

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glutathione S-transferase omega-1 (Homo sapiens (Human)) | BDBM50458516 (CHEMBL4210652) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University Curated by ChEMBL | Assay Description Inhibition of recombinant GSTO1-1 (unknown origin) expressed in Escherichia coli assessed as inhibitor constant using S-(4-nitrophenacyl)glutathione ... | J Med Chem 63: 2894-2914 (2020) Article DOI: 10.1021/acs.jmedchem.9b01391 BindingDB Entry DOI: 10.7270/Q24Q7ZDQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutathione S-transferase omega-1 (Homo sapiens (Human)) | BDBM50527166 (CHEMBL4589666) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University Curated by ChEMBL | Assay Description Inhibition of recombinant GSTO1-1 (unknown origin) expressed in Escherichia coli assessed as inhibitor constant using S-(4-nitrophenacyl)glutathione ... | J Med Chem 63: 2894-2914 (2020) Article DOI: 10.1021/acs.jmedchem.9b01391 BindingDB Entry DOI: 10.7270/Q24Q7ZDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50170576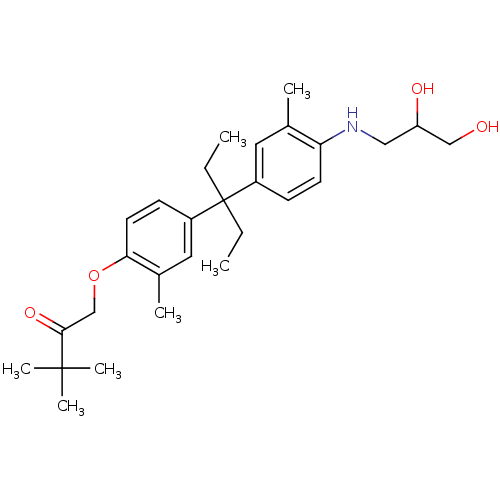 (1-(4-{1-[4-(2,3-Dihydroxy-propylamino)-3-methyl-ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Binding affinity to androgen receptor by incubation with GST-hARLBD and [3H]-testosterone | Bioorg Med Chem Lett 15: 4327-31 (2005) Article DOI: 10.1016/j.bmcl.2005.06.044 BindingDB Entry DOI: 10.7270/Q2SX6CS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase omega-1 (Homo sapiens (Human)) | BDBM50527182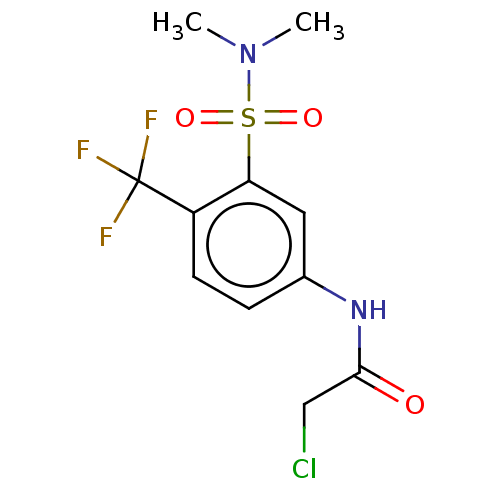 (CHEMBL4458115) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University Curated by ChEMBL | Assay Description Inhibition of recombinant GSTO1-1 (unknown origin) expressed in Escherichia coli assessed as inhibitor constant using S-(4-nitrophenacyl)glutathione ... | J Med Chem 63: 2894-2914 (2020) Article DOI: 10.1021/acs.jmedchem.9b01391 BindingDB Entry DOI: 10.7270/Q24Q7ZDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50170573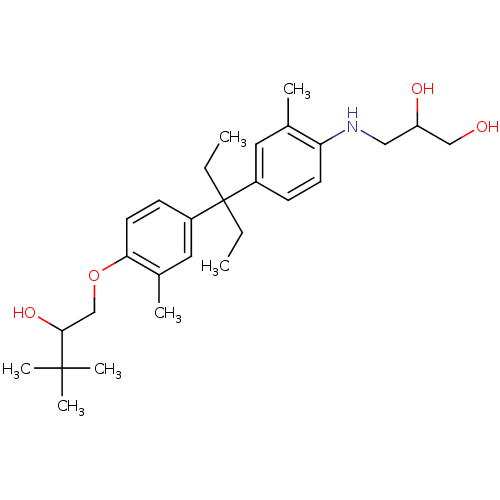 (3-(4-{1-Ethyl-1-[4-(2-hydroxy-3,3-dimethyl-butoxy)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Binding affinity to androgen receptor by incubation with GST-hARLBD and [3H]-testosterone | Bioorg Med Chem Lett 15: 4327-31 (2005) Article DOI: 10.1016/j.bmcl.2005.06.044 BindingDB Entry DOI: 10.7270/Q2SX6CS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase omega-1 (Homo sapiens (Human)) | BDBM50527163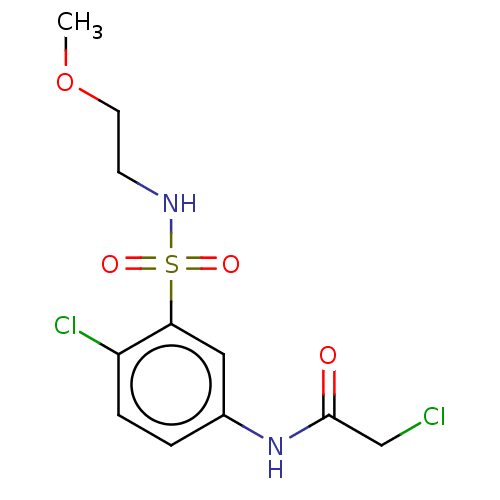 (CHEMBL4445050) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University Curated by ChEMBL | Assay Description Inhibition of recombinant GSTO1-1 (unknown origin) expressed in Escherichia coli assessed as inhibitor constant using S-(4-nitrophenacyl)glutathione ... | J Med Chem 63: 2894-2914 (2020) Article DOI: 10.1021/acs.jmedchem.9b01391 BindingDB Entry DOI: 10.7270/Q24Q7ZDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50170578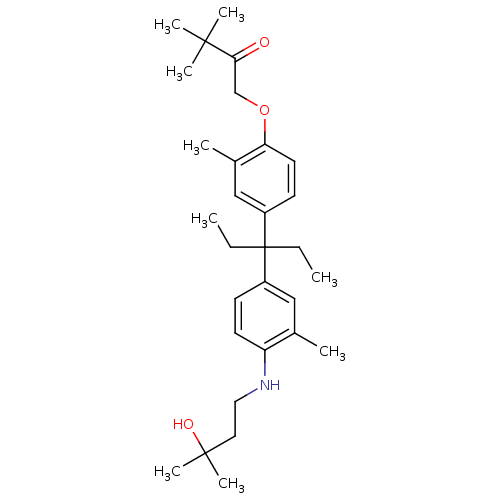 (1-(4-{1-Ethyl-1-[4-(3-hydroxy-3-methyl-butylamino)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Binding affinity to androgen receptor by incubation with GST-hARLBD and [3H]-testosterone | Bioorg Med Chem Lett 15: 4327-31 (2005) Article DOI: 10.1016/j.bmcl.2005.06.044 BindingDB Entry DOI: 10.7270/Q2SX6CS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50170582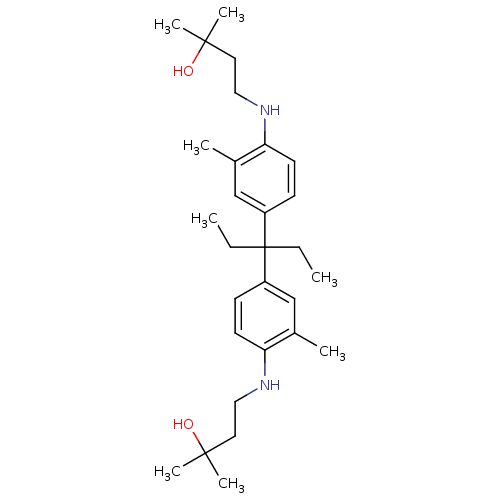 (4-(4-{1-Ethyl-1-[4-(3-hydroxy-3-methyl-butylamino)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Binding affinity to androgen receptor by incubation with GST-hARLBD and [3H]-testosterone | Bioorg Med Chem Lett 15: 4327-31 (2005) Article DOI: 10.1016/j.bmcl.2005.06.044 BindingDB Entry DOI: 10.7270/Q2SX6CS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase omega-1 (Homo sapiens (Human)) | BDBM50527184 (CHEMBL4454026) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University Curated by ChEMBL | Assay Description Inhibition of recombinant GSTO1-1 (unknown origin) expressed in Escherichia coli assessed as inhibitor constant using S-(4-nitrophenacyl)glutathione ... | J Med Chem 63: 2894-2914 (2020) Article DOI: 10.1021/acs.jmedchem.9b01391 BindingDB Entry DOI: 10.7270/Q24Q7ZDQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutathione S-transferase omega-1 (Homo sapiens (Human)) | BDBM50527185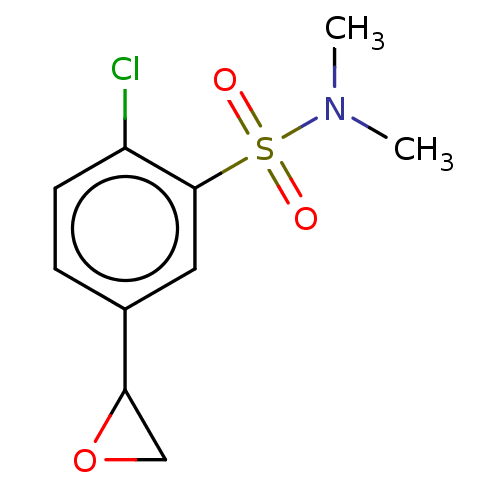 (CHEMBL4580168) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University Curated by ChEMBL | Assay Description Inhibition of recombinant GSTO1-1 (unknown origin) expressed in Escherichia coli assessed as inhibitor constant using S-(4-nitrophenacyl)glutathione ... | J Med Chem 63: 2894-2914 (2020) Article DOI: 10.1021/acs.jmedchem.9b01391 BindingDB Entry DOI: 10.7270/Q24Q7ZDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase omega-1 (Homo sapiens (Human)) | BDBM50527167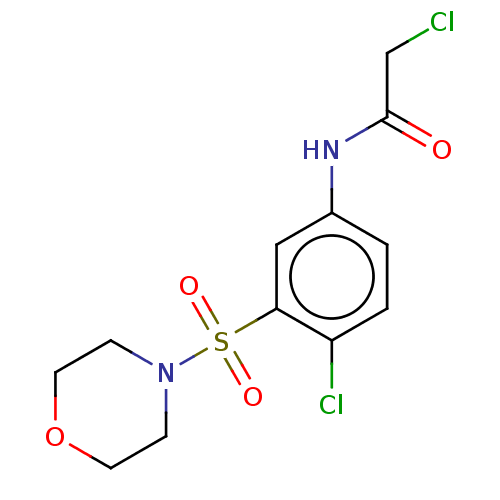 (CHEMBL4445668) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University Curated by ChEMBL | Assay Description Inhibition of recombinant GSTO1-1 (unknown origin) expressed in Escherichia coli assessed as inhibitor constant using S-(4-nitrophenacyl)glutathione ... | J Med Chem 63: 2894-2914 (2020) Article DOI: 10.1021/acs.jmedchem.9b01391 BindingDB Entry DOI: 10.7270/Q24Q7ZDQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutathione S-transferase omega-1 (Homo sapiens (Human)) | BDBM50527162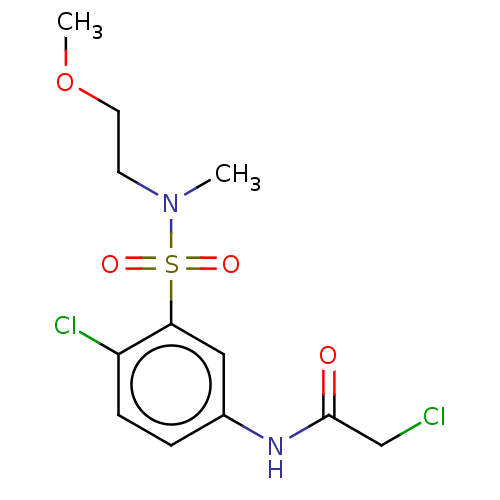 (CHEMBL4458365) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University Curated by ChEMBL | Assay Description Inhibition of recombinant GSTO1-1 (unknown origin) expressed in Escherichia coli assessed as inhibitor constant using S-(4-nitrophenacyl)glutathione ... | J Med Chem 63: 2894-2914 (2020) Article DOI: 10.1021/acs.jmedchem.9b01391 BindingDB Entry DOI: 10.7270/Q24Q7ZDQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutathione S-transferase omega-1 (Homo sapiens (Human)) | BDBM50527165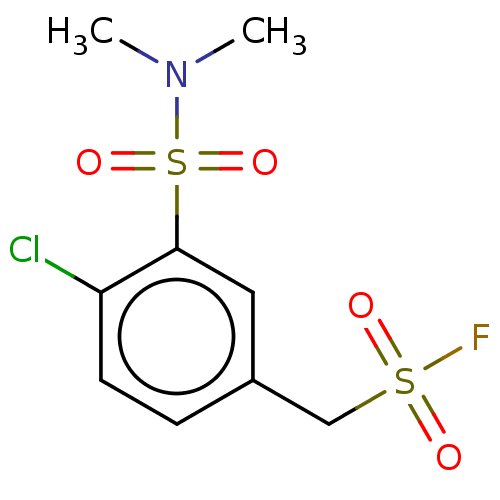 (CHEMBL4554461) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University Curated by ChEMBL | Assay Description Inhibition of recombinant GSTO1-1 (unknown origin) expressed in Escherichia coli assessed as inhibitor constant using S-(4-nitrophenacyl)glutathione ... | J Med Chem 63: 2894-2914 (2020) Article DOI: 10.1021/acs.jmedchem.9b01391 BindingDB Entry DOI: 10.7270/Q24Q7ZDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase omega-1 (Homo sapiens (Human)) | BDBM50527169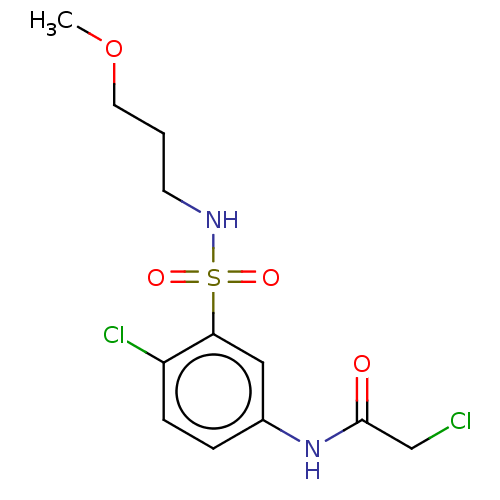 (CHEMBL4437777) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 9.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University Curated by ChEMBL | Assay Description Inhibition of recombinant GSTO1-1 (unknown origin) expressed in Escherichia coli assessed as inhibitor constant using S-(4-nitrophenacyl)glutathione ... | J Med Chem 63: 2894-2914 (2020) Article DOI: 10.1021/acs.jmedchem.9b01391 BindingDB Entry DOI: 10.7270/Q24Q7ZDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM451408 (US10710967, Example 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
ASKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description Microsomes were prepared from COS-1 cells transiently transfected with a plasmid containing human mPGES-1 cDNA, and used as mPGES-1 enzyme. The mPGES... | US Patent US10710967 (2020) BindingDB Entry DOI: 10.7270/Q2NZ8BP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM451407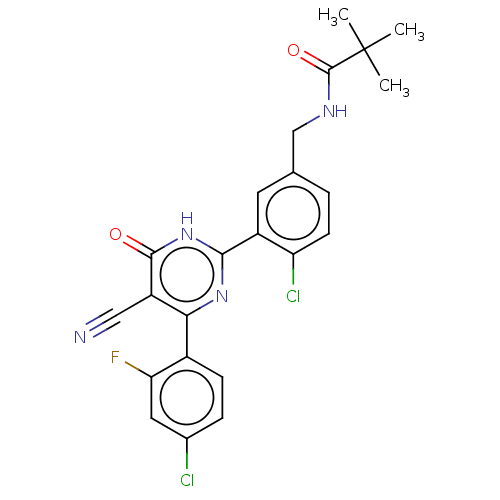 (US10710967, Example 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
ASKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description Microsomes were prepared from COS-1 cells transiently transfected with a plasmid containing human mPGES-1 cDNA, and used as mPGES-1 enzyme. The mPGES... | US Patent US10710967 (2020) BindingDB Entry DOI: 10.7270/Q2NZ8BP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM451438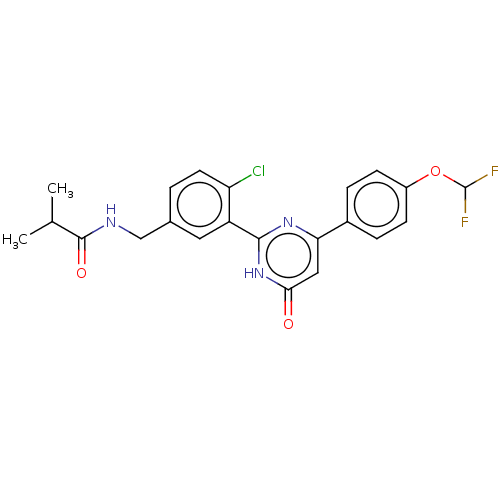 (US10710967, Example 20) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
ASKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description Microsomes were prepared from COS-1 cells transiently transfected with a plasmid containing human mPGES-1 cDNA, and used as mPGES-1 enzyme. The mPGES... | US Patent US10710967 (2020) BindingDB Entry DOI: 10.7270/Q2NZ8BP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM451477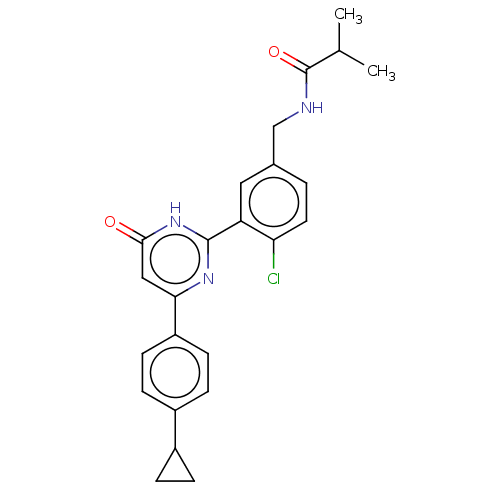 (US10710967, Example 74) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
ASKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description Microsomes were prepared from COS-1 cells transiently transfected with a plasmid containing human mPGES-1 cDNA, and used as mPGES-1 enzyme. The mPGES... | US Patent US10710967 (2020) BindingDB Entry DOI: 10.7270/Q2NZ8BP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM451427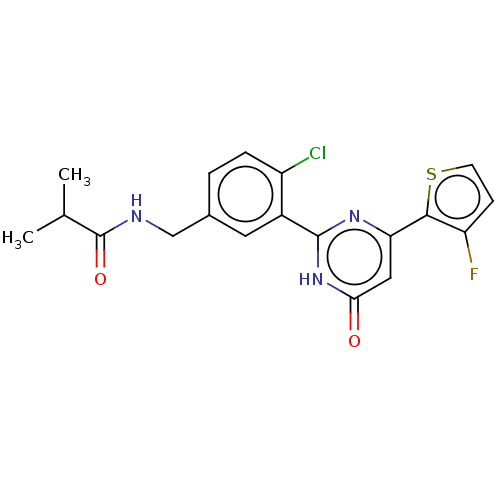 (US10710967, Example 22) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
ASKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description Microsomes were prepared from COS-1 cells transiently transfected with a plasmid containing human mPGES-1 cDNA, and used as mPGES-1 enzyme. The mPGES... | US Patent US10710967 (2020) BindingDB Entry DOI: 10.7270/Q2NZ8BP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM451447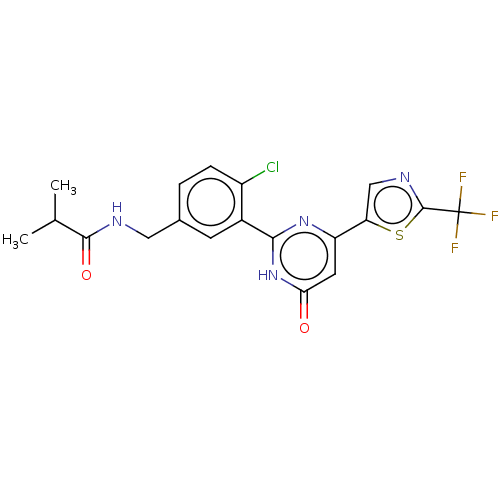 (US10710967, Example 43) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
ASKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description Microsomes were prepared from COS-1 cells transiently transfected with a plasmid containing human mPGES-1 cDNA, and used as mPGES-1 enzyme. The mPGES... | US Patent US10710967 (2020) BindingDB Entry DOI: 10.7270/Q2NZ8BP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM451418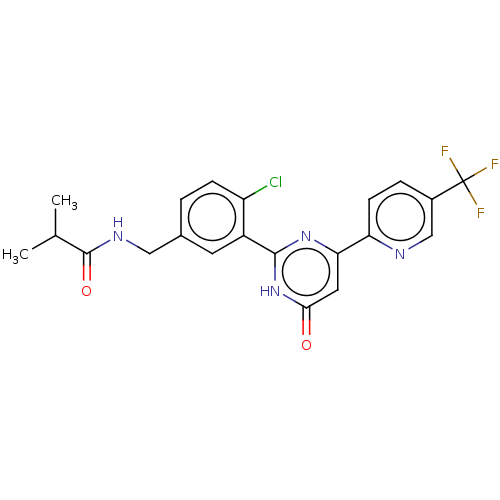 (US10710967, Example 12) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
ASKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description Microsomes were prepared from COS-1 cells transiently transfected with a plasmid containing human mPGES-1 cDNA, and used as mPGES-1 enzyme. The mPGES... | US Patent US10710967 (2020) BindingDB Entry DOI: 10.7270/Q2NZ8BP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM451429 (US10710967, Example 24) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
ASKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description Microsomes were prepared from COS-1 cells transiently transfected with a plasmid containing human mPGES-1 cDNA, and used as mPGES-1 enzyme. The mPGES... | US Patent US10710967 (2020) BindingDB Entry DOI: 10.7270/Q2NZ8BP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50383116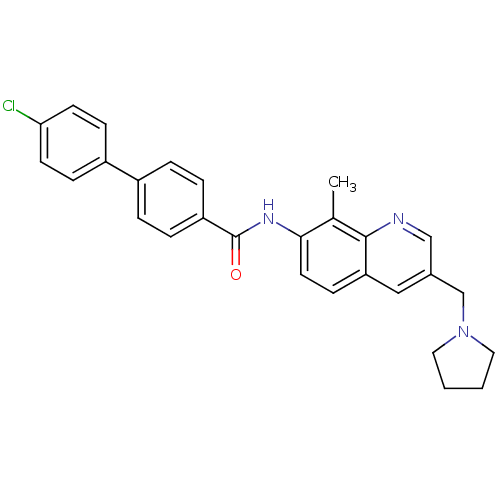 (CHEMBL2031736) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]MCH from human MCHR1 expressed in CHO cells after 1 hr by scintillation counting | J Med Chem 55: 2353-66 (2012) Article DOI: 10.1021/jm201596h BindingDB Entry DOI: 10.7270/Q2GH9JZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM451413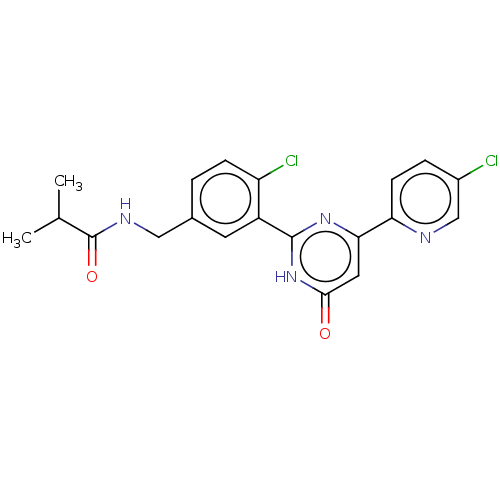 (US10710967, Example 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
ASKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description Microsomes were prepared from COS-1 cells transiently transfected with a plasmid containing human mPGES-1 cDNA, and used as mPGES-1 enzyme. The mPGES... | US Patent US10710967 (2020) BindingDB Entry DOI: 10.7270/Q2NZ8BP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM451409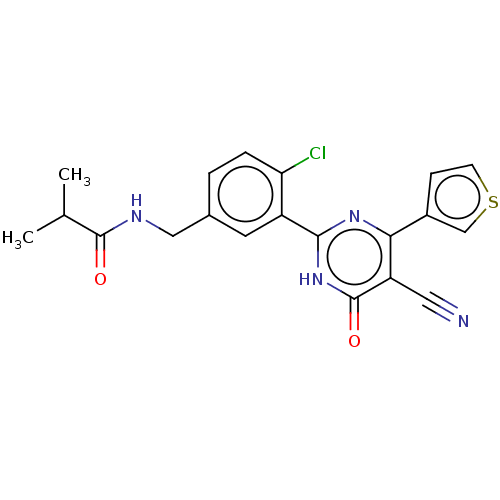 (US10710967, Example 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
ASKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description Microsomes were prepared from COS-1 cells transiently transfected with a plasmid containing human mPGES-1 cDNA, and used as mPGES-1 enzyme. The mPGES... | US Patent US10710967 (2020) BindingDB Entry DOI: 10.7270/Q2NZ8BP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Gallus gallus) | BDBM50145539 ((2S,4R)-2,4-Dihydroxy-6-[2-[(R)-1-((R)-5-hydroxy-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo Curated by ChEMBL | Assay Description In vitro relative binding affinity for chick intestinal vitamin D3 receptor compared to [3H]-1 | Bioorg Med Chem Lett 14: 2579-83 (2004) Article DOI: 10.1016/j.bmcl.2004.02.076 BindingDB Entry DOI: 10.7270/Q20C4V6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50383114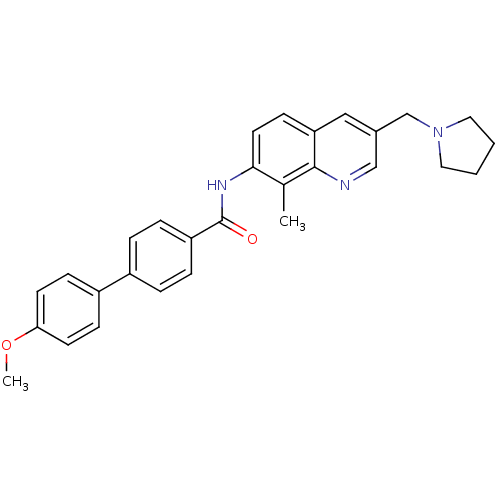 (CHEMBL2031734) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]MCH from human MCHR1 expressed in CHO cells after 1 hr by scintillation counting | J Med Chem 55: 2353-66 (2012) Article DOI: 10.1021/jm201596h BindingDB Entry DOI: 10.7270/Q2GH9JZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50351324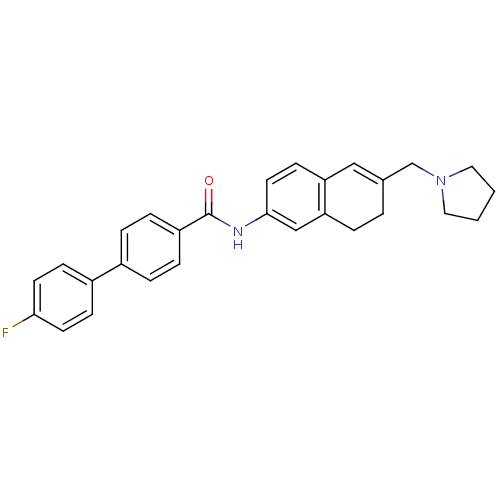 (CHEMBL1818901) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [3H]mesulergine from human 5HT2C receptor expressed in CHO cells after 1 hr by scintillation counting | J Med Chem 55: 2353-66 (2012) Article DOI: 10.1021/jm201596h BindingDB Entry DOI: 10.7270/Q2GH9JZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50383112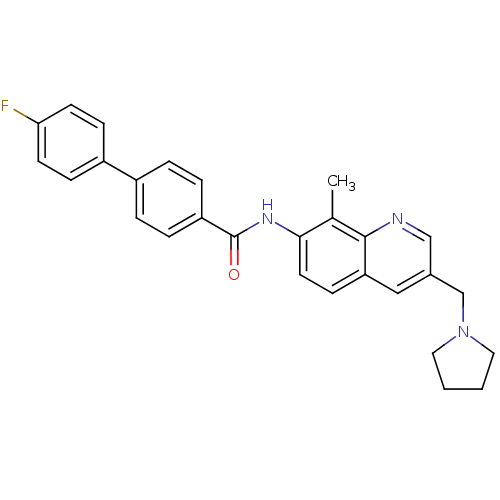 (CHEMBL2029372) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Displacement of [125I]MCH from human MCHR1 expressed in CHO cells after 60 mins by liquid scintillation counting | J Med Chem 55: 4336-51 (2012) Article DOI: 10.1021/jm300167z BindingDB Entry DOI: 10.7270/Q2JS9RHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50383112 (CHEMBL2029372) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]MCH from human MCHR1 expressed in CHO cells after 1 hr by scintillation counting | J Med Chem 55: 2353-66 (2012) Article DOI: 10.1021/jm201596h BindingDB Entry DOI: 10.7270/Q2GH9JZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50383096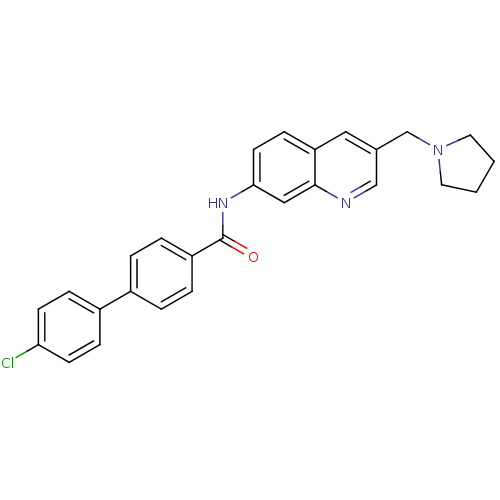 (CHEMBL2031716) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]MCH from human MCHR1 expressed in CHO cells after 1 hr by scintillation counting | J Med Chem 55: 2353-66 (2012) Article DOI: 10.1021/jm201596h BindingDB Entry DOI: 10.7270/Q2GH9JZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50383091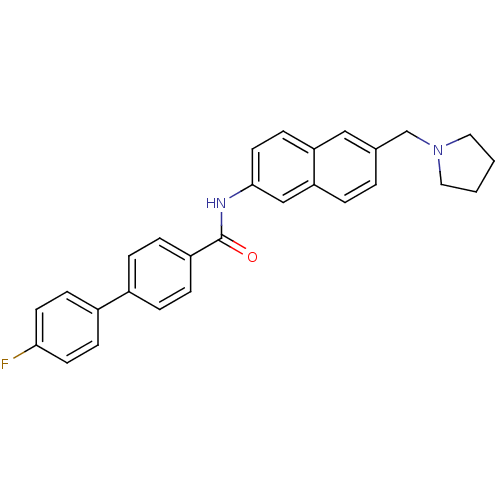 (CHEMBL2031573) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]MCH from human MCHR1 expressed in CHO cells after 1 hr by scintillation counting | J Med Chem 55: 2353-66 (2012) Article DOI: 10.1021/jm201596h BindingDB Entry DOI: 10.7270/Q2GH9JZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM451449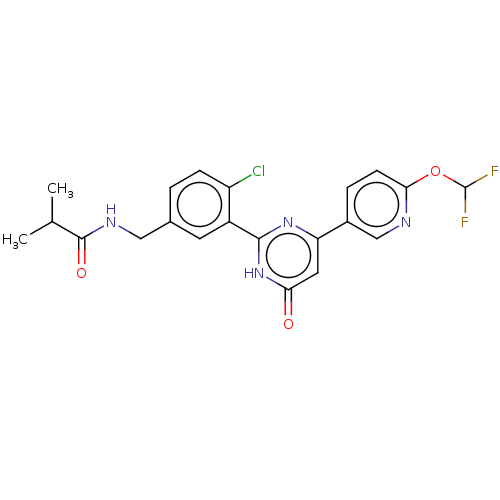 (US10710967, Example 45) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
ASKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description Microsomes were prepared from COS-1 cells transiently transfected with a plasmid containing human mPGES-1 cDNA, and used as mPGES-1 enzyme. The mPGES... | US Patent US10710967 (2020) BindingDB Entry DOI: 10.7270/Q2NZ8BP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM451558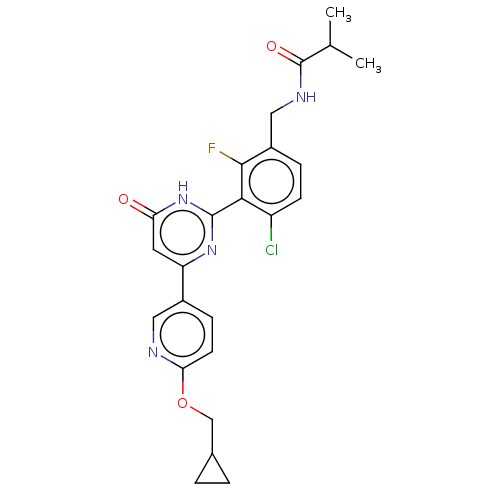 (US10710967, Example 157) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a |
ASKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description Microsomes were prepared from COS-1 cells transiently transfected with a plasmid containing human mPGES-1 cDNA, and used as mPGES-1 enzyme. The mPGES... | US Patent US10710967 (2020) BindingDB Entry DOI: 10.7270/Q2NZ8BP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM451423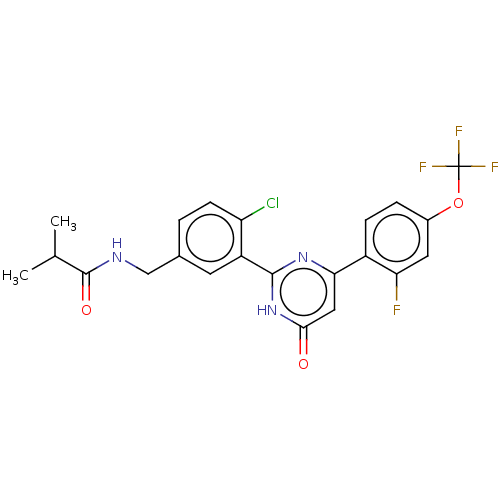 (US10710967, Example 17) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a |
ASKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description Microsomes were prepared from COS-1 cells transiently transfected with a plasmid containing human mPGES-1 cDNA, and used as mPGES-1 enzyme. The mPGES... | US Patent US10710967 (2020) BindingDB Entry DOI: 10.7270/Q2NZ8BP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM451426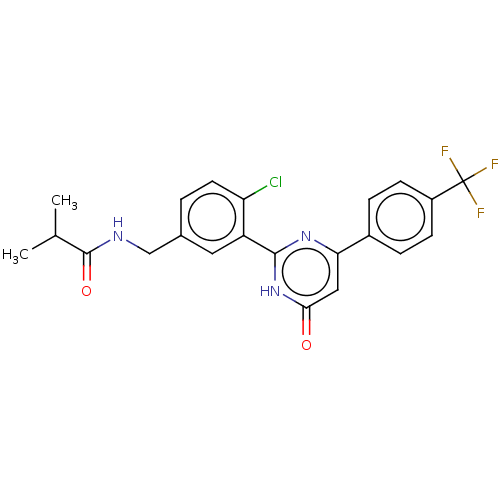 (US10710967, Example 21) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.880 | n/a | n/a | n/a | n/a | n/a | n/a |
ASKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description Microsomes were prepared from COS-1 cells transiently transfected with a plasmid containing human mPGES-1 cDNA, and used as mPGES-1 enzyme. The mPGES... | US Patent US10710967 (2020) BindingDB Entry DOI: 10.7270/Q2NZ8BP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50383092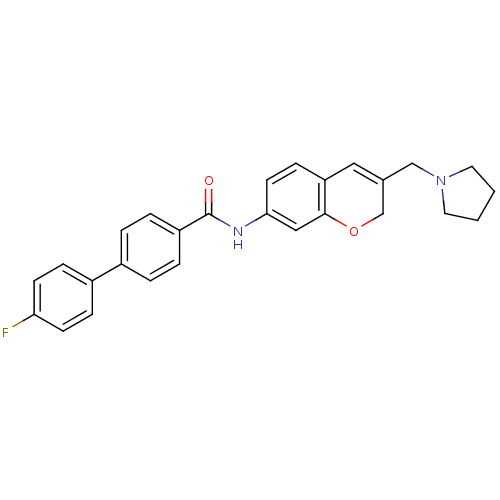 (CHEMBL2031574) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]MCH from human MCHR1 expressed in CHO cells after 1 hr by scintillation counting | J Med Chem 55: 2353-66 (2012) Article DOI: 10.1021/jm201596h BindingDB Entry DOI: 10.7270/Q2GH9JZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50383107 (CHEMBL2031727) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]MCH from human MCHR1 expressed in CHO cells after 1 hr by scintillation counting | J Med Chem 55: 2353-66 (2012) Article DOI: 10.1021/jm201596h BindingDB Entry DOI: 10.7270/Q2GH9JZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50357496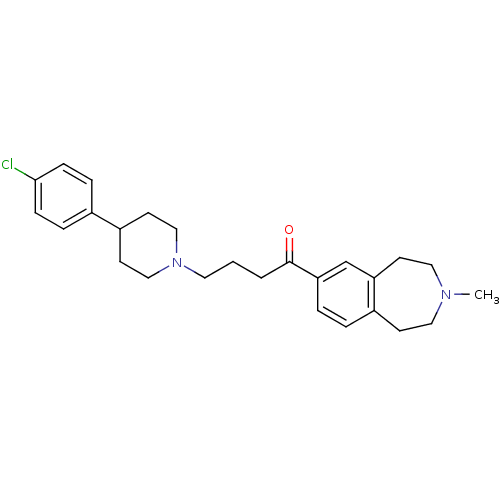 (CHEMBL1914623) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [125I]-MCH(4-19) from human MCHR1 expressed in CHO cells | Bioorg Med Chem 19: 6261-73 (2011) Article DOI: 10.1016/j.bmc.2011.09.007 BindingDB Entry DOI: 10.7270/Q26T0N2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM451642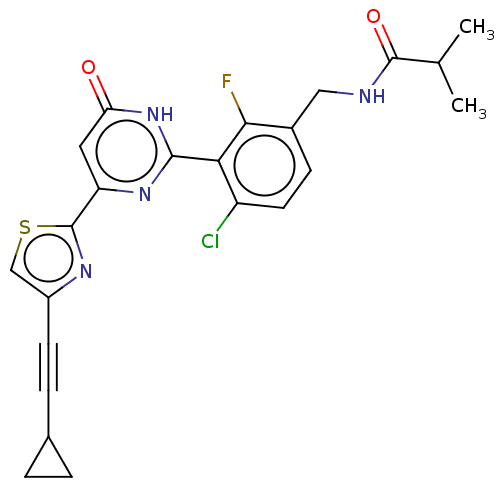 (US10710967, Example 240) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
ASKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description Microsomes were prepared from COS-1 cells transiently transfected with a plasmid containing human mPGES-1 cDNA, and used as mPGES-1 enzyme. The mPGES... | US Patent US10710967 (2020) BindingDB Entry DOI: 10.7270/Q2NZ8BP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM451657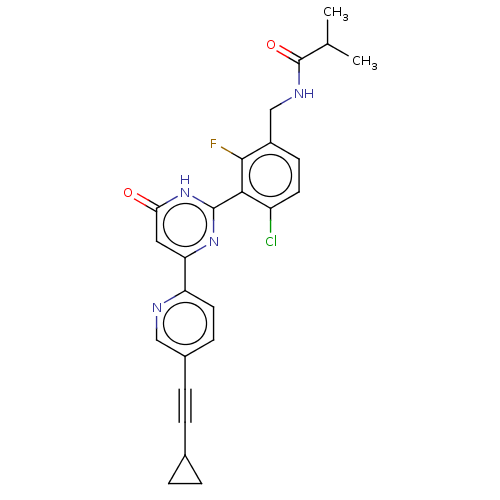 (US10710967, Example 255) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
ASKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description Microsomes were prepared from COS-1 cells transiently transfected with a plasmid containing human mPGES-1 cDNA, and used as mPGES-1 enzyme. The mPGES... | US Patent US10710967 (2020) BindingDB Entry DOI: 10.7270/Q2NZ8BP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM451658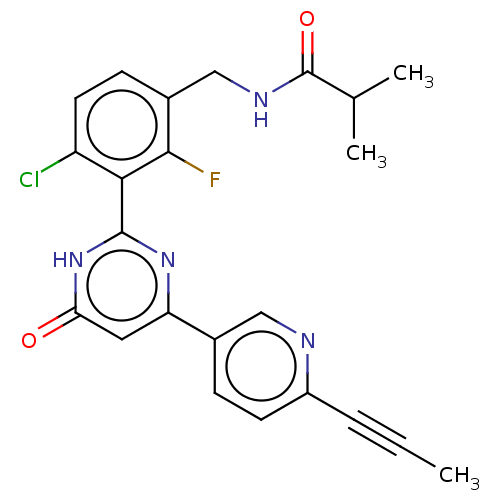 (US10710967, Example 256) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
ASKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description Microsomes were prepared from COS-1 cells transiently transfected with a plasmid containing human mPGES-1 cDNA, and used as mPGES-1 enzyme. The mPGES... | US Patent US10710967 (2020) BindingDB Entry DOI: 10.7270/Q2NZ8BP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM451665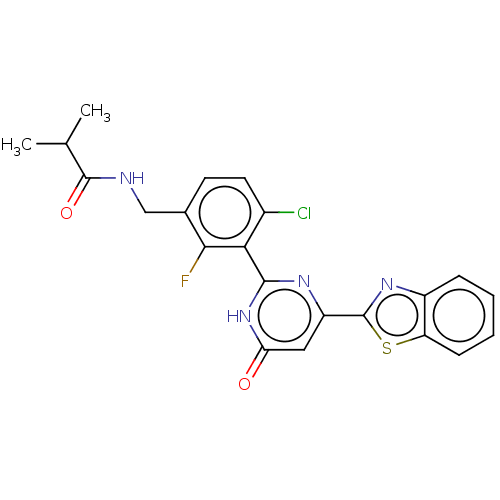 (US10710967, Example 263) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
ASKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description Microsomes were prepared from COS-1 cells transiently transfected with a plasmid containing human mPGES-1 cDNA, and used as mPGES-1 enzyme. The mPGES... | US Patent US10710967 (2020) BindingDB Entry DOI: 10.7270/Q2NZ8BP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM451668 (US10710967, Example 266) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
ASKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description Microsomes were prepared from COS-1 cells transiently transfected with a plasmid containing human mPGES-1 cDNA, and used as mPGES-1 enzyme. The mPGES... | US Patent US10710967 (2020) BindingDB Entry DOI: 10.7270/Q2NZ8BP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM451683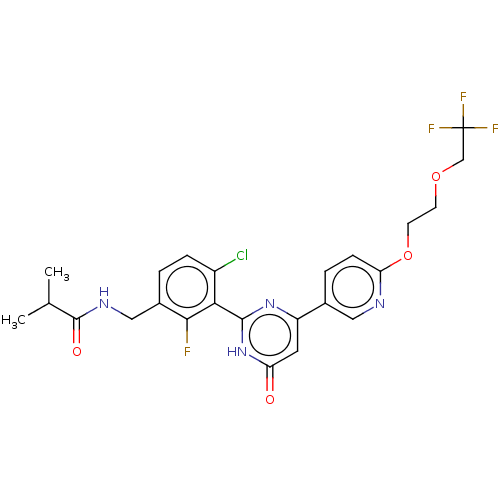 (US10710967, Example 278) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
ASKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description Microsomes were prepared from COS-1 cells transiently transfected with a plasmid containing human mPGES-1 cDNA, and used as mPGES-1 enzyme. The mPGES... | US Patent US10710967 (2020) BindingDB Entry DOI: 10.7270/Q2NZ8BP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM451508 (US10710967, Example 105) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
ASKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description Microsomes were prepared from COS-1 cells transiently transfected with a plasmid containing human mPGES-1 cDNA, and used as mPGES-1 enzyme. The mPGES... | US Patent US10710967 (2020) BindingDB Entry DOI: 10.7270/Q2NZ8BP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM451529 (US10710967, Example 127) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
ASKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description Microsomes were prepared from COS-1 cells transiently transfected with a plasmid containing human mPGES-1 cDNA, and used as mPGES-1 enzyme. The mPGES... | US Patent US10710967 (2020) BindingDB Entry DOI: 10.7270/Q2NZ8BP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM451563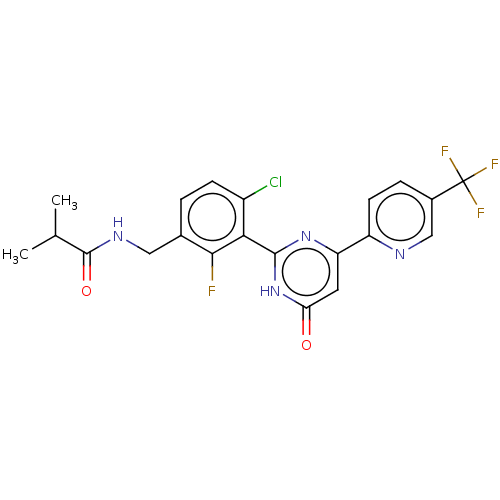 (US10710967, Example 162) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
ASKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description Microsomes were prepared from COS-1 cells transiently transfected with a plasmid containing human mPGES-1 cDNA, and used as mPGES-1 enzyme. The mPGES... | US Patent US10710967 (2020) BindingDB Entry DOI: 10.7270/Q2NZ8BP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM451579 (US10710967, Example 178) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
ASKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description Microsomes were prepared from COS-1 cells transiently transfected with a plasmid containing human mPGES-1 cDNA, and used as mPGES-1 enzyme. The mPGES... | US Patent US10710967 (2020) BindingDB Entry DOI: 10.7270/Q2NZ8BP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50383115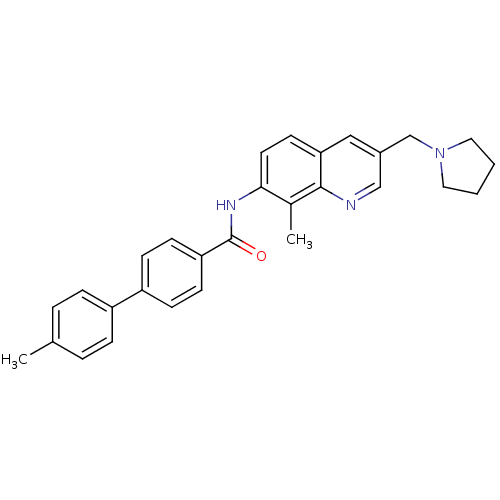 (CHEMBL2031735) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of [125I]MCH from human MCHR1 expressed in CHO cells after 1 hr by scintillation counting | J Med Chem 55: 2353-66 (2012) Article DOI: 10.1021/jm201596h BindingDB Entry DOI: 10.7270/Q2GH9JZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 788 total ) | Next | Last >> |