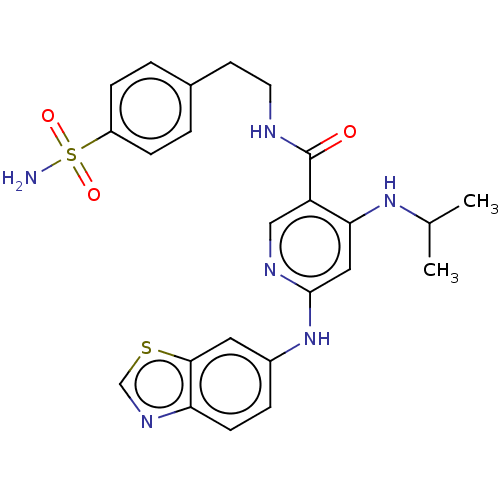
(Homo sapiens (Human)) | BDBM50544198
(CHEMBL4636136)Show SMILES CC(C)Nc1cc(Nc2ccc3ncsc3c2)ncc1C(=O)NCCc1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C24H26N6O3S2/c1-15(2)29-21-12-23(30-17-5-8-20-22(11-17)34-14-28-20)27-13-19(21)24(31)26-10-9-16-3-6-18(7-4-16)35(25,32)33/h3-8,11-15H,9-10H2,1-2H3,(H,26,31)(H2,25,32,33)(H2,27,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Biocon Bristol Myers Squibb Research Center
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
ACS Med Chem Lett 11: 1402-1409 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00082
BindingDB Entry DOI: 10.7270/Q2542S4M |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50400778
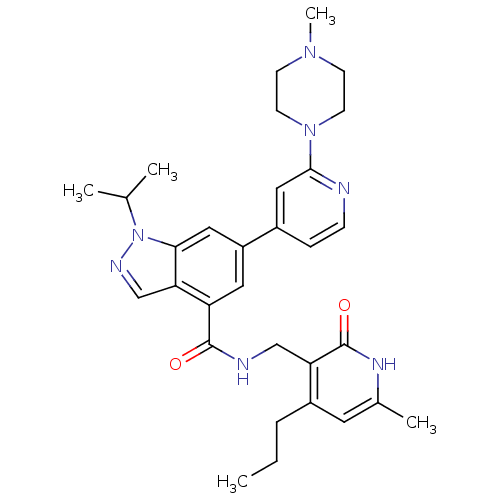
(CHEMBL2204995)Show SMILES CCCc1cc(C)[nH]c(=O)c1CNC(=O)c1cc(cc2n(ncc12)C(C)C)-c1ccnc(c1)N1CCN(C)CC1 Show InChI InChI=1S/C31H39N7O2/c1-6-7-23-14-21(4)35-31(40)26(23)18-33-30(39)25-15-24(16-28-27(25)19-34-38(28)20(2)3)22-8-9-32-29(17-22)37-12-10-36(5)11-13-37/h8-9,14-17,19-20H,6-7,10-13,18H2,1-5H3,(H,33,39)(H,35,40) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01208
BindingDB Entry DOI: 10.7270/Q25X2DZR |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50110357
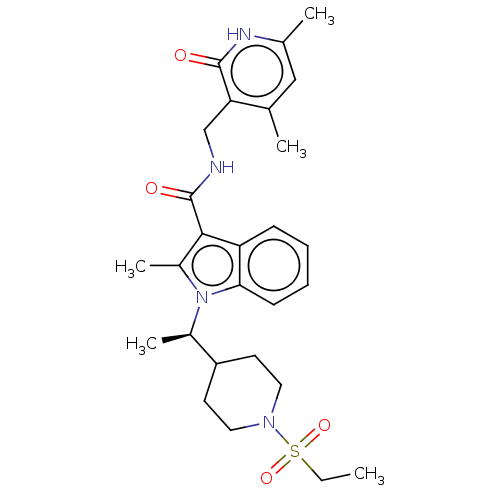
(CHEMBL3605455)Show SMILES CCS(=O)(=O)N1CCC(CC1)[C@@H](C)n1c(C)c(C(=O)NCc2c(C)cc(C)[nH]c2=O)c2ccccc12 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01208
BindingDB Entry DOI: 10.7270/Q25X2DZR |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data