 Found 29114 hits with Last Name = 'na' and Initial = 'y'
Found 29114 hits with Last Name = 'na' and Initial = 'y' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50300196

(10-(4-hydroxyphenyl)-8,13,15-triazatetracyclo[9.6....)Show SMILES Oc1ccc(cc1)C1c2c[nH]c3nccc(-c4ccccc4NC1=O)c23 Show InChI InChI=1S/C21H15N3O2/c25-13-7-5-12(6-8-13)18-16-11-23-20-19(16)15(9-10-22-20)14-3-1-2-4-17(14)24-21(18)26/h1-11,18,25H,(H,22,23)(H,24,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant JAK2 |
J Med Chem 52: 7938-41 (2009)
Checked by Author
Article DOI: 10.1021/jm901383u
BindingDB Entry DOI: 10.7270/Q2GF0TK0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50480930
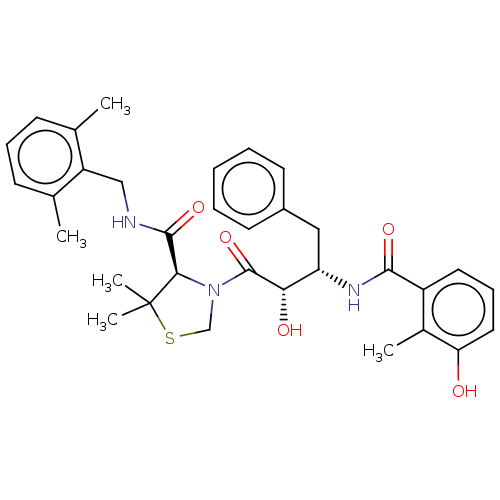
(CHEMBL584130 | KNI-814)Show SMILES Cc1cccc(C)c1CNC(=O)[C@H]1N(CSC1(C)C)C(=O)[C@@H](O)[C@H](Cc1ccccc1)NC(=O)c1cccc(O)c1C |r| Show InChI InChI=1S/C33H39N3O5S/c1-20-11-9-12-21(2)25(20)18-34-31(40)29-33(4,5)42-19-36(29)32(41)28(38)26(17-23-13-7-6-8-14-23)35-30(39)24-15-10-16-27(37)22(24)3/h6-16,26,28-29,37-38H,17-19H2,1-5H3,(H,34,40)(H,35,39)/t26-,28-,29+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 recombinant protease after 15 mins by fluorescence assay |
J Med Chem 52: 7604-17 (2009)
Article DOI: 10.1021/jm9005115
BindingDB Entry DOI: 10.7270/Q2FR00F2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50300196

(10-(4-hydroxyphenyl)-8,13,15-triazatetracyclo[9.6....)Show SMILES Oc1ccc(cc1)C1c2c[nH]c3nccc(-c4ccccc4NC1=O)c23 Show InChI InChI=1S/C21H15N3O2/c25-13-7-5-12(6-8-13)18-16-11-23-20-19(16)15(9-10-22-20)14-3-1-2-4-17(14)24-21(18)26/h1-11,18,25H,(H,22,23)(H,24,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant JAK3 |
J Med Chem 52: 7938-41 (2009)
Checked by Author
Article DOI: 10.1021/jm901383u
BindingDB Entry DOI: 10.7270/Q2GF0TK0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50300196

(10-(4-hydroxyphenyl)-8,13,15-triazatetracyclo[9.6....)Show SMILES Oc1ccc(cc1)C1c2c[nH]c3nccc(-c4ccccc4NC1=O)c23 Show InChI InChI=1S/C21H15N3O2/c25-13-7-5-12(6-8-13)18-16-11-23-20-19(16)15(9-10-22-20)14-3-1-2-4-17(14)24-21(18)26/h1-11,18,25H,(H,22,23)(H,24,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant JAK3 |
J Med Chem 52: 7938-41 (2009)
Checked by Author
Article DOI: 10.1021/jm901383u
BindingDB Entry DOI: 10.7270/Q2GF0TK0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM602107

(US11643417, Ex. No. 120)Show SMILES OCC1(O)CCC(CC1)C(F)(F)CN1CCNC(=O)c2oc3ccc(cc3c12)C(F)(F)F |(7.13,.13,;5.59,.13,;4.82,1.46,;6.31,1.86,;3.67,.45,;2.21,.95,;1.91,2.46,;3.07,3.47,;4.53,2.98,;.45,2.96,;-.56,4.12,;.95,4.42,;-.71,1.94,;-2.16,2.44,;-2.35,3.97,;-3.66,4.78,;-5.11,4.26,;-5.61,2.8,;-7.13,2.62,;-4.78,1.51,;-5.4,.1,;-4.26,-.93,;-4.26,-2.47,;-2.92,-3.24,;-1.59,-2.47,;-1.59,-.93,;-2.92,-.16,;-3.25,1.35,;-.26,-3.24,;1.08,-2.47,;-.26,-4.78,;1.08,-4.01,)| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2R49VQ0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM602075
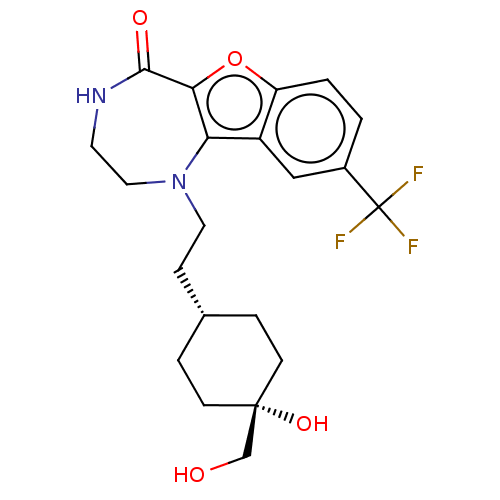
(US11643417, Ex. No. 90)Show SMILES OC[C@@]1(O)CC[C@H](CCN2CCNC(=O)c3oc4ccc(cc4c23)C(F)(F)F)CC1 |r,wU:6.6,2.2,wD:2.1,(7.13,.13,;5.59,.13,;4.82,1.46,;6.16,2.23,;4.53,2.98,;3.07,3.47,;1.91,2.46,;.45,2.96,;-.71,1.94,;-2.16,2.44,;-2.35,3.97,;-3.66,4.78,;-5.11,4.26,;-5.61,2.8,;-7.13,2.62,;-4.78,1.51,;-5.4,.1,;-4.26,-.93,;-4.26,-2.47,;-2.92,-3.24,;-1.59,-2.47,;-1.59,-.93,;-2.92,-.16,;-3.25,1.35,;-.26,-3.24,;1.08,-2.47,;-.26,-4.78,;1.08,-4.01,;2.21,.95,;3.67,.45,)| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2R49VQ0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM602103

(US11643417, Ex. No. 116)Show SMILES OCC1(O)CCC(CCN2CC3(CC3)NC(=O)c3oc4ccc(cc4c23)C(F)(F)F)CC1 |(7.29,1.28,;5.83,1.77,;4.67,.76,;6.13,.26,;3.51,-.25,;2.05,.25,;1.76,1.76,;.3,2.25,;-.86,1.24,;-2.32,1.74,;-2.5,3.27,;-3.81,4.08,;-3.19,5.48,;-4.72,5.32,;-5.26,3.56,;-5.76,2.1,;-7.29,1.92,;-4.93,.8,;-5.56,-.6,;-4.41,-1.63,;-4.41,-3.17,;-3.08,-3.94,;-1.74,-3.17,;-1.74,-1.63,;-3.08,-.86,;-3.4,.64,;-.41,-3.94,;.92,-3.17,;-.41,-5.48,;-.41,-2.4,;2.92,2.77,;4.37,2.27,)| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2R49VQ0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM84928
(CAS_125368 | FK 1052 | NSC_125368)Show InChI InChI=1S/C18H19N3O/c1-11-14-5-3-4-6-17(14)21-16(11)8-7-13(18(21)22)9-15-12(2)19-10-20-15/h3-6,10,13H,7-9H2,1-2H3,(H,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 265: 752-8 (1993)
BindingDB Entry DOI: 10.7270/Q2CV4G73 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM84928

(CAS_125368 | FK 1052 | NSC_125368)Show InChI InChI=1S/C18H19N3O/c1-11-14-5-3-4-6-17(14)21-16(11)8-7-13(18(21)22)9-15-12(2)19-10-20-15/h3-6,10,13H,7-9H2,1-2H3,(H,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 265: 752-8 (1993)
BindingDB Entry DOI: 10.7270/Q2CV4G73 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM602160
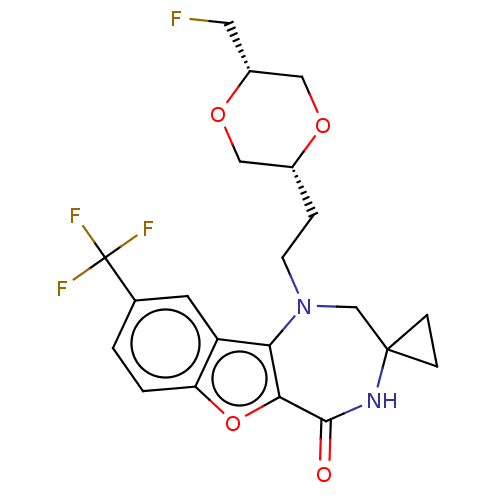
(US11643417, Ex. No. 156)Show SMILES FC[C@@H]1CO[C@H](CCN2CC3(CC3)NC(=O)c3oc4ccc(cc4c23)C(F)(F)F)CO1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2R49VQ0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM602097
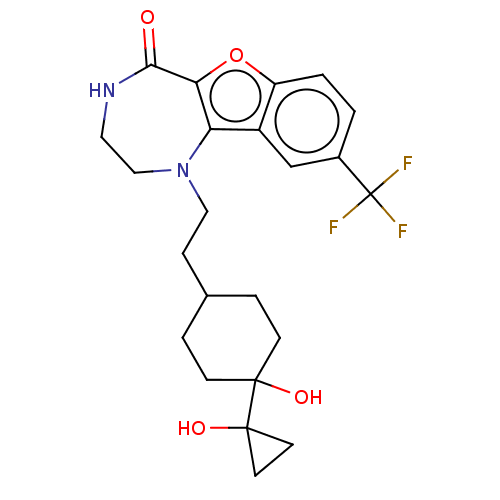
(US11643417, Ex. No. 111)Show SMILES OC1(CC1)C1(O)CCC(CCN2CCNC(=O)c3oc4ccc(cc4c23)C(F)(F)F)CC1 |(7.29,1.98,;6.13,.97,;5.63,-.49,;7.14,-.19,;4.67,1.46,;5.44,2.8,;3.51,.45,;2.05,.95,;1.76,2.46,;.3,2.96,;-.86,1.94,;-2.32,2.44,;-2.5,3.97,;-3.81,4.78,;-5.26,4.26,;-5.76,2.8,;-7.29,2.62,;-4.93,1.51,;-5.56,.1,;-4.41,-.93,;-4.41,-2.47,;-3.08,-3.24,;-1.74,-2.47,;-1.74,-.93,;-3.08,-.16,;-3.4,1.35,;-.41,-3.24,;.92,-2.47,;-.41,-4.78,;.92,-4.01,;2.92,3.47,;4.37,2.98,)| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2R49VQ0 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Mus musculus (Mouse)) | BDBM50366138
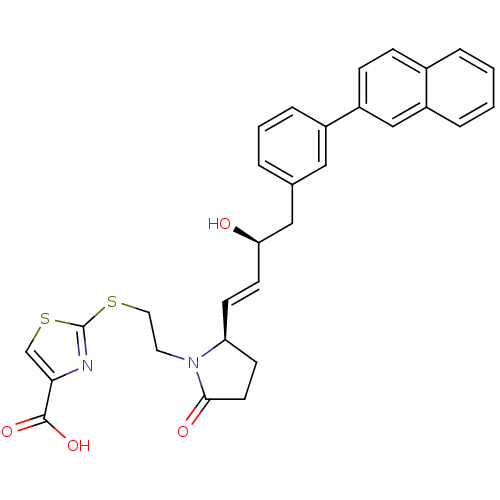
(CHEMBL1957437)Show SMILES O[C@@H](Cc1cccc(c1)-c1ccc2ccccc2c1)\C=C\[C@H]1CCC(=O)N1CCSc1nc(cs1)C(O)=O |r| Show InChI InChI=1S/C30H28N2O4S2/c33-26(17-20-4-3-7-22(16-20)24-9-8-21-5-1-2-6-23(21)18-24)12-10-25-11-13-28(34)32(25)14-15-37-30-31-27(19-38-30)29(35)36/h1-10,12,16,18-19,25-26,33H,11,13-15,17H2,(H,35,36)/b12-10+/t25-,26+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PGE2 from mouse EP4 receptor expressed in CHO cells after 60 mins by scintillation counting |
Bioorg Med Chem 20: 2235-51 (2012)
Article DOI: 10.1016/j.bmc.2012.02.018
BindingDB Entry DOI: 10.7270/Q2542P2G |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Mus musculus (Mouse)) | BDBM50366138

(CHEMBL1957437)Show SMILES O[C@@H](Cc1cccc(c1)-c1ccc2ccccc2c1)\C=C\[C@H]1CCC(=O)N1CCSc1nc(cs1)C(O)=O |r| Show InChI InChI=1S/C30H28N2O4S2/c33-26(17-20-4-3-7-22(16-20)24-9-8-21-5-1-2-6-23(21)18-24)12-10-25-11-13-28(34)32(25)14-15-37-30-31-27(19-38-30)29(35)36/h1-10,12,16,18-19,25-26,33H,11,13-15,17H2,(H,35,36)/b12-10+/t25-,26+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from mouse EP4 receptor expressed in CHO cells after 60 mins by scintillation counting |
Bioorg Med Chem 20: 3502-22 (2012)
Article DOI: 10.1016/j.bmc.2012.04.008
BindingDB Entry DOI: 10.7270/Q2D50P0B |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM602126
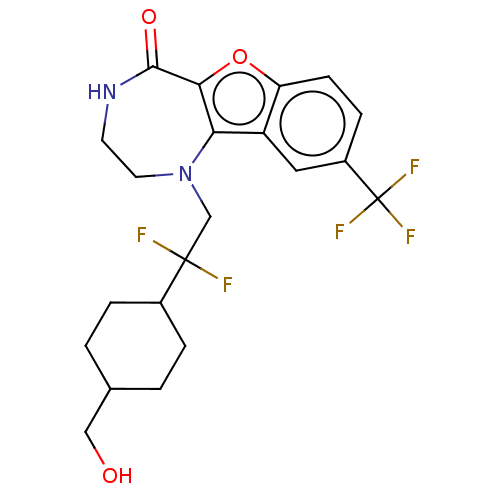
(US11643417, Ex. No. 138)Show SMILES OCC1CCC(CC1)C(F)(F)CN1CCNC(=O)c2oc3ccc(cc3c12)C(F)(F)F |(7.29,1.98,;6.13,.97,;4.67,1.46,;3.51,.45,;2.05,.95,;1.76,2.46,;2.92,3.47,;4.37,2.98,;.3,2.96,;0,4.47,;1.46,3.97,;-.86,1.94,;-2.32,2.44,;-2.5,3.97,;-3.81,4.78,;-5.26,4.26,;-5.76,2.8,;-7.29,2.62,;-4.93,1.51,;-5.56,.1,;-4.41,-.93,;-4.41,-2.47,;-3.08,-3.24,;-1.74,-2.47,;-1.74,-.93,;-3.08,-.16,;-3.4,1.35,;-.41,-3.24,;.92,-2.47,;-.41,-4.78,;.92,-4.01,)| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2R49VQ0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM601978
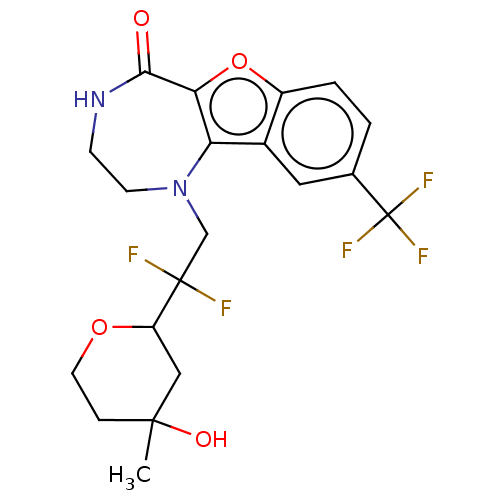
(US11643417, Ex. No. 11 | US11643417, Ex. No. 12 | ...)Show SMILES CC1(O)CCOC(C1)C(F)(F)CN1CCNC(=O)c2oc3ccc(cc3c12)C(F)(F)F | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2R49VQ0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM602169

(US11643417, Ex. No. 164)Show SMILES OC[C@@H]1CO[C@H](CO1)C(F)(F)CN1CC2(CC2)NC(=O)c2oc3ccc(cc3c12)C(F)(F)F |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2R49VQ0 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50083552

(1,9-di{4-[5-amino(imino)methylbenzo[b]furan-2-ylca...)Show SMILES NC(=N)c1ccc2oc(cc2c1)C(=O)N1CCN(CC1)C(=O)CCCCCCCC(=O)N1CCN(CC1)C(=O)c1cc2cc(ccc2o1)C(N)=N Show InChI InChI=1S/C37H44N8O6/c38-34(39)24-8-10-28-26(20-24)22-30(50-28)36(48)44-16-12-42(13-17-44)32(46)6-4-2-1-3-5-7-33(47)43-14-18-45(19-15-43)37(49)31-23-27-21-25(35(40)41)9-11-29(27)51-31/h8-11,20-23H,1-7,12-19H2,(H3,38,39)(H3,40,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, LTD.
Curated by ChEMBL
| Assay Description
Inhibition of tryptase activity |
Bioorg Med Chem Lett 9: 3285-90 (2000)
BindingDB Entry DOI: 10.7270/Q2TX3DK3 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50325534
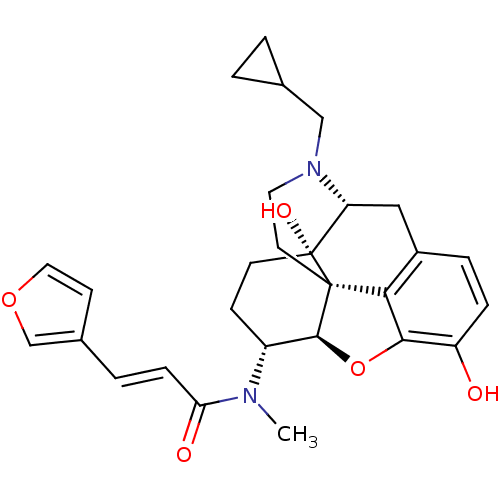
(CHEMBL267495 | nalfurafine)Show SMILES CN([C@@H]1CC[C@@]2(O)[C@H]3Cc4ccc(O)c5O[C@@H]1[C@]2(CCN3CC1CC1)c45)C(=O)\C=C\c1ccoc1 Show InChI InChI=1S/C28H32N2O5/c1-29(23(32)7-4-18-9-13-34-16-18)20-8-10-28(33)22-14-19-5-6-21(31)25-24(19)27(28,26(20)35-25)11-12-30(22)15-17-2-3-17/h4-7,9,13,16-17,20,22,26,31,33H,2-3,8,10-12,14-15H2,1H3/b7-4+/t20-,22-,26+,27+,28-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128527
BindingDB Entry DOI: 10.7270/Q2HT2TDM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM601981
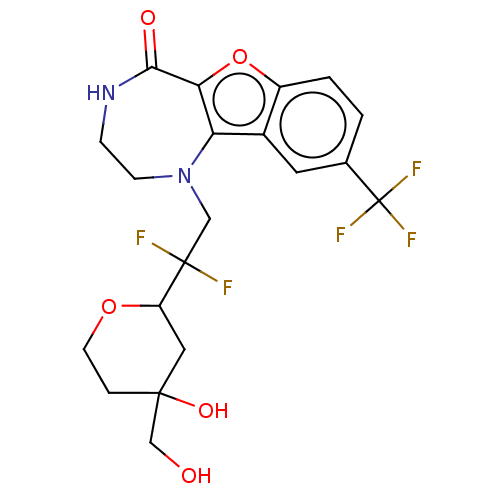
(US11643417, Ex. No. 13)Show SMILES OCC1(O)CCOC(C1)C(F)(F)CN1CCNC(=O)c2oc3ccc(cc3c12)C(F)(F)F | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2R49VQ0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM602002
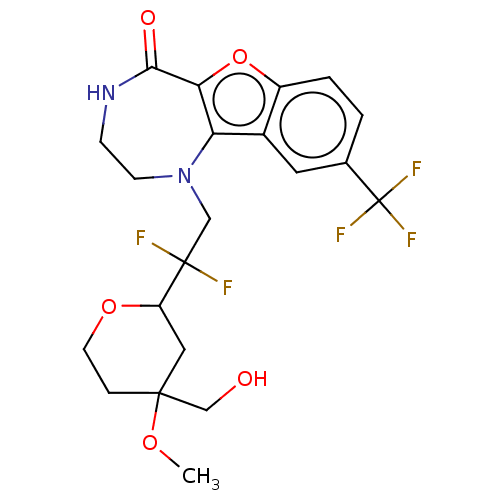
(US11643417, Ex. No. 34)Show SMILES COC1(CO)CCOC(C1)C(F)(F)CN1CCNC(=O)c2oc3ccc(cc3c12)C(F)(F)F | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2R49VQ0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM602134
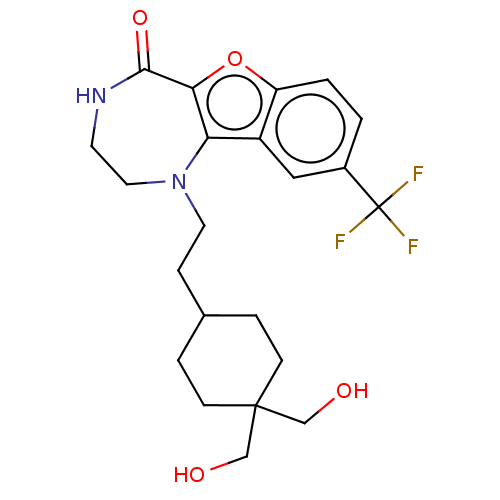
(US11643417, Ex. No. 146)Show SMILES OCC1(CO)CCC(CCN2CCNC(=O)c3oc4ccc(cc4c23)C(F)(F)F)CC1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2R49VQ0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM601982
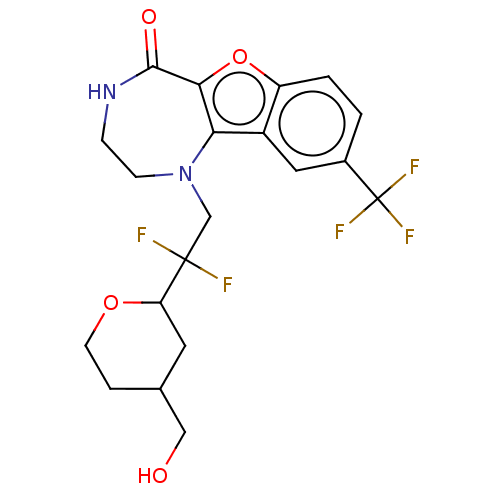
(US11643417, Ex. No. 14 | US11643417, Ex. No. 15 | ...)Show SMILES OCC1CCOC(C1)C(F)(F)CN1CCNC(=O)c2oc3ccc(cc3c12)C(F)(F)F | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2R49VQ0 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50596292

(CHEMBL5185211)Show SMILES [H][C@]12Oc3c4c(C[C@]5([H])C(CC[C@H]1N(C)C(=O)\C=C\c1ccoc1)[C@]24CCN5CC1CC1)ccc3O |r,THB:28:27:9:4.5.6| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0272 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128527
BindingDB Entry DOI: 10.7270/Q2HT2TDM |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM601978

(US11643417, Ex. No. 11 | US11643417, Ex. No. 12 | ...)Show SMILES CC1(O)CCOC(C1)C(F)(F)CN1CCNC(=O)c2oc3ccc(cc3c12)C(F)(F)F | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.0280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2R49VQ0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM602081
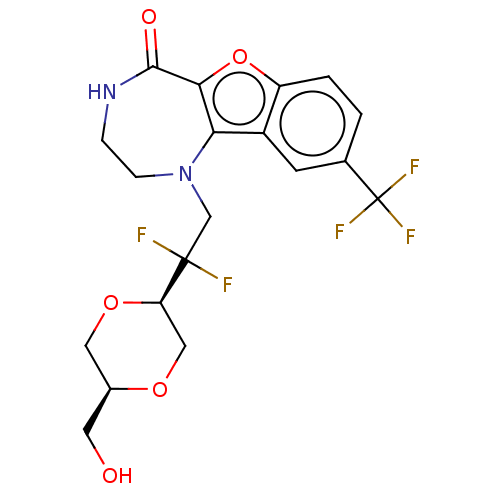
(US11643417, Ex. No. 96)Show SMILES OC[C@H]1CO[C@H](CO1)C(F)(F)CN1CCNC(=O)c2oc3ccc(cc3c12)C(F)(F)F |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.0280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2R49VQ0 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50083561

(1-{4-[5-amino(imino)methylbenzo[b]thiophen-2-ylcar...)Show SMILES NC(=N)c1ccc2sc(cc2c1)C(=O)N1CCN(CC1)C(=O)COc1ccc(OCC(=O)N2CCN(CC2)C(=O)c2cc3cc(ccc3s2)C(N)=N)cc1 Show InChI InChI=1S/C38H38N8O6S2/c39-35(40)23-1-7-29-25(17-23)19-31(53-29)37(49)45-13-9-43(10-14-45)33(47)21-51-27-3-5-28(6-4-27)52-22-34(48)44-11-15-46(16-12-44)38(50)32-20-26-18-24(36(41)42)2-8-30(26)54-32/h1-8,17-20H,9-16,21-22H2,(H3,39,40)(H3,41,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.0280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, LTD.
Curated by ChEMBL
| Assay Description
Inhibition of tryptase activity |
Bioorg Med Chem Lett 9: 3285-90 (2000)
BindingDB Entry DOI: 10.7270/Q2TX3DK3 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM601982

(US11643417, Ex. No. 14 | US11643417, Ex. No. 15 | ...)Show SMILES OCC1CCOC(C1)C(F)(F)CN1CCNC(=O)c2oc3ccc(cc3c12)C(F)(F)F | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.0290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2R49VQ0 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50083556
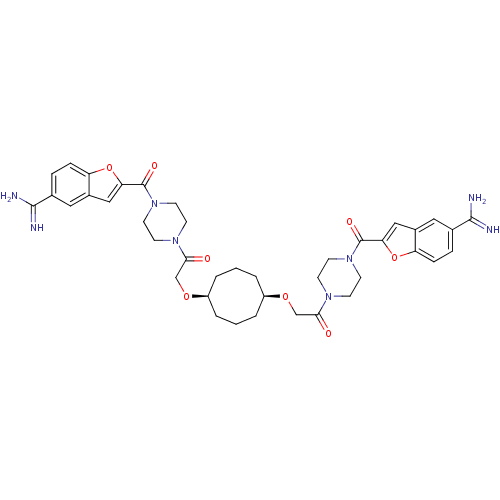
(1-{4-[5-amino(imino)methylbenzo[b]furan-2-ylcarbon...)Show SMILES NC(=N)c1ccc2oc(cc2c1)C(=O)N1CCN(CC1)C(=O)CO[C@H]1CCC[C@H](CCC1)OCC(=O)N1CCN(CC1)C(=O)c1cc2cc(ccc2o1)C(N)=N Show InChI InChI=1S/C40H48N8O8/c41-37(42)25-7-9-31-27(19-25)21-33(55-31)39(51)47-15-11-45(12-16-47)35(49)23-53-29-3-1-4-30(6-2-5-29)54-24-36(50)46-13-17-48(18-14-46)40(52)34-22-28-20-26(38(43)44)8-10-32(28)56-34/h7-10,19-22,29-30H,1-6,11-18,23-24H2,(H3,41,42)(H3,43,44)/t29-,30+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.0290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, LTD.
Curated by ChEMBL
| Assay Description
Inhibition of tryptase activity |
Bioorg Med Chem Lett 9: 3285-90 (2000)
BindingDB Entry DOI: 10.7270/Q2TX3DK3 |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM580

((4R)-3-[(2S,3S)-2-hydroxy-3-[(3-hydroxy-2-methylph...)Show SMILES Cc1ccccc1CNC(=O)[C@H]1N(CSC1(C)C)C(=O)[C@@H](O)[C@H](Cc1ccccc1)NC(=O)c1cccc(O)c1C |r| Show InChI InChI=1S/C32H37N3O5S/c1-20-11-8-9-14-23(20)18-33-30(39)28-32(3,4)41-19-35(28)31(40)27(37)25(17-22-12-6-5-7-13-22)34-29(38)24-15-10-16-26(36)21(24)2/h5-16,25,27-28,36-37H,17-19H2,1-4H3,(H,33,39)(H,34,38)/t25-,27-,28+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 recombinant protease after 15 mins by fluorescence assay |
J Med Chem 52: 7604-17 (2009)
Article DOI: 10.1021/jm9005115
BindingDB Entry DOI: 10.7270/Q2FR00F2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM602114
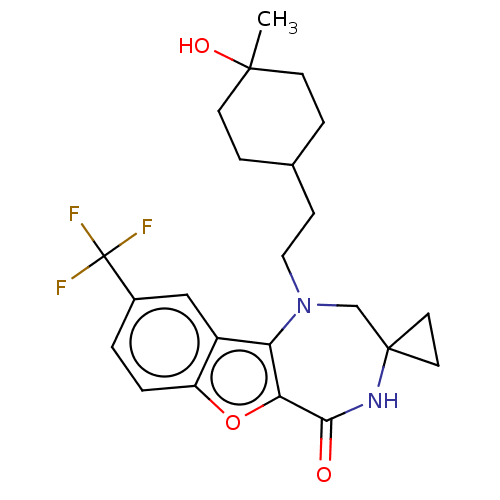
(US11643417, Ex. No. 127 | US11643417, Ex. No. 128)Show SMILES CC1(O)CCC(CCN2CC3(CC3)NC(=O)c3oc4ccc(cc4c23)C(F)(F)F)CC1 |(6.41,1.77,;5.25,.76,;6.71,.26,;4.09,-.25,;2.63,.25,;2.34,1.76,;.88,2.25,;-.28,1.24,;-1.74,1.74,;-1.92,3.27,;-3.23,4.08,;-2.61,5.48,;-4.14,5.32,;-4.68,3.56,;-5.18,2.1,;-6.71,1.92,;-4.35,.8,;-4.98,-.6,;-3.83,-1.63,;-3.83,-3.17,;-2.5,-3.94,;-1.16,-3.17,;-1.16,-1.63,;-2.5,-.86,;-2.82,.64,;.17,-3.94,;1.5,-3.17,;.17,-5.48,;.17,-2.4,;3.5,2.77,;4.95,2.27,)| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2R49VQ0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM602074
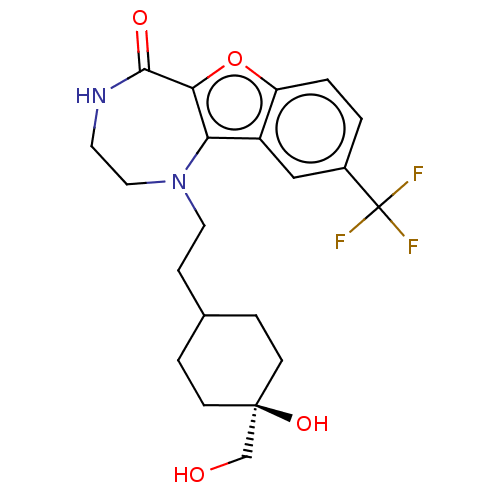
(US11643417, Ex. No. 89)Show SMILES OC[C@]1(O)CCC(CCN2CCNC(=O)c3oc4ccc(cc4c23)C(F)(F)F)CC1 |r,wU:2.1,wD:2.2,(7.13,.13,;5.59,.13,;4.82,1.46,;6.16,2.23,;4.53,2.98,;3.07,3.47,;1.91,2.46,;.45,2.96,;-.71,1.94,;-2.16,2.44,;-2.35,3.97,;-3.66,4.78,;-5.11,4.26,;-5.61,2.8,;-7.13,2.62,;-4.78,1.51,;-5.4,.1,;-4.26,-.93,;-4.26,-2.47,;-2.92,-3.24,;-1.59,-2.47,;-1.59,-.93,;-2.92,-.16,;-3.25,1.35,;-.26,-3.24,;1.08,-2.47,;-.26,-4.78,;1.08,-4.01,;2.21,.95,;3.67,.45,)| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.0330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2R49VQ0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM601982

(US11643417, Ex. No. 14 | US11643417, Ex. No. 15 | ...)Show SMILES OCC1CCOC(C1)C(F)(F)CN1CCNC(=O)c2oc3ccc(cc3c12)C(F)(F)F | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.0330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2R49VQ0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM601977
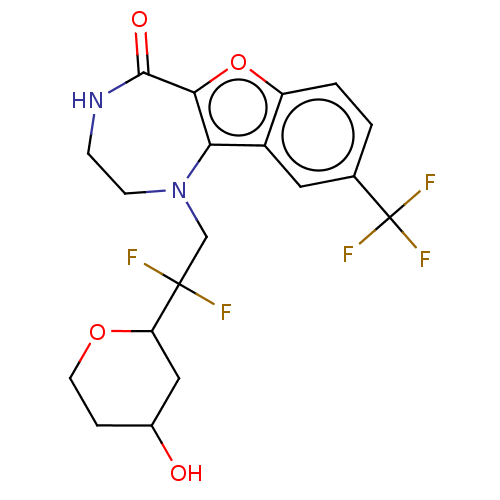
(US11643417, Ex. No. 10 | US11643417, Ex. No. 51)Show SMILES OC1CCOC(C1)C(F)(F)CN1CCNC(=O)c2oc3ccc(cc3c12)C(F)(F)F | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.0330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2R49VQ0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM602139
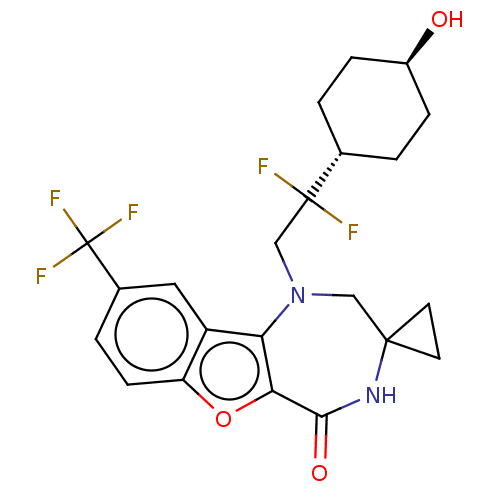
(US11643417, Ex. No. 149)Show SMILES O[C@H]1CC[C@@H](CC1)C(F)(F)CN1CC2(CC2)NC(=O)c2oc3ccc(cc3c12)C(F)(F)F |r,wU:4.7,wD:1.0,(6.71,.26,;5.25,.76,;4.95,2.27,;3.5,2.77,;2.34,1.76,;2.63,.25,;4.09,-.25,;.88,2.25,;-.13,3.41,;1.38,3.71,;-.28,1.24,;-1.74,1.74,;-1.92,3.27,;-3.23,4.08,;-2.61,5.48,;-4.14,5.32,;-4.68,3.56,;-5.18,2.1,;-6.71,1.92,;-4.35,.8,;-4.98,-.6,;-3.83,-1.63,;-3.83,-3.17,;-2.5,-3.94,;-1.16,-3.17,;-1.16,-1.63,;-2.5,-.86,;-2.82,.64,;.17,-3.94,;1.5,-3.17,;.17,-5.48,;1.5,-4.71,)| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2R49VQ0 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50596293
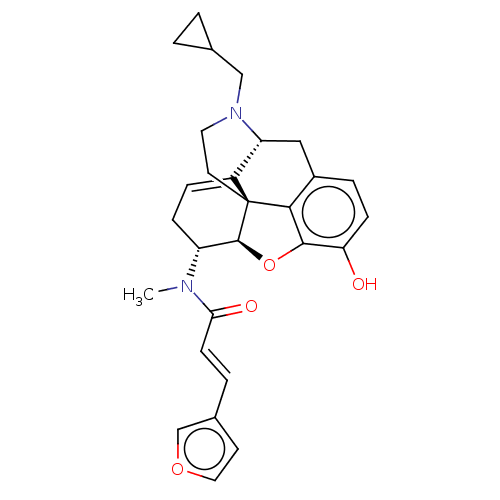
(CHEMBL5188658)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14C5=CC[C@H]2N(C)C(=O)\C=C\c1ccoc1)ccc3O |r,t:21,THB:10:9:17:4.5.6| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0356 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128527
BindingDB Entry DOI: 10.7270/Q2HT2TDM |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50491882
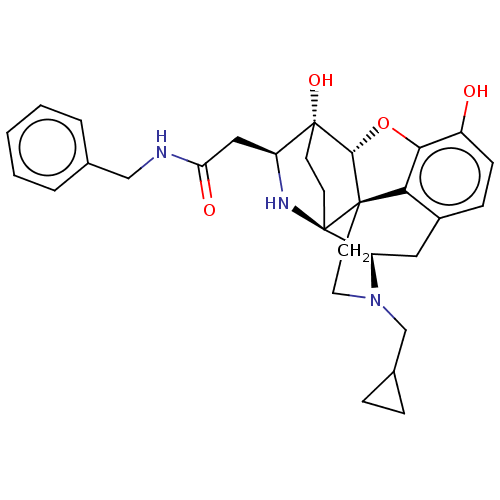
(CHEMBL3215908)Show SMILES Cl.Cl.[H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@]14[C@@]51CC[C@@]2(O)[C@H](CC(=O)NCc2ccccc2)N1)ccc3O |r,THB:25:24:3.18:20.21,12:11:19:6.8.7| Show InChI InChI=1S/C30H35N3O4.2ClH/c34-21-9-8-20-14-23-30-11-10-29(36,22(32-30)15-24(35)31-16-18-4-2-1-3-5-18)27-28(30,25(20)26(21)37-27)12-13-33(23)17-19-6-7-19;;/h1-5,8-9,19,22-23,27,32,34,36H,6-7,10-17H2,(H,31,35);2*1H/t22-,23+,27+,28+,29+,30+;;/m0../s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]U-69,593 from kappa opioid receptor in guinea pig cerebellum |
Bioorg Med Chem 21: 3032-50 (2013)
Article DOI: 10.1016/j.bmc.2013.03.026
BindingDB Entry DOI: 10.7270/Q2JQ13XJ |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50102826
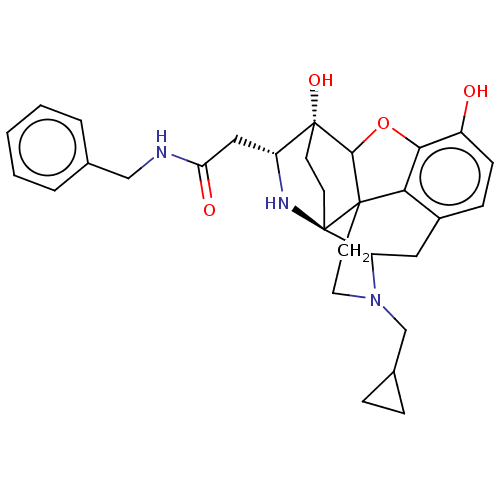
(CHEMBL3339374)Show SMILES Oc1ccc2CC3N(CC4CC4)CCC45C(Oc1c24)[C@@]1(O)CC[C@@]35N[C@@H]1CC(=O)NCc1ccccc1 |r,TLB:26:25:14.15:22.21,THB:3:4:23:7.12.13,17:18:23:7.12.13,22:23:4.18.5:7.12.13,24:23:4.18.5:7.12.13,8:7:4.18.5:23| Show InChI InChI=1S/C30H35N3O4/c34-21-9-8-20-14-23-30-11-10-29(36,22(32-30)15-24(35)31-16-18-4-2-1-3-5-18)27-28(30,25(20)26(21)37-27)12-13-33(23)17-19-6-7-19/h1-5,8-9,19,22-23,27,32,34,36H,6-7,10-17H2,(H,31,35)/t22-,23?,27?,28?,29-,30-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]U-69593 from kappa opioid receptor in guinea pig cerebellum |
Bioorg Med Chem Lett 24: 4980-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.029
BindingDB Entry DOI: 10.7270/Q2154JTJ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM601978

(US11643417, Ex. No. 11 | US11643417, Ex. No. 12 | ...)Show SMILES CC1(O)CCOC(C1)C(F)(F)CN1CCNC(=O)c2oc3ccc(cc3c12)C(F)(F)F | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2R49VQ0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM602032

(US11643417, Ex. No. 49)Show SMILES CC(C)(O)C1CCOC(C1)C(F)(F)CN1CCNC(=O)c2oc3ccc(cc3c12)C(F)(F)F | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2R49VQ0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM602044
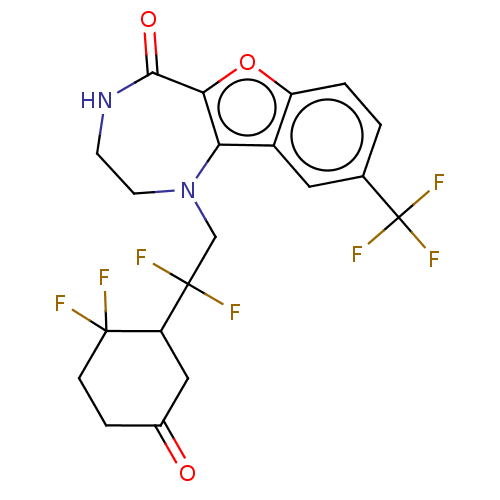
(US11643417, Ex. No. 61)Show SMILES FC(F)(CN1CCNC(=O)c2oc3ccc(cc3c12)C(F)(F)F)C1CC(=O)CCC1(F)F | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2R49VQ0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM50399614
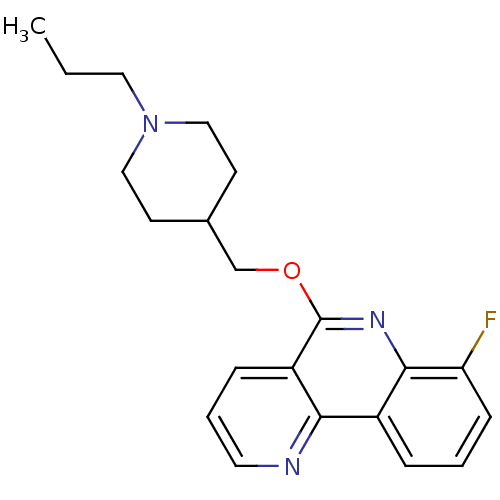
(CHEMBL2181170)Show InChI InChI=1S/C21H24FN3O/c1-2-11-25-12-8-15(9-13-25)14-26-21-17-6-4-10-23-19(17)16-5-3-7-18(22)20(16)24-21/h3-7,10,15H,2,8-9,11-14H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Caen Basse-Normandie
Curated by ChEMBL
| Assay Description
Displacement of radioligand from human 5HT4R by Cerep protocol based assay |
J Med Chem 55: 9693-707 (2012)
Article DOI: 10.1021/jm300943r
BindingDB Entry DOI: 10.7270/Q2ZC8417 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM50399614

(CHEMBL2181170)Show InChI InChI=1S/C21H24FN3O/c1-2-11-25-12-8-15(9-13-25)14-26-21-17-6-4-10-23-19(17)16-5-3-7-18(22)20(16)24-21/h3-7,10,15H,2,8-9,11-14H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Caen Basse-Normandie
Curated by ChEMBL
| Assay Description
Displacement of radioligand from human 5HT4R by Cerep protocol based assay |
J Med Chem 55: 9693-707 (2012)
Article DOI: 10.1021/jm300943r
BindingDB Entry DOI: 10.7270/Q2ZC8417 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM602092
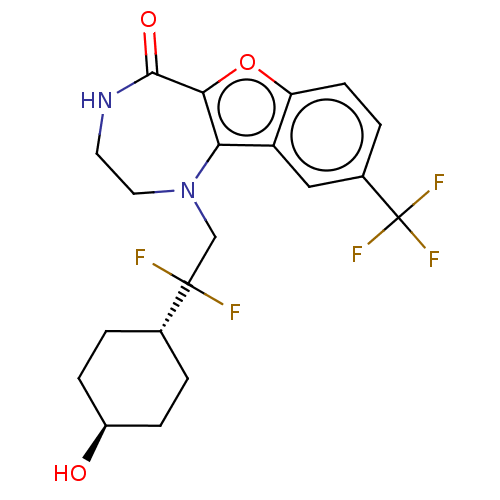
(US11643417, Ex. No. 106)Show SMILES O[C@H]1CC[C@@H](CC1)C(F)(F)CN1CCNC(=O)c2oc3ccc(cc3c12)C(F)(F)F |r,wU:4.7,wD:1.0,(6.71,.97,;5.25,1.46,;4.95,2.98,;3.5,3.47,;2.34,2.46,;2.63,.95,;4.09,.45,;.88,2.96,;1.38,4.42,;-.13,4.12,;-.28,1.94,;-1.74,2.44,;-1.92,3.97,;-3.23,4.78,;-4.68,4.26,;-5.18,2.8,;-6.71,2.62,;-4.35,1.51,;-4.98,.1,;-3.83,-.93,;-3.83,-2.47,;-2.5,-3.24,;-1.16,-2.47,;-1.16,-.93,;-2.5,-.16,;-2.82,1.35,;.17,-3.24,;1.5,-2.47,;.17,-4.78,;1.5,-4.01,)| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.0410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2R49VQ0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM601977

(US11643417, Ex. No. 10 | US11643417, Ex. No. 51)Show SMILES OC1CCOC(C1)C(F)(F)CN1CCNC(=O)c2oc3ccc(cc3c12)C(F)(F)F | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.0410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2R49VQ0 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50123490
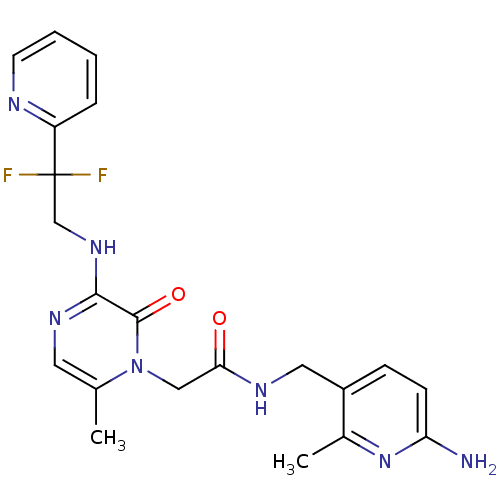
(CHEMBL143418 | N-(6-Amino-2-methyl-pyridin-3-ylmet...)Show SMILES Cc1cnc(NCC(F)(F)c2ccccn2)c(=O)n1CC(=O)NCc1ccc(N)nc1C Show InChI InChI=1S/C21H23F2N7O2/c1-13-9-27-19(28-12-21(22,23)16-5-3-4-8-25-16)20(32)30(13)11-18(31)26-10-15-6-7-17(24)29-14(15)2/h3-9H,10-12H2,1-2H3,(H2,24,29)(H,26,31)(H,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory constant against thrombin (IIa) |
J Med Chem 46: 461-73 (2003)
Article DOI: 10.1021/jm020311f
BindingDB Entry DOI: 10.7270/Q2W958J5 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50102833
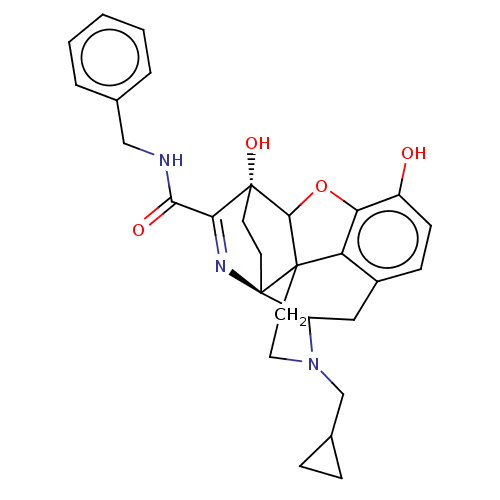
(CHEMBL3339378)Show SMILES Oc1ccc2CC3N(CC4CC4)CCC45C(Oc1c24)[C@@]1(O)CC[C@@]35N=C1C(=O)NCc1ccccc1 |r,c:30,TLB:26:25:14.15:22.21,THB:3:4:23:7.12.13,8:7:4.18.5:23,17:18:23:7.12.13,24:23:4.18.5:7.12.13,22:23:4.18.5:7.12.13| Show InChI InChI=1S/C29H31N3O4/c33-20-9-8-19-14-21-29-11-10-28(35,24(31-29)25(34)30-15-17-4-2-1-3-5-17)26-27(29,22(19)23(20)36-26)12-13-32(21)16-18-6-7-18/h1-5,8-9,18,21,26,33,35H,6-7,10-16H2,(H,30,34)/t21?,26?,27?,28-,29-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]U-69593 from kappa opioid receptor in guinea pig cerebellum |
Bioorg Med Chem Lett 24: 4980-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.029
BindingDB Entry DOI: 10.7270/Q2154JTJ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM602062

(US11643417, Ex. No. 77)Show SMILES OC[C@H]1CO[C@@H](CO1)C(F)(F)CN1CCNC(=O)c2oc3ccc(cc3c12)C(F)(F)F |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.0440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2R49VQ0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM602168
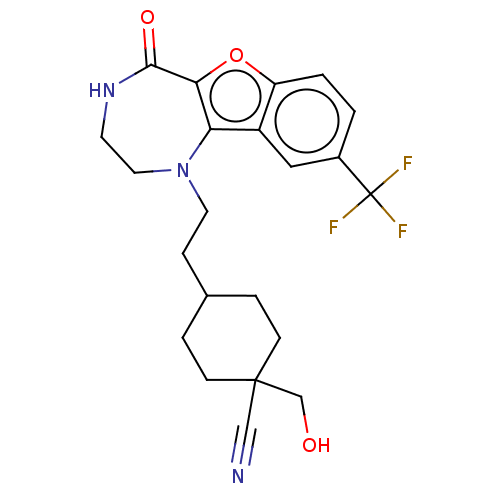
(US11643417, Ex. No. 163)Show SMILES OCC1(CCC(CCN2CCNC(=O)c3oc4ccc(cc4c23)C(F)(F)F)CC1)C#N |(4.98,-1.15,;5.48,.31,;4.47,1.46,;3.31,.45,;1.85,.95,;1.55,2.46,;.1,2.96,;-1.06,1.94,;-2.52,2.44,;-2.7,3.97,;-4.01,4.78,;-5.46,4.26,;-5.96,2.8,;-7.49,2.62,;-5.13,1.51,;-5.76,.1,;-4.61,-.93,;-4.61,-2.47,;-3.28,-3.24,;-1.95,-2.47,;-1.95,-.93,;-3.28,-.16,;-3.6,1.35,;-.61,-3.24,;.72,-2.47,;-.61,-4.78,;.72,-4.01,;2.71,3.47,;4.17,2.98,;5.98,1.76,;7.49,2.06,)| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.0440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2R49VQ0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM602080

(US11643417, Ex. No. 95)Show SMILES OC[C@@H]1CO[C@H](CO1)C(F)(F)CN1CCNC(=O)c2oc3ccc(cc3c12)C(F)(F)F |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.0440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2R49VQ0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM602040
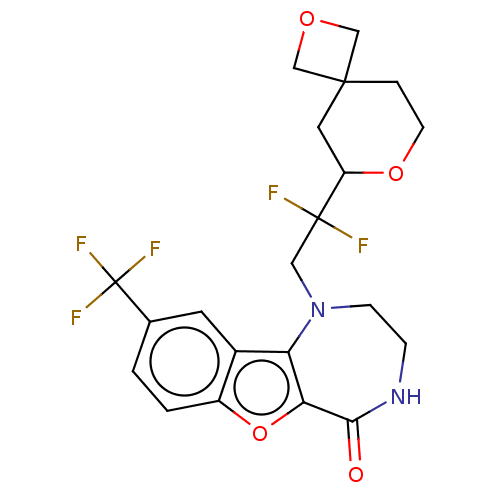
(US11643417, Ex. No. 57)Show SMILES FC(F)(F)c1ccc2oc3c(N(CC(F)(F)C4CC5(COC5)CCO4)CCNC3=O)c2c1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2R49VQ0 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data