

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine deaminase (Homo sapiens (Human)) | BDBM22925 ((8R)-3-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)oxol...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 0.0330 | -59.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd. | Assay Description The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... | Bioorg Med Chem Lett 13: 1115-8 (2003) Article DOI: 10.1016/S0960-894X(03)00026-X BindingDB Entry DOI: 10.7270/Q29Z936D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Homo sapiens (Human)) | BDBM22947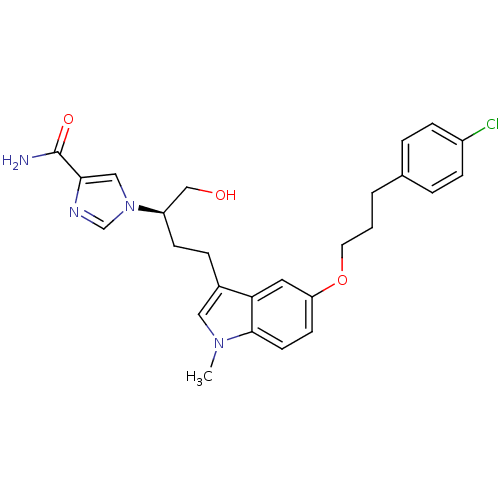 (1-[(2R)-4-{5-[3-(4-chlorophenyl)propoxy]-1-methyl-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.90 | -47.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd. | Assay Description The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... | J Med Chem 47: 3730-43 (2004) Article DOI: 10.1021/jm0306374 BindingDB Entry DOI: 10.7270/Q2668BG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Homo sapiens (Human)) | BDBM22937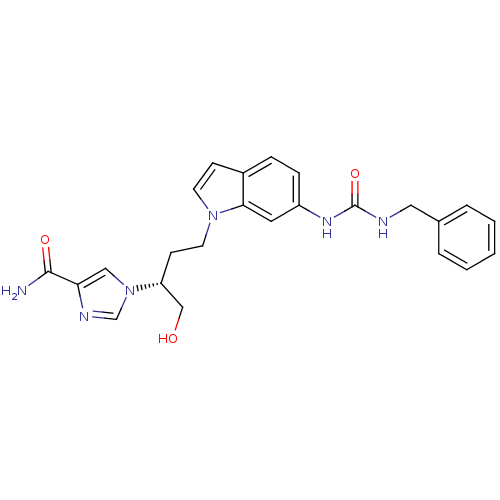 (1-[(2R)-4-{6-[(benzylcarbamoyl)amino]-1H-indol-1-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 7.5 | -45.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd. | Assay Description The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... | J Med Chem 47: 3730-43 (2004) Article DOI: 10.1021/jm0306374 BindingDB Entry DOI: 10.7270/Q2668BG0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine deaminase (Homo sapiens (Human)) | BDBM22920 (1-[(2R)-1-hydroxy-4-{6-[3-(1-methyl-1H-1,3-benzodi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 7.70 | -45.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd. | Assay Description The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... | J Med Chem 47: 2728-31 (2004) Article DOI: 10.1021/jm0499559 BindingDB Entry DOI: 10.7270/Q22F7KQT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine deaminase (Homo sapiens (Human)) | BDBM22920 (1-[(2R)-1-hydroxy-4-{6-[3-(1-methyl-1H-1,3-benzodi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 7.70 | -45.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd. | Assay Description The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... | J Am Chem Soc 126: 34-5 (2004) Article DOI: 10.1021/ja038606l BindingDB Entry DOI: 10.7270/Q2FQ9TWW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine deaminase (Homo sapiens (Human)) | BDBM22920 (1-[(2R)-1-hydroxy-4-{6-[3-(1-methyl-1H-1,3-benzodi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 7.70 | -45.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd. | Assay Description The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... | J Med Chem 47: 3730-43 (2004) Article DOI: 10.1021/jm0306374 BindingDB Entry DOI: 10.7270/Q2668BG0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine deaminase (Homo sapiens (Human)) | BDBM22950 (1-[(3R,4S)-4-hydroxy-1-(naphthalen-2-yloxy)pentan-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem | MMDB PDB Article PubMed | 9.80 | -45.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd. | Assay Description The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... | J Med Chem 47: 2728-31 (2004) Article DOI: 10.1021/jm0499559 BindingDB Entry DOI: 10.7270/Q22F7KQT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine deaminase (Homo sapiens (Human)) | BDBM22948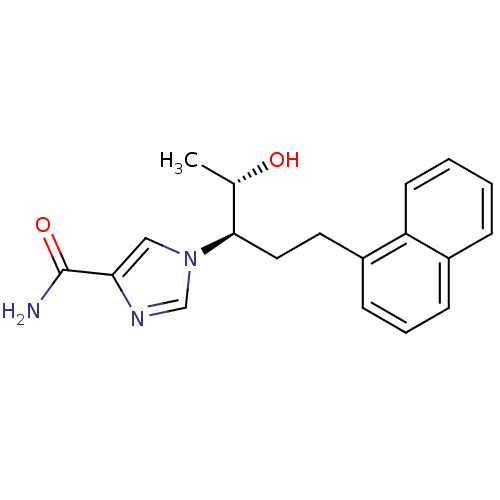 (1-[(1R,2S)-2-hydroxy-1-(2-naphthalen-1-ylethyl)pro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 11 | -45.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd. | Assay Description The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... | J Med Chem 47: 2728-31 (2004) Article DOI: 10.1021/jm0499559 BindingDB Entry DOI: 10.7270/Q22F7KQT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine deaminase (Homo sapiens (Human)) | BDBM22931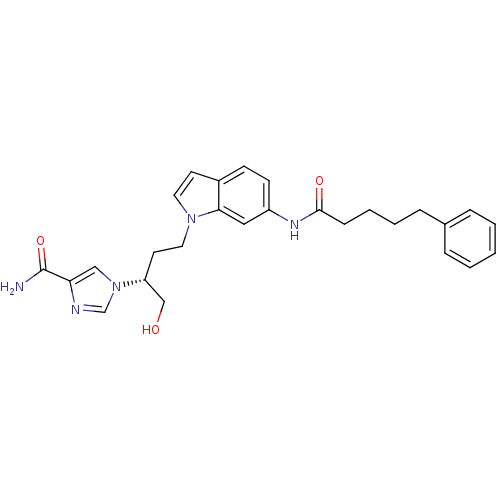 (1-[(2R)-1-hydroxy-4-[6-(5-phenylpentanamido)-1H-in...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 11 | -45.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd. | Assay Description The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... | J Med Chem 47: 3730-43 (2004) Article DOI: 10.1021/jm0306374 BindingDB Entry DOI: 10.7270/Q2668BG0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine deaminase (Homo sapiens (Human)) | BDBM22939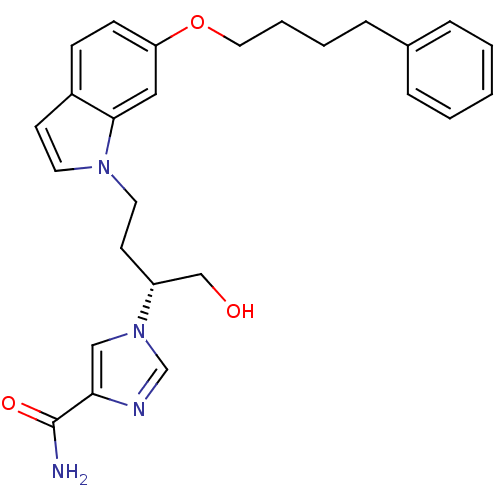 (1-[(2R)-1-hydroxy-4-[6-(4-phenylbutoxy)-1H-indol-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 12 | -44.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd. | Assay Description The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... | J Med Chem 47: 3730-43 (2004) Article DOI: 10.1021/jm0306374 BindingDB Entry DOI: 10.7270/Q2668BG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Homo sapiens (Human)) | BDBM22942 (1-[(2R)-4-{6-[3-(4-chlorophenyl)propoxy]-1H-indol-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 13 | -44.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd. | Assay Description The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... | J Med Chem 47: 3730-43 (2004) Article DOI: 10.1021/jm0306374 BindingDB Entry DOI: 10.7270/Q2668BG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Homo sapiens (Human)) | BDBM22946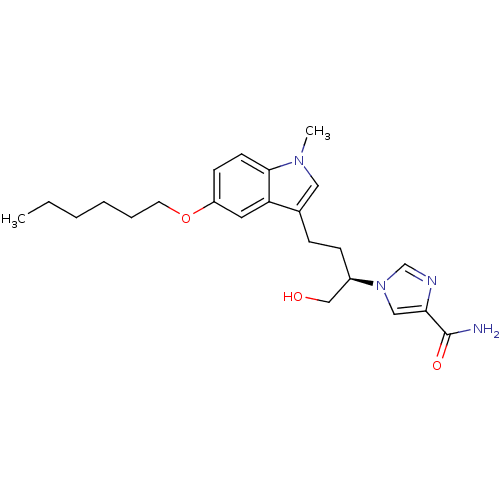 (1-[(2R)-4-[5-(hexyloxy)-1-methyl-1H-indol-3-yl]-1-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 13 | -44.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd. | Assay Description The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... | J Med Chem 47: 3730-43 (2004) Article DOI: 10.1021/jm0306374 BindingDB Entry DOI: 10.7270/Q2668BG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Homo sapiens (Human)) | BDBM22949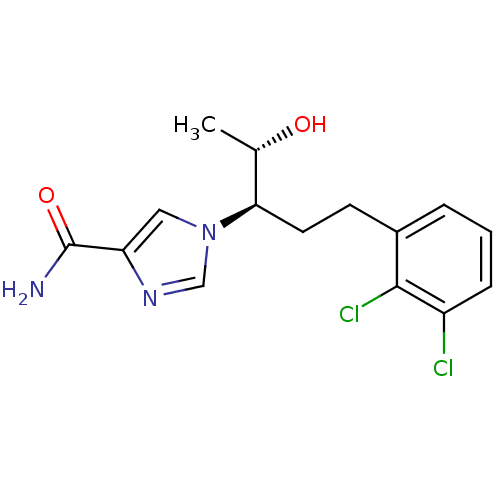 (1-[(3R,4S)-1-(2,3-dichlorophenyl)-4-hydroxypentan-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents | MMDB PDB Article PubMed | 13 | -44.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd. | Assay Description The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... | J Med Chem 47: 2728-31 (2004) Article DOI: 10.1021/jm0499559 BindingDB Entry DOI: 10.7270/Q22F7KQT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine deaminase (Homo sapiens (Human)) | BDBM22930 (1-[(2R)-1-hydroxy-4-[6-(4-phenylbutanamido)-1H-ind...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 16 | -44.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd. | Assay Description The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... | J Med Chem 47: 3730-43 (2004) Article DOI: 10.1021/jm0306374 BindingDB Entry DOI: 10.7270/Q2668BG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Homo sapiens (Human)) | BDBM22938 (1-[(2R)-1-hydroxy-4-[6-(3-phenylpropoxy)-1H-indol-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 17 | -43.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd. | Assay Description The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... | J Med Chem 47: 3730-43 (2004) Article DOI: 10.1021/jm0306374 BindingDB Entry DOI: 10.7270/Q2668BG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Homo sapiens (Human)) | BDBM22936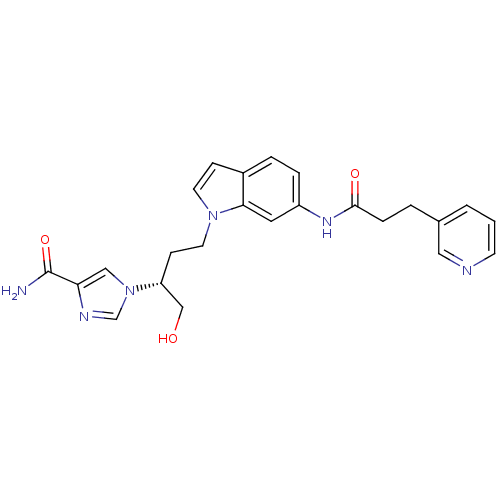 (1-[(2R)-1-hydroxy-4-{6-[3-(pyridin-3-yl)propanamid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 17 | -43.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd. | Assay Description The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... | J Med Chem 47: 3730-43 (2004) Article DOI: 10.1021/jm0306374 BindingDB Entry DOI: 10.7270/Q2668BG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Homo sapiens (Human)) | BDBM22928 (1-[(2R)-4-(6-hexanamido-1H-indol-1-yl)-1-hydroxybu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 24 | -43.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd. | Assay Description The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... | J Med Chem 47: 3730-43 (2004) Article DOI: 10.1021/jm0306374 BindingDB Entry DOI: 10.7270/Q2668BG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Homo sapiens (Human)) | BDBM22945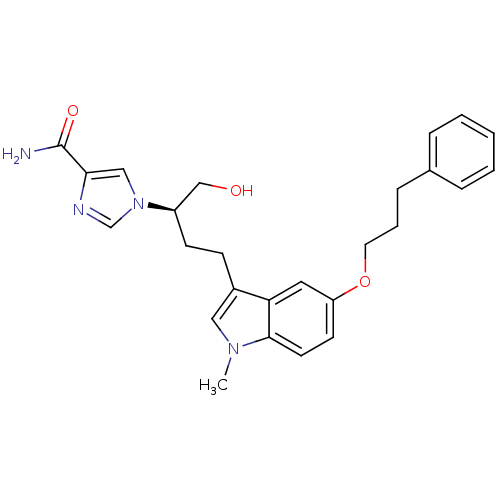 (1-[(2R)-1-hydroxy-4-[1-methyl-5-(3-phenylpropoxy)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 26 | -42.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd. | Assay Description The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... | J Med Chem 47: 3730-43 (2004) Article DOI: 10.1021/jm0306374 BindingDB Entry DOI: 10.7270/Q2668BG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Homo sapiens (Human)) | BDBM22929 (1-[(2R)-1-hydroxy-4-[6-(3-phenylpropanamido)-1H-in...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 30 | -42.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd. | Assay Description The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... | J Med Chem 47: 3730-43 (2004) Article DOI: 10.1021/jm0306374 BindingDB Entry DOI: 10.7270/Q2668BG0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine deaminase (Homo sapiens (Human)) | BDBM22935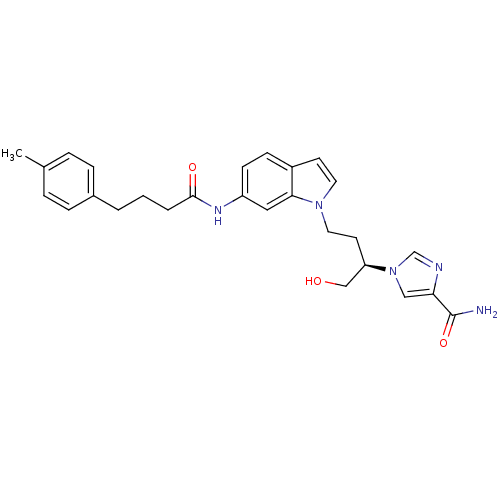 (1-[(2R)-1-hydroxy-4-{6-[4-(4-methylphenyl)butanami...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 34 | -42.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd. | Assay Description The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... | J Med Chem 47: 3730-43 (2004) Article DOI: 10.1021/jm0306374 BindingDB Entry DOI: 10.7270/Q2668BG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Homo sapiens (Human)) | BDBM22926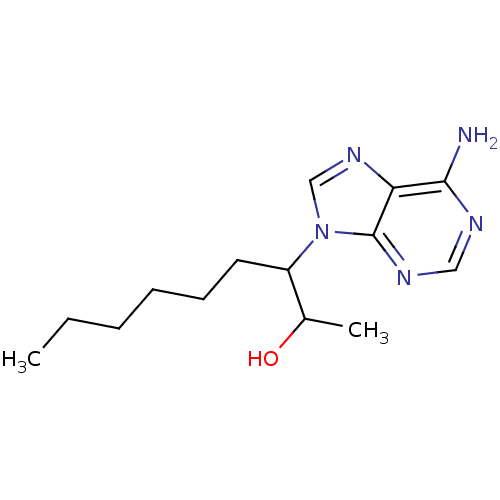 (3-(6-amino-9H-purin-9-yl)nonan-2-ol | EHNA | Eryth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 37 | -42.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd. | Assay Description The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... | Bioorg Med Chem Lett 13: 1115-8 (2003) Article DOI: 10.1016/S0960-894X(03)00026-X BindingDB Entry DOI: 10.7270/Q29Z936D | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine deaminase (Homo sapiens (Human)) | BDBM22933 (1-[(2R)-1-hydroxy-4-{6-[3-(4-methylphenyl)propanam...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 38 | -41.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd. | Assay Description The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... | J Med Chem 47: 3730-43 (2004) Article DOI: 10.1021/jm0306374 BindingDB Entry DOI: 10.7270/Q2668BG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50175277 (CHEMBL3809675) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Pharmaceutical Sciences, Kyoto University, Sakyo-ku, Kyoto 606-8501, Japan. Electronic address: hohno@pharm.kyoto-u.ac.jp. Curated by ChEMBL | Assay Description Inhibition of recombinant human SphK1 expressed in baculovirus infected sf9 cells using D-erythro-sphingosine as substrate in presence of [gamma-33P]... | Bioorg Med Chem 25: 3046-3052 (2017) Article DOI: 10.1016/j.bmc.2017.03.059 BindingDB Entry DOI: 10.7270/Q2XD144Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Homo sapiens (Human)) | BDBM22940 (1-[(2R)-4-[6-(hexyloxy)-1H-indol-1-yl]-1-hydroxybu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 55 | -41.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd. | Assay Description The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... | J Med Chem 47: 3730-43 (2004) Article DOI: 10.1021/jm0306374 BindingDB Entry DOI: 10.7270/Q2668BG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Homo sapiens (Human)) | BDBM22934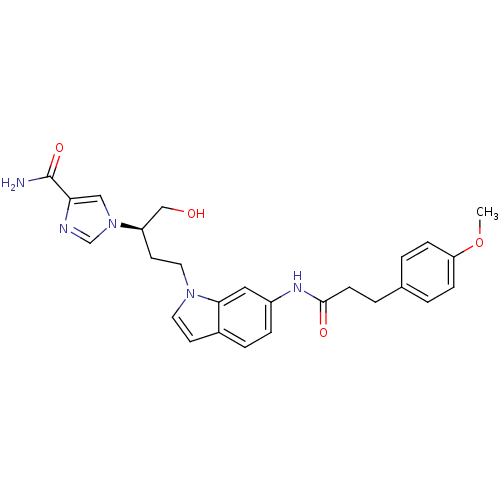 (1-[(2R)-1-hydroxy-4-{6-[3-(4-methoxyphenyl)propana...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 57 | -40.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd. | Assay Description The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... | J Med Chem 47: 3730-43 (2004) Article DOI: 10.1021/jm0306374 BindingDB Entry DOI: 10.7270/Q2668BG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Homo sapiens (Human)) | BDBM22932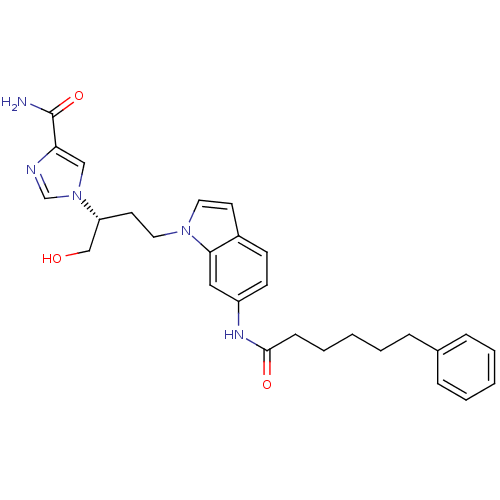 (1-[(2R)-1-hydroxy-4-[6-(6-phenylhexanamido)-1H-ind...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 91 | -39.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd. | Assay Description The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... | J Med Chem 47: 3730-43 (2004) Article DOI: 10.1021/jm0306374 BindingDB Entry DOI: 10.7270/Q2668BG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maltase-glucoamylase (Homo sapiens (Human)) | BDBM50330955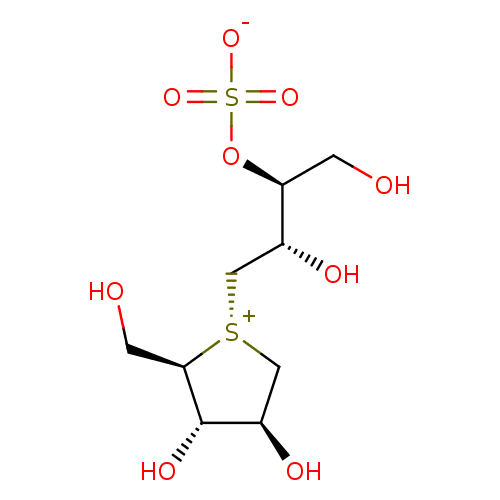 ((1S,2S)-3-[(2R,3S,4S)-3,4-dihydroxy-2-(hydroxymeth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human maltase-glucoamylase using p-nitrophenyl-alpha-D-glucopyranoside as substrate incubated for 35 mins by microtiter pla... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127751 BindingDB Entry DOI: 10.7270/Q22B92NF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Homo sapiens (Human)) | BDBM22941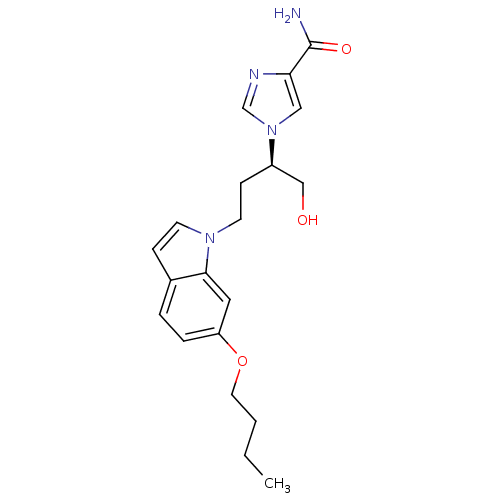 (1-[(2R)-4-(6-butoxy-1H-indol-1-yl)-1-hydroxybutan-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 240 | -37.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd. | Assay Description The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... | J Med Chem 47: 3730-43 (2004) Article DOI: 10.1021/jm0306374 BindingDB Entry DOI: 10.7270/Q2668BG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Homo sapiens (Human)) | BDBM22919 (1-[1-hydroxy-4-(naphthalen-1-yl)butan-2-yl]-1H-imi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 680 | -34.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd. | Assay Description The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... | J Am Chem Soc 126: 34-5 (2004) Article DOI: 10.1021/ja038606l BindingDB Entry DOI: 10.7270/Q2FQ9TWW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine deaminase (Homo sapiens (Human)) | BDBM22918 (2-(4-{5-[(1H-1,3-benzodiazol-2-ylamino)methyl]thio...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 1.20E+3 | -33.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd. | Assay Description The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... | J Am Chem Soc 126: 34-5 (2004) Article DOI: 10.1021/ja038606l BindingDB Entry DOI: 10.7270/Q2FQ9TWW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sphingosine kinase 2 (Homo sapiens (Human)) | BDBM174110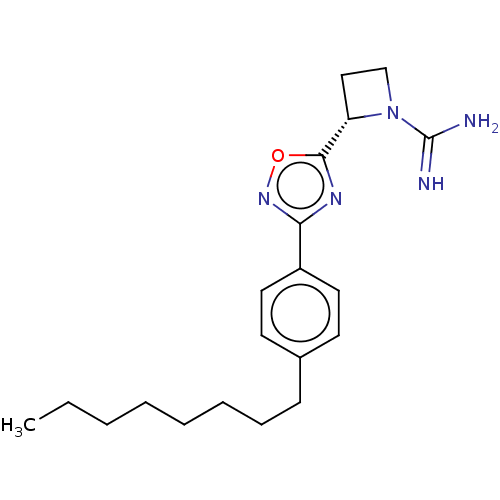 ((S)-2-(3-(4-octylphenyl)-1,2,4- oxadiazol-5-yl)aze...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Pharmaceutical Sciences, Kyoto University, Sakyo-ku, Kyoto 606-8501, Japan. Electronic address: hohno@pharm.kyoto-u.ac.jp. Curated by ChEMBL | Assay Description Inhibition of recombinant human SphK2 expressed in baculovirus infected sf9 cells using D-erythro-sphingosine as substrate after 20 mins in presence ... | Bioorg Med Chem 25: 3046-3052 (2017) Article DOI: 10.1016/j.bmc.2017.03.059 BindingDB Entry DOI: 10.7270/Q2XD144Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Homo sapiens (Human)) | BDBM22927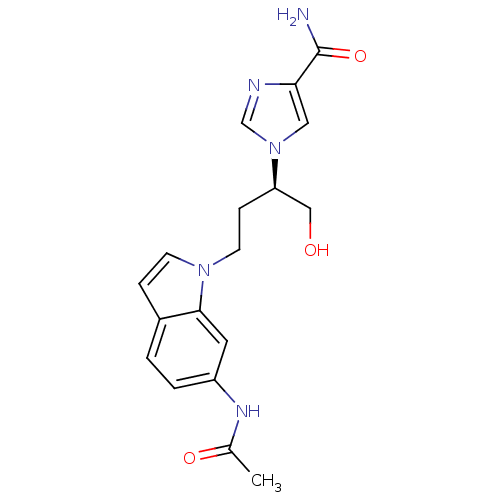 (1-[(2R)-4-(6-acetamido-1H-indol-1-yl)-1-hydroxybut...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.30E+3 | -33.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd. | Assay Description The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... | J Med Chem 47: 3730-43 (2004) Article DOI: 10.1021/jm0306374 BindingDB Entry DOI: 10.7270/Q2668BG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Homo sapiens (Human)) | BDBM22943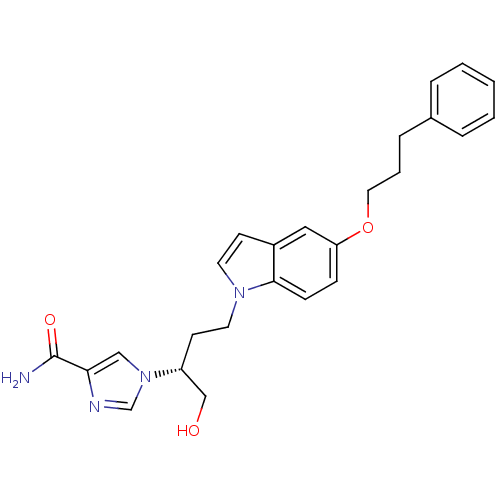 (1-[(2R)-1-hydroxy-4-[5-(3-phenylpropoxy)-1H-indol-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.90E+3 | -32.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd. | Assay Description The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... | J Med Chem 47: 3730-43 (2004) Article DOI: 10.1021/jm0306374 BindingDB Entry DOI: 10.7270/Q2668BG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Homo sapiens (Human)) | BDBM22917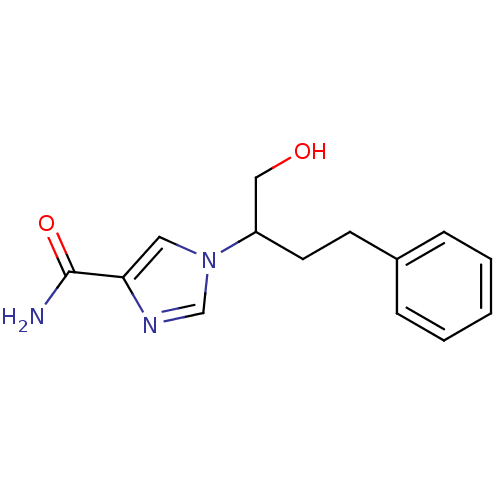 (1-(1-hydroxy-4-phenylbutan-2-yl)-1H-imidazole-4-ca...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 5.90E+3 | -29.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd. | Assay Description The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... | Bioorg Med Chem Lett 13: 1115-8 (2003) Article DOI: 10.1016/S0960-894X(03)00026-X BindingDB Entry DOI: 10.7270/Q29Z936D | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine deaminase (Homo sapiens (Human)) | BDBM22917 (1-(1-hydroxy-4-phenylbutan-2-yl)-1H-imidazole-4-ca...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 5.90E+3 | -29.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd. | Assay Description The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... | J Am Chem Soc 126: 34-5 (2004) Article DOI: 10.1021/ja038606l BindingDB Entry DOI: 10.7270/Q2FQ9TWW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Maltase-glucoamylase (Homo sapiens (Human)) | BDBM50549692 (CHEMBL4749306) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human maltase-glucoamylase using p-nitrophenyl-alpha-D-glucopyranoside as substrate incubated for 35 mins by microtiter pla... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127751 BindingDB Entry DOI: 10.7270/Q22B92NF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Homo sapiens (Human)) | BDBM22944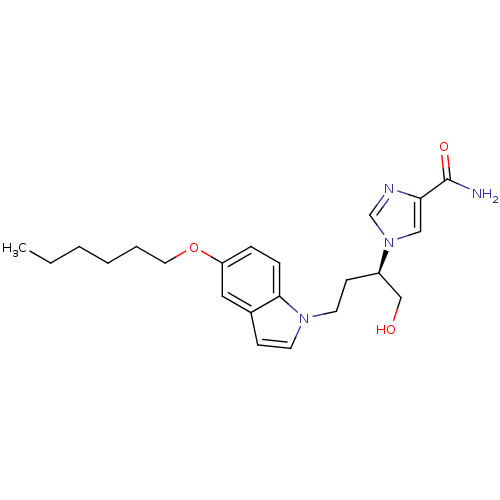 (1-[(2R)-4-[5-(hexyloxy)-1H-indol-1-yl]-1-hydroxybu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.20E+3 | -29.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd. | Assay Description The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... | J Med Chem 47: 3730-43 (2004) Article DOI: 10.1021/jm0306374 BindingDB Entry DOI: 10.7270/Q2668BG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM174110 ((S)-2-(3-(4-octylphenyl)-1,2,4- oxadiazol-5-yl)aze...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Pharmaceutical Sciences, Kyoto University, Sakyo-ku, Kyoto 606-8501, Japan. Electronic address: hohno@pharm.kyoto-u.ac.jp. Curated by ChEMBL | Assay Description Inhibition of recombinant human SphK1 expressed in baculovirus infected sf9 cells using D-erythro-sphingosine as substrate after 20 mins in presence ... | Bioorg Med Chem 25: 3046-3052 (2017) Article DOI: 10.1016/j.bmc.2017.03.059 BindingDB Entry DOI: 10.7270/Q2XD144Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 2 (Mus musculus (Mouse)) | BDBM50175277 (CHEMBL3809675) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.41E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Pharmaceutical Sciences, Kyoto University, Sakyo-ku, Kyoto 606-8501, Japan. Electronic address: hohno@pharm.kyoto-u.ac.jp. Curated by ChEMBL | Assay Description Inhibition of recombinant mouse SphK2 expressed in baculovirus infected sf9 cells using D-erythro-sphingosine as substrate in presence of [gamma-33P]... | Bioorg Med Chem 25: 3046-3052 (2017) Article DOI: 10.1016/j.bmc.2017.03.059 BindingDB Entry DOI: 10.7270/Q2XD144Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maltase-glucoamylase (Homo sapiens (Human)) | BDBM50549691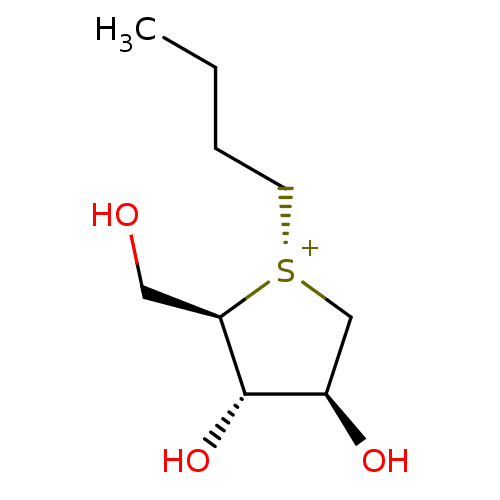 (CHEMBL4754034) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human maltase-glucoamylase using p-nitrophenyl-alpha-D-glucopyranoside as substrate incubated for 35 mins by microtiter pla... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127751 BindingDB Entry DOI: 10.7270/Q22B92NF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maltase-glucoamylase (Homo sapiens (Human)) | BDBM50549693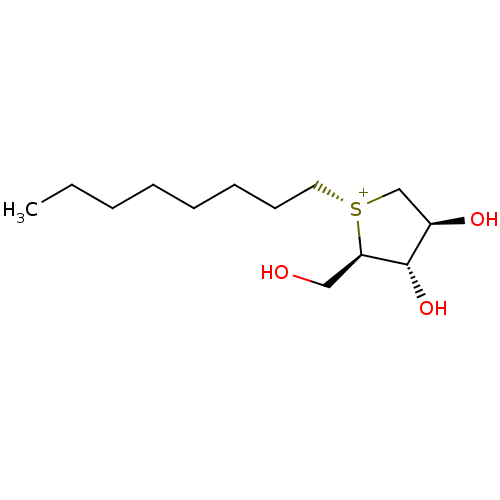 (CHEMBL4750690) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human maltase-glucoamylase using p-nitrophenyl-alpha-D-glucopyranoside as substrate incubated for 35 mins by microtiter pla... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127751 BindingDB Entry DOI: 10.7270/Q22B92NF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Homo sapiens (Human)) | BDBM22921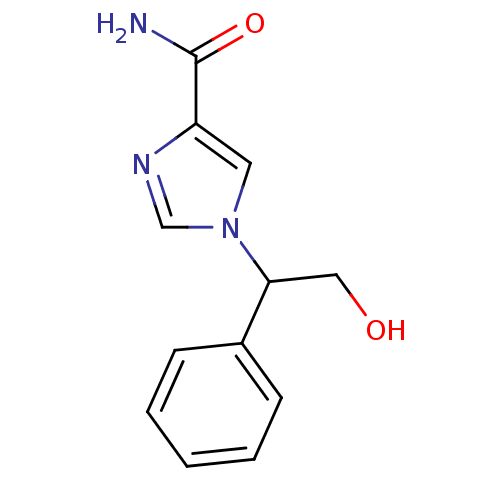 (1-(2-hydroxy-1-phenylethyl)-1H-imidazole-4-carboxa...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 5.40E+4 | -24.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd. | Assay Description The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... | Bioorg Med Chem Lett 13: 1115-8 (2003) Article DOI: 10.1016/S0960-894X(03)00026-X BindingDB Entry DOI: 10.7270/Q29Z936D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Homo sapiens (Human)) | BDBM22922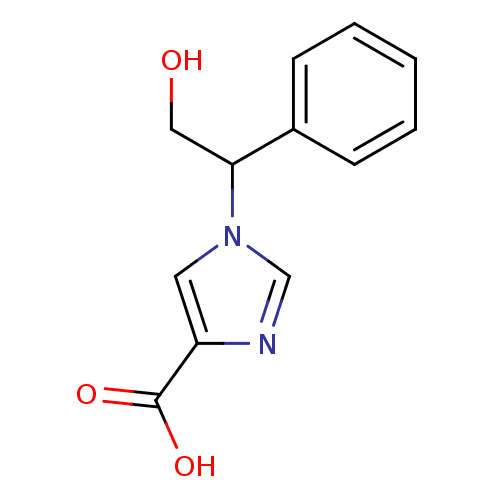 (1-(2-hydroxy-1-phenylethyl)-1H-imidazole-4-carboxy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | >-22.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd. | Assay Description The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... | Bioorg Med Chem Lett 13: 1115-8 (2003) Article DOI: 10.1016/S0960-894X(03)00026-X BindingDB Entry DOI: 10.7270/Q29Z936D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Homo sapiens (Human)) | BDBM22923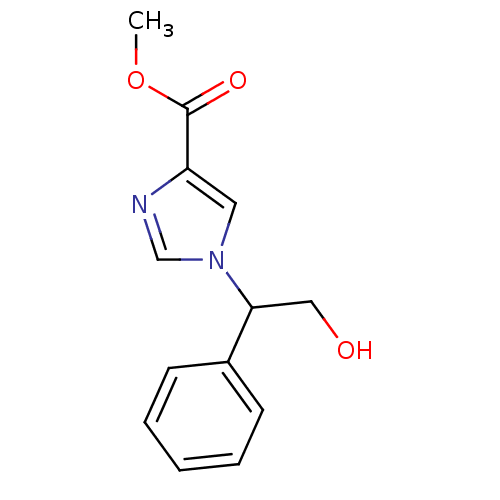 (imidazole-4-carboxylate compound, 5 | methyl 1-(2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | >-22.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd. | Assay Description The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... | Bioorg Med Chem Lett 13: 1115-8 (2003) Article DOI: 10.1016/S0960-894X(03)00026-X BindingDB Entry DOI: 10.7270/Q29Z936D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Homo sapiens (Human)) | BDBM22924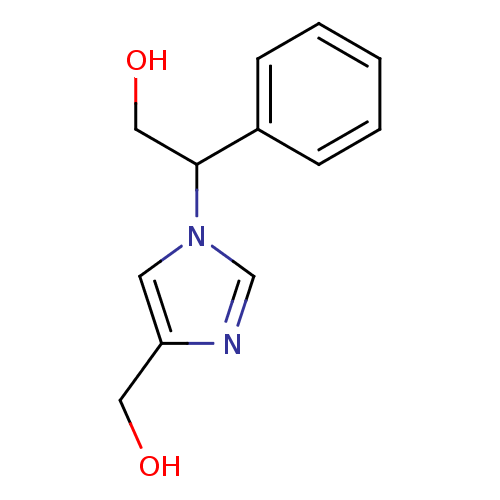 (2-[4-(hydroxymethyl)-1H-imidazol-1-yl]-2-phenyleth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | >-22.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd. | Assay Description The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... | Bioorg Med Chem Lett 13: 1115-8 (2003) Article DOI: 10.1016/S0960-894X(03)00026-X BindingDB Entry DOI: 10.7270/Q29Z936D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50070467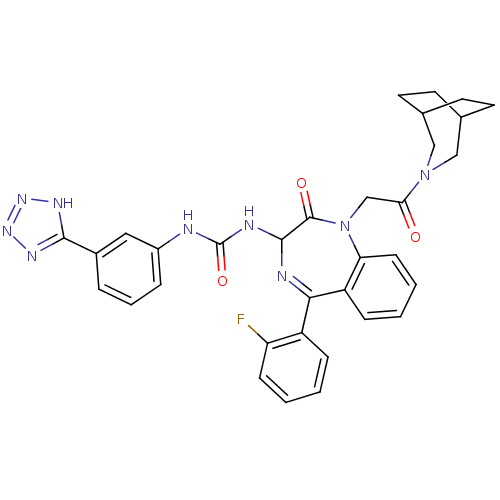 (1-[1-[2-(3-Aza-bicyclo[3.2.2]non-3-yl)-2-oxo-ethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.0870 | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of 124 I-CCK-8 binding at Cholecystokinin type B receptor on guinea pig cerebral cortical membranes | Bioorg Med Chem Lett 8: 1449-54 (1999) BindingDB Entry DOI: 10.7270/Q28P5ZMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50070464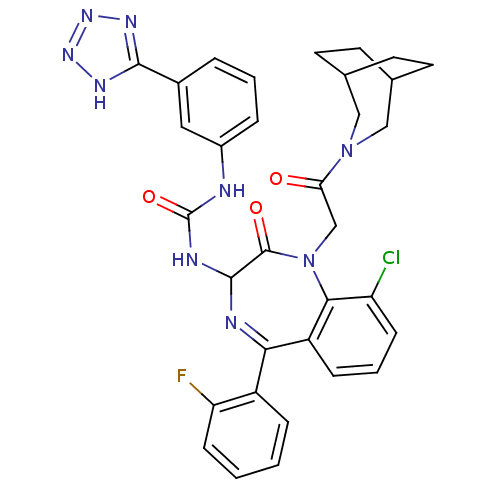 (1-[1-[2-(3-Aza-bicyclo[3.2.2]non-3-yl)-2-oxo-ethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of 124 I-CCK-8 binding at CCK-B receptorCholecystokinin type B receptorcerebral cortical membranes | Bioorg Med Chem Lett 8: 1449-54 (1999) BindingDB Entry DOI: 10.7270/Q28P5ZMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50070466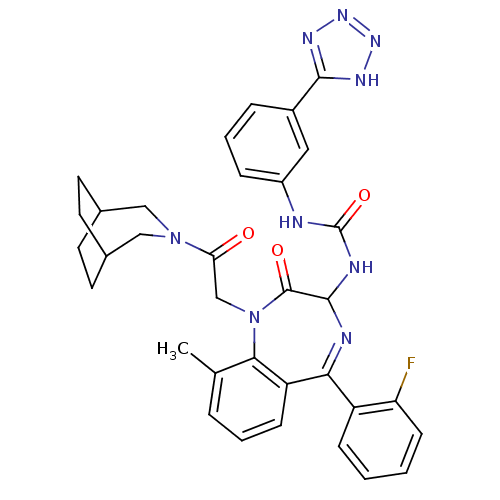 (1-[1-[2-(3-Aza-bicyclo[3.2.2]non-3-yl)-2-oxo-ethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of 124 I-CCK-8 binding at Cholecystokinin type B receptor on guinea pig cerebral cortical membranes | Bioorg Med Chem Lett 8: 1449-54 (1999) BindingDB Entry DOI: 10.7270/Q28P5ZMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50070465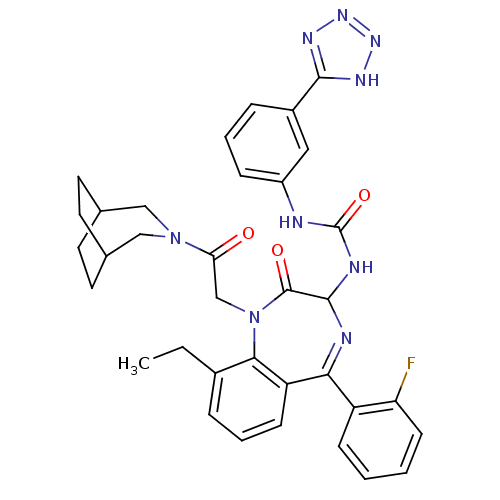 (1-[1-[2-(3-Aza-bicyclo[3.2.2]non-3-yl)-2-oxo-ethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of 124 I-CCK-8 binding at Cholecystokinin type B receptor on guinea pig cerebral cortical membranes | Bioorg Med Chem Lett 8: 1449-54 (1999) BindingDB Entry DOI: 10.7270/Q28P5ZMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50041978 (CHEMBL3134157) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Pharmaceutical Sciences, Kyoto University, Sakyo-ku, Kyoto 606-8501, Japan. Electronic address: hohno@pharm.kyoto-u.ac.jp. Curated by ChEMBL | Assay Description Inhibition of recombinant C-terminal His6-tagged human SphK1 expressed in baculovirus infected sf21 cells using FITC-sphingosine as substrate after 1... | Bioorg Med Chem 25: 3046-3052 (2017) Article DOI: 10.1016/j.bmc.2017.03.059 BindingDB Entry DOI: 10.7270/Q2XD144Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 405 total ) | Next | Last >> |