

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein kinase C eta type (Homo sapiens (Human)) | BDBM50097750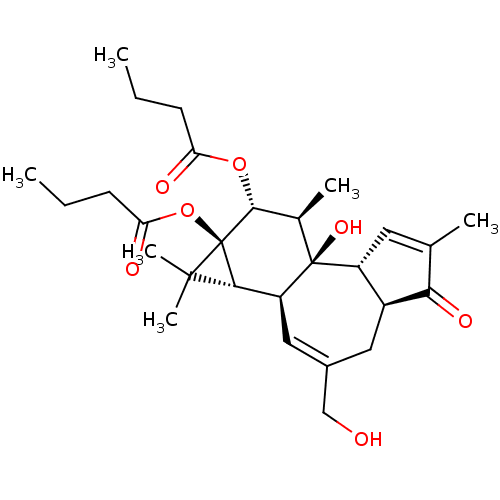 (Butyric acid (1aR,1bS,4aS,7aR,7bR,8R,9R,9aS)-9-but...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Displacement of 3[H]PDBu from Protein kinase C eta C1b domain | Bioorg Med Chem Lett 11: 719-22 (2001) BindingDB Entry DOI: 10.7270/Q2VM4BHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C delta type (Homo sapiens (Human)) | BDBM50097750 (Butyric acid (1aR,1bS,4aS,7aR,7bR,8R,9R,9aS)-9-but...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Displacement of 3[H]PDBu from Protein kinase C delta C1a domain | Bioorg Med Chem Lett 11: 719-22 (2001) BindingDB Entry DOI: 10.7270/Q2VM4BHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C epsilon type (Homo sapiens (Human)) | BDBM50097750 (Butyric acid (1aR,1bS,4aS,7aR,7bR,8R,9R,9aS)-9-but...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Displacement of 3[H]PDBu from Protein kinase C epsilon C1b domain | Bioorg Med Chem Lett 11: 719-22 (2001) BindingDB Entry DOI: 10.7270/Q2VM4BHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM50097750 (Butyric acid (1aR,1bS,4aS,7aR,7bR,8R,9R,9aS)-9-but...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Displacement of 3[H]PDBu from Protein kinase C theta C1b domain | Bioorg Med Chem Lett 11: 719-22 (2001) BindingDB Entry DOI: 10.7270/Q2VM4BHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C eta type (Homo sapiens (Human)) | BDBM50057512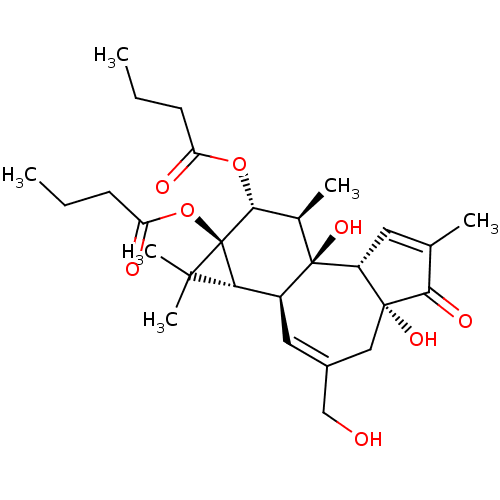 ((1aR,1bS,4aR,7aS,7bS,8R,9R,9aS)-4a,7b-dihydroxy-3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Displacement of 3[H]PDBu from Protein kinase C eta C1b domain | Bioorg Med Chem Lett 11: 719-22 (2001) BindingDB Entry DOI: 10.7270/Q2VM4BHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C delta type (Homo sapiens (Human)) | BDBM50057512 ((1aR,1bS,4aR,7aS,7bS,8R,9R,9aS)-4a,7b-dihydroxy-3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Displacement of 3[H]PDBu from Protein kinase C delta C1a domain | Bioorg Med Chem Lett 11: 719-22 (2001) BindingDB Entry DOI: 10.7270/Q2VM4BHZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protein kinase C epsilon type (Homo sapiens (Human)) | BDBM50057512 ((1aR,1bS,4aR,7aS,7bS,8R,9R,9aS)-4a,7b-dihydroxy-3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Displacement of 3[H]PDBu from Protein kinase C epsilon C1b domain | Bioorg Med Chem Lett 11: 719-22 (2001) BindingDB Entry DOI: 10.7270/Q2VM4BHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26976 (2-benzoylquinazoline analogue, (1R, 2S)-17 | N-(4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.40 | -50.5 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Nippon Shinyaku Co. | Assay Description Competitive binding displacement analysis was performed with membranes prepared from CHO-K1 cells stably expressing receptors. After incubation, samp... | Bioorg Med Chem 17: 119-32 (2009) Article DOI: 10.1016/j.bmc.2008.11.012 BindingDB Entry DOI: 10.7270/Q2Q52MX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM50057512 ((1aR,1bS,4aR,7aS,7bS,8R,9R,9aS)-4a,7b-dihydroxy-3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Displacement of 3[H]PDBu from Protein kinase C theta C1b domain | Bioorg Med Chem Lett 11: 719-22 (2001) BindingDB Entry DOI: 10.7270/Q2VM4BHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM50057512 ((1aR,1bS,4aR,7aS,7bS,8R,9R,9aS)-4a,7b-dihydroxy-3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Displacement of 3[H]PDBu from Protein kinase C alpha C1a domain | Bioorg Med Chem Lett 11: 719-22 (2001) BindingDB Entry DOI: 10.7270/Q2VM4BHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C gamma type (Homo sapiens (Human)) | BDBM50057512 ((1aR,1bS,4aR,7aS,7bS,8R,9R,9aS)-4a,7b-dihydroxy-3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Displacement of 3[H]PDBu from Protein kinase C eta C1a domain | Bioorg Med Chem Lett 11: 719-22 (2001) BindingDB Entry DOI: 10.7270/Q2VM4BHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C gamma type (Homo sapiens (Human)) | BDBM50097750 (Butyric acid (1aR,1bS,4aS,7aR,7bR,8R,9R,9aS)-9-but...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Displacement of 3[H]PDBu from Protein kinase C eta C1a domain | Bioorg Med Chem Lett 11: 719-22 (2001) BindingDB Entry DOI: 10.7270/Q2VM4BHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Homo sapiens (Human)) | BDBM50057512 ((1aR,1bS,4aR,7aS,7bS,8R,9R,9aS)-4a,7b-dihydroxy-3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Displacement of 3[H]PDBu from Protein kinase C epsilon C1a domain | Bioorg Med Chem Lett 11: 719-22 (2001) BindingDB Entry DOI: 10.7270/Q2VM4BHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Homo sapiens (Human)) | BDBM50057512 ((1aR,1bS,4aR,7aS,7bS,8R,9R,9aS)-4a,7b-dihydroxy-3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Displacement of 3[H]PDBu from Protein kinase C beta C1a domain | Bioorg Med Chem Lett 11: 719-22 (2001) BindingDB Entry DOI: 10.7270/Q2VM4BHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolactin-releasing peptide receptor (Homo sapiens (Human)) | BDBM195644 (US9206173, 2406) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2 | -49.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
OTSUKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description Receptor-expressing HEK 293 cell membrane fraction was analyzed by modifying the methods of C. J. Langmead et al. (see Langmead C J, Szekeres P G; Ch... | US Patent US9206173 (2015) BindingDB Entry DOI: 10.7270/Q23X85FS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolactin-releasing peptide receptor (Homo sapiens (Human)) | BDBM195624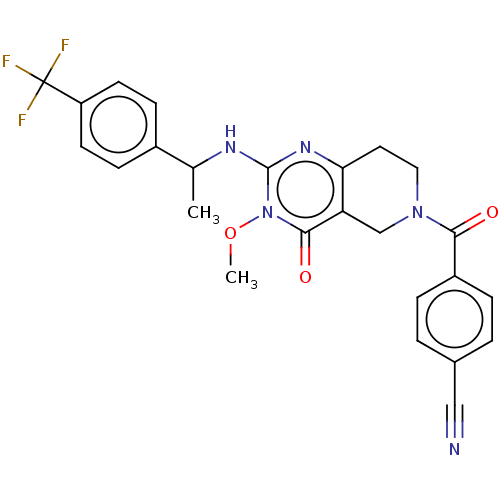 (US9206173, 2386) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2 | -49.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
OTSUKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description Receptor-expressing HEK 293 cell membrane fraction was analyzed by modifying the methods of C. J. Langmead et al. (see Langmead C J, Szekeres P G; Ch... | US Patent US9206173 (2015) BindingDB Entry DOI: 10.7270/Q23X85FS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolactin-releasing peptide receptor (Homo sapiens (Human)) | BDBM195673 (US9206173, 2435) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2 | -49.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
OTSUKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description Receptor-expressing HEK 293 cell membrane fraction was analyzed by modifying the methods of C. J. Langmead et al. (see Langmead C J, Szekeres P G; Ch... | US Patent US9206173 (2015) BindingDB Entry DOI: 10.7270/Q23X85FS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26975 (2-benzoylquinazoline analogue, (rac)-17 | N-{4-[(2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.10 | -49.5 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Nippon Shinyaku Co. | Assay Description Competitive binding displacement analysis was performed with membranes prepared from CHO-K1 cells stably expressing receptors. After incubation, samp... | Bioorg Med Chem 17: 119-32 (2009) Article DOI: 10.1016/j.bmc.2008.11.012 BindingDB Entry DOI: 10.7270/Q2Q52MX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Homo sapiens (Human)) | BDBM50097750 (Butyric acid (1aR,1bS,4aS,7aR,7bR,8R,9R,9aS)-9-but...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Displacement of 3[H]PDBu from Protein kinase C beta C1a domain | Bioorg Med Chem Lett 11: 719-22 (2001) BindingDB Entry DOI: 10.7270/Q2VM4BHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Homo sapiens (Human)) | BDBM50097750 (Butyric acid (1aR,1bS,4aS,7aR,7bR,8R,9R,9aS)-9-but...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Displacement of 3[H]PDBu from Protein kinase C gamma C1b domain | Bioorg Med Chem Lett 11: 719-22 (2001) BindingDB Entry DOI: 10.7270/Q2VM4BHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C gamma type (Homo sapiens (Human)) | BDBM50057512 ((1aR,1bS,4aR,7aS,7bS,8R,9R,9aS)-4a,7b-dihydroxy-3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Displacement of 3[H]PDBu from Protein kinase C gamma C1a domain | Bioorg Med Chem Lett 11: 719-22 (2001) BindingDB Entry DOI: 10.7270/Q2VM4BHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolactin-releasing peptide receptor (Homo sapiens (Human)) | BDBM195733 (US9206173, 2495) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3 | -48.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
OTSUKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description Receptor-expressing HEK 293 cell membrane fraction was analyzed by modifying the methods of C. J. Langmead et al. (see Langmead C J, Szekeres P G; Ch... | US Patent US9206173 (2015) BindingDB Entry DOI: 10.7270/Q23X85FS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolactin-releasing peptide receptor (Homo sapiens (Human)) | BDBM195701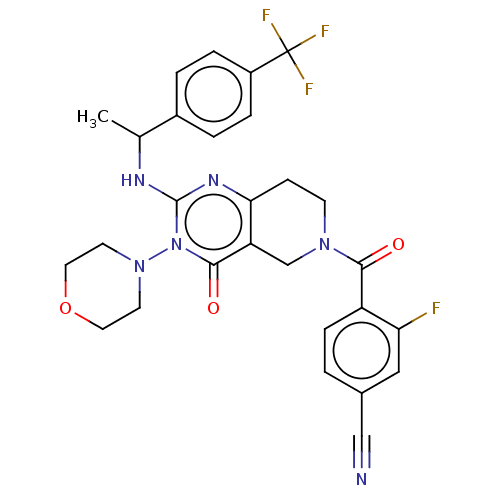 (US9206173, 2463) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3 | -48.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
OTSUKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description Receptor-expressing HEK 293 cell membrane fraction was analyzed by modifying the methods of C. J. Langmead et al. (see Langmead C J, Szekeres P G; Ch... | US Patent US9206173 (2015) BindingDB Entry DOI: 10.7270/Q23X85FS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolactin-releasing peptide receptor (Homo sapiens (Human)) | BDBM195690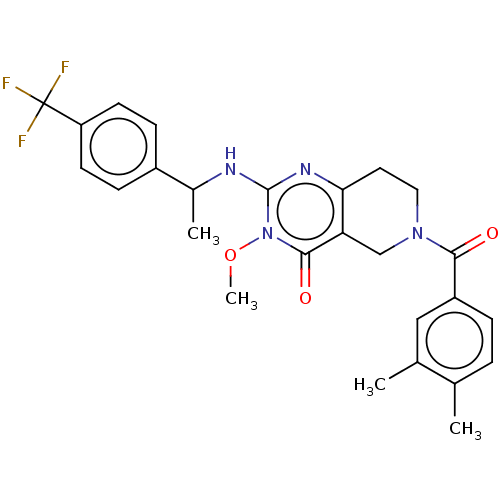 (US9206173, 2452) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4 | -47.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
OTSUKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description Receptor-expressing HEK 293 cell membrane fraction was analyzed by modifying the methods of C. J. Langmead et al. (see Langmead C J, Szekeres P G; Ch... | US Patent US9206173 (2015) BindingDB Entry DOI: 10.7270/Q23X85FS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM50097750 (Butyric acid (1aR,1bS,4aS,7aR,7bR,8R,9R,9aS)-9-but...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Displacement of 3[H]PDBu from Protein kinase C delta C1a domain | Bioorg Med Chem Lett 11: 719-22 (2001) BindingDB Entry DOI: 10.7270/Q2VM4BHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C epsilon type (Homo sapiens (Human)) | BDBM50097750 (Butyric acid (1aR,1bS,4aS,7aR,7bR,8R,9R,9aS)-9-but...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Displacement of 3[H]PDBu from Protein kinase C beta C1a domain | Bioorg Med Chem Lett 11: 719-22 (2001) BindingDB Entry DOI: 10.7270/Q2VM4BHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolactin-releasing peptide receptor (Homo sapiens (Human)) | BDBM195613 (US9206173, 1) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 5 | -47.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
OTSUKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description Receptor-expressing HEK 293 cell membrane fraction was analyzed by modifying the methods of C. J. Langmead et al. (see Langmead C J, Szekeres P G; Ch... | US Patent US9206173 (2015) BindingDB Entry DOI: 10.7270/Q23X85FS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolactin-releasing peptide receptor (Homo sapiens (Human)) | BDBM195749 (US9206173, 95) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 5 | -47.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
OTSUKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description Receptor-expressing HEK 293 cell membrane fraction was analyzed by modifying the methods of C. J. Langmead et al. (see Langmead C J, Szekeres P G; Ch... | US Patent US9206173 (2015) BindingDB Entry DOI: 10.7270/Q23X85FS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolactin-releasing peptide receptor (Homo sapiens (Human)) | BDBM195671 (US9206173, 2433) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 5 | -47.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
OTSUKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description Receptor-expressing HEK 293 cell membrane fraction was analyzed by modifying the methods of C. J. Langmead et al. (see Langmead C J, Szekeres P G; Ch... | US Patent US9206173 (2015) BindingDB Entry DOI: 10.7270/Q23X85FS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C gamma type (Homo sapiens (Human)) | BDBM50097750 (Butyric acid (1aR,1bS,4aS,7aR,7bR,8R,9R,9aS)-9-but...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Displacement of 3[H]PDBu from Protein kinase C gamma C1a domain | Bioorg Med Chem Lett 11: 719-22 (2001) BindingDB Entry DOI: 10.7270/Q2VM4BHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C eta type (Homo sapiens (Human)) | BDBM50057511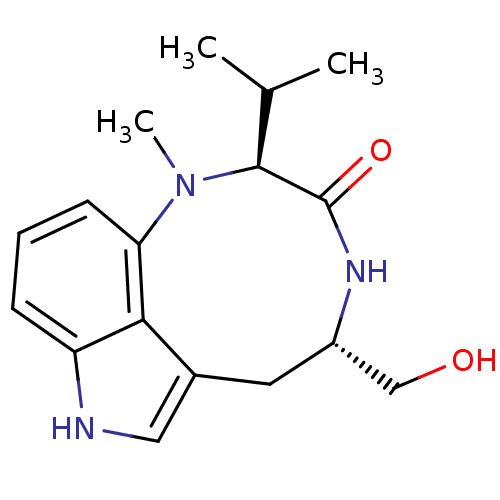 ((+/-)-indolactum-V 13-Hydroxymethyl-10-isopropyl-9...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibition of [3H]phorbol-12,13-dibutyrate (PDBu) binding to Protein kinase C eta C1b domain | Bioorg Med Chem Lett 11: 723-8 (2001) BindingDB Entry DOI: 10.7270/Q2QV3KRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolactin-releasing peptide receptor (Homo sapiens (Human)) | BDBM195694 (US9206173, 2456) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 6 | -46.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
OTSUKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description Receptor-expressing HEK 293 cell membrane fraction was analyzed by modifying the methods of C. J. Langmead et al. (see Langmead C J, Szekeres P G; Ch... | US Patent US9206173 (2015) BindingDB Entry DOI: 10.7270/Q23X85FS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolactin-releasing peptide receptor (Homo sapiens (Human)) | BDBM195706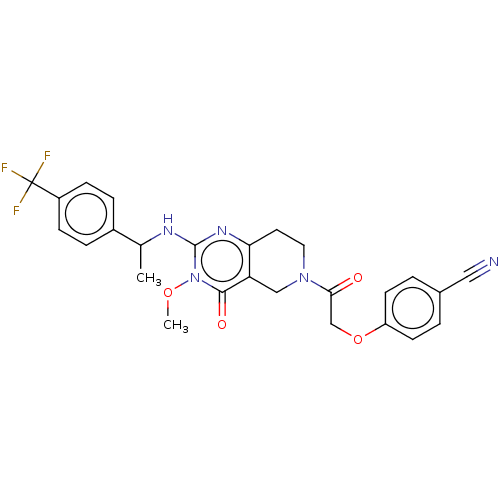 (US9206173, 2468) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 6 | -46.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
OTSUKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description Receptor-expressing HEK 293 cell membrane fraction was analyzed by modifying the methods of C. J. Langmead et al. (see Langmead C J, Szekeres P G; Ch... | US Patent US9206173 (2015) BindingDB Entry DOI: 10.7270/Q23X85FS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolactin-releasing peptide receptor (Homo sapiens (Human)) | BDBM195640 (US9206173, 2402) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 6 | -46.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
OTSUKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description Receptor-expressing HEK 293 cell membrane fraction was analyzed by modifying the methods of C. J. Langmead et al. (see Langmead C J, Szekeres P G; Ch... | US Patent US9206173 (2015) BindingDB Entry DOI: 10.7270/Q23X85FS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolactin-releasing peptide receptor (Homo sapiens (Human)) | BDBM195722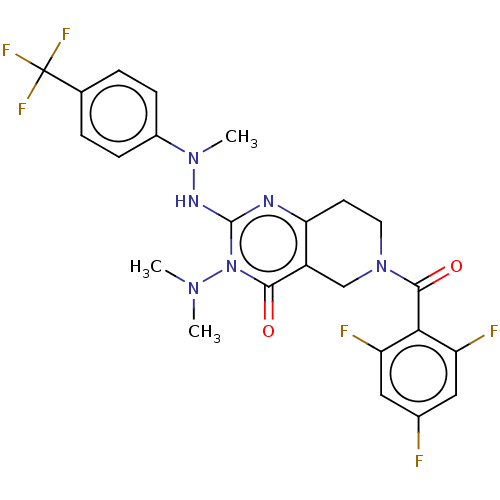 (US9206173, 2484) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 6 | -46.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
OTSUKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description Receptor-expressing HEK 293 cell membrane fraction was analyzed by modifying the methods of C. J. Langmead et al. (see Langmead C J, Szekeres P G; Ch... | US Patent US9206173 (2015) BindingDB Entry DOI: 10.7270/Q23X85FS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolactin-releasing peptide receptor (Homo sapiens (Human)) | BDBM195753 (US9206173, 166) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 6 | -46.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
OTSUKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description Receptor-expressing HEK 293 cell membrane fraction was analyzed by modifying the methods of C. J. Langmead et al. (see Langmead C J, Szekeres P G; Ch... | US Patent US9206173 (2015) BindingDB Entry DOI: 10.7270/Q23X85FS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C eta type (Homo sapiens (Human)) | BDBM50057510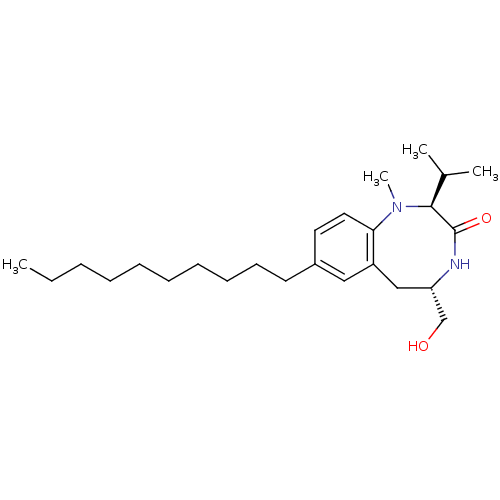 ((2S,5S)-8-Decyl-5-hydroxymethyl-2-isopropyl-1-meth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibition of [3H]phorbol-12,13-dibutyrate (PDBu) binding to Protein kinase C eta C1b domain | Bioorg Med Chem Lett 11: 723-8 (2001) BindingDB Entry DOI: 10.7270/Q2QV3KRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolactin-releasing peptide receptor (Homo sapiens (Human)) | BDBM195657 (US9206173, 2419) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 7 | -46.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
OTSUKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description Receptor-expressing HEK 293 cell membrane fraction was analyzed by modifying the methods of C. J. Langmead et al. (see Langmead C J, Szekeres P G; Ch... | US Patent US9206173 (2015) BindingDB Entry DOI: 10.7270/Q23X85FS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C epsilon type (Homo sapiens (Human)) | BDBM50057511 ((+/-)-indolactum-V 13-Hydroxymethyl-10-isopropyl-9...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibition of [3H]phorbol-12,13-dibutyrate (PDBu) binding to Protein kinase C epsilon C1b domain | Bioorg Med Chem Lett 11: 723-8 (2001) BindingDB Entry DOI: 10.7270/Q2QV3KRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolactin-releasing peptide receptor (Homo sapiens (Human)) | BDBM195695 (US9206173, 2457) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 8 | -46.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
OTSUKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description Receptor-expressing HEK 293 cell membrane fraction was analyzed by modifying the methods of C. J. Langmead et al. (see Langmead C J, Szekeres P G; Ch... | US Patent US9206173 (2015) BindingDB Entry DOI: 10.7270/Q23X85FS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolactin-releasing peptide receptor (Homo sapiens (Human)) | BDBM195617 (US9206173, 5) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 8 | -46.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
OTSUKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description Receptor-expressing HEK 293 cell membrane fraction was analyzed by modifying the methods of C. J. Langmead et al. (see Langmead C J, Szekeres P G; Ch... | US Patent US9206173 (2015) BindingDB Entry DOI: 10.7270/Q23X85FS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolactin-releasing peptide receptor (Homo sapiens (Human)) | BDBM195720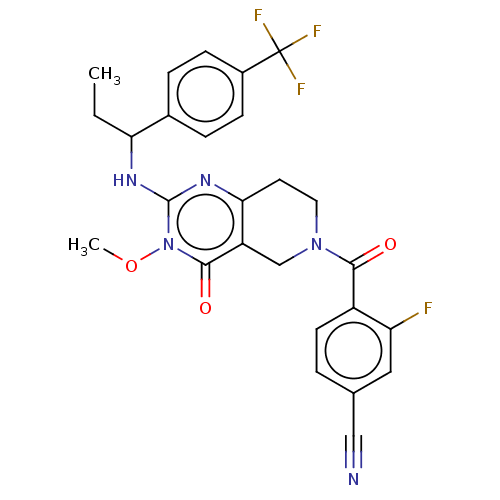 (US9206173, 2482) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 8 | -46.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
OTSUKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description Receptor-expressing HEK 293 cell membrane fraction was analyzed by modifying the methods of C. J. Langmead et al. (see Langmead C J, Szekeres P G; Ch... | US Patent US9206173 (2015) BindingDB Entry DOI: 10.7270/Q23X85FS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolactin-releasing peptide receptor (Homo sapiens (Human)) | BDBM195650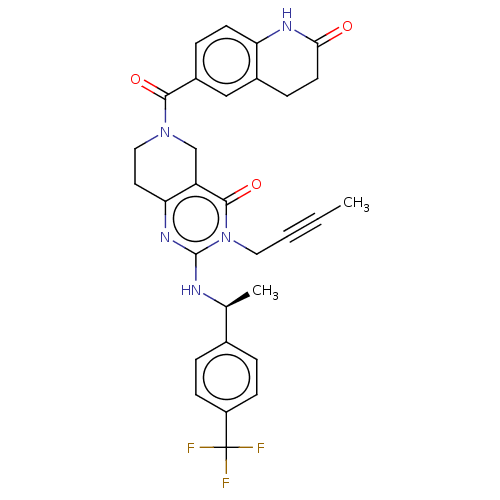 (US9206173, 2412) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 8 | -46.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
OTSUKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description Receptor-expressing HEK 293 cell membrane fraction was analyzed by modifying the methods of C. J. Langmead et al. (see Langmead C J, Szekeres P G; Ch... | US Patent US9206173 (2015) BindingDB Entry DOI: 10.7270/Q23X85FS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolactin-releasing peptide receptor (Homo sapiens (Human)) | BDBM195656 (US9206173, 2418) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 8 | -46.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
OTSUKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description Receptor-expressing HEK 293 cell membrane fraction was analyzed by modifying the methods of C. J. Langmead et al. (see Langmead C J, Szekeres P G; Ch... | US Patent US9206173 (2015) BindingDB Entry DOI: 10.7270/Q23X85FS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolactin-releasing peptide receptor (Homo sapiens (Human)) | BDBM195627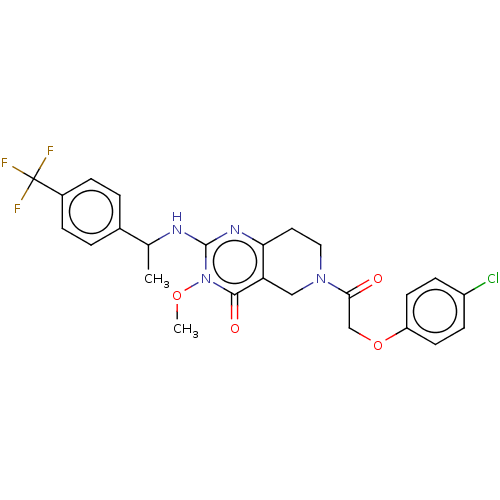 (US9206173, 2389) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 8 | -46.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
OTSUKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description Receptor-expressing HEK 293 cell membrane fraction was analyzed by modifying the methods of C. J. Langmead et al. (see Langmead C J, Szekeres P G; Ch... | US Patent US9206173 (2015) BindingDB Entry DOI: 10.7270/Q23X85FS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolactin-releasing peptide receptor (Homo sapiens (Human)) | BDBM195628 (US9206173, 2390) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 8 | -46.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
OTSUKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description Receptor-expressing HEK 293 cell membrane fraction was analyzed by modifying the methods of C. J. Langmead et al. (see Langmead C J, Szekeres P G; Ch... | US Patent US9206173 (2015) BindingDB Entry DOI: 10.7270/Q23X85FS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolactin-releasing peptide receptor (Homo sapiens (Human)) | BDBM195645 (US9206173, 2407) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 8 | -46.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
OTSUKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description Receptor-expressing HEK 293 cell membrane fraction was analyzed by modifying the methods of C. J. Langmead et al. (see Langmead C J, Szekeres P G; Ch... | US Patent US9206173 (2015) BindingDB Entry DOI: 10.7270/Q23X85FS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C delta type (Homo sapiens (Human)) | BDBM50057511 ((+/-)-indolactum-V 13-Hydroxymethyl-10-isopropyl-9...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibition of [3H]phorbol-12,13-dibutyrate (PDBu) binding to Protein kinase C epsilon C1b domain | Bioorg Med Chem Lett 11: 723-8 (2001) BindingDB Entry DOI: 10.7270/Q2QV3KRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM50057511 ((+/-)-indolactum-V 13-Hydroxymethyl-10-isopropyl-9...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibition of [3H]phorbol-12,13-dibutyrate (PDBu) binding to Protein kinase C theta C1b domain | Bioorg Med Chem Lett 11: 723-8 (2001) BindingDB Entry DOI: 10.7270/Q2QV3KRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolactin-releasing peptide receptor (Homo sapiens (Human)) | BDBM195689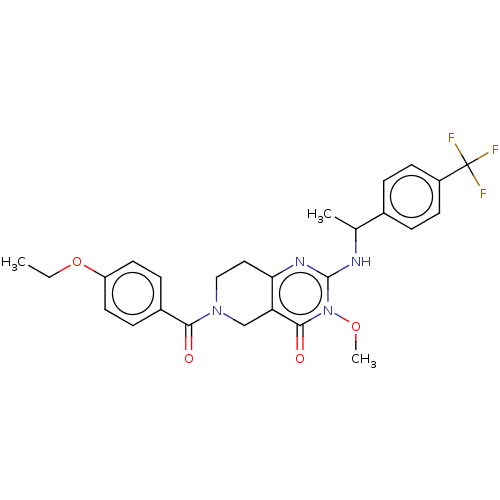 (US9206173, 2451) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 9 | -45.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
OTSUKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description Receptor-expressing HEK 293 cell membrane fraction was analyzed by modifying the methods of C. J. Langmead et al. (see Langmead C J, Szekeres P G; Ch... | US Patent US9206173 (2015) BindingDB Entry DOI: 10.7270/Q23X85FS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2284 total ) | Next | Last >> |