

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50146461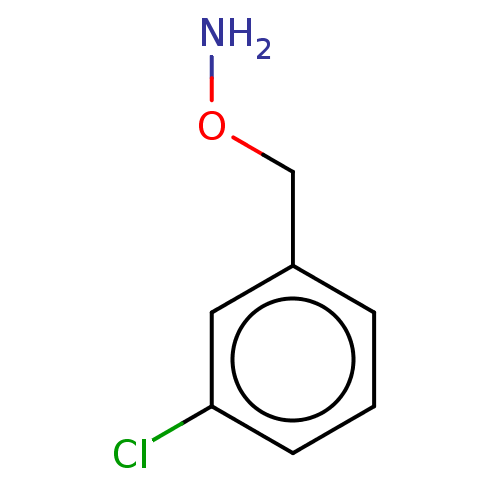 (CHEMBL3765807) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 154 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bryn Mawr College Curated by ChEMBL | Assay Description Inhibition of recombinant human IDO1 assessed as reduction in kynurenine production using L-tryptophan as substrate after 60 mins by Michaelis-Menton... | Eur J Med Chem 108: 564-76 (2016) Article DOI: 10.1016/j.ejmech.2015.12.028 BindingDB Entry DOI: 10.7270/Q26Q203T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50146460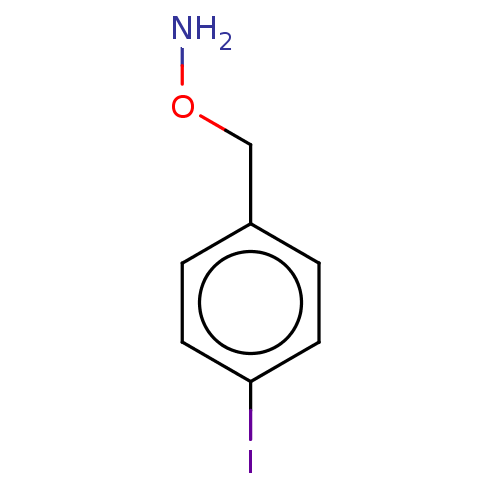 (CHEMBL3763688) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 164 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bryn Mawr College Curated by ChEMBL | Assay Description Inhibition of recombinant human IDO1 assessed as reduction in kynurenine production using L-tryptophan as substrate after 60 mins by Michaelis-Menton... | Eur J Med Chem 108: 564-76 (2016) Article DOI: 10.1016/j.ejmech.2015.12.028 BindingDB Entry DOI: 10.7270/Q26Q203T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24666 (4-(1H-imidazol-4-yl)benzene-1-thiol | 4-(1H-imidaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.80E+3 | -31.6 | 7.70E+3 | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 51: 4968-77 (2008) Article DOI: 10.1021/jm800512z BindingDB Entry DOI: 10.7270/Q2154FB1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24665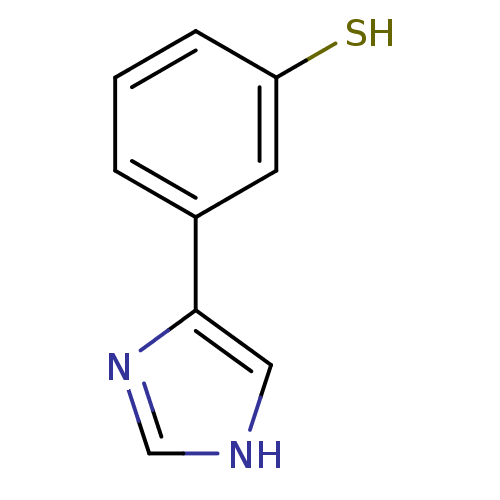 (3-(1H-imidazol-4-yl)benzene-1-thiol | 3-(1H-imidaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.30E+3 | -31.3 | 7.60E+3 | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 51: 4968-77 (2008) Article DOI: 10.1021/jm800512z BindingDB Entry DOI: 10.7270/Q2154FB1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24663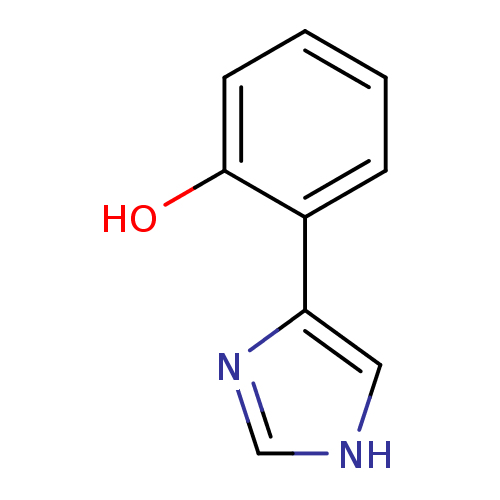 (2-(1H-imidazol-4-yl)phenol | 2-(1H-imidazol-4-yl)p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 8.90E+3 | -30.0 | 4.80E+3 | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 51: 4968-77 (2008) Article DOI: 10.1021/jm800512z BindingDB Entry DOI: 10.7270/Q2154FB1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24828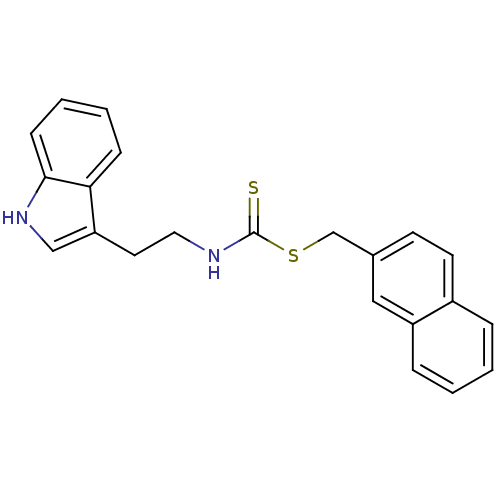 (Brassinin derivative, 16 | N-[2-(1H-indol-3-yl)eth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.16E+4 | -29.3 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 49: 684-92 (2006) Article DOI: 10.1021/jm0508888 BindingDB Entry DOI: 10.7270/Q2QR4VDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24825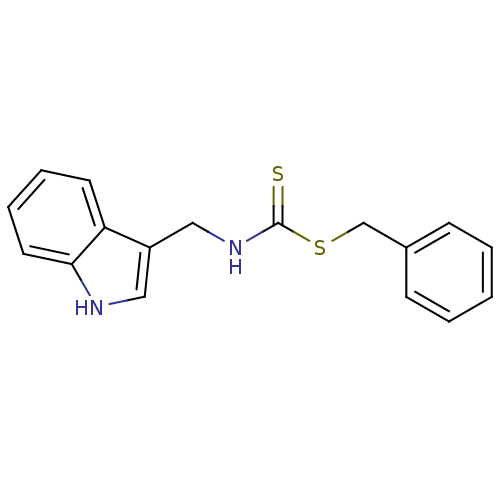 ((benzylsulfanyl)-N-(1H-indol-3-ylmethyl)carbothioa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.32E+4 | -29.0 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 49: 684-92 (2006) Article DOI: 10.1021/jm0508888 BindingDB Entry DOI: 10.7270/Q2QR4VDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24827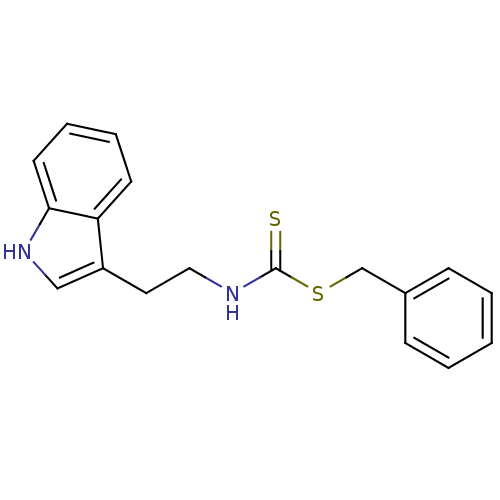 ((benzylsulfanyl)-N-[2-(1H-indol-3-yl)ethyl]carboth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.72E+4 | -28.3 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 49: 684-92 (2006) Article DOI: 10.1021/jm0508888 BindingDB Entry DOI: 10.7270/Q2QR4VDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24830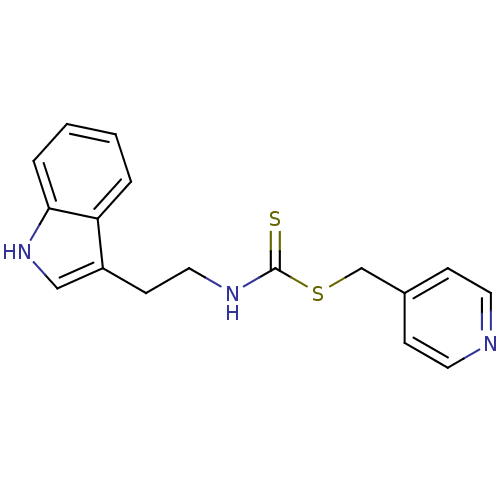 (Brassinin derivative, 18 | N-[2-(1H-indol-3-yl)eth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.05E+4 | -27.8 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 49: 684-92 (2006) Article DOI: 10.1021/jm0508888 BindingDB Entry DOI: 10.7270/Q2QR4VDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24829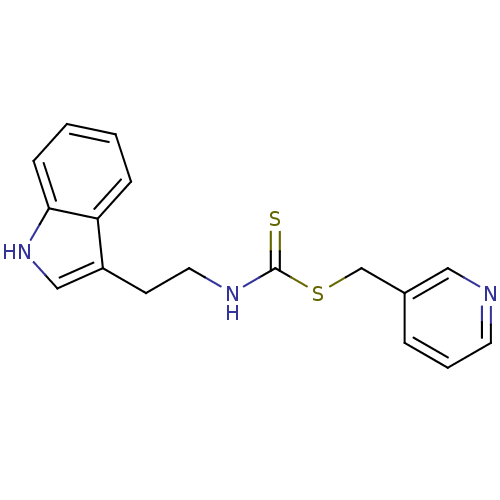 (Brassinin derivative, 17 | N-[2-(1H-indol-3-yl)eth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.84E+4 | -27.0 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 49: 684-92 (2006) Article DOI: 10.1021/jm0508888 BindingDB Entry DOI: 10.7270/Q2QR4VDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24816 (Brassinin derivative, 4 | N-[3-(1H-indol-3-yl)prop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.40E+4 | -26.5 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 49: 684-92 (2006) Article DOI: 10.1021/jm0508888 BindingDB Entry DOI: 10.7270/Q2QR4VDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24824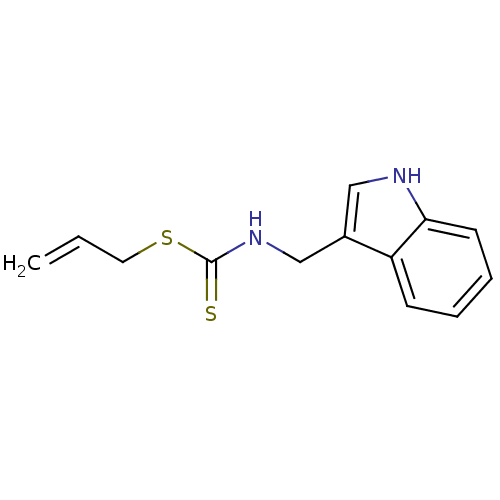 (Brassinin derivative, 12 | N-(1H-indol-3-ylmethyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 3.70E+4 | -26.3 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 49: 684-92 (2006) Article DOI: 10.1021/jm0508888 BindingDB Entry DOI: 10.7270/Q2QR4VDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24815 (Brassinin derivative, 3 | N-[2-(1-benzothiophen-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 4.10E+4 | -26.1 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 49: 684-92 (2006) Article DOI: 10.1021/jm0508888 BindingDB Entry DOI: 10.7270/Q2QR4VDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24817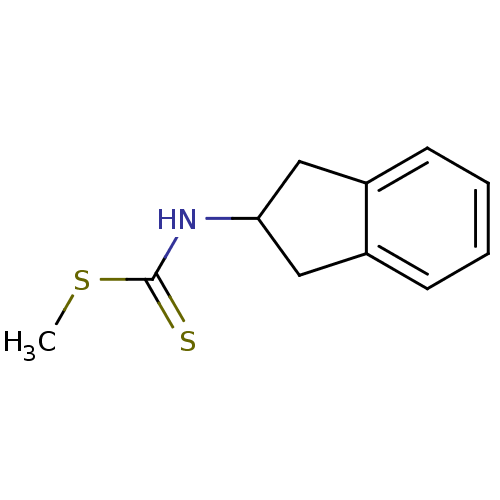 (Brassinin derivative, 5 | N-(2,3-dihydro-1H-inden-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 4.21E+4 | -26.0 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 49: 684-92 (2006) Article DOI: 10.1021/jm0508888 BindingDB Entry DOI: 10.7270/Q2QR4VDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24819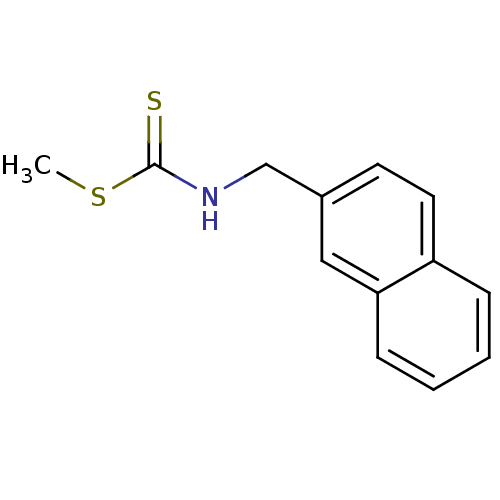 ((methylsulfanyl)-N-(naphthalen-2-ylmethyl)carbothi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.76E+4 | -25.7 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 49: 684-92 (2006) Article DOI: 10.1021/jm0508888 BindingDB Entry DOI: 10.7270/Q2QR4VDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24821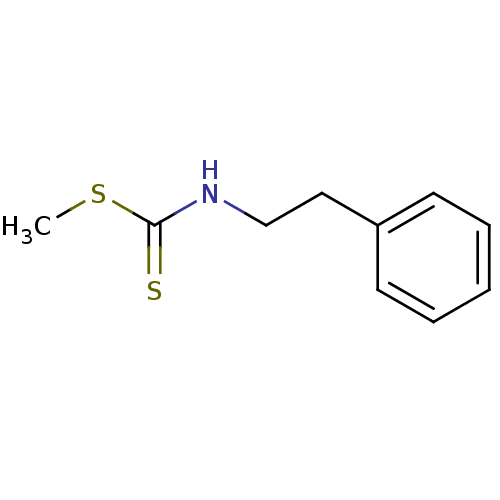 ((methylsulfanyl)-N-(2-phenylethyl)carbothioamide |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 6.24E+4 | -25.0 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 49: 684-92 (2006) Article DOI: 10.1021/jm0508888 BindingDB Entry DOI: 10.7270/Q2QR4VDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24820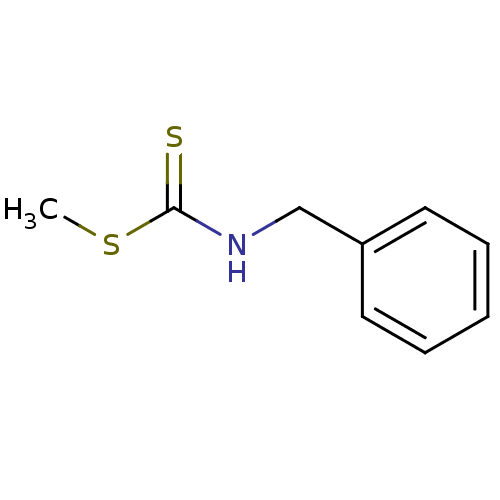 (Brassinin derivative, 8 | N-benzyl(methylsulfanyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 7.24E+4 | -24.6 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 49: 684-92 (2006) Article DOI: 10.1021/jm0508888 BindingDB Entry DOI: 10.7270/Q2QR4VDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24814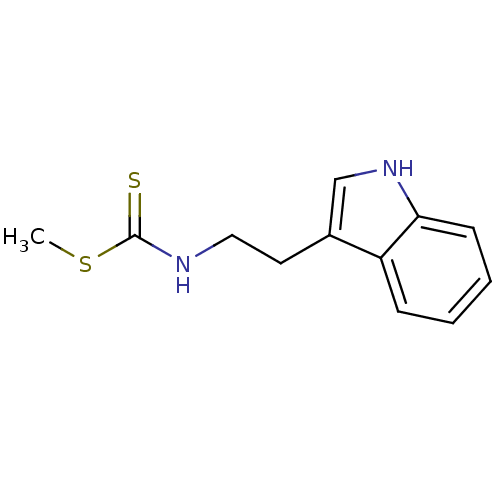 (Brassinin derivative, 2 | N-[2-(1H-indol-3-yl)ethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8.25E+4 | -24.2 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 49: 684-92 (2006) Article DOI: 10.1021/jm0508888 BindingDB Entry DOI: 10.7270/Q2QR4VDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24813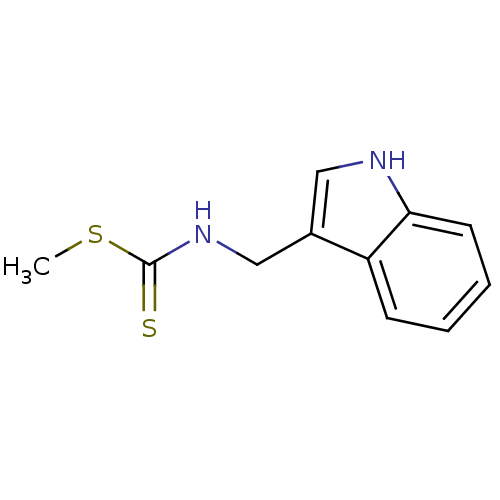 (Brassinin, 1 | N-(1H-indol-3-ylmethyl)(methylsulfa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 9.77E+4 | -23.8 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 49: 684-92 (2006) Article DOI: 10.1021/jm0508888 BindingDB Entry DOI: 10.7270/Q2QR4VDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24822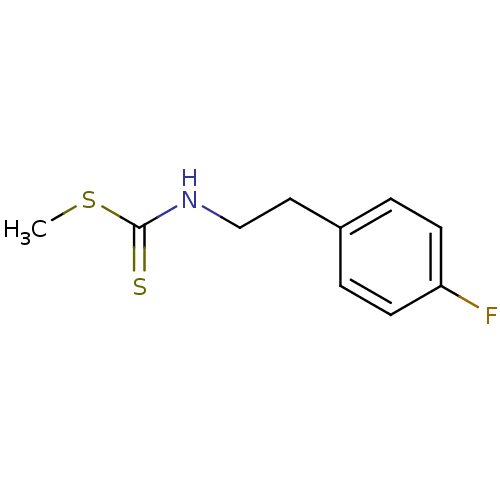 (Brassinin derivative, 10 | N-[2-(4-fluorophenyl)et...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.49E+5 | -22.7 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 49: 684-92 (2006) Article DOI: 10.1021/jm0508888 BindingDB Entry DOI: 10.7270/Q2QR4VDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24818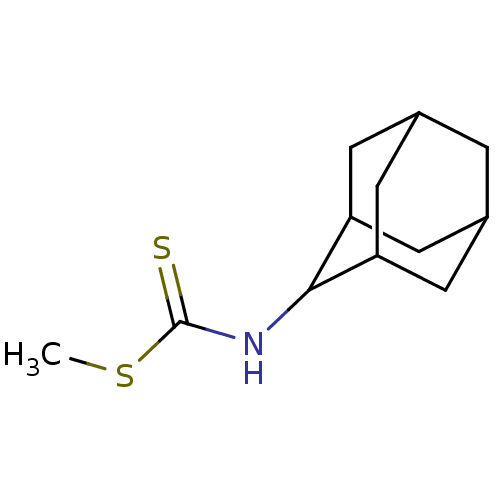 (Brassinin derivative, 6 | N-(adamantan-2-yl)(methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.80E+5 | -22.2 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 49: 684-92 (2006) Article DOI: 10.1021/jm0508888 BindingDB Entry DOI: 10.7270/Q2QR4VDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24834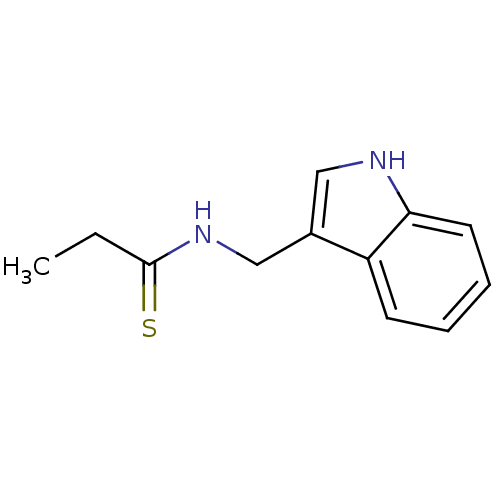 (N-(1H-indol-3-ylmethyl)propanethioamide | thioamid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.02E+5 | -21.9 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 49: 684-92 (2006) Article DOI: 10.1021/jm0508888 BindingDB Entry DOI: 10.7270/Q2QR4VDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24836 (4-(1H-indol-3-ylmethyl)-2-methyl-1,3-thiazole | th...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.29E+5 | -20.7 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 49: 684-92 (2006) Article DOI: 10.1021/jm0508888 BindingDB Entry DOI: 10.7270/Q2QR4VDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24832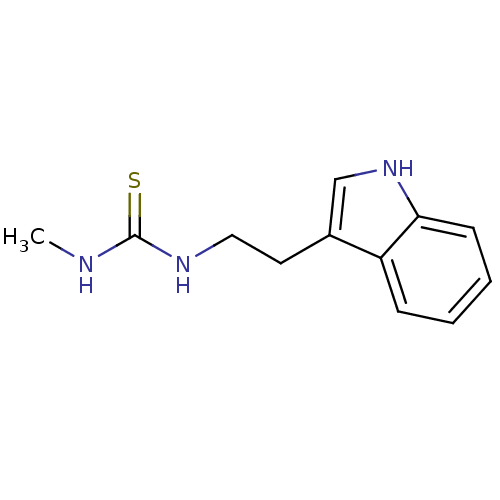 (1-[2-(1H-indol-3-yl)ethyl]-3-methylthiourea | thio...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 3.42E+5 | -20.6 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 49: 684-92 (2006) Article DOI: 10.1021/jm0508888 BindingDB Entry DOI: 10.7270/Q2QR4VDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24826 ((hexylsulfanyl)-N-(1H-indol-3-ylmethyl)carbothioam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.64E+5 | -20.4 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 49: 684-92 (2006) Article DOI: 10.1021/jm0508888 BindingDB Entry DOI: 10.7270/Q2QR4VDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24823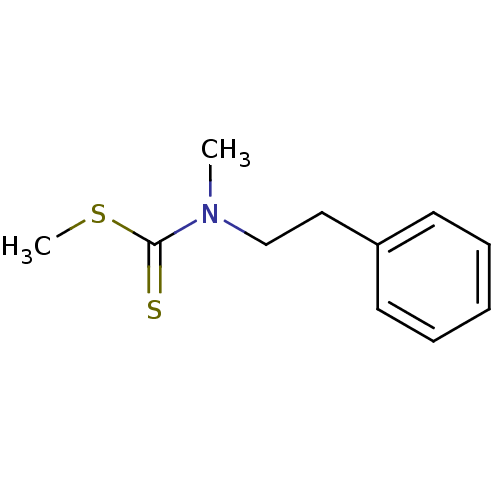 (Brassinin derivative, 11 | N-methyl(methylsulfanyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.27E+6 | -17.2 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 49: 684-92 (2006) Article DOI: 10.1021/jm0508888 BindingDB Entry DOI: 10.7270/Q2QR4VDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24835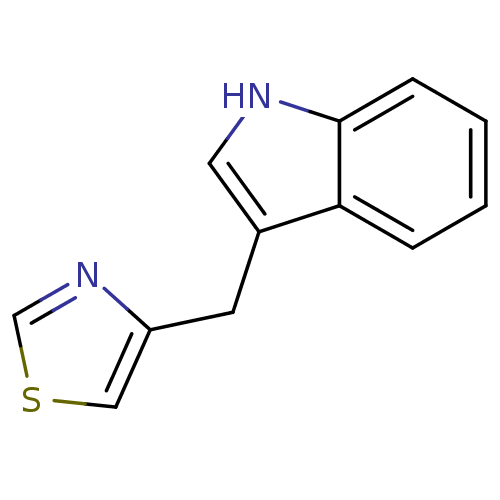 (4-(1H-indol-3-ylmethyl)-1,3-thiazole | thiazole, 2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.29E+6 | -17.2 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 49: 684-92 (2006) Article DOI: 10.1021/jm0508888 BindingDB Entry DOI: 10.7270/Q2QR4VDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24797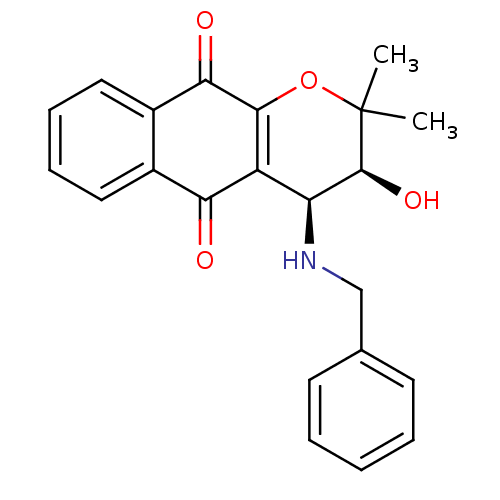 ((3S,4S)-4-(benzylamino)-3-hydroxy-2,2-dimethyl-2H,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 51: 1706-18 (2008) Article DOI: 10.1021/jm7014155 BindingDB Entry DOI: 10.7270/Q2VD6WSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50126143 (Epacadostat | INCB-024360) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Bryn Mawr College Curated by ChEMBL | Assay Description Inhibition of IDO1 in human HeLa cells using L-tryptophan as substrate after 20 hrs | Eur J Med Chem 162: 455-464 (2019) Article DOI: 10.1016/j.ejmech.2018.11.010 BindingDB Entry DOI: 10.7270/Q2959MV6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50146461 (CHEMBL3765807) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
Bryn Mawr College Curated by ChEMBL | Assay Description Inhibition of doxycycline-induced human IDO1 expressed in Trex cells assessed as reduction in kynurenine production treated with 5-fold serial diluti... | Eur J Med Chem 108: 564-76 (2016) Article DOI: 10.1016/j.ejmech.2015.12.028 BindingDB Entry DOI: 10.7270/Q26Q203T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24802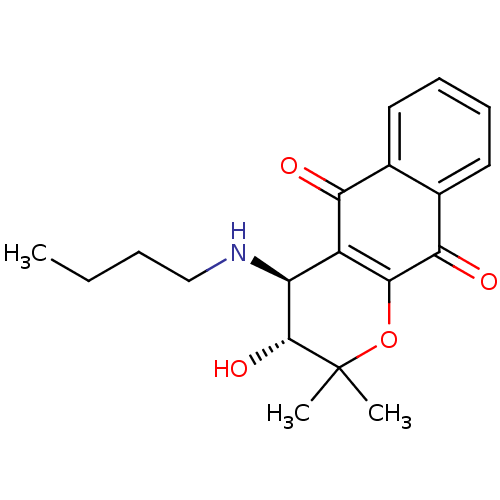 ((3R,4S)-4-(butylamino)-3-hydroxy-2,2-dimethyl-2H,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 51: 1706-18 (2008) Article DOI: 10.1021/jm7014155 BindingDB Entry DOI: 10.7270/Q2VD6WSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50146460 (CHEMBL3763688) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
Bryn Mawr College Curated by ChEMBL | Assay Description Inhibition of IFN gamma induced human IDO1 in HeLa cells assessed as reduction in kynurenine production treated with 5-fold serial dilution beginning... | Eur J Med Chem 108: 564-76 (2016) Article DOI: 10.1016/j.ejmech.2015.12.028 BindingDB Entry DOI: 10.7270/Q26Q203T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50146460 (CHEMBL3763688) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
Bryn Mawr College Curated by ChEMBL | Assay Description Inhibition of IFN gamma induced human IDO1 in HeLa cells assessed as reduction in kynurenine production treated with 3-fold serial dilution beginning... | Eur J Med Chem 108: 564-76 (2016) Article DOI: 10.1016/j.ejmech.2015.12.028 BindingDB Entry DOI: 10.7270/Q26Q203T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50146461 (CHEMBL3765807) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 114 | n/a | n/a | n/a | n/a | n/a | n/a |
Bryn Mawr College Curated by ChEMBL | Assay Description Inhibition of IFN gamma induced human IDO1 in HeLa cells assessed as reduction in kynurenine production treated with 5-fold serial dilution beginning... | Eur J Med Chem 108: 564-76 (2016) Article DOI: 10.1016/j.ejmech.2015.12.028 BindingDB Entry DOI: 10.7270/Q26Q203T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24794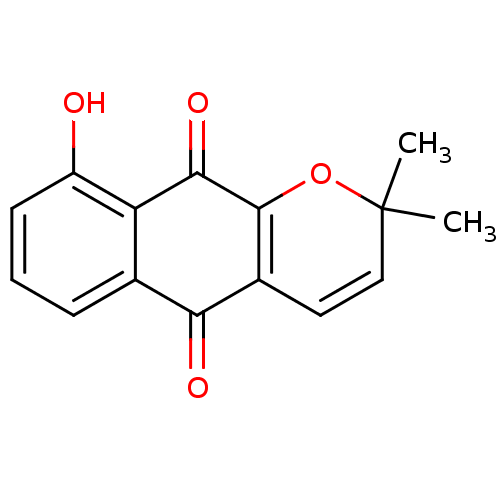 (9-hydroxy-2,2-dimethyl-2H,5H,10H-benzo[g]chromene-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 121 | n/a | n/a | n/a | n/a | n/a | n/a |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 51: 1706-18 (2008) Article DOI: 10.1021/jm7014155 BindingDB Entry DOI: 10.7270/Q2VD6WSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24801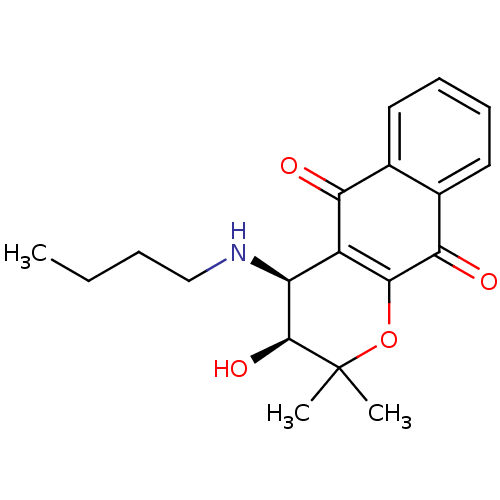 ((3S,4S)-4-(butylamino)-3-hydroxy-2,2-dimethyl-2H,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 51: 1706-18 (2008) Article DOI: 10.1021/jm7014155 BindingDB Entry DOI: 10.7270/Q2VD6WSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50146461 (CHEMBL3765807) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 134 | n/a | n/a | n/a | n/a | n/a | n/a |
Bryn Mawr College Curated by ChEMBL | Assay Description Inhibition of IFN gamma induced human IDO1 in HeLa cells assessed as reduction in kynurenine production treated with 3-fold serial dilution beginning... | Eur J Med Chem 108: 564-76 (2016) Article DOI: 10.1016/j.ejmech.2015.12.028 BindingDB Entry DOI: 10.7270/Q26Q203T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50146460 (CHEMBL3763688) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Bryn Mawr College Curated by ChEMBL | Assay Description Inhibition of doxycycline-induced human IDO1 expressed in Trex cells assessed as reduction in kynurenine production treated with 5-fold serial diluti... | Eur J Med Chem 108: 564-76 (2016) Article DOI: 10.1016/j.ejmech.2015.12.028 BindingDB Entry DOI: 10.7270/Q26Q203T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24800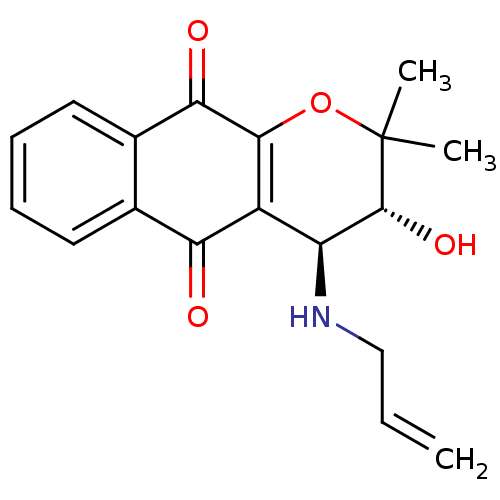 ((3R,4S)-3-hydroxy-2,2-dimethyl-4-(prop-2-en-1-ylam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 183 | n/a | n/a | n/a | n/a | n/a | n/a |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 51: 1706-18 (2008) Article DOI: 10.1021/jm7014155 BindingDB Entry DOI: 10.7270/Q2VD6WSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24799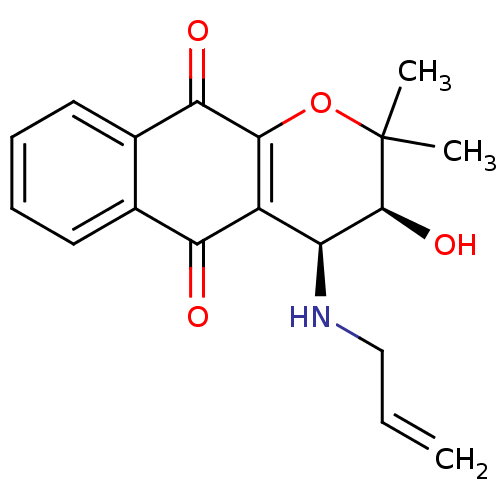 ((3S,4S)-3-hydroxy-2,2-dimethyl-4-(prop-2-en-1-ylam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 186 | n/a | n/a | n/a | n/a | n/a | n/a |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 51: 1706-18 (2008) Article DOI: 10.1021/jm7014155 BindingDB Entry DOI: 10.7270/Q2VD6WSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24788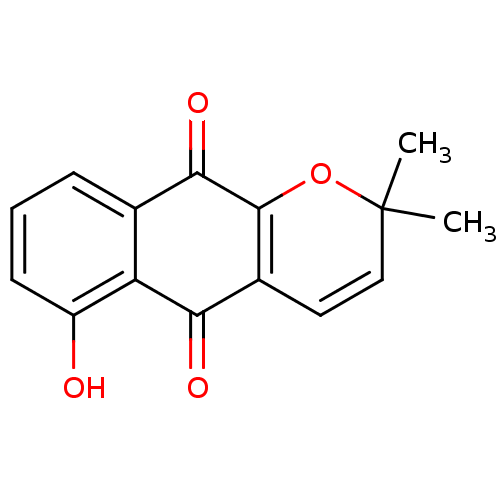 (6-hydroxy-2,2-dimethyl-2H,5H,10H-benzo[g]chromene-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 51: 1706-18 (2008) Article DOI: 10.1021/jm7014155 BindingDB Entry DOI: 10.7270/Q2VD6WSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50146460 (CHEMBL3763688) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Bryn Mawr College Curated by ChEMBL | Assay Description Inhibition of recombinant human IDO1 assessed as reduction in kynurenine production using L-tryptophan as substrate after 60 mins by microplate reade... | Eur J Med Chem 108: 564-76 (2016) Article DOI: 10.1016/j.ejmech.2015.12.028 BindingDB Entry DOI: 10.7270/Q26Q203T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM309529 (3-(5-fluoro-1H-indol-3- yl)pyrrolidine-2,5-dione |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Bryn Mawr College Curated by ChEMBL | Assay Description Inhibition of IDO1 in human HeLa cells using L-tryptophan as substrate after 20 hrs | Eur J Med Chem 162: 455-464 (2019) Article DOI: 10.1016/j.ejmech.2018.11.010 BindingDB Entry DOI: 10.7270/Q2959MV6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24786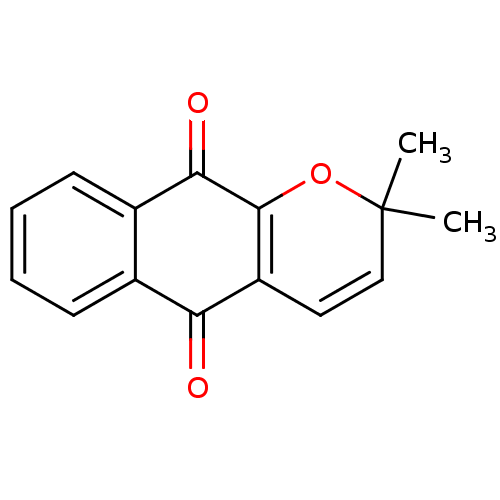 (2,2-dimethyl-2H,5H,10H-benzo[g]chromene-5,10-dione...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 214 | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 51: 1706-18 (2008) Article DOI: 10.1021/jm7014155 BindingDB Entry DOI: 10.7270/Q2VD6WSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50444455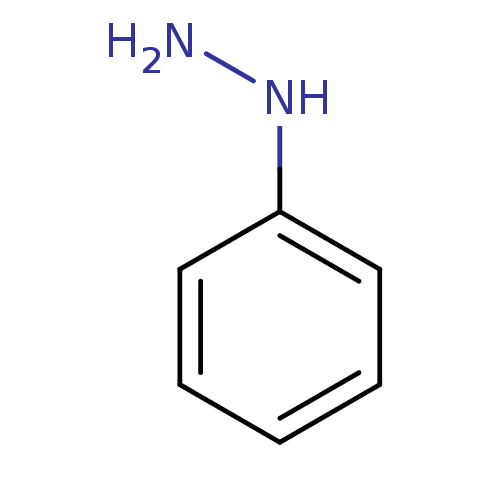 (CHEBI:27924 | Phenylhydrazine) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Bryn Mawr College Curated by ChEMBL | Assay Description Inhibition of recombinant human IDO1 assessed as reduction in kynurenine production using L-tryptophan as substrate after 60 mins by microplate reade... | Eur J Med Chem 108: 564-76 (2016) Article DOI: 10.1016/j.ejmech.2015.12.028 BindingDB Entry DOI: 10.7270/Q26Q203T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24787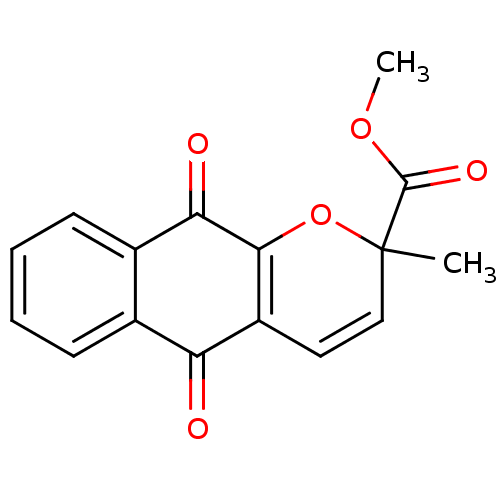 (Pyranonaphthoquinone derivative, 24 | methyl 2-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 247 | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 51: 1706-18 (2008) Article DOI: 10.1021/jm7014155 BindingDB Entry DOI: 10.7270/Q2VD6WSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24798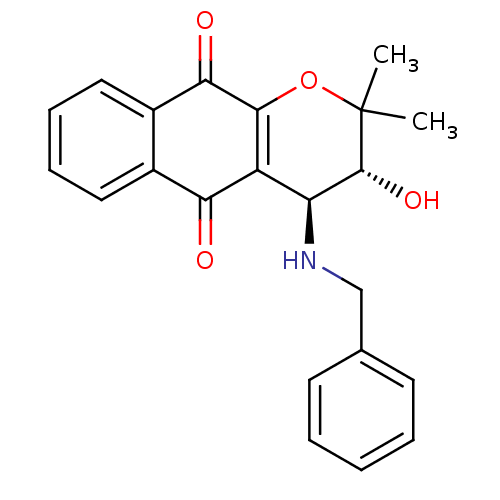 ((3R,4S)-4-(benzylamino)-3-hydroxy-2,2-dimethyl-2H,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 252 | n/a | n/a | n/a | n/a | n/a | n/a |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 51: 1706-18 (2008) Article DOI: 10.1021/jm7014155 BindingDB Entry DOI: 10.7270/Q2VD6WSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24774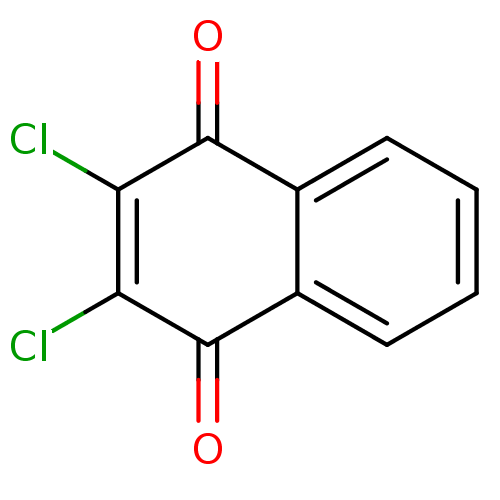 (2,3-dichloro-1,4-dihydronaphthalene-1,4-dione | 2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 51: 1706-18 (2008) Article DOI: 10.1021/jm7014155 BindingDB Entry DOI: 10.7270/Q2VD6WSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50146461 (CHEMBL3765807) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bryn Mawr College Curated by ChEMBL | Assay Description Inhibition of recombinant human IDO1 assessed as reduction in kynurenine production using L-tryptophan as substrate after 60 mins by microplate reade... | Eur J Med Chem 108: 564-76 (2016) Article DOI: 10.1016/j.ejmech.2015.12.028 BindingDB Entry DOI: 10.7270/Q26Q203T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50146407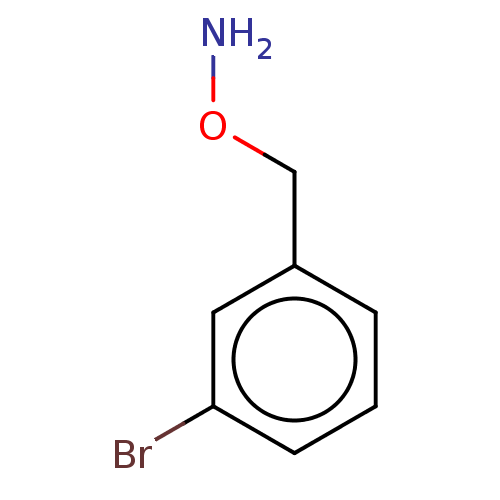 (CHEMBL3763469) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Bryn Mawr College Curated by ChEMBL | Assay Description Inhibition of recombinant human IDO1 assessed as reduction in kynurenine production using L-tryptophan as substrate after 60 mins by microplate reade... | Eur J Med Chem 108: 564-76 (2016) Article DOI: 10.1016/j.ejmech.2015.12.028 BindingDB Entry DOI: 10.7270/Q26Q203T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 275 total ) | Next | Last >> |