 Found 106 hits with Last Name = 'naito' and Initial = 's'
Found 106 hits with Last Name = 'naito' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50238110
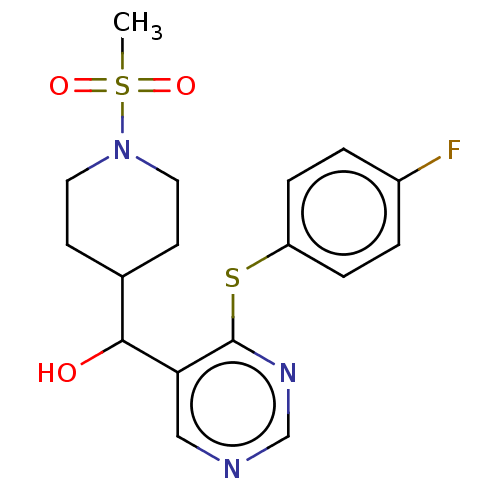
(CHEMBL4088766)Show SMILES CS(=O)(=O)N1CCC(CC1)C(O)c1cncnc1Sc1ccc(F)cc1 Show InChI InChI=1S/C17H20FN3O3S2/c1-26(23,24)21-8-6-12(7-9-21)16(22)15-10-19-11-20-17(15)25-14-4-2-13(18)3-5-14/h2-5,10-12,16,22H,6-9H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in HEK293A cells using deoxycorticosterone as substrate pretreated for 1 hr followed by substrate addition meas... |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50238110

(CHEMBL4088766)Show SMILES CS(=O)(=O)N1CCC(CC1)C(O)c1cncnc1Sc1ccc(F)cc1 Show InChI InChI=1S/C17H20FN3O3S2/c1-26(23,24)21-8-6-12(7-9-21)16(22)15-10-19-11-20-17(15)25-14-4-2-13(18)3-5-14/h2-5,10-12,16,22H,6-9H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B1 expressed in HEK293A cells |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50238108
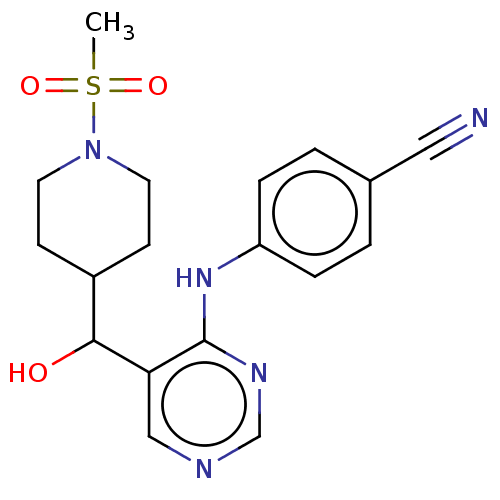
(CHEMBL4070230)Show SMILES CS(=O)(=O)N1CCC(CC1)C(O)c1cncnc1Nc1ccc(cc1)C#N Show InChI InChI=1S/C18H21N5O3S/c1-27(25,26)23-8-6-14(7-9-23)17(24)16-11-20-12-21-18(16)22-15-4-2-13(10-19)3-5-15/h2-5,11-12,14,17,24H,6-9H2,1H3,(H,20,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50238115
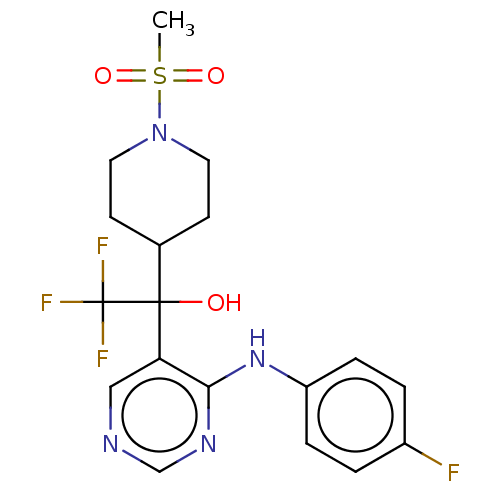
(CHEMBL4082403)Show SMILES CS(=O)(=O)N1CCC(CC1)C(O)(c1cncnc1Nc1ccc(F)cc1)C(F)(F)F Show InChI InChI=1S/C18H20F4N4O3S/c1-30(28,29)26-8-6-12(7-9-26)17(27,18(20,21)22)15-10-23-11-24-16(15)25-14-4-2-13(19)3-5-14/h2-5,10-12,27H,6-9H2,1H3,(H,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B1 expressed in HEK293A cells |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50238115

(CHEMBL4082403)Show SMILES CS(=O)(=O)N1CCC(CC1)C(O)(c1cncnc1Nc1ccc(F)cc1)C(F)(F)F Show InChI InChI=1S/C18H20F4N4O3S/c1-30(28,29)26-8-6-12(7-9-26)17(27,18(20,21)22)15-10-23-11-24-16(15)25-14-4-2-13(19)3-5-14/h2-5,10-12,27H,6-9H2,1H3,(H,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50238103

(CHEMBL4070323)Show SMILES CC(O)(C1CCN(CC1)S(C)(=O)=O)c1cncnc1Nc1ccc(F)cc1 Show InChI InChI=1S/C18H23FN4O3S/c1-18(24,13-7-9-23(10-8-13)27(2,25)26)16-11-20-12-21-17(16)22-15-5-3-14(19)4-6-15/h3-6,11-13,24H,7-10H2,1-2H3,(H,20,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50179532

(1-(biphenyl-3-ylamino)-cyclohexanecarboxylic acid ...)Show SMILES CC[C@@H](CNc1ccc(OC)cc1)NC(=O)C1(CCCCC1)Nc1cccc(c1)-c1ccccc1 Show InChI InChI=1S/C30H37N3O2/c1-3-25(22-31-26-15-17-28(35-2)18-16-26)32-29(34)30(19-8-5-9-20-30)33-27-14-10-13-24(21-27)23-11-6-4-7-12-23/h4,6-7,10-18,21,25,31,33H,3,5,8-9,19-20,22H2,1-2H3,(H,32,34)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Cathepsin K |
Bioorg Med Chem Lett 16: 1502-5 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.053
BindingDB Entry DOI: 10.7270/Q2T154FS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50238105

(CHEMBL4099824)Show SMILES CS(=O)(=O)N1CCC(CC1)C(O)c1cncnc1Nc1ccc(F)cc1 Show InChI InChI=1S/C17H21FN4O3S/c1-26(24,25)22-8-6-12(7-9-22)16(23)15-10-19-11-20-17(15)21-14-4-2-13(18)3-5-14/h2-5,10-12,16,23H,6-9H2,1H3,(H,19,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50238109
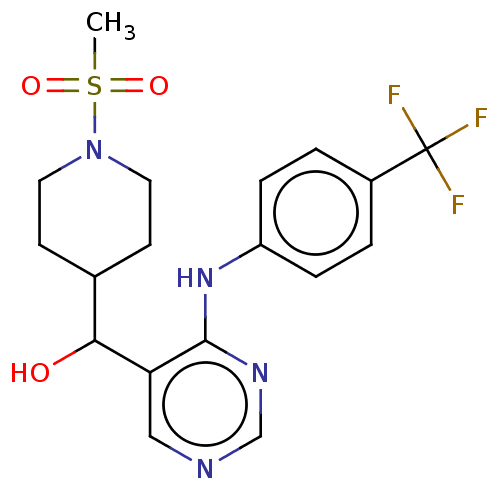
(CHEMBL4091167)Show SMILES CS(=O)(=O)N1CCC(CC1)C(O)c1cncnc1Nc1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C18H21F3N4O3S/c1-29(27,28)25-8-6-12(7-9-25)16(26)15-10-22-11-23-17(15)24-14-4-2-13(3-5-14)18(19,20)21/h2-5,10-12,16,26H,6-9H2,1H3,(H,22,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50179555
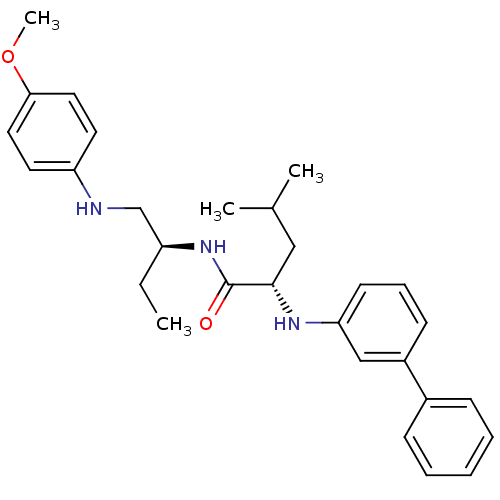
((S)-2-(biphenyl-3-ylamino)-4-methyl-pentanoic acid...)Show SMILES CC[C@@H](CNc1ccc(OC)cc1)NC(=O)[C@H](CC(C)C)Nc1cccc(c1)-c1ccccc1 Show InChI InChI=1S/C29H37N3O2/c1-5-24(20-30-25-14-16-27(34-4)17-15-25)32-29(33)28(18-21(2)3)31-26-13-9-12-23(19-26)22-10-7-6-8-11-22/h6-17,19,21,24,28,30-31H,5,18,20H2,1-4H3,(H,32,33)/t24-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Cathepsin K |
Bioorg Med Chem Lett 16: 1502-5 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.053
BindingDB Entry DOI: 10.7270/Q2T154FS |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50179548

(1-(biphenyl-3-ylamino)-cyclohexanecarboxylic acid ...)Show SMILES CCC[C@@H](CNc1ccc(OC)cc1)NC(=O)C1(CCCCC1)Nc1cccc(c1)-c1ccccc1 Show InChI InChI=1S/C31H39N3O2/c1-3-11-28(23-32-26-16-18-29(36-2)19-17-26)33-30(35)31(20-8-5-9-21-31)34-27-15-10-14-25(22-27)24-12-6-4-7-13-24/h4,6-7,10,12-19,22,28,32,34H,3,5,8-9,11,20-21,23H2,1-2H3,(H,33,35)/t28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Cathepsin K |
Bioorg Med Chem Lett 16: 1502-5 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.053
BindingDB Entry DOI: 10.7270/Q2T154FS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50238115

(CHEMBL4082403)Show SMILES CS(=O)(=O)N1CCC(CC1)C(O)(c1cncnc1Nc1ccc(F)cc1)C(F)(F)F Show InChI InChI=1S/C18H20F4N4O3S/c1-30(28,29)26-8-6-12(7-9-26)17(27,18(20,21)22)15-10-23-11-24-16(15)25-14-4-2-13(19)3-5-14/h2-5,10-12,27H,6-9H2,1H3,(H,23,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50238115

(CHEMBL4082403)Show SMILES CS(=O)(=O)N1CCC(CC1)C(O)(c1cncnc1Nc1ccc(F)cc1)C(F)(F)F Show InChI InChI=1S/C18H20F4N4O3S/c1-30(28,29)26-8-6-12(7-9-26)17(27,18(20,21)22)15-10-23-11-24-16(15)25-14-4-2-13(19)3-5-14/h2-5,10-12,27H,6-9H2,1H3,(H,23,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in HEK293A cells using deoxycorticosterone as substrate pretreated for 1 hr followed by substrate addition meas... |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50047262
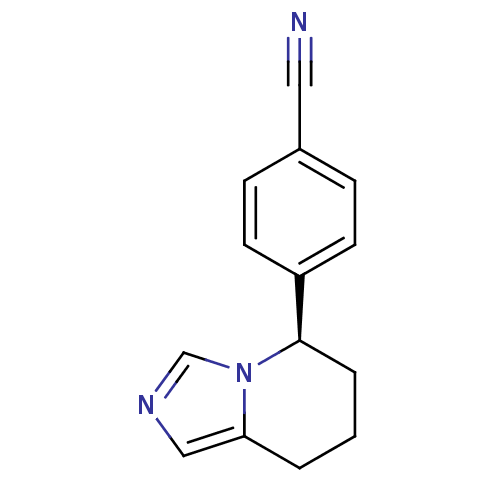
((R)-4-(5,6,7,8-tetrahydroimidazo[1,5-a]pyridin-5-y...)Show InChI InChI=1S/C14H13N3/c15-8-11-4-6-12(7-5-11)14-3-1-2-13-9-16-10-17(13)14/h4-7,9-10,14H,1-3H2/t14-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in HEK293A cells using deoxycorticosterone as substrate pretreated for 1 hr followed by substrate addition meas... |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cathepsin K
(Homo sapiens (Human)) | BDBM50179543
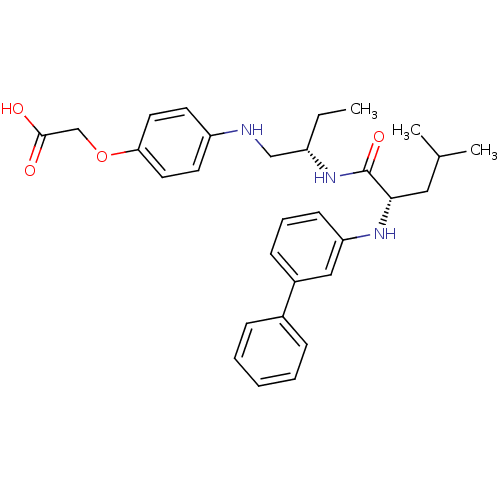
((4-{(S)-2-[(S)-2-(biphenyl-3-ylamino)-4-methyl-pen...)Show SMILES CC[C@@H](CNc1ccc(OCC(O)=O)cc1)NC(=O)[C@H](CC(C)C)Nc1cccc(c1)-c1ccccc1 Show InChI InChI=1S/C30H37N3O4/c1-4-24(19-31-25-13-15-27(16-14-25)37-20-29(34)35)33-30(36)28(17-21(2)3)32-26-12-8-11-23(18-26)22-9-6-5-7-10-22/h5-16,18,21,24,28,31-32H,4,17,19-20H2,1-3H3,(H,33,36)(H,34,35)/t24-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Cathepsin K |
Bioorg Med Chem Lett 16: 1502-5 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.053
BindingDB Entry DOI: 10.7270/Q2T154FS |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 8
(Homo sapiens (Human)) | BDBM50579915
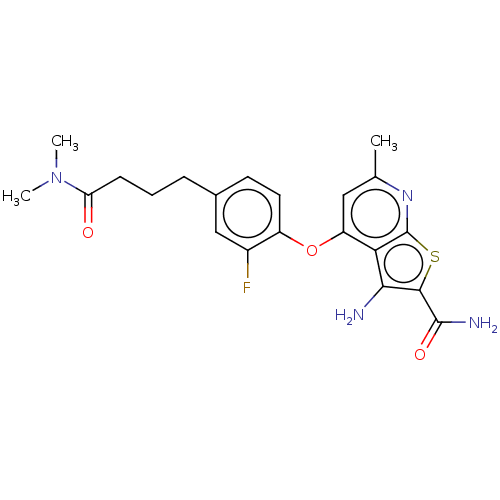
(CHEMBL4442570)Show SMILES CN(C)C(=O)CCCc1ccc(Oc2cc(C)nc3sc(C(N)=O)c(N)c23)c(F)c1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CDK8 in human HEK293 cells measured by NanoBRET assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128440
BindingDB Entry DOI: 10.7270/Q2PK0M1K |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50179547
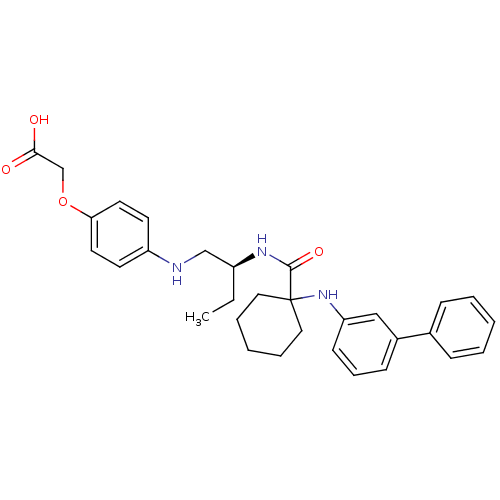
(CHEMBL557986 | [4-((S)-2-{[1-(biphenyl-3-ylamino)-...)Show SMILES CC[C@@H](CNc1ccc(OCC(O)=O)cc1)NC(=O)C1(CCCCC1)Nc1cccc(c1)-c1ccccc1 Show InChI InChI=1S/C31H37N3O4/c1-2-25(21-32-26-14-16-28(17-15-26)38-22-29(35)36)33-30(37)31(18-7-4-8-19-31)34-27-13-9-12-24(20-27)23-10-5-3-6-11-23/h3,5-6,9-17,20,25,32,34H,2,4,7-8,18-19,21-22H2,1H3,(H,33,37)(H,35,36)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Cathepsin K |
Bioorg Med Chem Lett 16: 1502-5 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.053
BindingDB Entry DOI: 10.7270/Q2T154FS |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50179539

(CHEMBL383551 | [4-((S)-2-{[1-(biphenyl-3-ylamino)-...)Show SMILES CC[C@@H](CNc1ccc(OCC(=O)OC(C)(C)C)cc1)NC(=O)C1(CCCCC1)Nc1cccc(c1)-c1ccccc1 Show InChI InChI=1S/C35H45N3O4/c1-5-28(24-36-29-17-19-31(20-18-29)41-25-32(39)42-34(2,3)4)37-33(40)35(21-10-7-11-22-35)38-30-16-12-15-27(23-30)26-13-8-6-9-14-26/h6,8-9,12-20,23,28,36,38H,5,7,10-11,21-22,24-25H2,1-4H3,(H,37,40)/t28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Cathepsin K |
Bioorg Med Chem Lett 16: 1502-5 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.053
BindingDB Entry DOI: 10.7270/Q2T154FS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50238114

(CHEMBL4093575)Show SMILES CC(C)C(O)(C1CCN(CC1)S(C)(=O)=O)c1cncnc1Nc1ccc(F)cc1 Show InChI InChI=1S/C20H27FN4O3S/c1-14(2)20(26,15-8-10-25(11-9-15)29(3,27)28)18-12-22-13-23-19(18)24-17-6-4-16(21)5-7-17/h4-7,12-15,26H,8-11H2,1-3H3,(H,22,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in HEK293A cells using deoxycorticosterone as substrate pretreated for 1 hr followed by substrate addition meas... |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50238106
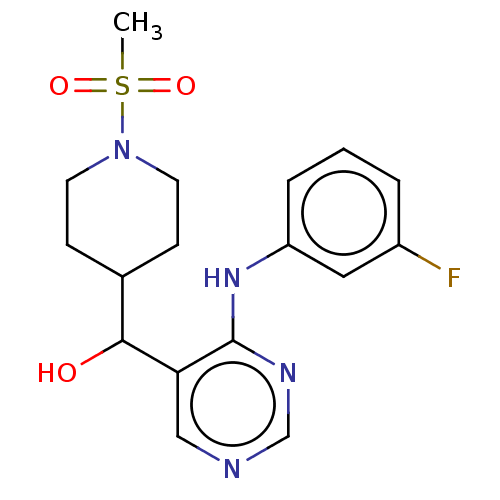
(CHEMBL4072943)Show SMILES CS(=O)(=O)N1CCC(CC1)C(O)c1cncnc1Nc1cccc(F)c1 Show InChI InChI=1S/C17H21FN4O3S/c1-26(24,25)22-7-5-12(6-8-22)16(23)15-10-19-11-20-17(15)21-14-4-2-3-13(18)9-14/h2-4,9-12,16,23H,5-8H2,1H3,(H,19,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50179542

(1-(biphenyl-3-ylamino)-cyclohexanecarboxylic acid ...)Show SMILES CCCC[C@@H](CNc1ccc(OC)cc1)NC(=O)C1(CCCCC1)Nc1cccc(c1)-c1ccccc1 Show InChI InChI=1S/C32H41N3O2/c1-3-4-15-29(24-33-27-17-19-30(37-2)20-18-27)34-31(36)32(21-9-6-10-22-32)35-28-16-11-14-26(23-28)25-12-7-5-8-13-25/h5,7-8,11-14,16-20,23,29,33,35H,3-4,6,9-10,15,21-22,24H2,1-2H3,(H,34,36)/t29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Cathepsin K |
Bioorg Med Chem Lett 16: 1502-5 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.053
BindingDB Entry DOI: 10.7270/Q2T154FS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50238103

(CHEMBL4070323)Show SMILES CC(O)(C1CCN(CC1)S(C)(=O)=O)c1cncnc1Nc1ccc(F)cc1 Show InChI InChI=1S/C18H23FN4O3S/c1-18(24,13-7-9-23(10-8-13)27(2,25)26)16-11-20-12-21-17(16)22-15-5-3-14(19)4-6-15/h3-6,11-13,24H,7-10H2,1-2H3,(H,20,21,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B1 expressed in HEK293A cells |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50179558
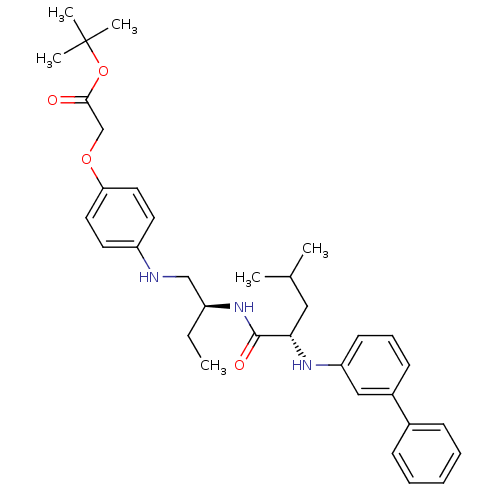
((4-{(S)-2-[(S)-2-(biphenyl-3-ylamino)-4-methyl-pen...)Show SMILES CC[C@@H](CNc1ccc(OCC(=O)OC(C)(C)C)cc1)NC(=O)[C@H](CC(C)C)Nc1cccc(c1)-c1ccccc1 Show InChI InChI=1S/C34H45N3O4/c1-7-27(22-35-28-16-18-30(19-17-28)40-23-32(38)41-34(4,5)6)37-33(39)31(20-24(2)3)36-29-15-11-14-26(21-29)25-12-9-8-10-13-25/h8-19,21,24,27,31,35-36H,7,20,22-23H2,1-6H3,(H,37,39)/t27-,31-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Cathepsin K |
Bioorg Med Chem Lett 16: 1502-5 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.053
BindingDB Entry DOI: 10.7270/Q2T154FS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50238120
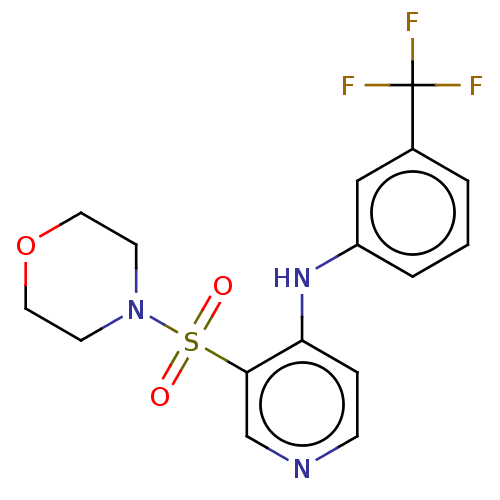
(CHEMBL4097020)Show SMILES FC(F)(F)c1cccc(Nc2ccncc2S(=O)(=O)N2CCOCC2)c1 Show InChI InChI=1S/C16H16F3N3O3S/c17-16(18,19)12-2-1-3-13(10-12)21-14-4-5-20-11-15(14)26(23,24)22-6-8-25-9-7-22/h1-5,10-11H,6-9H2,(H,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in HEK293A cells using deoxycorticosterone as substrate pretreated for 1 hr followed by substrate addition meas... |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50238117
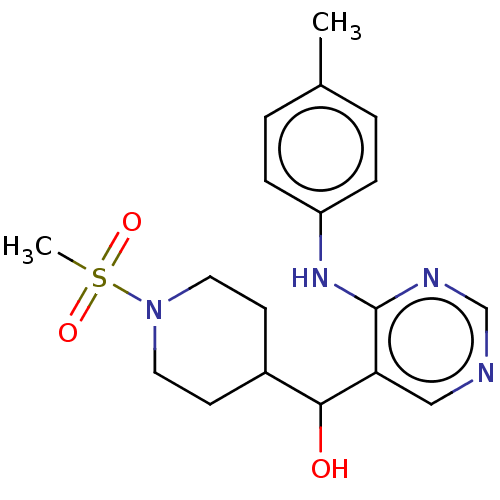
(CHEMBL4092081)Show SMILES Cc1ccc(Nc2ncncc2C(O)C2CCN(CC2)S(C)(=O)=O)cc1 Show InChI InChI=1S/C18H24N4O3S/c1-13-3-5-15(6-4-13)21-18-16(11-19-12-20-18)17(23)14-7-9-22(10-8-14)26(2,24)25/h3-6,11-12,14,17,23H,7-10H2,1-2H3,(H,19,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50238123

(CHEMBL4061565)Show SMILES CS(=O)(=O)N1CCC(CC1)C(O)c1cncnc1Nc1cccc2ccccc12 Show InChI InChI=1S/C21H24N4O3S/c1-29(27,28)25-11-9-16(10-12-25)20(26)18-13-22-14-23-21(18)24-19-8-4-6-15-5-2-3-7-17(15)19/h2-8,13-14,16,20,26H,9-12H2,1H3,(H,22,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in HEK293A cells using deoxycorticosterone as substrate pretreated for 1 hr followed by substrate addition meas... |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50179551

(1-(biphenyl-3-ylamino)-cyclohexanecarboxylic acid ...)Show SMILES COc1ccc(NC[C@H](C)NC(=O)C2(CCCCC2)Nc2cccc(c2)-c2ccccc2)cc1 Show InChI InChI=1S/C29H35N3O2/c1-22(21-30-25-14-16-27(34-2)17-15-25)31-28(33)29(18-7-4-8-19-29)32-26-13-9-12-24(20-26)23-10-5-3-6-11-23/h3,5-6,9-17,20,22,30,32H,4,7-8,18-19,21H2,1-2H3,(H,31,33)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Cathepsin K |
Bioorg Med Chem Lett 16: 1502-5 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.053
BindingDB Entry DOI: 10.7270/Q2T154FS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50238105

(CHEMBL4099824)Show SMILES CS(=O)(=O)N1CCC(CC1)C(O)c1cncnc1Nc1ccc(F)cc1 Show InChI InChI=1S/C17H21FN4O3S/c1-26(24,25)22-8-6-12(7-9-22)16(23)15-10-19-11-20-17(15)21-14-4-2-13(18)3-5-14/h2-5,10-12,16,23H,6-9H2,1H3,(H,19,20,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Mus musculus) | BDBM50238115

(CHEMBL4082403)Show SMILES CS(=O)(=O)N1CCC(CC1)C(O)(c1cncnc1Nc1ccc(F)cc1)C(F)(F)F Show InChI InChI=1S/C18H20F4N4O3S/c1-30(28,29)26-8-6-12(7-9-26)17(27,18(20,21)22)15-10-23-11-24-16(15)25-14-4-2-13(19)3-5-14/h2-5,10-12,27H,6-9H2,1H3,(H,23,24,25) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of mouse CYP11B2 expressed in HEK293 cells using deoxycorticosterone as substrate pretreated for 1 hr followed by substrate addition after... |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Mus musculus) | BDBM50238115

(CHEMBL4082403)Show SMILES CS(=O)(=O)N1CCC(CC1)C(O)(c1cncnc1Nc1ccc(F)cc1)C(F)(F)F Show InChI InChI=1S/C18H20F4N4O3S/c1-30(28,29)26-8-6-12(7-9-26)17(27,18(20,21)22)15-10-23-11-24-16(15)25-14-4-2-13(19)3-5-14/h2-5,10-12,27H,6-9H2,1H3,(H,23,24,25) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of mouse CYP11B2 expressed in HEK293 cells using deoxycorticosterone as substrate pretreated for 1 hr followed by substrate addition after... |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50179559
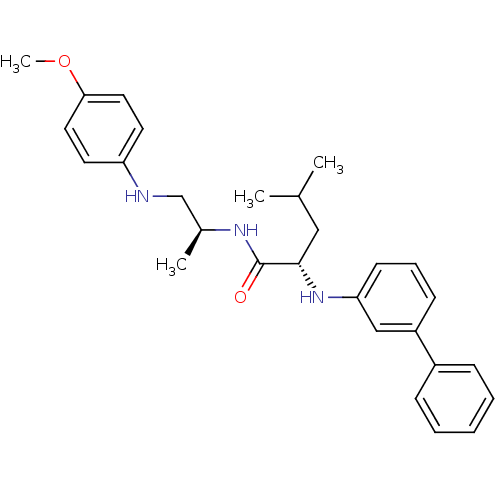
((S)-2-(biphenyl-3-ylamino)-4-methyl-pentanoic acid...)Show SMILES COc1ccc(NC[C@H](C)NC(=O)[C@H](CC(C)C)Nc2cccc(c2)-c2ccccc2)cc1 Show InChI InChI=1S/C28H35N3O2/c1-20(2)17-27(31-25-12-8-11-23(18-25)22-9-6-5-7-10-22)28(32)30-21(3)19-29-24-13-15-26(33-4)16-14-24/h5-16,18,20-21,27,29,31H,17,19H2,1-4H3,(H,30,32)/t21-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Cathepsin K |
Bioorg Med Chem Lett 16: 1502-5 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.053
BindingDB Entry DOI: 10.7270/Q2T154FS |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50179538

((S)-2-(biphenyl-3-ylamino)-4-methyl-pentanoic acid...)Show SMILES CC[C@@H](CNc1ccc(OCc2nnn[nH]2)cc1)NC(=O)[C@H](CC(C)C)Nc1cccc(c1)-c1ccccc1 Show InChI InChI=1S/C30H37N7O2/c1-4-24(19-31-25-13-15-27(16-14-25)39-20-29-34-36-37-35-29)33-30(38)28(17-21(2)3)32-26-12-8-11-23(18-26)22-9-6-5-7-10-22/h5-16,18,21,24,28,31-32H,4,17,19-20H2,1-3H3,(H,33,38)(H,34,35,36,37)/t24-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Cathepsin K |
Bioorg Med Chem Lett 16: 1502-5 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.053
BindingDB Entry DOI: 10.7270/Q2T154FS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50238108

(CHEMBL4070230)Show SMILES CS(=O)(=O)N1CCC(CC1)C(O)c1cncnc1Nc1ccc(cc1)C#N Show InChI InChI=1S/C18H21N5O3S/c1-27(25,26)23-8-6-14(7-9-23)17(24)16-11-20-12-21-18(16)22-15-4-2-13(10-19)3-5-15/h2-5,11-12,14,17,24H,6-9H2,1H3,(H,20,21,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B1 expressed in HEK293A cells |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50238106

(CHEMBL4072943)Show SMILES CS(=O)(=O)N1CCC(CC1)C(O)c1cncnc1Nc1cccc(F)c1 Show InChI InChI=1S/C17H21FN4O3S/c1-26(24,25)22-7-5-12(6-8-22)16(23)15-10-19-11-20-17(15)21-14-4-2-3-13(18)9-14/h2-4,9-12,16,23H,5-8H2,1H3,(H,19,20,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50238114

(CHEMBL4093575)Show SMILES CC(C)C(O)(C1CCN(CC1)S(C)(=O)=O)c1cncnc1Nc1ccc(F)cc1 Show InChI InChI=1S/C20H27FN4O3S/c1-14(2)20(26,15-8-10-25(11-9-15)29(3,27)28)18-12-22-13-23-19(18)24-17-6-4-16(21)5-7-17/h4-7,12-15,26H,8-11H2,1-3H3,(H,22,23,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B1 expressed in HEK293A cells |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50238107

(CHEMBL4064367)Show SMILES COc1ccc(Nc2ncncc2C(O)C2CCN(CC2)S(C)(=O)=O)cc1 Show InChI InChI=1S/C18H24N4O4S/c1-26-15-5-3-14(4-6-15)21-18-16(11-19-12-20-18)17(23)13-7-9-22(10-8-13)27(2,24)25/h3-6,11-13,17,23H,7-10H2,1-2H3,(H,19,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in HEK293A cells using deoxycorticosterone as substrate pretreated for 1 hr followed by substrate addition meas... |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50238116
(CHEMBL4079006)Show InChI InChI=1S/C17H22N4O3S/c1-25(23,24)21-9-7-13(8-10-21)16(22)15-11-18-12-19-17(15)20-14-5-3-2-4-6-14/h2-6,11-13,16,22H,7-10H2,1H3,(H,18,19,20) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50238109

(CHEMBL4091167)Show SMILES CS(=O)(=O)N1CCC(CC1)C(O)c1cncnc1Nc1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C18H21F3N4O3S/c1-29(27,28)25-8-6-12(7-9-25)16(26)15-10-22-11-23-17(15)24-14-4-2-13(3-5-14)18(19,20)21/h2-5,10-12,16,26H,6-9H2,1H3,(H,22,23,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50238117

(CHEMBL4092081)Show SMILES Cc1ccc(Nc2ncncc2C(O)C2CCN(CC2)S(C)(=O)=O)cc1 Show InChI InChI=1S/C18H24N4O3S/c1-13-3-5-15(6-4-13)21-18-16(11-19-12-20-18)17(23)14-7-9-22(10-8-14)26(2,24)25/h3-6,11-12,14,17,23H,7-10H2,1-2H3,(H,19,20,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Mus musculus) | BDBM50238114

(CHEMBL4093575)Show SMILES CC(C)C(O)(C1CCN(CC1)S(C)(=O)=O)c1cncnc1Nc1ccc(F)cc1 Show InChI InChI=1S/C20H27FN4O3S/c1-14(2)20(26,15-8-10-25(11-9-15)29(3,27)28)18-12-22-13-23-19(18)24-17-6-4-16(21)5-7-17/h4-7,12-15,26H,8-11H2,1-3H3,(H,22,23,24) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
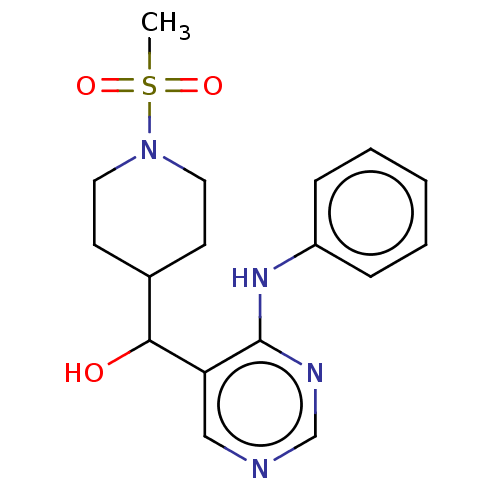
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50238102
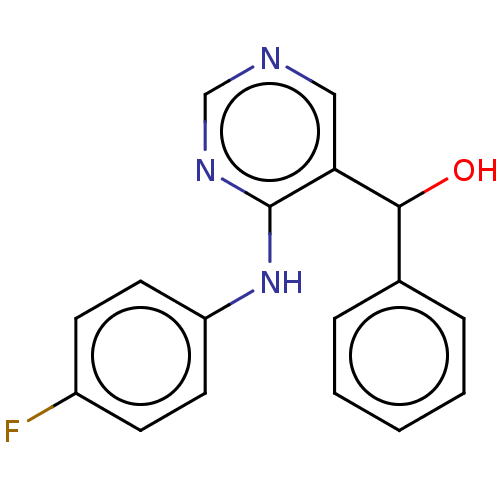
(CHEMBL4065476)Show InChI InChI=1S/C17H14FN3O/c18-13-6-8-14(9-7-13)21-17-15(10-19-11-20-17)16(22)12-4-2-1-3-5-12/h1-11,16,22H,(H,19,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in HEK293A cells using deoxycorticosterone as substrate pretreated for 1 hr followed by substrate addition meas... |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 8
(Homo sapiens (Human)) | BDBM50579909
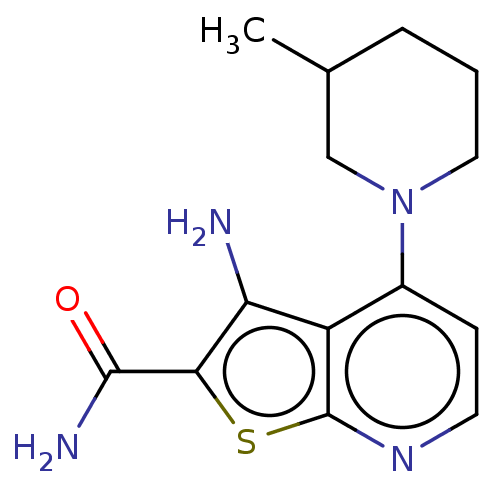
(CHEMBL5076378) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CDK8 in human HEK293 cells measured by NanoBRET assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128440
BindingDB Entry DOI: 10.7270/Q2PK0M1K |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 8
(Homo sapiens (Human)) | BDBM576465

(US11471446, Compound DBA-6) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CDK8 in human HEK293 cells measured by NanoBRET assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128440
BindingDB Entry DOI: 10.7270/Q2PK0M1K |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 8
(Homo sapiens (Human)) | BDBM50579914

(CHEMBL5071605)Show SMILES CCOCCOC[C@H]1CCCN(C1)c1ccnc2sc(C(N)=O)c(N)c12 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CDK8 in human HEK293 cells measured by NanoBRET assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128440
BindingDB Entry DOI: 10.7270/Q2PK0M1K |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 8
(Homo sapiens (Human)) | BDBM50579911
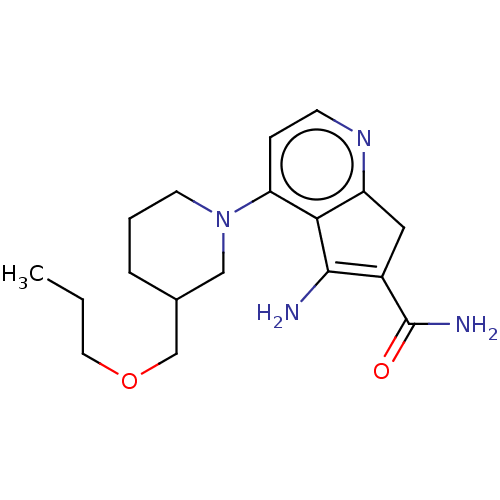
(CHEMBL5090279)Show SMILES CCCOCC1CCCN(C1)c1ccnc2CC(C(N)=O)=C(N)c12 |t:21| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CDK8 in human HEK293 cells measured by NanoBRET assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128440
BindingDB Entry DOI: 10.7270/Q2PK0M1K |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 8
(Homo sapiens (Human)) | BDBM50579912

(CHEMBL5083027)Show SMILES COC[C@H]1CCCN(C1)c1ccnc2sc(C(N)=O)c(N)c12 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CDK8 in human HEK293 cells measured by NanoBRET assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128440
BindingDB Entry DOI: 10.7270/Q2PK0M1K |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50179549

((S)-2-(biphenyl-3-ylamino)-4-methyl-pentanoic acid...)Show SMILES COc1ccc(NCCNC(=O)[C@H](CC(C)C)Nc2cccc(c2)-c2ccccc2)cc1 Show InChI InChI=1S/C27H33N3O2/c1-20(2)18-26(27(31)29-17-16-28-23-12-14-25(32-3)15-13-23)30-24-11-7-10-22(19-24)21-8-5-4-6-9-21/h4-15,19-20,26,28,30H,16-18H2,1-3H3,(H,29,31)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Cathepsin K |
Bioorg Med Chem Lett 16: 1502-5 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.053
BindingDB Entry DOI: 10.7270/Q2T154FS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50238116

(CHEMBL4079006)Show InChI InChI=1S/C17H22N4O3S/c1-25(23,24)21-9-7-13(8-10-21)16(22)15-11-18-12-19-17(15)20-14-5-3-2-4-6-14/h2-6,11-13,16,22H,7-10H2,1H3,(H,18,19,20) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50238104

(CHEMBL4072871)Show SMILES CCCCC(O)(C1CCN(CC1)S(C)(=O)=O)c1cncnc1Nc1ccc(F)cc1 Show InChI InChI=1S/C21H29FN4O3S/c1-3-4-11-21(27,16-9-12-26(13-10-16)30(2,28)29)19-14-23-15-24-20(19)25-18-7-5-17(22)6-8-18/h5-8,14-16,27H,3-4,9-13H2,1-2H3,(H,23,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi-Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in HEK293A cells using deoxycorticosterone as substrate pretreated for 1 hr followed by substrate addition meas... |
Bioorg Med Chem Lett 27: 1902-1906 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.034
BindingDB Entry DOI: 10.7270/Q2KS6TTS |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50179556

((S)-2-(3-cyclohexylphenylamino)-N-(2-(4-methoxyphe...)Show SMILES COc1ccc(NCCNC(=O)[C@H](CC(C)C)Nc2cccc(c2)C2CCCCC2)cc1 Show InChI InChI=1S/C27H39N3O2/c1-20(2)18-26(27(31)29-17-16-28-23-12-14-25(32-3)15-13-23)30-24-11-7-10-22(19-24)21-8-5-4-6-9-21/h7,10-15,19-21,26,28,30H,4-6,8-9,16-18H2,1-3H3,(H,29,31)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Cathepsin K |
Bioorg Med Chem Lett 16: 1502-5 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.053
BindingDB Entry DOI: 10.7270/Q2T154FS |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data