 Found 265 hits with Last Name = 'murphy' and Initial = 'pv'
Found 265 hits with Last Name = 'murphy' and Initial = 'pv' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM21393

(7-(dipropylamino)-5,6,7,8-tetrahydronaphthalen-1-o...)Show InChI InChI=1S/C16H25NO/c1-3-10-17(11-4-2)14-9-8-13-6-5-7-16(18)15(13)12-14/h5-7,14,18H,3-4,8-12H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Ireland Galway
Curated by ChEMBL
| Assay Description
Displacement of [3H]OH-DPAT from human recombinant 5-HT1A receptor measured after 60 mins by scintillation counter method |
Eur J Med Chem 176: 292-309 (2019)
Article DOI: 10.1016/j.ejmech.2019.04.064
BindingDB Entry DOI: 10.7270/Q2NP27V8 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM21395

(3-(2-(4-(4-Fluorobenzoyl)piperidinol)ethyl)-2,4(1H...)Show SMILES Fc1ccc(cc1)C(=O)C1CCN(CCn2c(=O)[nH]c3ccccc3c2=O)CC1 Show InChI InChI=1S/C22H22FN3O3/c23-17-7-5-15(6-8-17)20(27)16-9-11-25(12-10-16)13-14-26-21(28)18-3-1-2-4-19(18)24-22(26)29/h1-8,16H,9-14H2,(H,24,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Ireland Galway
Curated by ChEMBL
| Assay Description
Displacement of [3H] ketanserin from human recombinant 5-HT2A receptor measured after 60 mins by scintillation counter method |
Eur J Med Chem 176: 292-309 (2019)
Article DOI: 10.1016/j.ejmech.2019.04.064
BindingDB Entry DOI: 10.7270/Q2NP27V8 |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50591659
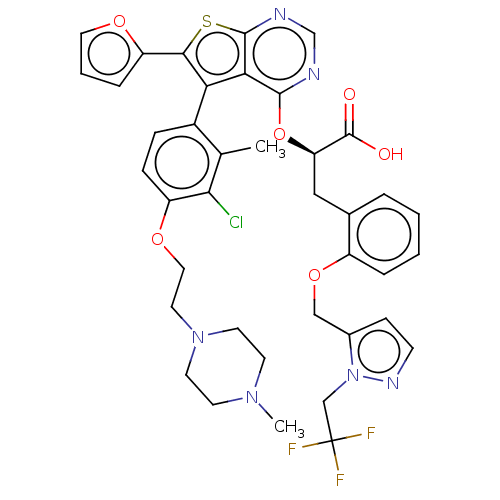
(CHEMBL5196373)Show SMILES CN1CCN(CCOc2ccc(-c3c(sc4ncnc(O[C@H](Cc5ccccc5OCc5ccnn5CC(F)(F)F)C(O)=O)c34)-c3ccco3)c(C)c2Cl)CC1 |r,wU:21.21,(9.12,2.97,;7.64,2.57,;6.55,3.66,;5.06,3.26,;4.66,1.78,;3.17,1.38,;2.08,2.47,;.6,2.07,;.2,.58,;-1.29,.18,;-1.69,-1.3,;-.6,-2.39,;-1,-3.88,;-.09,-5.12,;-1,-6.37,;-2.46,-5.89,;-3.79,-6.66,;-5.13,-5.9,;-5.13,-4.35,;-3.79,-3.58,;-3.79,-2.04,;-5.13,-1.27,;-5.13,.27,;-6.46,1.04,;-7.79,.27,;-9.12,1.03,;-9.12,2.57,;-7.8,3.34,;-6.46,2.58,;-5.13,3.35,;-3.79,2.58,;-2.46,3.35,;-1.05,2.73,;-.02,3.87,;-.79,5.2,;-2.3,4.88,;-3.39,5.97,;-4.87,5.57,;-5.96,6.66,;-5.96,4.48,;-4.48,4.09,;-6.46,-2.04,;-7.79,-1.27,;-6.46,-3.58,;-2.46,-4.35,;1.45,-5.12,;2.35,-3.88,;3.81,-4.35,;3.81,-5.89,;2.35,-6.37,;.89,-1.99,;1.98,-3.08,;1.28,-.5,;2.77,-.1,;5.75,.69,;7.24,1.09,)| | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113038
BindingDB Entry DOI: 10.7270/Q2B28081 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50010859
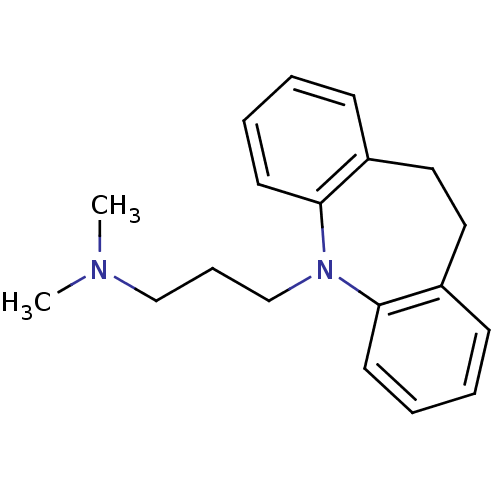
(CHEMBL11 | IMIPRAMINE HYDROCHLORIDE | IMIPRAMINE P...)Show InChI InChI=1S/C19H24N2/c1-20(2)14-7-15-21-18-10-5-3-8-16(18)12-13-17-9-4-6-11-19(17)21/h3-6,8-11H,7,12-15H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Ireland Galway
Curated by ChEMBL
| Assay Description
Displacement of [3H] imipramine from human recombinant 5-HT transporter measured after 60 mins by scintillation counter method |
Eur J Med Chem 176: 292-309 (2019)
Article DOI: 10.1016/j.ejmech.2019.04.064
BindingDB Entry DOI: 10.7270/Q2NP27V8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50024204

(1H-imidazo[4,5-c]pyridine derivative | 2N-[4,7-dim...)Show SMILES CN(C)S(=O)(=O)N[C@H]1C[C@H]2[C@@H](Cc3cn(C)c4cccc2c34)N(C)C1 Show InChI InChI=1S/C18H26N4O2S/c1-20(2)25(23,24)19-13-9-15-14-6-5-7-16-18(14)12(10-21(16)3)8-17(15)22(4)11-13/h5-7,10,13,15,17,19H,8-9,11H2,1-4H3/t13-,15+,17+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Ireland Galway
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant 5-HT2B receptor in CHOK1 cells measured after 30 mins by HTRF assay |
Eur J Med Chem 176: 292-309 (2019)
Article DOI: 10.1016/j.ejmech.2019.04.064
BindingDB Entry DOI: 10.7270/Q2NP27V8 |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50330247
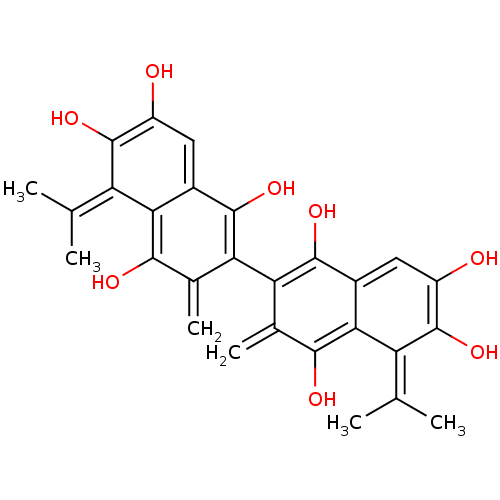
(6,6',7,7'-Tetrahydroxy-5,5'-diisopropyl-3,3'-dimet...)Show SMILES CC(C)=c1c(O)c(O)cc2c(O)c(-c3c(O)c4cc(O)c(O)c(=C(C)C)c4c(O)c3=C)c(=C)c(O)c12 |(3.81,-6.1,;2.48,-6.86,;1.15,-6.08,;2.46,-8.4,;3.8,-9.19,;5.14,-8.43,;3.78,-10.73,;5.1,-11.52,;2.43,-11.49,;1.11,-10.71,;-.21,-11.46,;-.21,-13,;-1.54,-10.69,;-2.88,-11.46,;-4.22,-10.68,;-4.22,-9.14,;-5.55,-11.46,;-6.89,-10.68,;-8.22,-11.46,;-9.55,-10.69,;-8.22,-13.01,;-9.55,-13.78,;-6.89,-13.78,;-6.89,-15.31,;-8.22,-16.08,;-5.56,-16.08,;-5.55,-13.01,;-4.21,-13.77,;-4.21,-15.31,;-2.87,-13,;-1.54,-13.77,;-1.55,-9.16,;-2.88,-8.39,;-.21,-8.39,;-.2,-6.85,;1.13,-9.16,)| Show InChI InChI=1S/C28H26O8/c1-9(2)17-21-13(7-15(29)27(17)35)25(33)19(11(5)23(21)31)20-12(6)24(32)22-14(26(20)34)8-16(30)28(36)18(22)10(3)4/h7-8,29-36H,5-6H2,1-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113038
BindingDB Entry DOI: 10.7270/Q2B28081 |
More data for this
Ligand-Target Pair | |
Bcl-2 homologous antagonist/killer
(Homo sapiens) | BDBM50303503

(CHEMBL4160782 | US10858316, Compound SF-5-141)Show SMILES Cc1cc(Oc2ccc(cc2)S(=O)(=O)N2CC(Cc3ccccc23)C(O)=O)cc(C)c1Cl Show InChI InChI=1S/C24H22ClNO5S/c1-15-11-20(12-16(2)23(15)25)31-19-7-9-21(10-8-19)32(29,30)26-14-18(24(27)28)13-17-5-3-4-6-22(17)26/h3-12,18H,13-14H2,1-2H3,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113038
BindingDB Entry DOI: 10.7270/Q2B28081 |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM50524648

(CHEMBL4579120)Show SMILES [H][C@]12OC(C)(C)O[C@@]1([H])[C@@]1([H])Cn3cc(COCCCCCCN(CCc4ccc5ccccc5c4)CC[C@@]([H])(O1)[C@@H]2OCCC(C)C)nn3 |r| Show InChI InChI=1S/C37H54N4O5/c1-27(2)17-22-43-34-32-16-20-40(19-15-28-13-14-29-11-7-8-12-30(29)23-28)18-9-5-6-10-21-42-26-31-24-41(39-38-31)25-33(44-32)35-36(34)46-37(3,4)45-35/h7-8,11-14,23-24,27,32-36H,5-6,9-10,15-22,25-26H2,1-4H3/t32-,33-,34+,35+,36-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Ireland Galway
Curated by ChEMBL
| Assay Description
Displacement of [125I]NKA from human recombinant NK2 receptor measured after 60 mins by scintillation counter method |
Eur J Med Chem 176: 292-309 (2019)
Article DOI: 10.1016/j.ejmech.2019.04.064
BindingDB Entry DOI: 10.7270/Q2NP27V8 |
More data for this
Ligand-Target Pair | |
Bcl-2 homologous antagonist/killer
(Homo sapiens) | BDBM50591668

(CHEMBL5198484)Show SMILES [H][C@]12C[C@H](CCOC(=O)c3ccc(Oc4cccnc4)cc3)C=C[C@]1([C@H](\C=C(/C)c1ccc(cc1)C(O)=O)C=C(C)C2)C(O)=O |r,c:24,t:42| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 153 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113038
BindingDB Entry DOI: 10.7270/Q2B28081 |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50013858
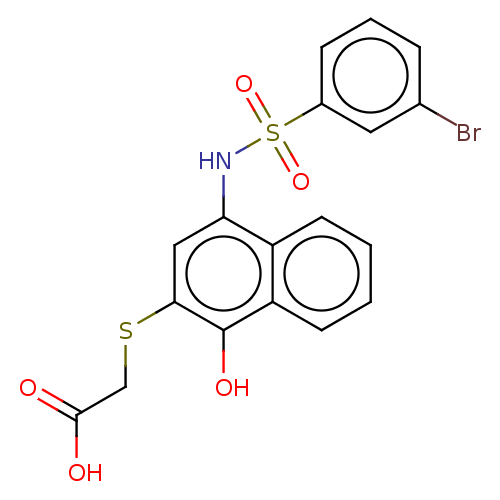
(CHEMBL3265291)Show SMILES OC(=O)CSc1cc(NS(=O)(=O)c2cccc(Br)c2)c2ccccc2c1O Show InChI InChI=1S/C18H14BrNO5S2/c19-11-4-3-5-12(8-11)27(24,25)20-15-9-16(26-10-17(21)22)18(23)14-7-2-1-6-13(14)15/h1-9,20,23H,10H2,(H,21,22) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113038
BindingDB Entry DOI: 10.7270/Q2B28081 |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50303352

(CHEMBL4164057)Show SMILES COc1cc(CC(=O)NCc2ccc(cc2)C(=O)N2CCN(Cc3ccccc3-c3ccccc3)CC2)cc(OC)c1OC Show InChI InChI=1S/C36H39N3O5/c1-42-32-21-27(22-33(43-2)35(32)44-3)23-34(40)37-24-26-13-15-29(16-14-26)36(41)39-19-17-38(18-20-39)25-30-11-7-8-12-31(30)28-9-5-4-6-10-28/h4-16,21-22H,17-20,23-25H2,1-3H3,(H,37,40) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113038
BindingDB Entry DOI: 10.7270/Q2B28081 |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50591660

(CHEMBL3818394)Show SMILES CCOC(=O)C1=C(C)N=c2s\c(=C/c3cn(Cc4ccc(cc4)-c4ccccc4)c4ccccc34)c(=O)n2C1c1ccccc1 |c:5,t:8| | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113038
BindingDB Entry DOI: 10.7270/Q2B28081 |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50581328
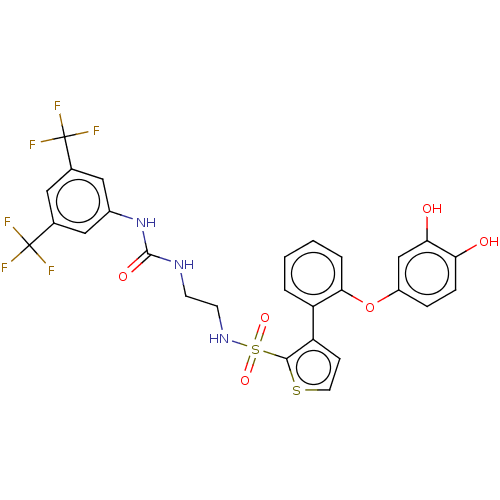
(CHEMBL5090868)Show SMILES Oc1ccc(Oc2ccccc2-c2ccsc2S(=O)(=O)NCCNC(=O)Nc2cc(cc(c2)C(F)(F)F)C(F)(F)F)cc1O | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 298 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113038
BindingDB Entry DOI: 10.7270/Q2B28081 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50524648

(CHEMBL4579120)Show SMILES [H][C@]12OC(C)(C)O[C@@]1([H])[C@@]1([H])Cn3cc(COCCCCCCN(CCc4ccc5ccccc5c4)CC[C@@]([H])(O1)[C@@H]2OCCC(C)C)nn3 |r| Show InChI InChI=1S/C37H54N4O5/c1-27(2)17-22-43-34-32-16-20-40(19-15-28-13-14-29-11-7-8-12-30(29)23-28)18-9-5-6-10-21-42-26-31-24-41(39-38-31)25-33(44-32)35-36(34)46-37(3,4)45-35/h7-8,11-14,23-24,27,32-36H,5-6,9-10,15-22,25-26H2,1-4H3/t32-,33-,34+,35+,36-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Ireland Galway
Curated by ChEMBL
| Assay Description
Displacement of [3H]OH-DPAT from human recombinant 5-HT1A receptor measured after 60 mins by scintillation counter method |
Eur J Med Chem 176: 292-309 (2019)
Article DOI: 10.1016/j.ejmech.2019.04.064
BindingDB Entry DOI: 10.7270/Q2NP27V8 |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50303139

(CHEMBL4171930)Show SMILES COc1cc(NC(=O)CCC(=O)N2CCN(Cc3cccc(c3)-c3ccc(Cl)cc3)CC2)cc(OC)c1OC Show InChI InChI=1S/C30H34ClN3O5/c1-37-26-18-25(19-27(38-2)30(26)39-3)32-28(35)11-12-29(36)34-15-13-33(14-16-34)20-21-5-4-6-23(17-21)22-7-9-24(31)10-8-22/h4-10,17-19H,11-16,20H2,1-3H3,(H,32,35) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113038
BindingDB Entry DOI: 10.7270/Q2B28081 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50524649

(CHEMBL4562303)Show SMILES [H][C@]12OC(C)(C)O[C@@]1([H])[C@@]1([H])COCc3cn(C[C@@]4([H])O[C@]([H])(CCN(CCc5ccc6ccccc6c5)CC[C@@]([H])(O1)[C@@H]2OCCC(C)C)[C@H](OCCC(C)C)[C@@]1([H])OC(C)(C)O[C@@]41[H])nn3 |r| Show InChI InChI=1S/C47H70N4O9/c1-30(2)18-23-53-40-36-16-21-50(20-15-32-13-14-33-11-9-10-12-34(33)25-32)22-17-37-41(54-24-19-31(3)4)45-43(58-47(7,8)60-45)39(56-37)29-52-28-35-26-51(49-48-35)27-38(55-36)42-44(40)59-46(5,6)57-42/h9-14,25-26,30-31,36-45H,15-24,27-29H2,1-8H3/t36-,37-,38-,39-,40+,41+,42+,43+,44-,45-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Ireland Galway
Curated by ChEMBL
| Assay Description
Displacement of [3H]OH-DPAT from human recombinant 5-HT1A receptor measured after 60 mins by scintillation counter method |
Eur J Med Chem 176: 292-309 (2019)
Article DOI: 10.1016/j.ejmech.2019.04.064
BindingDB Entry DOI: 10.7270/Q2NP27V8 |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50591661
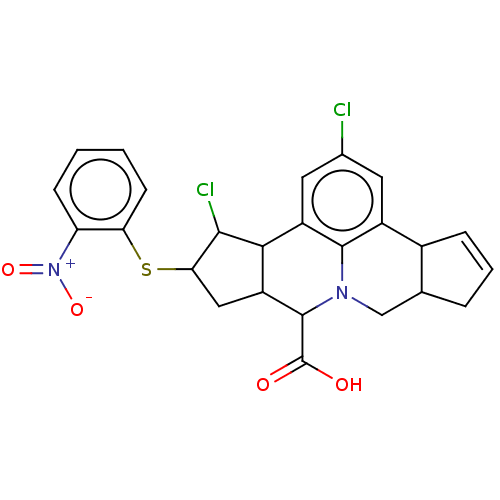
(CHEMBL5186810)Show SMILES OC(=O)C1C2CC(Sc3ccccc3[N+]([O-])=O)C(Cl)C2c2cc(Cl)cc3C4C=CCC4CN1c23 |c:29| | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113038
BindingDB Entry DOI: 10.7270/Q2B28081 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM50507589
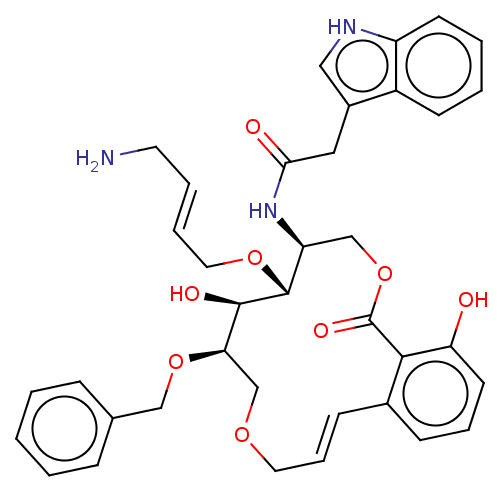
(CHEMBL4586779)Show SMILES NC\C=C\CO[C@@H]1[C@H](COC(=O)c2c(O)cccc2\C=C\COC[C@@H](OCc2ccccc2)[C@H]1O)NC(=O)Cc1c[nH]c2ccccc12 |r,t:20| Show InChI InChI=1S/C37H41N3O8/c38-17-6-7-19-46-36-30(40-33(42)20-27-21-39-29-15-5-4-14-28(27)29)23-48-37(44)34-26(12-8-16-31(34)41)13-9-18-45-24-32(35(36)43)47-22-25-10-2-1-3-11-25/h1-16,21,30,32,35-36,39,41,43H,17-20,22-24,38H2,(H,40,42)/b7-6+,13-9+/t30-,32+,35+,36+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Ireland Galway
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR4 expressed in African green monkey COS1 cell membranes after 2 hrs by scintillation cou... |
Eur J Med Chem 163: 148-159 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.030
BindingDB Entry DOI: 10.7270/Q2DR2ZT1 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50330247

(6,6',7,7'-Tetrahydroxy-5,5'-diisopropyl-3,3'-dimet...)Show SMILES CC(C)=c1c(O)c(O)cc2c(O)c(-c3c(O)c4cc(O)c(O)c(=C(C)C)c4c(O)c3=C)c(=C)c(O)c12 |(3.81,-6.1,;2.48,-6.86,;1.15,-6.08,;2.46,-8.4,;3.8,-9.19,;5.14,-8.43,;3.78,-10.73,;5.1,-11.52,;2.43,-11.49,;1.11,-10.71,;-.21,-11.46,;-.21,-13,;-1.54,-10.69,;-2.88,-11.46,;-4.22,-10.68,;-4.22,-9.14,;-5.55,-11.46,;-6.89,-10.68,;-8.22,-11.46,;-9.55,-10.69,;-8.22,-13.01,;-9.55,-13.78,;-6.89,-13.78,;-6.89,-15.31,;-8.22,-16.08,;-5.56,-16.08,;-5.55,-13.01,;-4.21,-13.77,;-4.21,-15.31,;-2.87,-13,;-1.54,-13.77,;-1.55,-9.16,;-2.88,-8.39,;-.21,-8.39,;-.2,-6.85,;1.13,-9.16,)| Show InChI InChI=1S/C28H26O8/c1-9(2)17-21-13(7-15(29)27(17)35)25(33)19(11(5)23(21)31)20-12(6)24(32)22-14(26(20)34)8-16(30)28(36)18(22)10(3)4/h7-8,29-36H,5-6H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113038
BindingDB Entry DOI: 10.7270/Q2B28081 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50524648

(CHEMBL4579120)Show SMILES [H][C@]12OC(C)(C)O[C@@]1([H])[C@@]1([H])Cn3cc(COCCCCCCN(CCc4ccc5ccccc5c4)CC[C@@]([H])(O1)[C@@H]2OCCC(C)C)nn3 |r| Show InChI InChI=1S/C37H54N4O5/c1-27(2)17-22-43-34-32-16-20-40(19-15-28-13-14-29-11-7-8-12-30(29)23-28)18-9-5-6-10-21-42-26-31-24-41(39-38-31)25-33(44-32)35-36(34)46-37(3,4)45-35/h7-8,11-14,23-24,27,32-36H,5-6,9-10,15-22,25-26H2,1-4H3/t32-,33-,34+,35+,36-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Ireland Galway
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant 5-HT2B receptor in CHOK1 cells measured after 30 mins by HTRF assay |
Eur J Med Chem 176: 292-309 (2019)
Article DOI: 10.1016/j.ejmech.2019.04.064
BindingDB Entry DOI: 10.7270/Q2NP27V8 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50524648

(CHEMBL4579120)Show SMILES [H][C@]12OC(C)(C)O[C@@]1([H])[C@@]1([H])Cn3cc(COCCCCCCN(CCc4ccc5ccccc5c4)CC[C@@]([H])(O1)[C@@H]2OCCC(C)C)nn3 |r| Show InChI InChI=1S/C37H54N4O5/c1-27(2)17-22-43-34-32-16-20-40(19-15-28-13-14-29-11-7-8-12-30(29)23-28)18-9-5-6-10-21-42-26-31-24-41(39-38-31)25-33(44-32)35-36(34)46-37(3,4)45-35/h7-8,11-14,23-24,27,32-36H,5-6,9-10,15-22,25-26H2,1-4H3/t32-,33-,34+,35+,36-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Ireland Galway
Curated by ChEMBL
| Assay Description
Displacement of [3H] ketanserin from human recombinant 5-HT2A receptor measured after 60 mins by scintillation counter method |
Eur J Med Chem 176: 292-309 (2019)
Article DOI: 10.1016/j.ejmech.2019.04.064
BindingDB Entry DOI: 10.7270/Q2NP27V8 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50507589

(CHEMBL4586779)Show SMILES NC\C=C\CO[C@@H]1[C@H](COC(=O)c2c(O)cccc2\C=C\COC[C@@H](OCc2ccccc2)[C@H]1O)NC(=O)Cc1c[nH]c2ccccc12 |r,t:20| Show InChI InChI=1S/C37H41N3O8/c38-17-6-7-19-46-36-30(40-33(42)20-27-21-39-29-15-5-4-14-28(27)29)23-48-37(44)34-26(12-8-16-31(34)41)13-9-18-45-24-32(35(36)43)47-22-25-10-2-1-3-11-25/h1-16,21,30,32,35-36,39,41,43H,17-20,22-24,38H2,(H,40,42)/b7-6+,13-9+/t30-,32+,35+,36+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Ireland Galway
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR5 expressed in African green monkey COS1 cell membranes after 2 hrs by scintillation cou... |
Eur J Med Chem 163: 148-159 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.030
BindingDB Entry DOI: 10.7270/Q2DR2ZT1 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM50051567
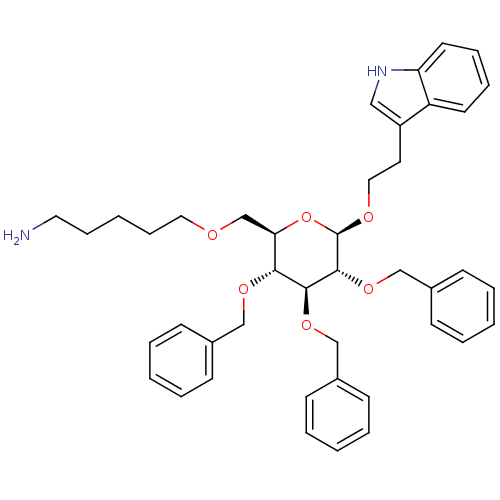
(5-{(2R,3R,4S,5R,6R)-3,4,5-Tris-benzyloxy-6-[2-(1H-...)Show SMILES NCCCCCOC[C@H]1O[C@@H](OCCc2c[nH]c3ccccc23)[C@H](OCc2ccccc2)[C@@H](OCc2ccccc2)[C@@H]1OCc1ccccc1 Show InChI InChI=1S/C42H50N2O6/c43-24-13-4-14-25-45-31-38-39(47-28-32-15-5-1-6-16-32)40(48-29-33-17-7-2-8-18-33)41(49-30-34-19-9-3-10-20-34)42(50-38)46-26-23-35-27-44-37-22-12-11-21-36(35)37/h1-3,5-12,15-22,27,38-42,44H,4,13-14,23-26,28-31,43H2/t38-,39-,40+,41-,42-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Ireland Galway
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR4 expressed in African green monkey COS1 cell membranes after 2 hrs by scintillation cou... |
Eur J Med Chem 163: 148-159 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.030
BindingDB Entry DOI: 10.7270/Q2DR2ZT1 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50507592
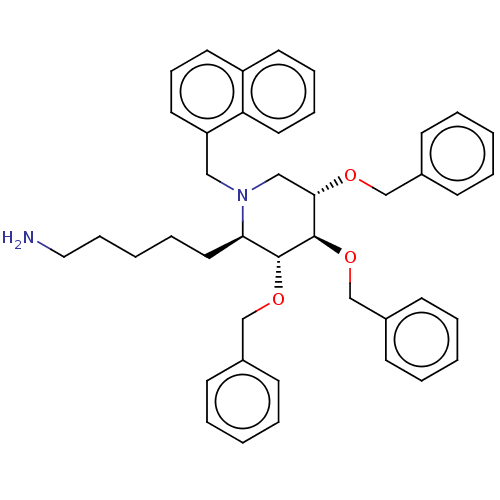
(CHEMBL4532486)Show SMILES NCCCCC[C@@H]1[C@@H](OCc2ccccc2)[C@H](OCc2ccccc2)[C@H](CN1Cc1cccc2ccccc12)OCc1ccccc1 |r| Show InChI InChI=1S/C42H48N2O3/c43-27-14-4-11-26-39-41(46-31-34-18-7-2-8-19-34)42(47-32-35-20-9-3-10-21-35)40(45-30-33-16-5-1-6-17-33)29-44(39)28-37-24-15-23-36-22-12-13-25-38(36)37/h1-3,5-10,12-13,15-25,39-42H,4,11,14,26-32,43H2/t39-,40+,41-,42-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Ireland Galway
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR5 expressed in African green monkey COS1 cell membranes after 2 hrs by scintillation cou... |
Eur J Med Chem 163: 148-159 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.030
BindingDB Entry DOI: 10.7270/Q2DR2ZT1 |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM50524649

(CHEMBL4562303)Show SMILES [H][C@]12OC(C)(C)O[C@@]1([H])[C@@]1([H])COCc3cn(C[C@@]4([H])O[C@]([H])(CCN(CCc5ccc6ccccc6c5)CC[C@@]([H])(O1)[C@@H]2OCCC(C)C)[C@H](OCCC(C)C)[C@@]1([H])OC(C)(C)O[C@@]41[H])nn3 |r| Show InChI InChI=1S/C47H70N4O9/c1-30(2)18-23-53-40-36-16-21-50(20-15-32-13-14-33-11-9-10-12-34(33)25-32)22-17-37-41(54-24-19-31(3)4)45-43(58-47(7,8)60-45)39(56-37)29-52-28-35-26-51(49-48-35)27-38(55-36)42-44(40)59-46(5,6)57-42/h9-14,25-26,30-31,36-45H,15-24,27-29H2,1-8H3/t36-,37-,38-,39-,40+,41+,42+,43+,44-,45-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Ireland Galway
Curated by ChEMBL
| Assay Description
Displacement of [125I]NKA from human recombinant NK2 receptor measured after 60 mins by scintillation counter method |
Eur J Med Chem 176: 292-309 (2019)
Article DOI: 10.1016/j.ejmech.2019.04.064
BindingDB Entry DOI: 10.7270/Q2NP27V8 |
More data for this
Ligand-Target Pair | |
Bcl-2 homologous antagonist/killer
(Homo sapiens) | BDBM50266958

(CHEMBL4077940)Show SMILES CC(C)Oc1cc(ccc1N)C(=O)Nc1ccc(nc1OC(C)C)C(=O)Nc1ccc(cc1OC(C)C)C(O)=O Show InChI InChI=1S/C29H34N4O7/c1-15(2)38-24-13-18(7-9-20(24)30)26(34)32-23-12-11-22(33-28(23)40-17(5)6)27(35)31-21-10-8-19(29(36)37)14-25(21)39-16(3)4/h7-17H,30H2,1-6H3,(H,31,35)(H,32,34)(H,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113038
BindingDB Entry DOI: 10.7270/Q2B28081 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM50507592

(CHEMBL4532486)Show SMILES NCCCCC[C@@H]1[C@@H](OCc2ccccc2)[C@H](OCc2ccccc2)[C@H](CN1Cc1cccc2ccccc12)OCc1ccccc1 |r| Show InChI InChI=1S/C42H48N2O3/c43-27-14-4-11-26-39-41(46-31-34-18-7-2-8-19-34)42(47-32-35-20-9-3-10-21-35)40(45-30-33-16-5-1-6-17-33)29-44(39)28-37-24-15-23-36-22-12-13-25-38(36)37/h1-3,5-10,12-13,15-25,39-42H,4,11,14,26-32,43H2/t39-,40+,41-,42-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Ireland Galway
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR4 expressed in African green monkey COS1 cell membranes after 2 hrs by scintillation cou... |
Eur J Med Chem 163: 148-159 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.030
BindingDB Entry DOI: 10.7270/Q2DR2ZT1 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM50507590
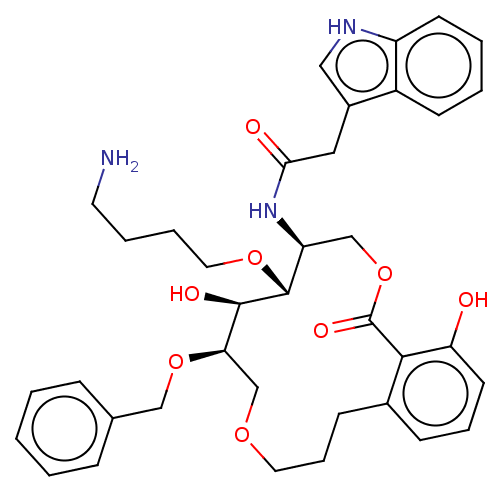
(CHEMBL4557933)Show SMILES NCCCCO[C@@H]1[C@H](COC(=O)c2c(O)cccc2CCCOC[C@@H](OCc2ccccc2)[C@H]1O)NC(=O)Cc1c[nH]c2ccccc12 |r| Show InChI InChI=1S/C37H45N3O8/c38-17-6-7-19-46-36-30(40-33(42)20-27-21-39-29-15-5-4-14-28(27)29)23-48-37(44)34-26(12-8-16-31(34)41)13-9-18-45-24-32(35(36)43)47-22-25-10-2-1-3-11-25/h1-5,8,10-12,14-16,21,30,32,35-36,39,41,43H,6-7,9,13,17-20,22-24,38H2,(H,40,42)/t30-,32+,35+,36+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Ireland Galway
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR4 expressed in African green monkey COS1 cell membranes after 2 hrs by scintillation cou... |
Eur J Med Chem 163: 148-159 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.030
BindingDB Entry DOI: 10.7270/Q2DR2ZT1 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM50507584

(CHEMBL4544582)Show SMILES Cc1ccc(S[C@H]2O[C@H](CO)[C@@H](O)[C@H](OC\C=C\CN)[C@@H]2NC(=O)Cc2c[nH]c3ccccc23)cc1 |r| Show InChI InChI=1S/C27H33N3O5S/c1-17-8-10-19(11-9-17)36-27-24(26(34-13-5-4-12-28)25(33)22(16-31)35-27)30-23(32)14-18-15-29-21-7-3-2-6-20(18)21/h2-11,15,22,24-27,29,31,33H,12-14,16,28H2,1H3,(H,30,32)/b5-4+/t22-,24+,25-,26-,27-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Ireland Galway
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR4 expressed in African green monkey COS1 cell membranes after 2 hrs by scintillation cou... |
Eur J Med Chem 163: 148-159 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.030
BindingDB Entry DOI: 10.7270/Q2DR2ZT1 |
More data for this
Ligand-Target Pair | |
Bcl-2 homologous antagonist/killer
(Homo sapiens) | BDBM50303215
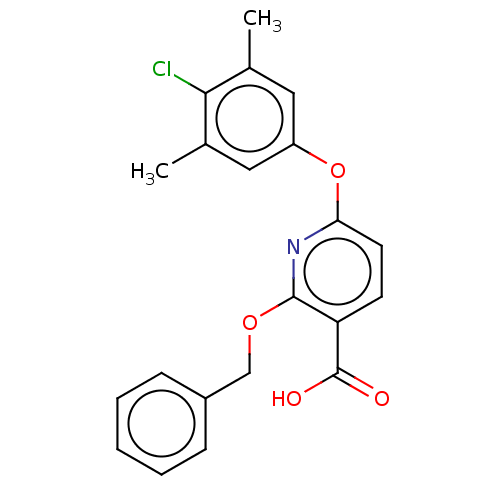
(CHEMBL4167501)Show SMILES Cc1cc(Oc2ccc(C(O)=O)c(OCc3ccccc3)n2)cc(C)c1Cl Show InChI InChI=1S/C21H18ClNO4/c1-13-10-16(11-14(2)19(13)22)27-18-9-8-17(21(24)25)20(23-18)26-12-15-6-4-3-5-7-15/h3-11H,12H2,1-2H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113038
BindingDB Entry DOI: 10.7270/Q2B28081 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50507590

(CHEMBL4557933)Show SMILES NCCCCO[C@@H]1[C@H](COC(=O)c2c(O)cccc2CCCOC[C@@H](OCc2ccccc2)[C@H]1O)NC(=O)Cc1c[nH]c2ccccc12 |r| Show InChI InChI=1S/C37H45N3O8/c38-17-6-7-19-46-36-30(40-33(42)20-27-21-39-29-15-5-4-14-28(27)29)23-48-37(44)34-26(12-8-16-31(34)41)13-9-18-45-24-32(35(36)43)47-22-25-10-2-1-3-11-25/h1-5,8,10-12,14-16,21,30,32,35-36,39,41,43H,6-7,9,13,17-20,22-24,38H2,(H,40,42)/t30-,32+,35+,36+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Ireland Galway
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR5 expressed in African green monkey COS1 cell membranes after 2 hrs by scintillation cou... |
Eur J Med Chem 163: 148-159 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.030
BindingDB Entry DOI: 10.7270/Q2DR2ZT1 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM50507581

(CHEMBL3589939)Show SMILES NCCCCCOC[C@@H]1[C@@H](O)[C@H](O)[C@@H](O)CN1CCc1c[nH]c2ccccc12 |r| Show InChI InChI=1S/C21H33N3O4/c22-9-4-1-5-11-28-14-18-20(26)21(27)19(25)13-24(18)10-8-15-12-23-17-7-3-2-6-16(15)17/h2-3,6-7,12,18-21,23,25-27H,1,4-5,8-11,13-14,22H2/t18-,19+,20-,21-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Ireland Galway
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR4 expressed in African green monkey COS1 cell membranes after 2 hrs by scintillation cou... |
Eur J Med Chem 163: 148-159 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.030
BindingDB Entry DOI: 10.7270/Q2DR2ZT1 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50507585
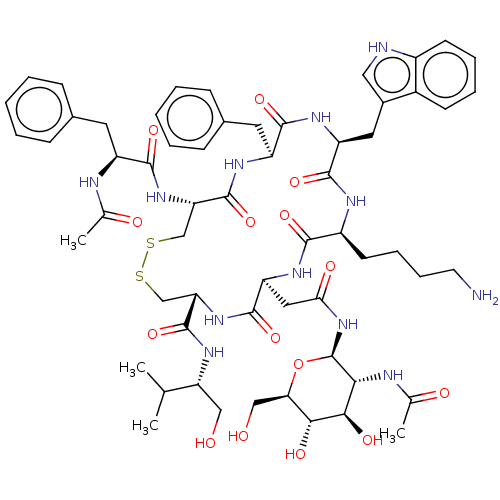
(CHEMBL4549840)Show SMILES CC(C)[C@@H](CO)NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@H](Cc2ccccc2)NC(C)=O)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(=O)N[C@@H]2O[C@H](CO)[C@@H](O)[C@H](O)[C@H]2NC(C)=O)C(=O)N1 |r| Show InChI InChI=1S/C60H82N12O15S2/c1-32(2)45(28-73)69-59(86)47-31-89-88-30-46(70-54(81)41(63-33(3)75)23-35-15-7-5-8-16-35)58(85)66-42(24-36-17-9-6-10-18-36)55(82)67-43(25-37-27-62-39-20-12-11-19-38(37)39)56(83)65-40(21-13-14-22-61)53(80)68-44(57(84)71-47)26-49(77)72-60-50(64-34(4)76)52(79)51(78)48(29-74)87-60/h5-12,15-20,27,32,40-48,50-52,60,62,73-74,78-79H,13-14,21-26,28-31,61H2,1-4H3,(H,63,75)(H,64,76)(H,65,83)(H,66,85)(H,67,82)(H,68,80)(H,69,86)(H,70,81)(H,71,84)(H,72,77)/t40-,41-,42-,43-,44-,45+,46-,47-,48+,50+,51+,52+,60+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Ireland Galway
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR5 expressed in African green monkey COS1 cell membranes after 2 hrs by scintillation cou... |
Eur J Med Chem 163: 148-159 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.030
BindingDB Entry DOI: 10.7270/Q2DR2ZT1 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50524648

(CHEMBL4579120)Show SMILES [H][C@]12OC(C)(C)O[C@@]1([H])[C@@]1([H])Cn3cc(COCCCCCCN(CCc4ccc5ccccc5c4)CC[C@@]([H])(O1)[C@@H]2OCCC(C)C)nn3 |r| Show InChI InChI=1S/C37H54N4O5/c1-27(2)17-22-43-34-32-16-20-40(19-15-28-13-14-29-11-7-8-12-30(29)23-28)18-9-5-6-10-21-42-26-31-24-41(39-38-31)25-33(44-32)35-36(34)46-37(3,4)45-35/h7-8,11-14,23-24,27,32-36H,5-6,9-10,15-22,25-26H2,1-4H3/t32-,33-,34+,35+,36-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Ireland Galway
Curated by ChEMBL
| Assay Description
Displacement of [3H] imipramine from human recombinant 5-HT transporter measured after 60 mins by scintillation counter method |
Eur J Med Chem 176: 292-309 (2019)
Article DOI: 10.1016/j.ejmech.2019.04.064
BindingDB Entry DOI: 10.7270/Q2NP27V8 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50507584

(CHEMBL4544582)Show SMILES Cc1ccc(S[C@H]2O[C@H](CO)[C@@H](O)[C@H](OC\C=C\CN)[C@@H]2NC(=O)Cc2c[nH]c3ccccc23)cc1 |r| Show InChI InChI=1S/C27H33N3O5S/c1-17-8-10-19(11-9-17)36-27-24(26(34-13-5-4-12-28)25(33)22(16-31)35-27)30-23(32)14-18-15-29-21-7-3-2-6-20(18)21/h2-11,15,22,24-27,29,31,33H,12-14,16,28H2,1H3,(H,30,32)/b5-4+/t22-,24+,25-,26-,27-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Ireland Galway
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR5 expressed in African green monkey COS1 cell membranes after 2 hrs by scintillation cou... |
Eur J Med Chem 163: 148-159 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.030
BindingDB Entry DOI: 10.7270/Q2DR2ZT1 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50507582
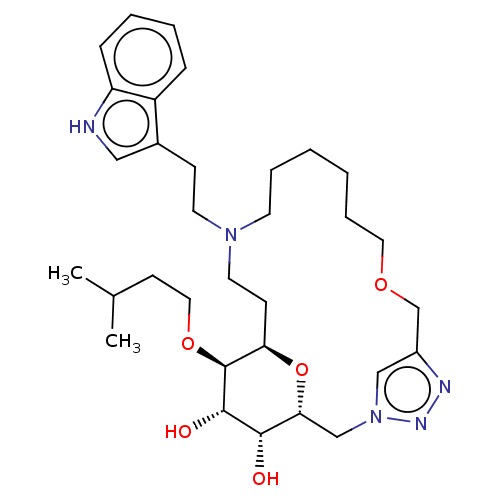
(CHEMBL4519358)Show SMILES CC(C)CCO[C@H]1[C@H]2CCN(CCc3c[nH]c4ccccc34)CCCCCCOCc3cn(C[C@@H](O2)[C@H](O)[C@@H]1O)nn3 |r| Show InChI InChI=1S/C32H49N5O5/c1-23(2)13-18-41-32-28-12-16-36(15-11-24-19-33-27-10-6-5-9-26(24)27)14-7-3-4-8-17-40-22-25-20-37(35-34-25)21-29(42-28)30(38)31(32)39/h5-6,9-10,19-20,23,28-33,38-39H,3-4,7-8,11-18,21-22H2,1-2H3/t28-,29-,30+,31+,32+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Ireland Galway
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR5 expressed in African green monkey COS1 cell membranes after 2 hrs by scintillation cou... |
Eur J Med Chem 163: 148-159 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.030
BindingDB Entry DOI: 10.7270/Q2DR2ZT1 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM50333650

(5-(((2R,3R,4R,5S)-1-(2-(1H-indol-3-yl)ethyl)-3,4,5...)Show SMILES NCCCCCOC[C@@H]1[C@@H](OCc2ccccc2)[C@H](OCc2ccccc2)[C@H](CN1CCc1c[nH]c2ccccc12)OCc1ccccc1 |r| Show InChI InChI=1S/C42H51N3O4/c43-24-13-4-14-26-46-32-39-41(48-30-34-17-7-2-8-18-34)42(49-31-35-19-9-3-10-20-35)40(47-29-33-15-5-1-6-16-33)28-45(39)25-23-36-27-44-38-22-12-11-21-37(36)38/h1-3,5-12,15-22,27,39-42,44H,4,13-14,23-26,28-32,43H2/t39-,40+,41-,42-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University School of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity to human SSTR4 |
Bioorg Med Chem Lett 21: 824-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.088
BindingDB Entry DOI: 10.7270/Q23N23NM |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM50333650

(5-(((2R,3R,4R,5S)-1-(2-(1H-indol-3-yl)ethyl)-3,4,5...)Show SMILES NCCCCCOC[C@@H]1[C@@H](OCc2ccccc2)[C@H](OCc2ccccc2)[C@H](CN1CCc1c[nH]c2ccccc12)OCc1ccccc1 |r| Show InChI InChI=1S/C42H51N3O4/c43-24-13-4-14-26-46-32-39-41(48-30-34-17-7-2-8-18-34)42(49-31-35-19-9-3-10-20-35)40(47-29-33-15-5-1-6-16-33)28-45(39)25-23-36-27-44-38-22-12-11-21-37(36)38/h1-3,5-12,15-22,27,39-42,44H,4,13-14,23-26,28-32,43H2/t39-,40+,41-,42-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Ireland Galway
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR4 expressed in African green monkey COS1 cell membranes after 2 hrs by scintillation cou... |
Eur J Med Chem 163: 148-159 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.030
BindingDB Entry DOI: 10.7270/Q2DR2ZT1 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50524649

(CHEMBL4562303)Show SMILES [H][C@]12OC(C)(C)O[C@@]1([H])[C@@]1([H])COCc3cn(C[C@@]4([H])O[C@]([H])(CCN(CCc5ccc6ccccc6c5)CC[C@@]([H])(O1)[C@@H]2OCCC(C)C)[C@H](OCCC(C)C)[C@@]1([H])OC(C)(C)O[C@@]41[H])nn3 |r| Show InChI InChI=1S/C47H70N4O9/c1-30(2)18-23-53-40-36-16-21-50(20-15-32-13-14-33-11-9-10-12-34(33)25-32)22-17-37-41(54-24-19-31(3)4)45-43(58-47(7,8)60-45)39(56-37)29-52-28-35-26-51(49-48-35)27-38(55-36)42-44(40)59-46(5,6)57-42/h9-14,25-26,30-31,36-45H,15-24,27-29H2,1-8H3/t36-,37-,38-,39-,40+,41+,42+,43+,44-,45-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Ireland Galway
Curated by ChEMBL
| Assay Description
Displacement of [3H] ketanserin from human recombinant 5-HT2A receptor measured after 60 mins by scintillation counter method |
Eur J Med Chem 176: 292-309 (2019)
Article DOI: 10.1016/j.ejmech.2019.04.064
BindingDB Entry DOI: 10.7270/Q2NP27V8 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50333650

(5-(((2R,3R,4R,5S)-1-(2-(1H-indol-3-yl)ethyl)-3,4,5...)Show SMILES NCCCCCOC[C@@H]1[C@@H](OCc2ccccc2)[C@H](OCc2ccccc2)[C@H](CN1CCc1c[nH]c2ccccc12)OCc1ccccc1 |r| Show InChI InChI=1S/C42H51N3O4/c43-24-13-4-14-26-46-32-39-41(48-30-34-17-7-2-8-18-34)42(49-31-35-19-9-3-10-20-35)40(47-29-33-15-5-1-6-16-33)28-45(39)25-23-36-27-44-38-22-12-11-21-37(36)38/h1-3,5-12,15-22,27,39-42,44H,4,13-14,23-26,28-32,43H2/t39-,40+,41-,42-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Ireland Galway
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR5 expressed in African green monkey COS1 cell membranes after 2 hrs by scintillation cou... |
Eur J Med Chem 163: 148-159 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.030
BindingDB Entry DOI: 10.7270/Q2DR2ZT1 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50333650

(5-(((2R,3R,4R,5S)-1-(2-(1H-indol-3-yl)ethyl)-3,4,5...)Show SMILES NCCCCCOC[C@@H]1[C@@H](OCc2ccccc2)[C@H](OCc2ccccc2)[C@H](CN1CCc1c[nH]c2ccccc12)OCc1ccccc1 |r| Show InChI InChI=1S/C42H51N3O4/c43-24-13-4-14-26-46-32-39-41(48-30-34-17-7-2-8-18-34)42(49-31-35-19-9-3-10-20-35)40(47-29-33-15-5-1-6-16-33)28-45(39)25-23-36-27-44-38-22-12-11-21-37(36)38/h1-3,5-12,15-22,27,39-42,44H,4,13-14,23-26,28-32,43H2/t39-,40+,41-,42-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University School of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity to human SSTR5 |
Bioorg Med Chem Lett 21: 824-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.088
BindingDB Entry DOI: 10.7270/Q23N23NM |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50507594
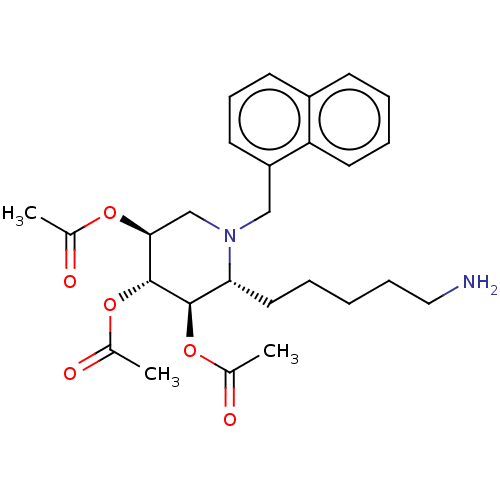
(CHEMBL4577766)Show SMILES CC(=O)O[C@H]1CN(Cc2cccc3ccccc23)[C@H](CCCCCN)[C@@H](OC(C)=O)[C@@H]1OC(C)=O |r| Show InChI InChI=1S/C27H36N2O6/c1-18(30)33-25-17-29(16-22-12-9-11-21-10-6-7-13-23(21)22)24(14-5-4-8-15-28)26(34-19(2)31)27(25)35-20(3)32/h6-7,9-13,24-27H,4-5,8,14-17,28H2,1-3H3/t24-,25+,26-,27-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Ireland Galway
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR5 expressed in African green monkey COS1 cell membranes after 2 hrs by scintillation cou... |
Eur J Med Chem 163: 148-159 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.030
BindingDB Entry DOI: 10.7270/Q2DR2ZT1 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM50507594

(CHEMBL4577766)Show SMILES CC(=O)O[C@H]1CN(Cc2cccc3ccccc23)[C@H](CCCCCN)[C@@H](OC(C)=O)[C@@H]1OC(C)=O |r| Show InChI InChI=1S/C27H36N2O6/c1-18(30)33-25-17-29(16-22-12-9-11-21-10-6-7-13-23(21)22)24(14-5-4-8-15-28)26(34-19(2)31)27(25)35-20(3)32/h6-7,9-13,24-27H,4-5,8,14-17,28H2,1-3H3/t24-,25+,26-,27-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Ireland Galway
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR4 expressed in African green monkey COS1 cell membranes after 2 hrs by scintillation cou... |
Eur J Med Chem 163: 148-159 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.030
BindingDB Entry DOI: 10.7270/Q2DR2ZT1 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM50507583
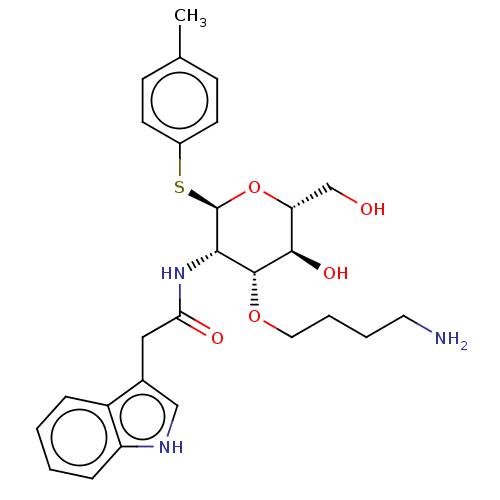
(CHEMBL4461404)Show SMILES Cc1ccc(S[C@H]2O[C@H](CO)[C@@H](O)[C@H](OCCCCN)[C@@H]2NC(=O)Cc2c[nH]c3ccccc23)cc1 |r| Show InChI InChI=1S/C27H35N3O5S/c1-17-8-10-19(11-9-17)36-27-24(26(34-13-5-4-12-28)25(33)22(16-31)35-27)30-23(32)14-18-15-29-21-7-3-2-6-20(18)21/h2-3,6-11,15,22,24-27,29,31,33H,4-5,12-14,16,28H2,1H3,(H,30,32)/t22-,24+,25-,26-,27-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Ireland Galway
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR4 expressed in African green monkey COS1 cell membranes after 2 hrs by scintillation cou... |
Eur J Med Chem 163: 148-159 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.030
BindingDB Entry DOI: 10.7270/Q2DR2ZT1 |
More data for this
Ligand-Target Pair | |
Bcl-2 homologous antagonist/killer
(Homo sapiens) | BDBM50308110
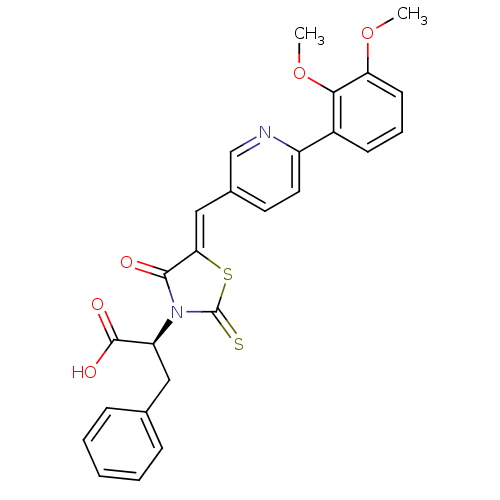
((S,Z)-2-(5-((6-(2,3-dimethoxyphenyl)pyridin-3-yl)m...)Show SMILES COc1cccc(c1OC)-c1ccc(\C=C2/SC(=S)N([C@@H](Cc3ccccc3)C(O)=O)C2=O)cn1 |r| Show InChI InChI=1S/C26H22N2O5S2/c1-32-21-10-6-9-18(23(21)33-2)19-12-11-17(15-27-19)14-22-24(29)28(26(34)35-22)20(25(30)31)13-16-7-4-3-5-8-16/h3-12,14-15,20H,13H2,1-2H3,(H,30,31)/b22-14-/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113038
BindingDB Entry DOI: 10.7270/Q2B28081 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM50507580

(CHEMBL4462329)Show SMILES CC(C)[C@@H](CO)NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@H](Cc2ccccc2)NC(C)=O)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(O)=O)C(=O)N1 |r| Show InChI InChI=1S/C52H68N10O11S2/c1-30(2)42(27-63)60-52(73)44-29-75-74-28-43(61-47(68)38(55-31(3)64)22-32-14-6-4-7-15-32)51(72)57-39(23-33-16-8-5-9-17-33)48(69)58-40(24-34-26-54-36-19-11-10-18-35(34)36)49(70)56-37(20-12-13-21-53)46(67)59-41(25-45(65)66)50(71)62-44/h4-11,14-19,26,30,37-44,54,63H,12-13,20-25,27-29,53H2,1-3H3,(H,55,64)(H,56,70)(H,57,72)(H,58,69)(H,59,67)(H,60,73)(H,61,68)(H,62,71)(H,65,66)/t37-,38-,39-,40-,41-,42+,43-,44-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Ireland Galway
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin-14 (Tyr11) from human SSTR4 expressed in African green monkey COS1 cell membranes after 2 hrs by scintillation cou... |
Eur J Med Chem 163: 148-159 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.030
BindingDB Entry DOI: 10.7270/Q2DR2ZT1 |
More data for this
Ligand-Target Pair | |
Bcl-2 homologous antagonist/killer
(Homo sapiens) | BDBM50591666
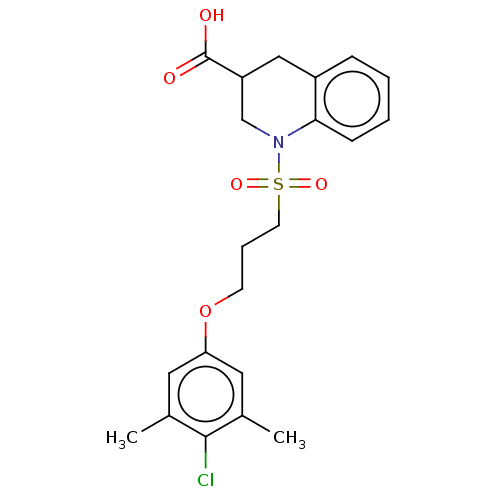
(CHEMBL5207001)Show SMILES Cc1cc(OCCCS(=O)(=O)N2CC(Cc3ccccc23)C(O)=O)cc(C)c1Cl | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113038
BindingDB Entry DOI: 10.7270/Q2B28081 |
More data for this
Ligand-Target Pair | |
Bcl-2 homologous antagonist/killer
(Homo sapiens) | BDBM50308111
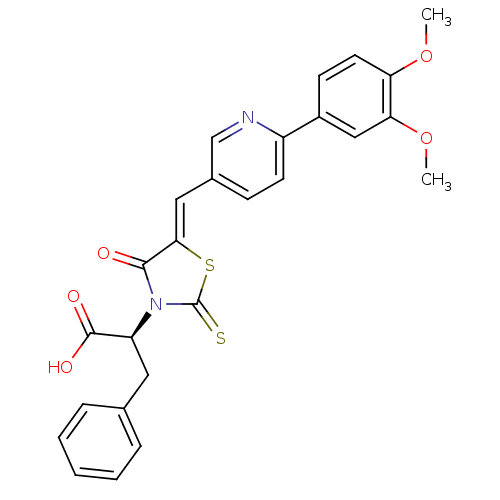
((S,Z)-2-(5-((6-(3,4-dimethoxyphenyl)pyridin-3-yl)m...)Show SMILES COc1ccc(cc1OC)-c1ccc(\C=C2/SC(=S)N([C@@H](Cc3ccccc3)C(O)=O)C2=O)cn1 |r| Show InChI InChI=1S/C26H22N2O5S2/c1-32-21-11-9-18(14-22(21)33-2)19-10-8-17(15-27-19)13-23-24(29)28(26(34)35-23)20(25(30)31)12-16-6-4-3-5-7-16/h3-11,13-15,20H,12H2,1-2H3,(H,30,31)/b23-13-/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113038
BindingDB Entry DOI: 10.7270/Q2B28081 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50524649

(CHEMBL4562303)Show SMILES [H][C@]12OC(C)(C)O[C@@]1([H])[C@@]1([H])COCc3cn(C[C@@]4([H])O[C@]([H])(CCN(CCc5ccc6ccccc6c5)CC[C@@]([H])(O1)[C@@H]2OCCC(C)C)[C@H](OCCC(C)C)[C@@]1([H])OC(C)(C)O[C@@]41[H])nn3 |r| Show InChI InChI=1S/C47H70N4O9/c1-30(2)18-23-53-40-36-16-21-50(20-15-32-13-14-33-11-9-10-12-34(33)25-32)22-17-37-41(54-24-19-31(3)4)45-43(58-47(7,8)60-45)39(56-37)29-52-28-35-26-51(49-48-35)27-38(55-36)42-44(40)59-46(5,6)57-42/h9-14,25-26,30-31,36-45H,15-24,27-29H2,1-8H3/t36-,37-,38-,39-,40+,41+,42+,43+,44-,45-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Ireland Galway
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant 5-HT2B receptor in CHOK1 cells measured after 30 mins by HTRF assay |
Eur J Med Chem 176: 292-309 (2019)
Article DOI: 10.1016/j.ejmech.2019.04.064
BindingDB Entry DOI: 10.7270/Q2NP27V8 |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50591659

(CHEMBL5196373)Show SMILES CN1CCN(CCOc2ccc(-c3c(sc4ncnc(O[C@H](Cc5ccccc5OCc5ccnn5CC(F)(F)F)C(O)=O)c34)-c3ccco3)c(C)c2Cl)CC1 |r,wU:21.21,(9.12,2.97,;7.64,2.57,;6.55,3.66,;5.06,3.26,;4.66,1.78,;3.17,1.38,;2.08,2.47,;.6,2.07,;.2,.58,;-1.29,.18,;-1.69,-1.3,;-.6,-2.39,;-1,-3.88,;-.09,-5.12,;-1,-6.37,;-2.46,-5.89,;-3.79,-6.66,;-5.13,-5.9,;-5.13,-4.35,;-3.79,-3.58,;-3.79,-2.04,;-5.13,-1.27,;-5.13,.27,;-6.46,1.04,;-7.79,.27,;-9.12,1.03,;-9.12,2.57,;-7.8,3.34,;-6.46,2.58,;-5.13,3.35,;-3.79,2.58,;-2.46,3.35,;-1.05,2.73,;-.02,3.87,;-.79,5.2,;-2.3,4.88,;-3.39,5.97,;-4.87,5.57,;-5.96,6.66,;-5.96,4.48,;-4.48,4.09,;-6.46,-2.04,;-7.79,-1.27,;-6.46,-3.58,;-2.46,-4.35,;1.45,-5.12,;2.35,-3.88,;3.81,-4.35,;3.81,-5.89,;2.35,-6.37,;.89,-1.99,;1.98,-3.08,;1.28,-.5,;2.77,-.1,;5.75,.69,;7.24,1.09,)| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113038
BindingDB Entry DOI: 10.7270/Q2B28081 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data