

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM50524862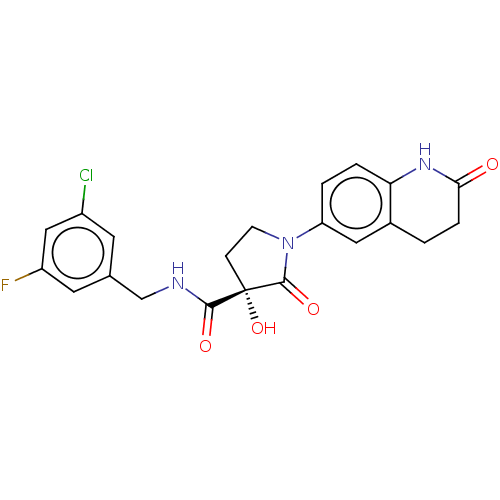 (CHEMBL4475680) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare Curated by ChEMBL | Assay Description Inhibition of 5-[(S)-3-(3-Chloro-5-fluoro-benzylcarbamoyl)-3-hydroxy-2-oxopyrrolidin-1-yl]-1H-indole-2-carboxylic Acid (3-Amino-propyl)-amide-Dy647 b... | J Med Chem 62: 11119-11134 (2019) Article DOI: 10.1021/acs.jmedchem.9b01070 BindingDB Entry DOI: 10.7270/Q26H4MXQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM50524862 (CHEMBL4475680) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare Curated by ChEMBL | Assay Description Inhibition of 5-[(S)-3-(3-Chloro-5-fluoro-benzylcarbamoyl)-3-hydroxy-2-oxopyrrolidin-1-yl]-1H-indole-2-carboxylic Acid (3-Amino-propyl)-amide-Dy647 b... | J Med Chem 62: 11119-11134 (2019) Article DOI: 10.1021/acs.jmedchem.9b01070 BindingDB Entry DOI: 10.7270/Q26H4MXQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM401307 (US10005756, Compound A78) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare Curated by ChEMBL | Assay Description Inhibition of 5-[(S)-3-(3-Chloro-5-fluoro-benzylcarbamoyl)-3-hydroxy-2-oxopyrrolidin-1-yl]-1H-indole-2-carboxylic Acid (3-Amino-propyl)-amide-Dy647 b... | J Med Chem 62: 11119-11134 (2019) Article DOI: 10.1021/acs.jmedchem.9b01070 BindingDB Entry DOI: 10.7270/Q26H4MXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM401307 (US10005756, Compound A78) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare Curated by ChEMBL | Assay Description Inhibition of 5-[(S)-3-(3-Chloro-5-fluoro-benzylcarbamoyl)-3-hydroxy-2-oxopyrrolidin-1-yl]-1H-indole-2-carboxylic Acid (3-Amino-propyl)-amide-Dy647 b... | J Med Chem 62: 11119-11134 (2019) Article DOI: 10.1021/acs.jmedchem.9b01070 BindingDB Entry DOI: 10.7270/Q26H4MXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50085448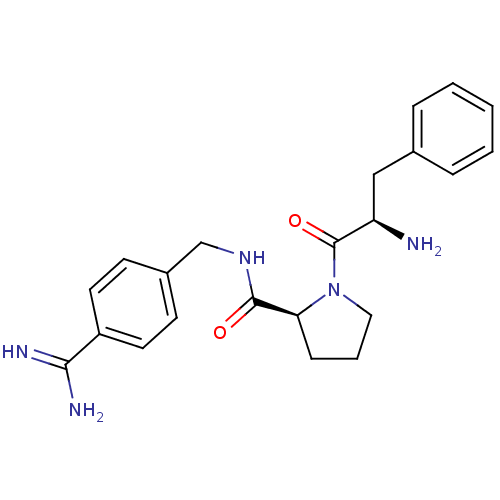 ((S)-1-((R)-2-Amino-3-phenyl-propionyl)-pyrrolidine...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description The compound was evaluated for binding affinity against Prothrombin | Bioorg Med Chem Lett 10: 243-7 (2000) BindingDB Entry DOI: 10.7270/Q2K9381W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM50531161 (CHEMBL4448724) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare Curated by ChEMBL | Assay Description Inhibition of 5-[(S)-3-(3-Chloro-5-fluoro-benzylcarbamoyl)-3-hydroxy-2-oxopyrrolidin-1-yl]-1H-indole-2-carboxylic Acid (3-Amino-propyl)-amide-Dy647 b... | J Med Chem 62: 11119-11134 (2019) Article DOI: 10.1021/acs.jmedchem.9b01070 BindingDB Entry DOI: 10.7270/Q26H4MXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM50531161 (CHEMBL4448724) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare Curated by ChEMBL | Assay Description Inhibition of 5-[(S)-3-(3-Chloro-5-fluoro-benzylcarbamoyl)-3-hydroxy-2-oxopyrrolidin-1-yl]-1H-indole-2-carboxylic Acid (3-Amino-propyl)-amide-Dy647 b... | J Med Chem 62: 11119-11134 (2019) Article DOI: 10.1021/acs.jmedchem.9b01070 BindingDB Entry DOI: 10.7270/Q26H4MXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM50531163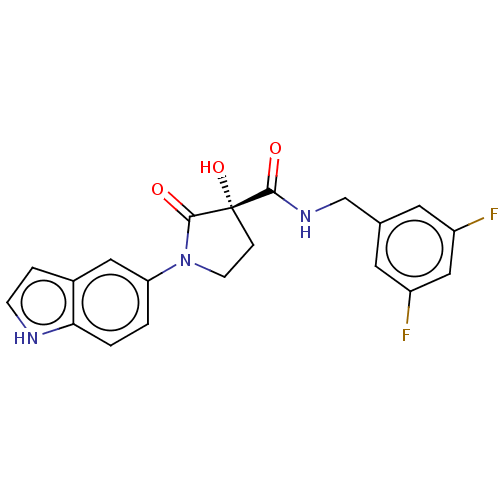 (CHEMBL4464946) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare Curated by ChEMBL | Assay Description Inhibition of 5-[(S)-3-(3-Chloro-5-fluoro-benzylcarbamoyl)-3-hydroxy-2-oxopyrrolidin-1-yl]-1H-indole-2-carboxylic Acid (3-Amino-propyl)-amide-Dy647 b... | J Med Chem 62: 11119-11134 (2019) Article DOI: 10.1021/acs.jmedchem.9b01070 BindingDB Entry DOI: 10.7270/Q26H4MXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM50531163 (CHEMBL4464946) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare Curated by ChEMBL | Assay Description Inhibition of 5-[(S)-3-(3-Chloro-5-fluoro-benzylcarbamoyl)-3-hydroxy-2-oxopyrrolidin-1-yl]-1H-indole-2-carboxylic Acid (3-Amino-propyl)-amide-Dy647 b... | J Med Chem 62: 11119-11134 (2019) Article DOI: 10.1021/acs.jmedchem.9b01070 BindingDB Entry DOI: 10.7270/Q26H4MXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50085451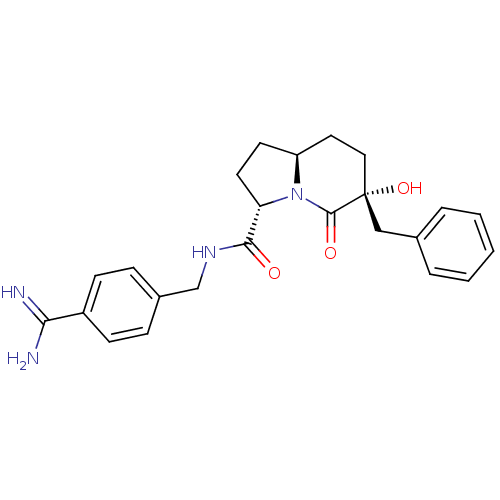 ((3S,6R,8aS)-6-Benzyl-6-hydroxy-5-oxo-octahydro-ind...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description The compound was evaluated for binding affinity against Prothrombin | Bioorg Med Chem Lett 10: 243-7 (2000) BindingDB Entry DOI: 10.7270/Q2K9381W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50085450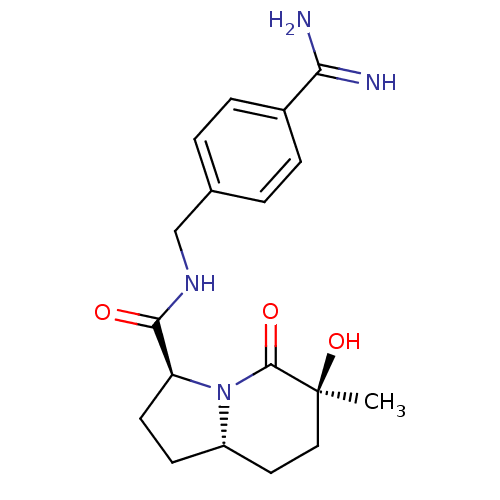 ((3S,6S,8aS)-6-Hydroxy-6-methyl-5-oxo-octahydro-ind...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 125 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description The compound was evaluated for binding affinity against Prothrombin | Bioorg Med Chem Lett 10: 243-7 (2000) BindingDB Entry DOI: 10.7270/Q2K9381W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50085449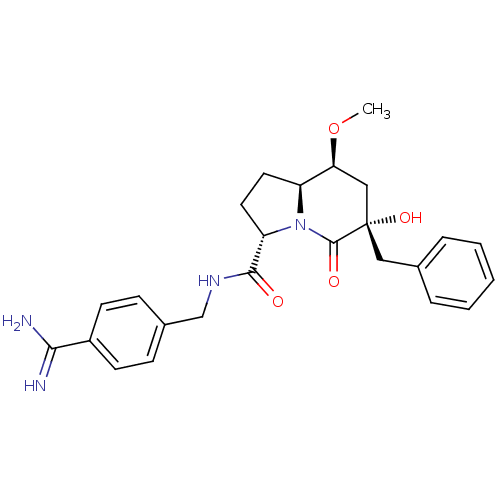 ((3S,6S,8S,8aS)-6-Benzyl-6-hydroxy-8-methoxy-5-oxo-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 125 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Concentration required for 50 percent inhibition of Prothrombin | Bioorg Med Chem Lett 10: 243-7 (2000) BindingDB Entry DOI: 10.7270/Q2K9381W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50505332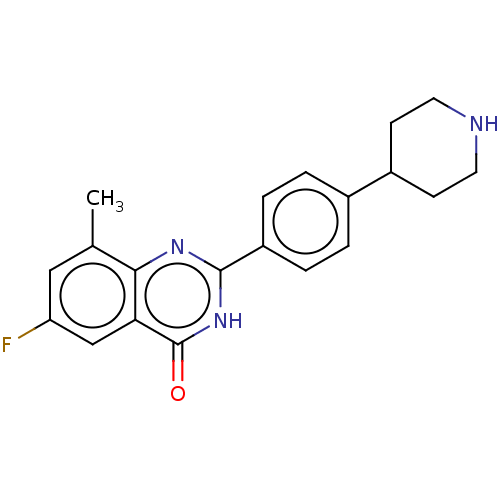 (CHEMBL4567515) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare KGaA Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in HEK293 cells at -80 mV holding potential by whole cell patch clamp method | J Med Chem 62: 7897-7909 (2019) Article DOI: 10.1021/acs.jmedchem.9b00656 BindingDB Entry DOI: 10.7270/Q2FJ2M28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50125842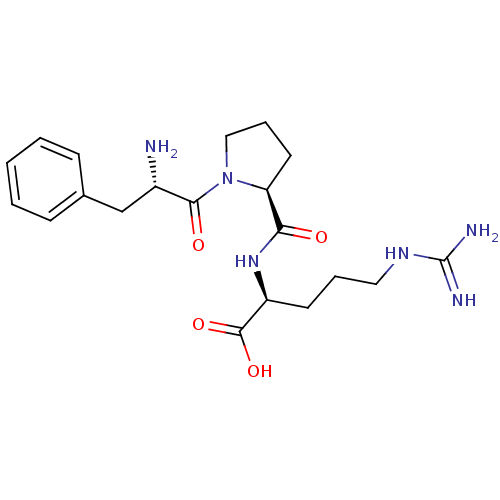 (5-amino(imino)methylamino-2-[1-[2-amino-3-phenyl-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.00170 | n/a | n/a | n/a | n/a | n/a | n/a |
Link£ping University Curated by ChEMBL | Assay Description In vitro inhibitory concentration of compound against human thrombin | J Med Chem 46: 1165-79 (2003) Article DOI: 10.1021/jm021065a BindingDB Entry DOI: 10.7270/Q2NG4RCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 8 (Homo sapiens (Human)) | BDBM50189412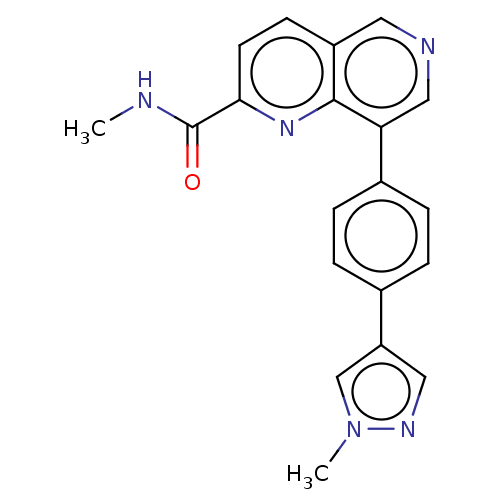 (CHEMBL3827983) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Binding affinity to CDK8 (unknown origin) expressed in human 7dF3 cells preincubated for 2 hrs followed by beta-oestradiol addition measured after 24... | ACS Med Chem Lett 7: 573-8 (2016) Article DOI: 10.1021/acsmedchemlett.6b00022 BindingDB Entry DOI: 10.7270/Q2N87CRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 8 (Homo sapiens (Human)) | BDBM50189417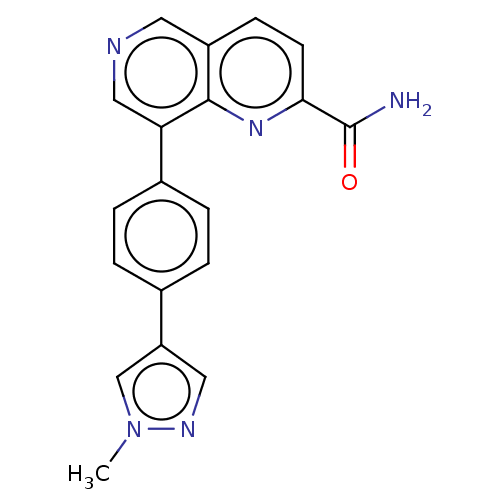 (CHEMBL3828116) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Binding affinity to CDK8 (unknown origin) expressed in human 7dF3 cells preincubated for 2 hrs followed by beta-oestradiol addition measured after 24... | ACS Med Chem Lett 7: 573-8 (2016) Article DOI: 10.1021/acsmedchemlett.6b00022 BindingDB Entry DOI: 10.7270/Q2N87CRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 (Homo sapiens (Human)) | BDBM50505331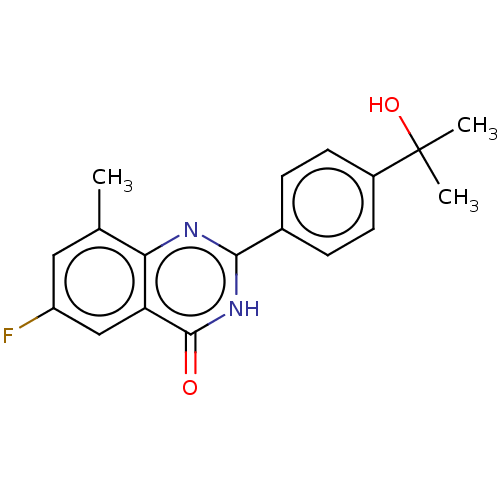 (CHEMBL4458129) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare KGaA Curated by ChEMBL | Assay Description Inhibition of GST tagged-TEV cleavage site-fused human recombinant TNKS2 (873 to 1166 residues) expressed in baculovirus infected sf9 cells assessed ... | J Med Chem 62: 7897-7909 (2019) Article DOI: 10.1021/acs.jmedchem.9b00656 BindingDB Entry DOI: 10.7270/Q2FJ2M28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 8 (Homo sapiens (Human)) | BDBM50189435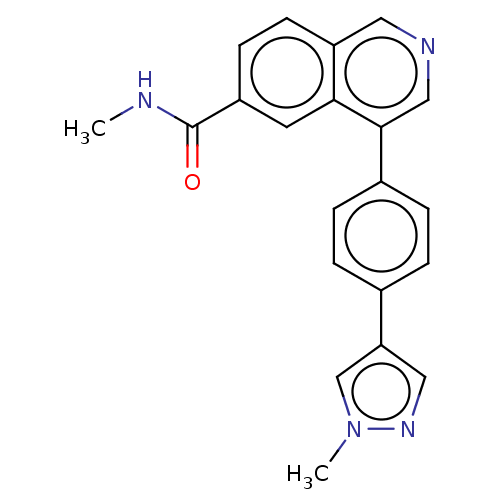 (CHEMBL3828003) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Binding affinity to CDK8 (unknown origin) expressed in human 7dF3 cells preincubated for 2 hrs followed by beta-oestradiol addition measured after 24... | ACS Med Chem Lett 7: 573-8 (2016) Article DOI: 10.1021/acsmedchemlett.6b00022 BindingDB Entry DOI: 10.7270/Q2N87CRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 8 (Homo sapiens (Human)) | BDBM50191472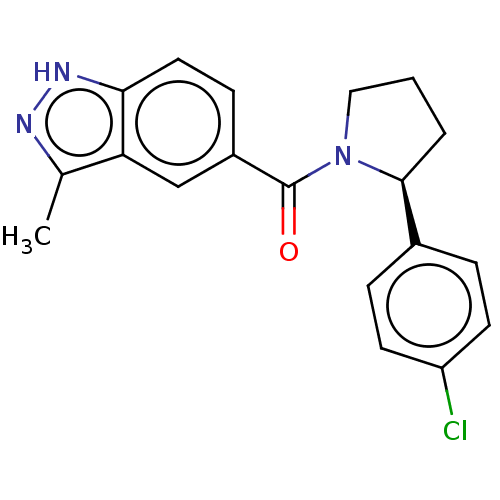 (CHEMBL3956719) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Inhibition of CDK8 in human 7dF3 cells preincubated for 2 hrs followed by beta-oestradiol addition measured after 24 hrs by luciferase reporter gene ... | J Med Chem 59: 9337-9349 (2016) BindingDB Entry DOI: 10.7270/Q2K64M1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-1 (Homo sapiens (Human)) | BDBM50593627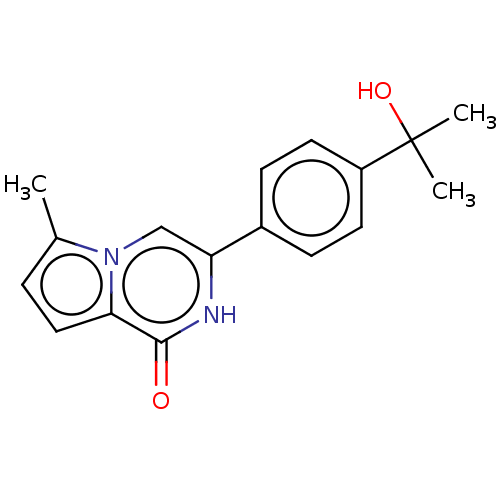 (CHEMBL5199558) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against dihydrofolate reductase (DHFR) from rat liver | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 (Homo sapiens (Human)) | BDBM50505332 (CHEMBL4567515) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare KGaA Curated by ChEMBL | Assay Description Inhibition of GST tagged-TEV cleavage site-fused human recombinant TNKS2 (873 to 1166 residues) expressed in baculovirus infected sf9 cells assessed ... | J Med Chem 62: 7897-7909 (2019) Article DOI: 10.1021/acs.jmedchem.9b00656 BindingDB Entry DOI: 10.7270/Q2FJ2M28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-C (Homo sapiens (Human)) | BDBM50189434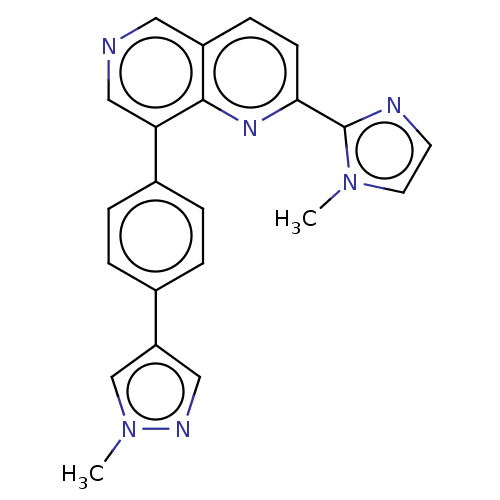 (CHEMBL3828221) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Competitive binding affinity to full length His-tagged human recombinant CDK8/cyclin C expressed in baculovirus after 20 mins in presence of Alexa647... | ACS Med Chem Lett 7: 573-8 (2016) Article DOI: 10.1021/acsmedchemlett.6b00022 BindingDB Entry DOI: 10.7270/Q2N87CRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-1 (Homo sapiens (Human)) | BDBM50505331 (CHEMBL4458129) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare KGaA Curated by ChEMBL | Assay Description Inhibition of GST tagged-TEV cleavage site-fused human recombinant TNKS1 (1023 to 1327 residues) expressed in baculovirus infected sf9 cells assessed... | J Med Chem 62: 7897-7909 (2019) Article DOI: 10.1021/acs.jmedchem.9b00656 BindingDB Entry DOI: 10.7270/Q2FJ2M28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-1 (Homo sapiens (Human)) | CHEMBL5272750 | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against dihydrofolate reductase (DHFR) of Neisseria gonorrhoea | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-1 (Homo sapiens (Human)) | CHEMBL5274873 | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against dihydrofolate reductase (DHFR) of Plasmodium berghei | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 (Homo sapiens (Human)) | BDBM50593627 (CHEMBL5199558) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against dihydrofolate reductase (DHFR) from Escherichia coli | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 (Homo sapiens (Human)) | CHEMBL5274873 | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against dihydrofolate reductase (DHFR) of Neisseria gonorrhoea | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-C (Homo sapiens (Human)) | BDBM50189415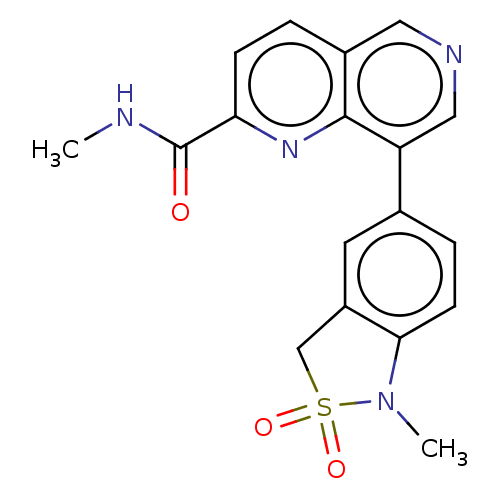 (CHEMBL3828637) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Competitive binding affinity to full length His-tagged human recombinant CDK8/cyclin C expressed in baculovirus after 20 mins in presence of Alexa647... | ACS Med Chem Lett 7: 573-8 (2016) Article DOI: 10.1021/acsmedchemlett.6b00022 BindingDB Entry DOI: 10.7270/Q2N87CRB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 (Homo sapiens (Human)) | CHEMBL5271127 | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against dihydrofolate reductase (DHFR) of Neisseria gonorrhoea | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-C (Homo sapiens (Human)) | BDBM50189428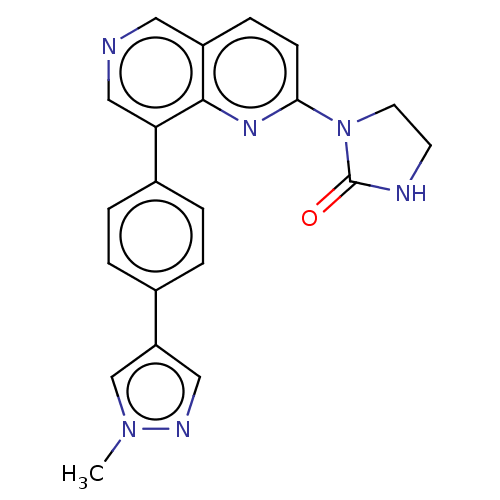 (CHEMBL3828458) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Competitive binding affinity to full length His-tagged human recombinant CDK8/cyclin C expressed in baculovirus after 20 mins in presence of Alexa647... | ACS Med Chem Lett 7: 573-8 (2016) Article DOI: 10.1021/acsmedchemlett.6b00022 BindingDB Entry DOI: 10.7270/Q2N87CRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 (Homo sapiens (Human)) | CHEMBL5272750 | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against dihydrofolate reductase (DHFR) from rat liver | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-1 (Homo sapiens (Human)) | CHEMBL5271127 | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against dihydrofolate reductase (DHFR) from rat liver | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 8 (Homo sapiens (Human)) | BDBM50189436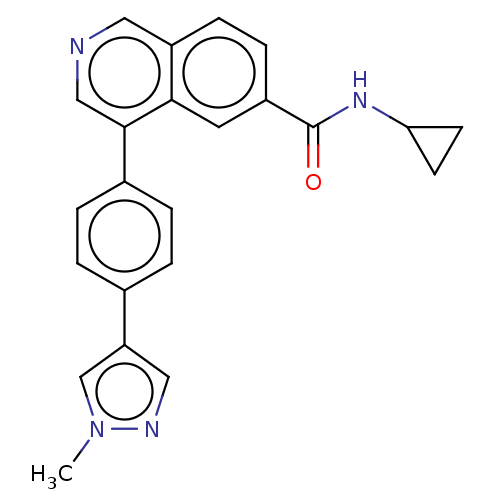 (CHEMBL3827327) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Binding affinity to CDK8 (unknown origin) expressed in human 7dF3 cells preincubated for 2 hrs followed by beta-oestradiol addition measured after 24... | ACS Med Chem Lett 7: 573-8 (2016) Article DOI: 10.1021/acsmedchemlett.6b00022 BindingDB Entry DOI: 10.7270/Q2N87CRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-C (Homo sapiens (Human)) | BDBM50189447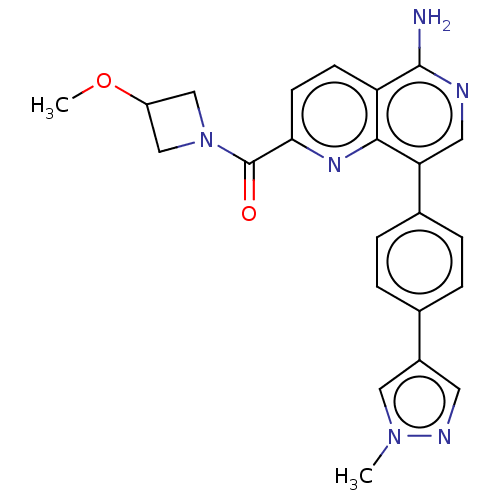 (CHEMBL3828553) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Competitive binding affinity to full length His-tagged human recombinant CDK8/cyclin C expressed in baculovirus after 20 mins in presence of Alexa647... | ACS Med Chem Lett 7: 573-8 (2016) Article DOI: 10.1021/acsmedchemlett.6b00022 BindingDB Entry DOI: 10.7270/Q2N87CRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-1 (Homo sapiens (Human)) | BDBM50505324 (CHEMBL4588170) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare KGaA Curated by ChEMBL | Assay Description Inhibition of GST tagged-TEV cleavage site-fused human recombinant TNKS1 (1023 to 1327 residues) expressed in baculovirus infected sf9 cells assessed... | J Med Chem 62: 7897-7909 (2019) Article DOI: 10.1021/acs.jmedchem.9b00656 BindingDB Entry DOI: 10.7270/Q2FJ2M28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-1 (Homo sapiens (Human)) | CHEMBL5284314 | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against dihydrofolate reductase (DHFR) of Plasmodium berghei | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-1 (Homo sapiens (Human)) | BDBM50593627 (CHEMBL5199558) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against dihydrofolate reductase (DHFR) from Escherichia coli | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-C (Homo sapiens (Human)) | BDBM50191414 (CHEMBL3947140) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Inhibition of Alexa647 tracer binding to full length recombinant human His-tagged CDK8/cyclin C expressed in Baculovirus expression system preincubat... | J Med Chem 59: 9337-9349 (2016) BindingDB Entry DOI: 10.7270/Q2K64M1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-1 (Homo sapiens (Human)) | BDBM50505332 (CHEMBL4567515) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare KGaA Curated by ChEMBL | Assay Description Inhibition of GST tagged-TEV cleavage site-fused human recombinant TNKS1 (1023 to 1327 residues) expressed in baculovirus infected sf9 cells assessed... | J Med Chem 62: 7897-7909 (2019) Article DOI: 10.1021/acs.jmedchem.9b00656 BindingDB Entry DOI: 10.7270/Q2FJ2M28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50069989 ((R)-3-methyl-1-((S)-3-phenyl-2-(pyrazine-2-carboxa...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00604 BindingDB Entry DOI: 10.7270/Q200064W | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-C (Homo sapiens (Human)) | BDBM50191472 (CHEMBL3956719) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Inhibition of Alexa647 tracer binding to full length recombinant human His-tagged CDK8/cyclin C expressed in Baculovirus expression system preincubat... | J Med Chem 59: 9337-9349 (2016) BindingDB Entry DOI: 10.7270/Q2K64M1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 (Homo sapiens (Human)) | BDBM50593627 (CHEMBL5199558) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against dihydrofolate reductase (DHFR) of Plasmodium berghei | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 (Homo sapiens (Human)) | BDBM50505324 (CHEMBL4588170) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare KGaA Curated by ChEMBL | Assay Description Inhibition of GST tagged-TEV cleavage site-fused human recombinant TNKS2 (873 to 1166 residues) expressed in baculovirus infected sf9 cells assessed ... | J Med Chem 62: 7897-7909 (2019) Article DOI: 10.1021/acs.jmedchem.9b00656 BindingDB Entry DOI: 10.7270/Q2FJ2M28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-1 (Homo sapiens (Human)) | BDBM50505335 (CHEMBL4584689) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare KGaA Curated by ChEMBL | Assay Description Inhibition of GST tagged-TEV cleavage site-fused human recombinant TNKS1 (1023 to 1327 residues) expressed in baculovirus infected sf9 cells assessed... | J Med Chem 62: 7897-7909 (2019) Article DOI: 10.1021/acs.jmedchem.9b00656 BindingDB Entry DOI: 10.7270/Q2FJ2M28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 (Homo sapiens (Human)) | BDBM50505334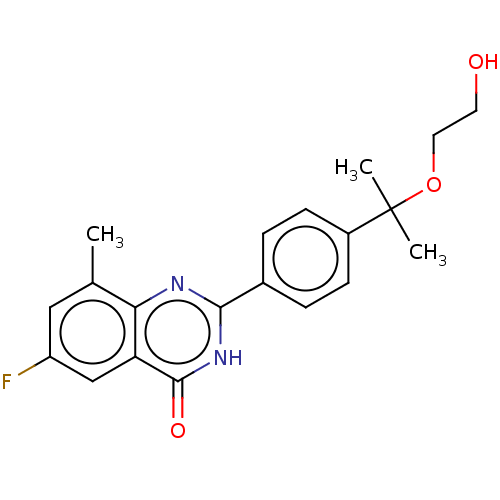 (CHEMBL4551200) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare KGaA Curated by ChEMBL | Assay Description Inhibition of GST tagged-TEV cleavage site-fused human recombinant TNKS2 (873 to 1166 residues) expressed in baculovirus infected sf9 cells assessed ... | J Med Chem 62: 7897-7909 (2019) Article DOI: 10.1021/acs.jmedchem.9b00656 BindingDB Entry DOI: 10.7270/Q2FJ2M28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-C (Homo sapiens (Human)) | BDBM50189417 (CHEMBL3828116) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Competitive binding affinity to full length His-tagged human recombinant CDK8/cyclin C expressed in baculovirus after 20 mins in presence of Alexa647... | ACS Med Chem Lett 7: 573-8 (2016) Article DOI: 10.1021/acsmedchemlett.6b00022 BindingDB Entry DOI: 10.7270/Q2N87CRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Focal adhesion kinase 1 (Homo sapiens (Human)) | BDBM50318884 (CHEMBL1084546 | CHEMBL2430359 | N-methyl-N-(3-((2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Competitive binding affinity to FAK kinase domain (410 to 689) (unknown origin) assessed as phosphorylation of p(Glu/Tyr) in presence of ATP | Bioorg Med Chem Lett 23: 5401-9 (2013) Article DOI: 10.1016/j.bmcl.2013.07.050 BindingDB Entry DOI: 10.7270/Q2891782 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 (Homo sapiens (Human)) | CHEMBL5281076 | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against dihydrofolate reductase (DHFR) from rat liver | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 (Homo sapiens (Human)) | CHEMBL5279709 | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL CHEMBL MCE PC cid PC sid UniChem Similars | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against dihydrofolate reductase (DHFR) from Escherichia coli | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 (Homo sapiens (Human)) | CHEMBL5280682 | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against dihydrofolate reductase (DHFR) of Neisseria gonorrhoea | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1504 total ) | Next | Last >> |