

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM50509592 (CHEMBL4460946) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Uncompetitive inhibition of SMYD3 (unknown origin) assessed as inhibitory constant incubated for 60 mins by Cheng-Prusoff equation analysis | ACS Med Chem Lett 11: 133-140 (2020) Article DOI: 10.1021/acsmedchemlett.9b00493 BindingDB Entry DOI: 10.7270/Q2W66Q2M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM50509581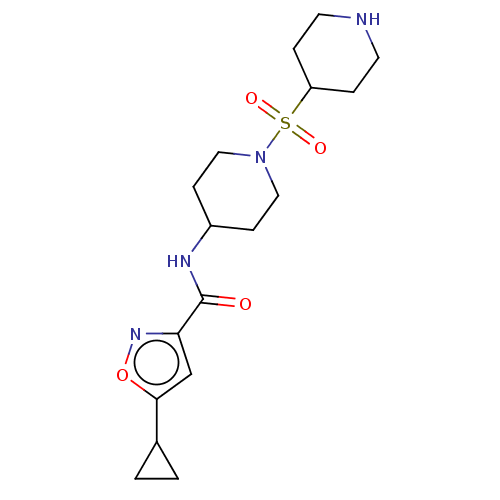 (CHEMBL4535915) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Uncompetitive inhibition of SMYD3 (unknown origin) assessed as inhibitory constant incubated for 60 mins by Cheng-Prusoff equation analysis | ACS Med Chem Lett 11: 133-140 (2020) Article DOI: 10.1021/acsmedchemlett.9b00493 BindingDB Entry DOI: 10.7270/Q2W66Q2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM50509609 (CHEMBL4476167) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Uncompetitive inhibition of SMYD3 (unknown origin) assessed as inhibitory constant incubated for 60 mins by Lineweaver-Burk plot analysis | ACS Med Chem Lett 11: 133-140 (2020) Article DOI: 10.1021/acsmedchemlett.9b00493 BindingDB Entry DOI: 10.7270/Q2W66Q2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM50509609 (CHEMBL4476167) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Uncompetitive inhibition of SMYD3 (unknown origin) assessed as inhibitory constant incubated for 60 mins by Cheng-Prusoff equation analysis | ACS Med Chem Lett 11: 133-140 (2020) Article DOI: 10.1021/acsmedchemlett.9b00493 BindingDB Entry DOI: 10.7270/Q2W66Q2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50401764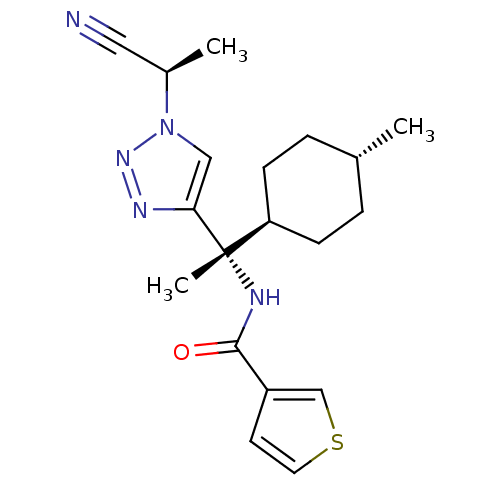 (CHEMBL2207564) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Inhibition of cathepsin S using FR-aminoluciferin as substrate preincubated for 15 mins before substrate addition measured after 1 hr by luminescence... | Bioorg Med Chem Lett 22: 7189-93 (2012) Article DOI: 10.1016/j.bmcl.2012.09.054 BindingDB Entry DOI: 10.7270/Q2R212JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50401763 (CHEMBL2207565) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Inhibition of cathepsin S using FR-aminoluciferin as substrate preincubated for 15 mins before substrate addition measured after 1 hr by luminescence... | Bioorg Med Chem Lett 22: 7189-93 (2012) Article DOI: 10.1016/j.bmcl.2012.09.054 BindingDB Entry DOI: 10.7270/Q2R212JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50249132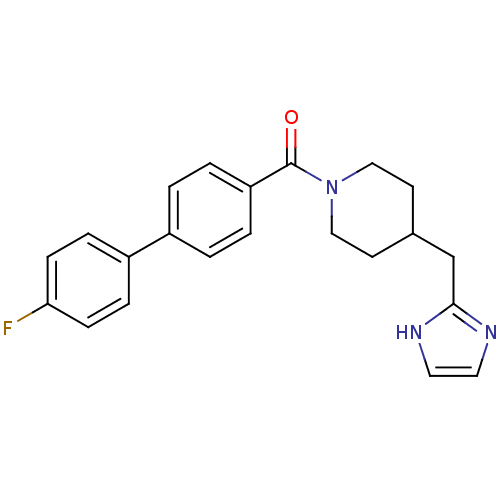 ((4-((1H-imidazol-2-yl)methyl)piperidin-1-yl)(4'-fl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Antagonist activity at 5-HT2B receptor (unknown origin) expressed in CHOK1 cells assessed as inhibition of serotonin-induced intracellular Ca2+ flux ... | Bioorg Med Chem Lett 19: 2206-10 (2009) Article DOI: 10.1016/j.bmcl.2009.02.126 BindingDB Entry DOI: 10.7270/Q20Z735D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50209479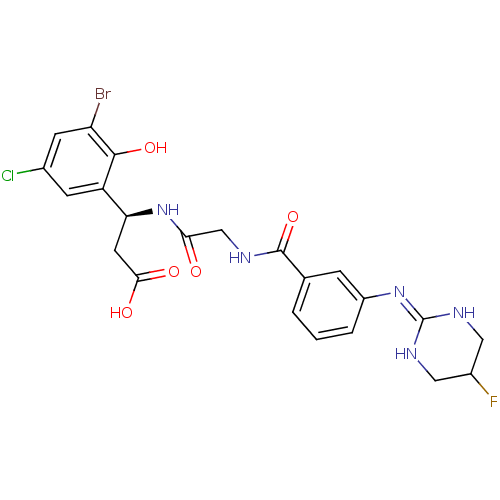 ((beta1S)-3-bromo-5-chloro-b-[[[[3-[(5-fluoro-1,4,5...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of alphaVbeta3 integrin receptor expressed in human 293 cells | Bioorg Med Chem 15: 3783-800 (2007) Article DOI: 10.1016/j.bmc.2007.03.034 BindingDB Entry DOI: 10.7270/Q25Q4VR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50209482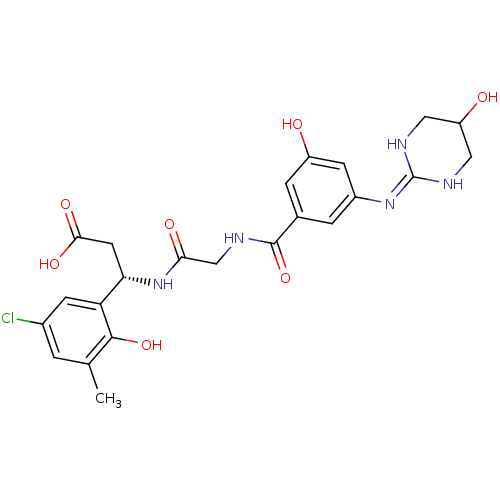 ((3S)-3-(5-chloro-2-hydroxy-3-methylphenyl)-3-(2-(3...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of alphaVbeta3 integrin receptor expressed in human 293 cells | Bioorg Med Chem 15: 3783-800 (2007) Article DOI: 10.1016/j.bmc.2007.03.034 BindingDB Entry DOI: 10.7270/Q25Q4VR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic (Homo sapiens (Human)) | BDBM279989 (US10028961, Compound 142 | US10172864, Compound 14...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Agios Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of IDH1 R132H mutant in glioma patient-derived human TS603 neurosphere cells assessed as reduction in 2-HG content using alpha-ketoglutara... | ACS Med Chem Lett 11: 101-107 (2020) Article DOI: 10.1021/acsmedchemlett.9b00509 BindingDB Entry DOI: 10.7270/Q28G8Q0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50401766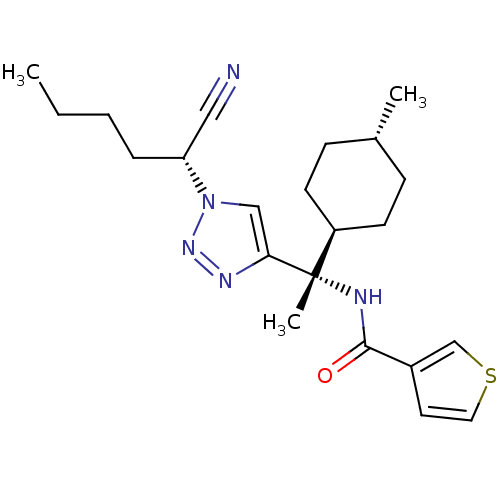 (CHEMBL2207562) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Inhibition of cathepsin S using FR-aminoluciferin as substrate preincubated for 15 mins before substrate addition measured after 1 hr by luminescence... | Bioorg Med Chem Lett 22: 7189-93 (2012) Article DOI: 10.1016/j.bmcl.2012.09.054 BindingDB Entry DOI: 10.7270/Q2R212JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50209446 ((3S)-3-(3,5-dichloro-2-hydroxyphenyl)-3-(2-(3-(5-h...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of alphaVbeta3 integrin receptor expressed in human 293 cells | Bioorg Med Chem 15: 3783-800 (2007) Article DOI: 10.1016/j.bmc.2007.03.034 BindingDB Entry DOI: 10.7270/Q25Q4VR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50209471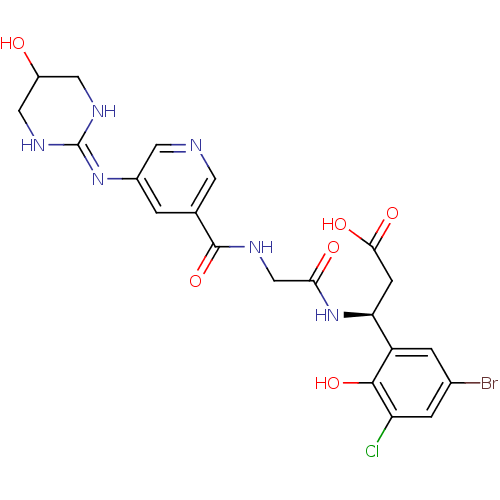 ((3S)-3-(5-bromo-3-chloro-2-hydroxyphenyl)-3-(2-(3-...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of alphaVbeta3 integrin receptor expressed in human 293 cells | Bioorg Med Chem 15: 3783-800 (2007) Article DOI: 10.1016/j.bmc.2007.03.034 BindingDB Entry DOI: 10.7270/Q25Q4VR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50249483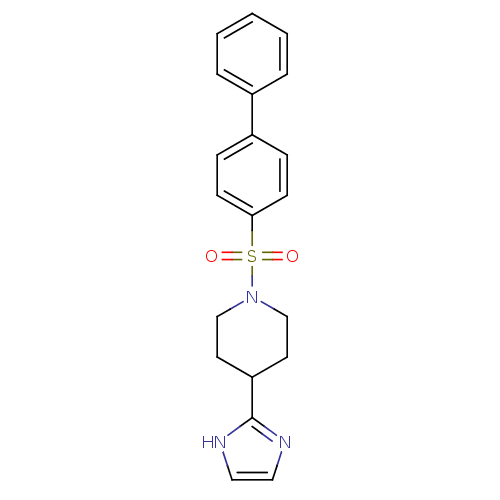 (1-(biphenyl-4-ylsulfonyl)-4-(1H-imidazol-2-yl)pipe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Antagonist activity at 5-HT2B receptor (unknown origin) expressed in CHOK1 cells assessed as inhibition of serotonin-induced intracellular Ca2+ flux ... | Bioorg Med Chem Lett 19: 2206-10 (2009) Article DOI: 10.1016/j.bmcl.2009.02.126 BindingDB Entry DOI: 10.7270/Q20Z735D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic (Homo sapiens (Human)) | BDBM279977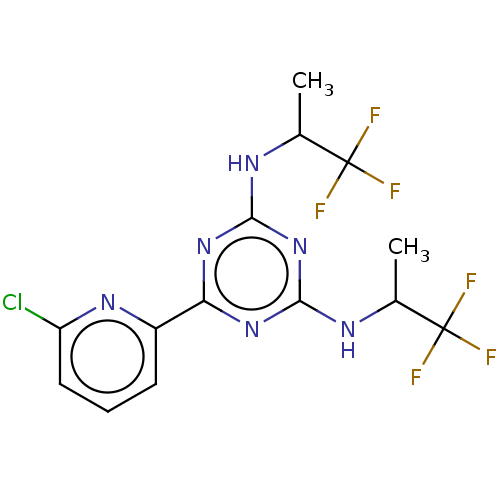 (US10028961, Compound 130 | US10172864, Compound 13...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Agios Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of IDH1 R132H mutant in glioma patient-derived human TS603 neurosphere cells assessed as reduction in 2-HG content using alpha-ketoglutara... | ACS Med Chem Lett 11: 101-107 (2020) Article DOI: 10.1021/acsmedchemlett.9b00509 BindingDB Entry DOI: 10.7270/Q28G8Q0N | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50209459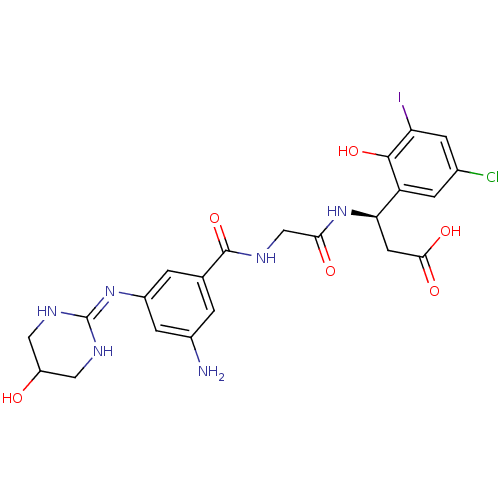 ((3R)-3-(2-(3-amino-5-(5-hydroxy-1,4,5,6-tetrahydro...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of alphaVbeta3 integrin receptor expressed in human 293 cells | Bioorg Med Chem 15: 3783-800 (2007) Article DOI: 10.1016/j.bmc.2007.03.034 BindingDB Entry DOI: 10.7270/Q25Q4VR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50209441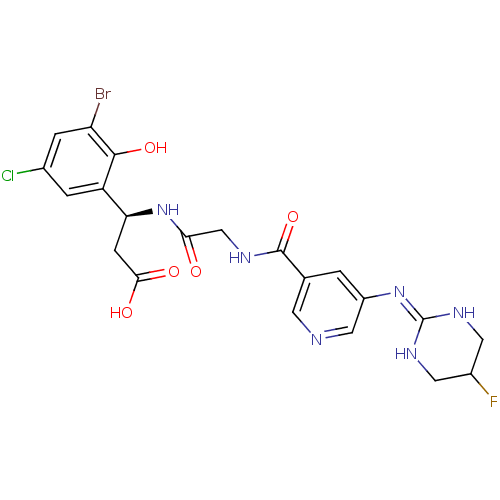 ((3S)-3-(3-bromo-5-chloro-2-hydroxyphenyl)-3-(2-(3-...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of alphaVbeta3 integrin receptor expressed in human 293 cells | Bioorg Med Chem 15: 3783-800 (2007) Article DOI: 10.1016/j.bmc.2007.03.034 BindingDB Entry DOI: 10.7270/Q25Q4VR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19502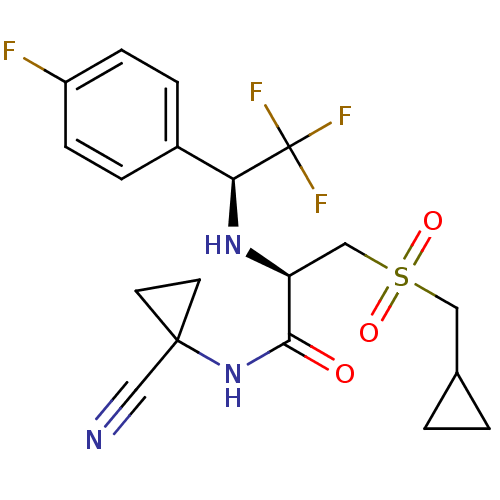 ((2R)-N-(1-cyanocyclopropyl)-3-(cyclopropylmethane)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Inhibition of cathepsin S using FR-aminoluciferin as substrate preincubated for 15 mins before substrate addition measured after 1 hr by luminescence... | Bioorg Med Chem Lett 22: 7189-93 (2012) Article DOI: 10.1016/j.bmcl.2012.09.054 BindingDB Entry DOI: 10.7270/Q2R212JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50209124 ((2,2-dimethyl-3-{4-[2-(5,6,7,8-tetrahydro-1,8-naph...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human intergrin alphavbeta3 receptor by solid phase receptor assay | Bioorg Med Chem 15: 3390-412 (2007) Article DOI: 10.1016/j.bmc.2007.03.020 BindingDB Entry DOI: 10.7270/Q2057FK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50209124 ((2,2-dimethyl-3-{4-[2-(5,6,7,8-tetrahydro-1,8-naph...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human intergrin alphavbeta3 receptor expressed in HEK293 cells | Bioorg Med Chem 15: 3390-412 (2007) Article DOI: 10.1016/j.bmc.2007.03.020 BindingDB Entry DOI: 10.7270/Q2057FK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50209124 ((2,2-dimethyl-3-{4-[2-(5,6,7,8-tetrahydro-1,8-naph...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human intergrin alphavbeta3 receptor expressed in HEK293 cells | Bioorg Med Chem 15: 3390-412 (2007) Article DOI: 10.1016/j.bmc.2007.03.020 BindingDB Entry DOI: 10.7270/Q2057FK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50209461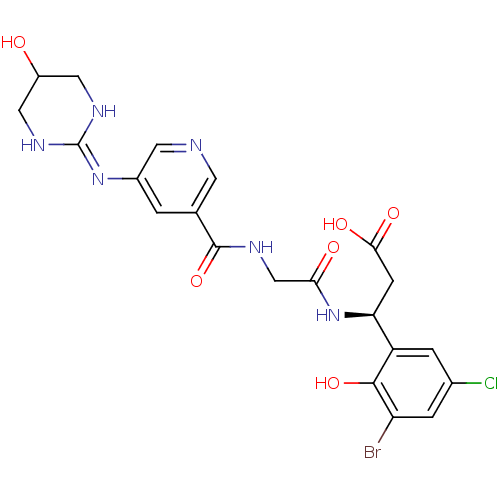 ((3S)-3-(3-bromo-5-chloro-2-hydroxyphenyl)-3-(2-(3-...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of alphaVbeta3 integrin receptor expressed in human 293 cells | Bioorg Med Chem 15: 3783-800 (2007) Article DOI: 10.1016/j.bmc.2007.03.034 BindingDB Entry DOI: 10.7270/Q25Q4VR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50249464 ((4-((1H-imidazol-2-yl)methyl)piperidin-1-yl)(biphe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Antagonist activity at 5-HT2B receptor (unknown origin) expressed in CHOK1 cells assessed as inhibition of serotonin-induced intracellular Ca2+ flux ... | Bioorg Med Chem Lett 19: 2206-10 (2009) Article DOI: 10.1016/j.bmcl.2009.02.126 BindingDB Entry DOI: 10.7270/Q20Z735D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50209132 ((2-{3-fluoro-4-[3-(5,6,7,8-tetrahydro-1,8-naphthyr...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human intergrin alphavbeta3 receptor by solid phase receptor assay | Bioorg Med Chem 15: 3390-412 (2007) Article DOI: 10.1016/j.bmc.2007.03.020 BindingDB Entry DOI: 10.7270/Q2057FK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50209131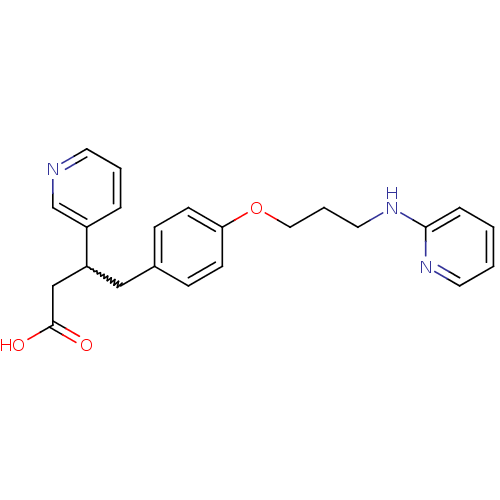 (4-[3-(2-N-pyridyl)amino]-1-propyloxyphenyl-3-(3-py...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human intergrin alphavbeta3 receptor by solid phase receptor assay | Bioorg Med Chem 15: 3390-412 (2007) Article DOI: 10.1016/j.bmc.2007.03.020 BindingDB Entry DOI: 10.7270/Q2057FK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50401765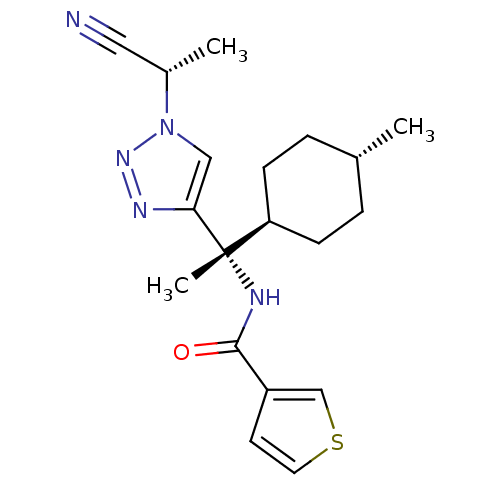 (CHEMBL2207563) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Inhibition of cathepsin S using FR-aminoluciferin as substrate preincubated for 15 mins before substrate addition measured after 1 hr by luminescence... | Bioorg Med Chem Lett 22: 7189-93 (2012) Article DOI: 10.1016/j.bmcl.2012.09.054 BindingDB Entry DOI: 10.7270/Q2R212JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50401814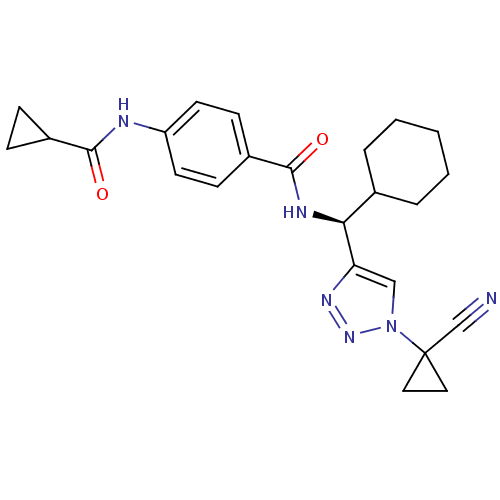 (CHEMBL2207591) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Inhibition of cathepsin S using FR-aminoluciferin as substrate preincubated for 15 mins before substrate addition measured after 1 hr by luminescence... | Bioorg Med Chem Lett 22: 7189-93 (2012) Article DOI: 10.1016/j.bmcl.2012.09.054 BindingDB Entry DOI: 10.7270/Q2R212JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50249131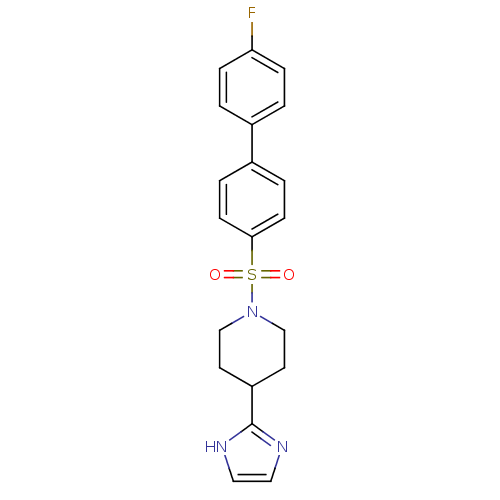 (1-(4'-fluorobiphenyl-4-ylsulfonyl)-4-(1H-imidazol-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Antagonist activity at 5-HT2B receptor (unknown origin) expressed in CHOK1 cells assessed as inhibition of serotonin-induced intracellular Ca2+ flux ... | Bioorg Med Chem Lett 19: 2206-10 (2009) Article DOI: 10.1016/j.bmcl.2009.02.126 BindingDB Entry DOI: 10.7270/Q20Z735D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50209121 ((2-{4-[2-(5,6,7,8-tetrahydro-1,8-naphthyridin-2-yl...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human intergrin alphavbeta3 receptor by solid phase receptor assay | Bioorg Med Chem 15: 3390-412 (2007) Article DOI: 10.1016/j.bmc.2007.03.020 BindingDB Entry DOI: 10.7270/Q2057FK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50209121 ((2-{4-[2-(5,6,7,8-tetrahydro-1,8-naphthyridin-2-yl...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human intergrin alphavbeta3 receptor expressed in HEK293 cells | Bioorg Med Chem 15: 3390-412 (2007) Article DOI: 10.1016/j.bmc.2007.03.020 BindingDB Entry DOI: 10.7270/Q2057FK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50401835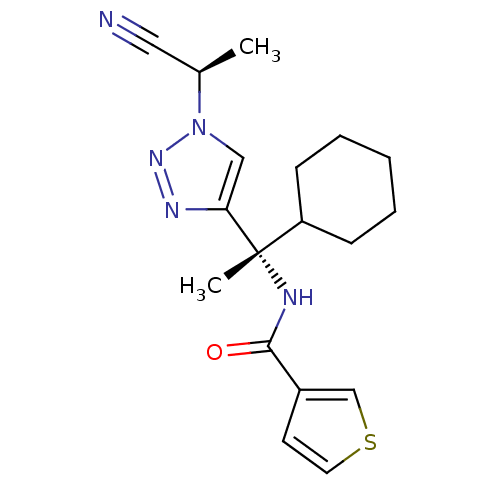 (CHEMBL2207570) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Inhibition of cathepsin S using FR-aminoluciferin as substrate preincubated for 15 mins before substrate addition measured after 1 hr by luminescence... | Bioorg Med Chem Lett 22: 7189-93 (2012) Article DOI: 10.1016/j.bmcl.2012.09.054 BindingDB Entry DOI: 10.7270/Q2R212JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50209456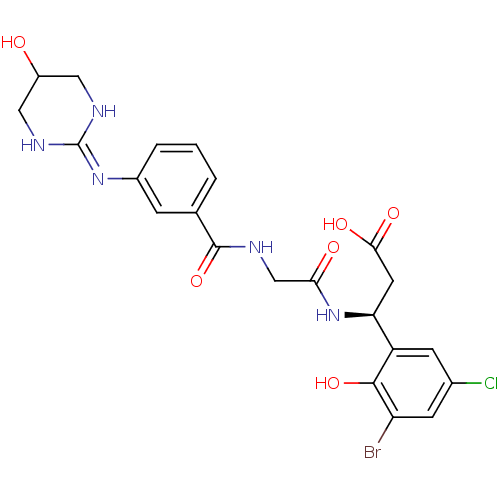 ((3S)-3-(3-bromo-5-chloro-2-hydroxyphenyl)-3-(2-(3-...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of alphaVbeta3 integrin receptor expressed in human 293 cells | Bioorg Med Chem 15: 3783-800 (2007) Article DOI: 10.1016/j.bmc.2007.03.034 BindingDB Entry DOI: 10.7270/Q25Q4VR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50209450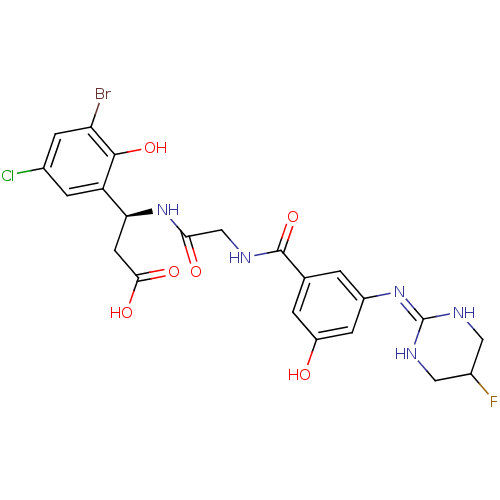 ((beta1R)-3-bromo-5-chloro-b-[[[[3-[(5-fluoro-1,4,5...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of alphaVbeta3 integrin receptor expressed in human 293 cells | Bioorg Med Chem 15: 3783-800 (2007) Article DOI: 10.1016/j.bmc.2007.03.034 BindingDB Entry DOI: 10.7270/Q25Q4VR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50401834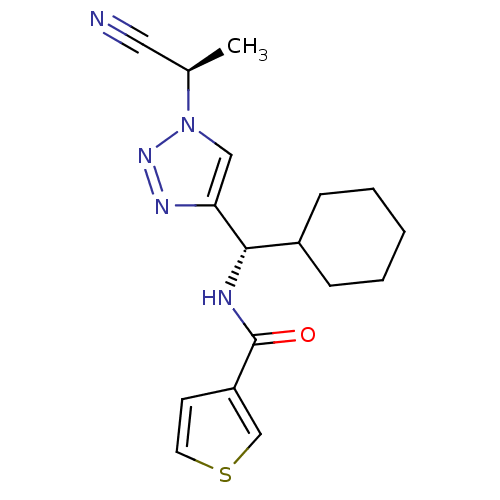 (CHEMBL2207571) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Inhibition of cathepsin S using FR-aminoluciferin as substrate preincubated for 15 mins before substrate addition measured after 1 hr by luminescence... | Bioorg Med Chem Lett 22: 7189-93 (2012) Article DOI: 10.1016/j.bmcl.2012.09.054 BindingDB Entry DOI: 10.7270/Q2R212JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50209475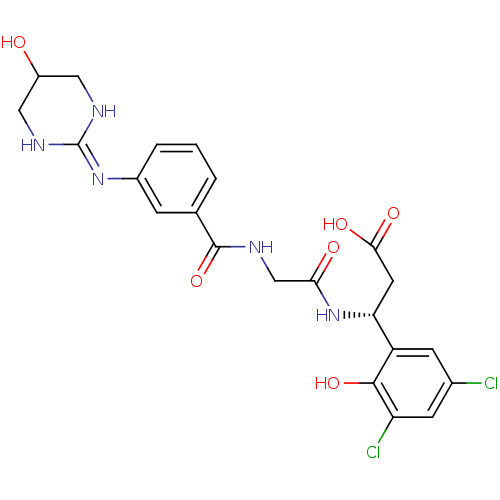 ((3R)-3-(3,5-dichloro-2-hydroxyphenyl)-3-(2-(3-(5-h...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of alphaVbeta3 integrin receptor expressed in human 293 cells | Bioorg Med Chem 15: 3783-800 (2007) Article DOI: 10.1016/j.bmc.2007.03.034 BindingDB Entry DOI: 10.7270/Q25Q4VR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50249134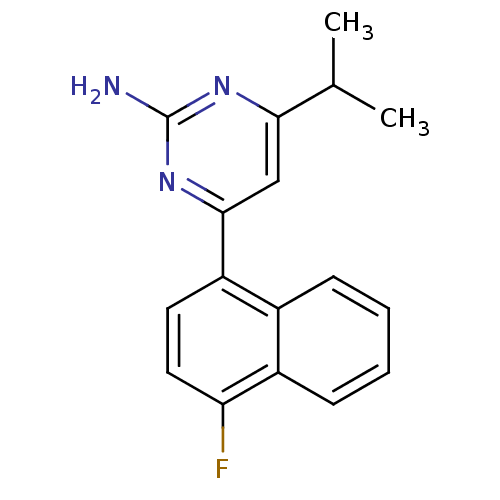 (4-(4-fluoronaphthalen-1-yl)-6-isopropylpyrimidin-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Antagonist activity at 5-HT2B receptor (unknown origin) expressed in CHOK1 cells assessed as inhibition of serotonin-induced intracellular Ca2+ flux ... | Bioorg Med Chem Lett 19: 2206-10 (2009) Article DOI: 10.1016/j.bmcl.2009.02.126 BindingDB Entry DOI: 10.7270/Q20Z735D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50401761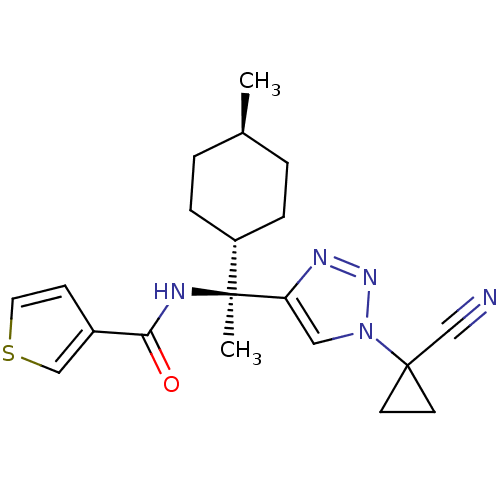 (CHEMBL2207567) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Inhibition of cathepsin S using FR-aminoluciferin as substrate preincubated for 15 mins before substrate addition measured after 1 hr by luminescence... | Bioorg Med Chem Lett 22: 7189-93 (2012) Article DOI: 10.1016/j.bmcl.2012.09.054 BindingDB Entry DOI: 10.7270/Q2R212JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic (Homo sapiens (Human)) | BDBM279960 (US10028961, Compound 113 | US10172864, Compound 11...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Agios Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of IDH1 R132H mutant in glioma patient-derived human TS603 neurosphere cells assessed as reduction in 2-HG content using alpha-ketoglutara... | ACS Med Chem Lett 11: 101-107 (2020) Article DOI: 10.1021/acsmedchemlett.9b00509 BindingDB Entry DOI: 10.7270/Q28G8Q0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50209428 ((3R)-3-(3-bromo-5-chloro-2-hydroxyphenyl)-3-(2-(3-...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of alphaVbeta3 integrin receptor expressed in human 293 cells | Bioorg Med Chem 15: 3783-800 (2007) Article DOI: 10.1016/j.bmc.2007.03.034 BindingDB Entry DOI: 10.7270/Q25Q4VR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50209463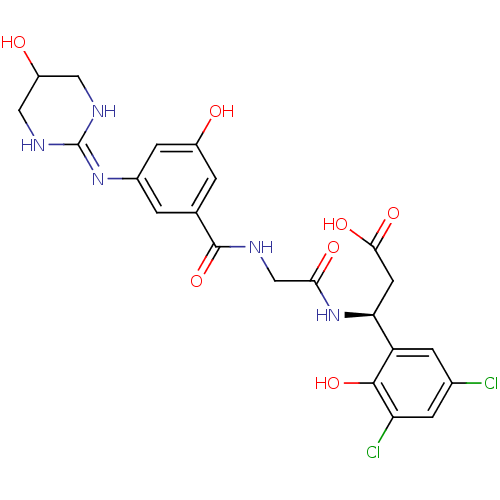 ((beta1S)-3,5-dichloro-b-[[[[3-[(5-hydroxy-1,4,5,6-...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of alphaVbeta3 integrin receptor expressed in human 293 cells | Bioorg Med Chem 15: 3783-800 (2007) Article DOI: 10.1016/j.bmc.2007.03.034 BindingDB Entry DOI: 10.7270/Q25Q4VR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50401770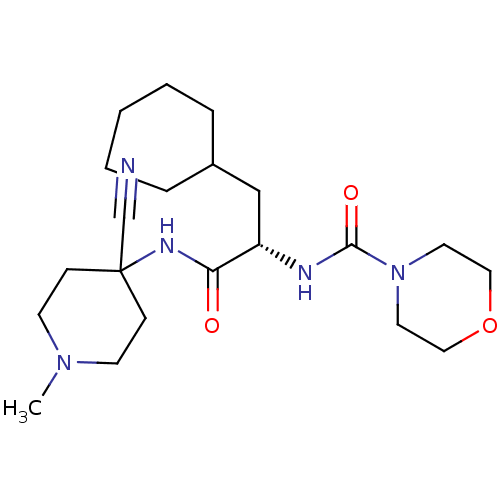 (CHEMBL1236882) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Inhibition of cathepsin S-mediated antigen presentation in B/T hybridoma cells assessed as IL-2 level | Bioorg Med Chem Lett 22: 7189-93 (2012) Article DOI: 10.1016/j.bmcl.2012.09.054 BindingDB Entry DOI: 10.7270/Q2R212JH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50209486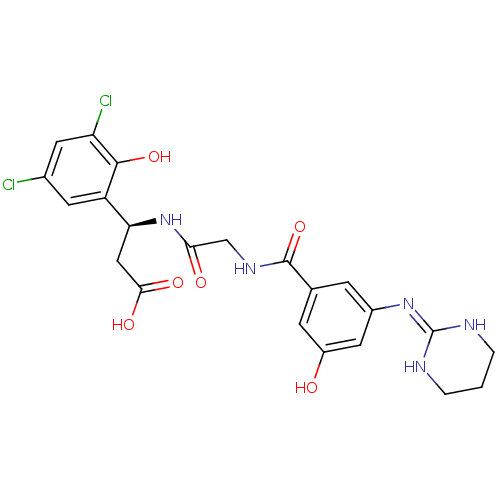 ((S)-3-(3,5-dichloro-2-hydroxyphenyl)-3-(2-(3-hydro...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of alphaVbeta3 integrin receptor expressed in human 293 cells | Bioorg Med Chem 15: 3783-800 (2007) Article DOI: 10.1016/j.bmc.2007.03.034 BindingDB Entry DOI: 10.7270/Q25Q4VR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50209120 ((2-{3-hydroxy-4-[2-(5,6,7,8-tetrahydro-1,8-naphthy...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human intergrin alphavbeta3 receptor expressed in HEK293 cells | Bioorg Med Chem 15: 3390-412 (2007) Article DOI: 10.1016/j.bmc.2007.03.020 BindingDB Entry DOI: 10.7270/Q2057FK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50209477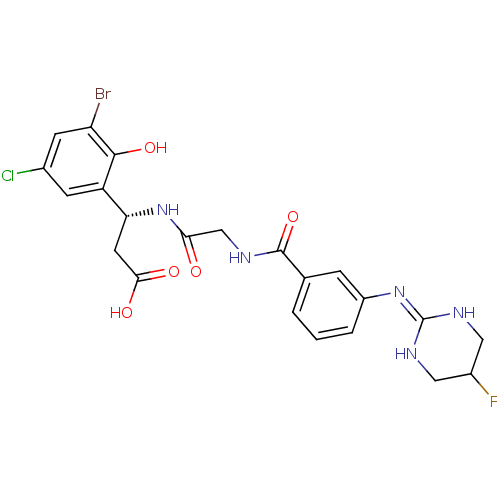 ((beta1R)-3-bromo-5-chloro-b-[[[[3-[(5-fluoro-1,4,5...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of alphaVbeta3 integrin receptor expressed in human 293 cells | Bioorg Med Chem 15: 3783-800 (2007) Article DOI: 10.1016/j.bmc.2007.03.034 BindingDB Entry DOI: 10.7270/Q25Q4VR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM50509589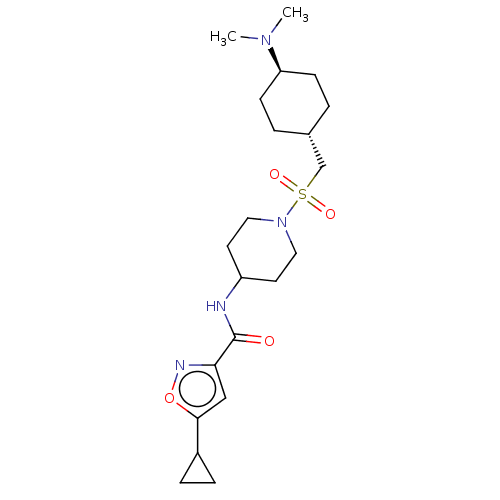 (CHEMBL4460836) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal FLAG/His-tagged SMYD3 (unknown origin) expressed in baculovirus infected insect cells using 3H-SAM as substrate ... | ACS Med Chem Lett 11: 133-140 (2020) Article DOI: 10.1021/acsmedchemlett.9b00493 BindingDB Entry DOI: 10.7270/Q2W66Q2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50209483 ((beta1R)-3-bromo-5-chloro-b-[[[[3-[(5-fluoro-1,4,5...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of alphaVbeta3 integrin receptor expressed in human 293 cells | Bioorg Med Chem 15: 3783-800 (2007) Article DOI: 10.1016/j.bmc.2007.03.034 BindingDB Entry DOI: 10.7270/Q25Q4VR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50209439 ((3R)-3-(5-chloro-2-hydroxy-3-iodophenyl)-3-(2-(3-(...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of alphaVbeta3 integrin receptor expressed in human 293 cells | Bioorg Med Chem 15: 3783-800 (2007) Article DOI: 10.1016/j.bmc.2007.03.034 BindingDB Entry DOI: 10.7270/Q25Q4VR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50209481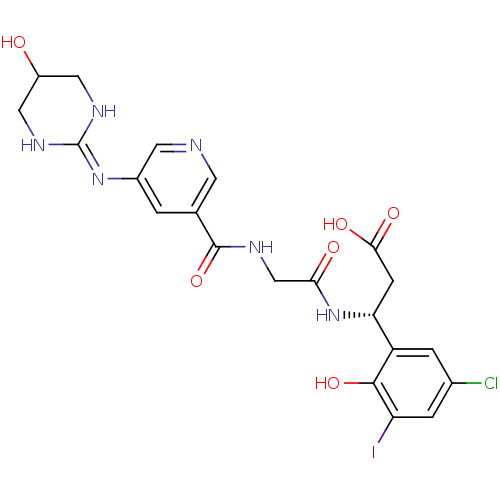 ((3R)-3-(5-chloro-2-hydroxy-3-iodophenyl)-3-(2-(3-(...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of alphaVbeta3 integrin receptor expressed in human 293 cells | Bioorg Med Chem 15: 3783-800 (2007) Article DOI: 10.1016/j.bmc.2007.03.034 BindingDB Entry DOI: 10.7270/Q25Q4VR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM50509577 (CHEMBL4472528) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal FLAG/His-tagged SMYD3 (unknown origin) expressed in baculovirus infected insect cells using 3H-SAM as substrate ... | ACS Med Chem Lett 11: 133-140 (2020) Article DOI: 10.1021/acsmedchemlett.9b00493 BindingDB Entry DOI: 10.7270/Q2W66Q2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM50509576 (CHEMBL4549988) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal FLAG/His-tagged SMYD3 (unknown origin) expressed in baculovirus infected insect cells using 3H-SAM as substrate ... | ACS Med Chem Lett 11: 133-140 (2020) Article DOI: 10.1021/acsmedchemlett.9b00493 BindingDB Entry DOI: 10.7270/Q2W66Q2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 634 total ) | Next | Last >> |