 Found 121 hits with Last Name = 'nakazawa' and Initial = 't'
Found 121 hits with Last Name = 'nakazawa' and Initial = 't' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Kappa-type opioid receptor
(Mus musculus (Mouse)) | BDBM50010542
(2-[5-Guanidino-2-(5-guanidino-2-{2-[2-(2-{2-[3-(4-...)Show SMILES CNC(Cc1ccc(O)cc1)C(=O)NCC(=O)NCC(=O)NC(Cc1ccc(cc1)[N+]([O-])=O)C(=O)NC(CC(C)C)C(=O)NC(CCC[N-]C(N)=[NH2+])C(=O)NC(CCC[N-]C(N)=[NH2+])C(=O)NC(CC(C)C)C(N)=O Show InChI InChI=1S/C47H74N16O11/c1-26(2)20-34(40(48)67)61-43(70)33(9-7-19-55-47(51)52)59-42(69)32(8-6-18-54-46(49)50)60-44(71)36(21-27(3)4)62-45(72)37(23-28-10-14-30(15-11-28)63(73)74)58-39(66)25-56-38(65)24-57-41(68)35(53-5)22-29-12-16-31(64)17-13-29/h10-17,26-27,32-37,53H,6-9,18-25H2,1-5H3,(H18,48,49,50,51,52,54,55,56,57,58,59,60,61,62,64,65,66,67,68,69,70,71,72) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinities against Opioid receptor kappa 1 of guinea pig brain membrane using [3H]U69,593 as the radioligand using competition binding assays... |
J Med Chem 33: 206-12 (1990)
BindingDB Entry DOI: 10.7270/Q2QV3KG6 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(GUINEA PIG) | BDBM50010532
(13-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]-7...)Show SMILES CCNC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCNC(N)=N)N(C)C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H]1CSSC[C@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@H](Cc2ccccc2)C(=O)N1 Show InChI InChI=1S/C47H73N15O9S2/c1-5-53-40(66)33(21-27(2)3)59-44(70)37(14-10-20-55-47(51)52)62(4)45(71)32(13-9-19-54-46(49)50)58-43(69)36-26-73-72-25-35(60-39(65)31(48)22-29-15-17-30(63)18-16-29)41(67)56-24-38(64)57-34(42(68)61-36)23-28-11-7-6-8-12-28/h6-8,11-12,15-18,27,31-37,63H,5,9-10,13-14,19-26,48H2,1-4H3,(H,53,66)(H,56,67)(H,57,64)(H,58,69)(H,59,70)(H,60,65)(H,61,68)(H4,49,50,54)(H4,51,52,55)/t31-,32-,33-,34+,35-,36+,37-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinities against Opioid receptor mu 1 of guinea pig brain membrane using [3H]-DAGO as the radioligand using competition binding assays. |
J Med Chem 33: 206-12 (1990)
BindingDB Entry DOI: 10.7270/Q2QV3KG6 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(GUINEA PIG) | BDBM50010542

(2-[5-Guanidino-2-(5-guanidino-2-{2-[2-(2-{2-[3-(4-...)Show SMILES CNC(Cc1ccc(O)cc1)C(=O)NCC(=O)NCC(=O)NC(Cc1ccc(cc1)[N+]([O-])=O)C(=O)NC(CC(C)C)C(=O)NC(CCC[N-]C(N)=[NH2+])C(=O)NC(CCC[N-]C(N)=[NH2+])C(=O)NC(CC(C)C)C(N)=O Show InChI InChI=1S/C47H74N16O11/c1-26(2)20-34(40(48)67)61-43(70)33(9-7-19-55-47(51)52)59-42(69)32(8-6-18-54-46(49)50)60-44(71)36(21-27(3)4)62-45(72)37(23-28-10-14-30(15-11-28)63(73)74)58-39(66)25-56-38(65)24-57-41(68)35(53-5)22-29-12-16-31(64)17-13-29/h10-17,26-27,32-37,53H,6-9,18-25H2,1-5H3,(H18,48,49,50,51,52,54,55,56,57,58,59,60,61,62,64,65,66,67,68,69,70,71,72) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinities against Opioid receptor mu 1 of guinea pig brain membrane using [3H]-DAGO as the radioligand using competition binding assays. |
J Med Chem 33: 206-12 (1990)
BindingDB Entry DOI: 10.7270/Q2QV3KG6 |
More data for this
Ligand-Target Pair | |
Promotilin
(RABBIT) | BDBM85614

([Leu13]motilin | [leu13]pMOT(1-22))Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccccc1)-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](-[#8])=O |r| Show InChI InChI=1S/C121H190N34O35/c1-10-65(8)98(153-115(185)87-32-23-53-155(87)118(188)97(64(6)7)152-100(170)71(124)56-67-24-13-11-14-25-67)116(186)151-85(57-68-26-15-12-16-27-68)114(184)154-99(66(9)156)117(187)150-84(58-69-33-35-70(157)36-34-69)102(172)136-60-92(162)137-76(40-46-94(164)165)106(176)148-83(55-63(4)5)112(182)145-77(37-43-88(125)158)107(177)141-74(30-21-51-133-120(129)130)104(174)147-82(54-62(2)3)111(181)146-78(38-44-89(126)159)108(178)144-80(42-48-96(168)169)109(179)140-73(29-18-20-50-123)103(173)143-79(41-47-95(166)167)110(180)142-75(31-22-52-134-121(131)132)105(175)149-86(59-91(128)161)113(183)139-72(28-17-19-49-122)101(171)135-61-93(163)138-81(119(189)190)39-45-90(127)160/h11-16,24-27,33-36,62-66,71-87,97-99,156-157H,10,17-23,28-32,37-61,122-124H2,1-9H3,(H2,125,158)(H2,126,159)(H2,127,160)(H2,128,161)(H,135,171)(H,136,172)(H,137,162)(H,138,163)(H,139,183)(H,140,179)(H,141,177)(H,142,180)(H,143,173)(H,144,178)(H,145,182)(H,146,181)(H,147,174)(H,148,176)(H,149,175)(H,150,187)(H,151,186)(H,152,170)(H,153,185)(H,154,184)(H,164,165)(H,166,167)(H,168,169)(H,189,190)(H4,129,130,133)(H4,131,132,134)/t65-,66+,71-,72-,73-,74-,75-,76-,77-,78-,79-,80-,81-,82-,83-,84-,85-,86-,87-,97-,98-,99-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanwa Kagaku Kenkyusho Co., Ltd.
Curated by PDSP Ki Database
| |
Pharmacology 60: 128-35 (2000)
Article DOI: 28357
BindingDB Entry DOI: 10.7270/Q2RV0M7N |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Mus musculus (Mouse)) | BDBM50010538
(2-[2-(2-{2-[2-(2-{2-[2-Amino-3-(4-hydroxy-phenyl)-...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(N)=O Show InChI InChI=1S/C46H73N15O9/c1-5-27(4)38(39(48)65)61-42(68)33(14-10-20-54-46(51)52)58-41(67)32(13-9-19-53-45(49)50)59-43(69)34(21-26(2)3)60-44(70)35(23-28-11-7-6-8-12-28)57-37(64)25-55-36(63)24-56-40(66)31(47)22-29-15-17-30(62)18-16-29/h6-8,11-12,15-18,26-27,31-35,38,62H,5,9-10,13-14,19-25,47H2,1-4H3,(H2,48,65)(H,55,63)(H,56,66)(H,57,64)(H,58,67)(H,59,69)(H,60,70)(H,61,68)(H4,49,50,53)(H4,51,52,54)/t27-,31-,32-,33-,34-,35+,38-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinities against Opioid receptor kappa 1 of guinea pig brain membrane using [3H]U69,593 as the radioligand using competition binding assays... |
J Med Chem 33: 206-12 (1990)
BindingDB Entry DOI: 10.7270/Q2QV3KG6 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(GUINEA PIG) | BDBM50010537
(2-[5-Guanidino-2-(5-guanidino-2-{2-[2-(2-{2-[3-(4-...)Show SMILES CN[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](C)C(=O)NCC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(N)=O Show InChI InChI=1S/C48H77N15O9/c1-27(2)22-35(40(49)66)62-43(69)34(15-11-21-56-48(52)53)60-42(68)33(14-10-20-55-47(50)51)61-45(71)37(23-28(3)4)63-46(72)38(25-30-12-8-7-9-13-30)59-39(65)26-57-41(67)29(5)58-44(70)36(54-6)24-31-16-18-32(64)19-17-31/h7-9,12-13,16-19,27-29,33-38,54,64H,10-11,14-15,20-26H2,1-6H3,(H2,49,66)(H,57,67)(H,58,70)(H,59,65)(H,60,68)(H,61,71)(H,62,69)(H,63,72)(H4,50,51,55)(H4,52,53,56)/t29-,33-,34-,35-,36-,37-,38-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinities against Opioid receptor mu 1 of guinea pig brain membrane using [3H]-DAGO as the radioligand using competition binding assays. |
J Med Chem 33: 206-12 (1990)
BindingDB Entry DOI: 10.7270/Q2QV3KG6 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Mus musculus (Mouse)) | BDBM50010535
(2-{5-Guanidino-2-[(5-guanidino-2-{2-[2-(2-{2-[3-(4...)Show SMILES CN[C@@H](Cc1ccc(O)cc1)C(=O)NCC(=O)NCC(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N(C)[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(N)=O Show InChI InChI=1S/C48H77N15O9/c1-28(2)22-34(41(49)67)61-45(71)38(15-11-21-56-48(52)53)63(6)46(72)33(14-10-20-55-47(50)51)60-43(69)36(23-29(3)4)62-44(70)37(25-30-12-8-7-9-13-30)59-40(66)27-57-39(65)26-58-42(68)35(54-5)24-31-16-18-32(64)19-17-31/h7-9,12-13,16-19,28-29,33-38,54,64H,10-11,14-15,20-27H2,1-6H3,(H2,49,67)(H,57,65)(H,58,68)(H,59,66)(H,60,69)(H,61,71)(H,62,70)(H4,50,51,55)(H4,52,53,56)/t33-,34-,35-,36-,37+,38-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinities against Opioid receptor kappa 1 of guinea pig brain membrane using [3H]U69,593 as the radioligand using competition binding assays... |
J Med Chem 33: 206-12 (1990)
BindingDB Entry DOI: 10.7270/Q2QV3KG6 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Mus musculus (Mouse)) | BDBM50010536
(2-[5-Guanidino-2-(5-guanidino-2-{2-[2-(2-{2-[3-(4-...)Show SMILES CN[C@@H](Cc1ccc(O)cc1)C(=O)NCC(=O)NCC(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(N)=O Show InChI InChI=1S/C47H75N15O9/c1-27(2)21-34(40(48)66)61-43(69)33(14-10-20-55-47(51)52)59-42(68)32(13-9-19-54-46(49)50)60-44(70)36(22-28(3)4)62-45(71)37(24-29-11-7-6-8-12-29)58-39(65)26-56-38(64)25-57-41(67)35(53-5)23-30-15-17-31(63)18-16-30/h6-8,11-12,15-18,27-28,32-37,53,63H,9-10,13-14,19-26H2,1-5H3,(H2,48,66)(H,56,64)(H,57,67)(H,58,65)(H,59,68)(H,60,70)(H,61,69)(H,62,71)(H4,49,50,54)(H4,51,52,55)/t32-,33-,34-,35-,36-,37+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinities against Opioid receptor kappa 1 of guinea pig brain membrane using [3H]U69,593 as the radioligand using competition binding assays... |
J Med Chem 33: 206-12 (1990)
BindingDB Entry DOI: 10.7270/Q2QV3KG6 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50010532

(13-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]-7...)Show SMILES CCNC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCNC(N)=N)N(C)C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H]1CSSC[C@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@H](Cc2ccccc2)C(=O)N1 Show InChI InChI=1S/C47H73N15O9S2/c1-5-53-40(66)33(21-27(2)3)59-44(70)37(14-10-20-55-47(51)52)62(4)45(71)32(13-9-19-54-46(49)50)58-43(69)36-26-73-72-25-35(60-39(65)31(48)22-29-15-17-30(63)18-16-29)41(67)56-24-38(64)57-34(42(68)61-36)23-28-11-7-6-8-12-28/h6-8,11-12,15-18,27,31-37,63H,5,9-10,13-14,19-26,48H2,1-4H3,(H,53,66)(H,56,67)(H,57,64)(H,58,69)(H,59,70)(H,60,65)(H,61,68)(H4,49,50,54)(H4,51,52,55)/t31-,32-,33-,34+,35-,36+,37-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinities against Opioid receptor delta 1 of guinea pig brain membrane using [3H]-DPDPE as the radioligand using competition binding assays. |
J Med Chem 33: 206-12 (1990)
BindingDB Entry DOI: 10.7270/Q2QV3KG6 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(GUINEA PIG) | BDBM50010545

(2-{5-Guanidino-2-[(5-guanidino-2-{2-[2-(2-{2-[3-(4...)Show SMILES CCNC(=O)C(CC(C)C)NC(=O)C(CCC[N-]C(N)=[NH2+])N(C)C(=O)C(CCC[N-]C(N)=[NH2+])NC(=O)C(CC(C)C)NC(=O)C(Cc1ccc(cc1)[N+]([O-])=O)NC(=O)CNC(=O)CNC(=O)C(Cc1ccc(O)cc1)NC Show InChI InChI=1S/C50H80N16O11/c1-8-56-44(71)37(23-29(2)3)64-47(74)40(12-10-22-58-50(53)54)65(7)48(75)35(11-9-21-57-49(51)52)62-45(72)38(24-30(4)5)63-46(73)39(26-31-13-17-33(18-14-31)66(76)77)61-42(69)28-59-41(68)27-60-43(70)36(55-6)25-32-15-19-34(67)20-16-32/h13-20,29-30,35-40,55H,8-12,21-28H2,1-7H3,(H16,51,52,53,54,56,57,58,59,60,61,62,63,64,67,68,69,70,71,72,73,74) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against Opioid receptor mu 1 of guinea pig ileum (GPI) |
J Med Chem 33: 206-12 (1990)
BindingDB Entry DOI: 10.7270/Q2QV3KG6 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Mus musculus (Mouse)) | BDBM50010534
(2-[2-(2-{2-[3-(4-Hydroxy-phenyl)-2-methylamino-pro...)Show SMILES CCNC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCNC(N)=N)N(C)C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@H](Cc1ccc(O)cc1)NC Show InChI InChI=1S/C50H81N15O9/c1-8-56-44(70)37(24-30(2)3)64-47(73)40(17-13-23-58-50(53)54)65(7)48(74)35(16-12-22-57-49(51)52)62-45(71)38(25-31(4)5)63-46(72)39(27-32-14-10-9-11-15-32)61-42(68)29-59-41(67)28-60-43(69)36(55-6)26-33-18-20-34(66)21-19-33/h9-11,14-15,18-21,30-31,35-40,55,66H,8,12-13,16-17,22-29H2,1-7H3,(H,56,70)(H,59,67)(H,60,69)(H,61,68)(H,62,71)(H,63,72)(H,64,73)(H4,51,52,57)(H4,53,54,58)/t35-,36-,37-,38-,39+,40-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinities against Opioid receptor kappa 1 of guinea pig brain membrane using [3H]U69,593 as the radioligand using competition binding assays... |
J Med Chem 33: 206-12 (1990)
BindingDB Entry DOI: 10.7270/Q2QV3KG6 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(GUINEA PIG) | BDBM50010536

(2-[5-Guanidino-2-(5-guanidino-2-{2-[2-(2-{2-[3-(4-...)Show SMILES CN[C@@H](Cc1ccc(O)cc1)C(=O)NCC(=O)NCC(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(N)=O Show InChI InChI=1S/C47H75N15O9/c1-27(2)21-34(40(48)66)61-43(69)33(14-10-20-55-47(51)52)59-42(68)32(13-9-19-54-46(49)50)60-44(70)36(22-28(3)4)62-45(71)37(24-29-11-7-6-8-12-29)58-39(65)26-56-38(64)25-57-41(67)35(53-5)23-30-15-17-31(63)18-16-30/h6-8,11-12,15-18,27-28,32-37,53,63H,9-10,13-14,19-26H2,1-5H3,(H2,48,66)(H,56,64)(H,57,67)(H,58,65)(H,59,68)(H,60,70)(H,61,69)(H,62,71)(H4,49,50,54)(H4,51,52,55)/t32-,33-,34-,35-,36-,37+/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinities against Opioid receptor mu 1 of guinea pig brain membrane using [3H]-DAGO as the radioligand using competition binding assays. |
J Med Chem 33: 206-12 (1990)
BindingDB Entry DOI: 10.7270/Q2QV3KG6 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Mus musculus (Mouse)) | BDBM50010532

(13-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]-7...)Show SMILES CCNC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCNC(N)=N)N(C)C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H]1CSSC[C@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@H](Cc2ccccc2)C(=O)N1 Show InChI InChI=1S/C47H73N15O9S2/c1-5-53-40(66)33(21-27(2)3)59-44(70)37(14-10-20-55-47(51)52)62(4)45(71)32(13-9-19-54-46(49)50)58-43(69)36-26-73-72-25-35(60-39(65)31(48)22-29-15-17-30(63)18-16-29)41(67)56-24-38(64)57-34(42(68)61-36)23-28-11-7-6-8-12-28/h6-8,11-12,15-18,27,31-37,63H,5,9-10,13-14,19-26,48H2,1-4H3,(H,53,66)(H,56,67)(H,57,64)(H,58,69)(H,59,70)(H,60,65)(H,61,68)(H4,49,50,54)(H4,51,52,55)/t31-,32-,33-,34+,35-,36+,37-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinities against Opioid receptor kappa 1 of guinea pig brain membrane using [3H]U69,593 as the radioligand using competition binding assays... |
J Med Chem 33: 206-12 (1990)
BindingDB Entry DOI: 10.7270/Q2QV3KG6 |
More data for this
Ligand-Target Pair | |
Promotilin
(RABBIT) | BDBM85389
(CAS_52906-92-0 | Motilin)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccccc1)-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#16]-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](-[#8])=O |r| Show InChI InChI=1S/C120H188N34O35S/c1-9-64(6)97(152-114(184)86-31-22-53-154(86)117(187)96(63(4)5)151-99(169)70(123)56-66-23-12-10-13-24-66)115(185)150-84(57-67-25-14-11-15-26-67)113(183)153-98(65(7)155)116(186)149-83(58-68-32-34-69(156)35-33-68)101(171)135-60-91(161)136-75(39-45-93(163)164)105(175)147-82(55-62(2)3)111(181)145-77(37-43-88(125)158)106(176)140-73(29-20-51-132-119(128)129)103(173)146-80(48-54-190-8)110(180)142-76(36-42-87(124)157)107(177)144-79(41-47-95(167)168)108(178)139-72(28-17-19-50-122)102(172)143-78(40-46-94(165)166)109(179)141-74(30-21-52-133-120(130)131)104(174)148-85(59-90(127)160)112(182)138-71(27-16-18-49-121)100(170)134-61-92(162)137-81(118(188)189)38-44-89(126)159/h10-15,23-26,32-35,62-65,70-86,96-98,155-156H,9,16-22,27-31,36-61,121-123H2,1-8H3,(H2,124,157)(H2,125,158)(H2,126,159)(H2,127,160)(H,134,170)(H,135,171)(H,136,161)(H,137,162)(H,138,182)(H,139,178)(H,140,176)(H,141,179)(H,142,180)(H,143,172)(H,144,177)(H,145,181)(H,146,173)(H,147,175)(H,148,174)(H,149,186)(H,150,185)(H,151,169)(H,152,184)(H,153,183)(H,163,164)(H,165,166)(H,167,168)(H,188,189)(H4,128,129,132)(H4,130,131,133)/t64-,65+,70-,71-,72-,73-,74-,75-,76-,77-,78-,79-,80-,81-,82-,83-,84-,85-,86-,96-,97-,98-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanwa Kagaku Kenkyusho Co., Ltd.
Curated by PDSP Ki Database
| |
Pharmacology 60: 128-35 (2000)
Article DOI: 28357
BindingDB Entry DOI: 10.7270/Q2RV0M7N |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(GUINEA PIG) | BDBM50010535

(2-{5-Guanidino-2-[(5-guanidino-2-{2-[2-(2-{2-[3-(4...)Show SMILES CN[C@@H](Cc1ccc(O)cc1)C(=O)NCC(=O)NCC(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N(C)[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(N)=O Show InChI InChI=1S/C48H77N15O9/c1-28(2)22-34(41(49)67)61-45(71)38(15-11-21-56-48(52)53)63(6)46(72)33(14-10-20-55-47(50)51)60-43(69)36(23-29(3)4)62-44(70)37(25-30-12-8-7-9-13-30)59-40(66)27-57-39(65)26-58-42(68)35(54-5)24-31-16-18-32(64)19-17-31/h7-9,12-13,16-19,28-29,33-38,54,64H,10-11,14-15,20-27H2,1-6H3,(H2,49,67)(H,57,65)(H,58,68)(H,59,66)(H,60,69)(H,61,71)(H,62,70)(H4,50,51,55)(H4,52,53,56)/t33-,34-,35-,36-,37+,38-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against Opioid receptor mu 1 of guinea pig ileum (GPI) |
J Med Chem 33: 206-12 (1990)
BindingDB Entry DOI: 10.7270/Q2QV3KG6 |
More data for this
Ligand-Target Pair | |
Promotilin
(RABBIT) | BDBM85615

(L-Phe-L-Val-L-Pro-L-Ile-L-Phe-L-Thr-L-Tyr-Gly-L-Gl...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccccc1)-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#8])-[#6](-[#8])=O Show InChI InChI=1S/C125H197N35O37/c1-10-67(8)101(158-119(192)90-32-23-54-160(90)122(195)100(66(6)7)157-103(176)73(128)58-69-24-13-11-14-25-69)120(193)156-88(59-70-26-15-12-16-27-70)118(191)159-102(68(9)162)121(194)155-87(60-71-33-35-72(163)36-34-71)105(178)140-63-96(169)142-79(40-46-97(170)171)110(183)153-86(57-65(4)5)116(189)149-80(38-44-92(130)165)111(184)145-76(30-21-52-137-124(133)134)107(180)152-85(56-64(2)3)115(188)150-81(39-45-93(131)166)112(185)148-83(42-48-99(174)175)113(186)144-75(29-18-20-51-127)106(179)147-82(41-47-98(172)173)114(187)146-77(31-22-53-138-125(135)136)108(181)154-89(61-94(132)167)117(190)143-74(28-17-19-50-126)104(177)139-62-95(168)141-78(37-43-91(129)164)109(182)151-84(49-55-161)123(196)197/h11-16,24-27,33-36,64-68,73-90,100-102,161-163H,10,17-23,28-32,37-63,126-128H2,1-9H3,(H2,129,164)(H2,130,165)(H2,131,166)(H2,132,167)(H,139,177)(H,140,178)(H,141,168)(H,142,169)(H,143,190)(H,144,186)(H,145,184)(H,146,187)(H,147,179)(H,148,185)(H,149,189)(H,150,188)(H,151,182)(H,152,180)(H,153,183)(H,154,181)(H,155,194)(H,156,193)(H,157,176)(H,158,192)(H,159,191)(H,170,171)(H,172,173)(H,174,175)(H,196,197)(H4,133,134,137)(H4,135,136,138)/t67-,68+,73-,74-,75-,76-,77-,78-,79-,80-,81-,82-,83-,84-,85-,86-,87-,88-,89-,90-,100-,101-,102-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanwa Kagaku Kenkyusho Co., Ltd.
Curated by PDSP Ki Database
| |
Pharmacology 60: 128-35 (2000)
Article DOI: 28357
BindingDB Entry DOI: 10.7270/Q2RV0M7N |
More data for this
Ligand-Target Pair | |
Promotilin
(RABBIT) | BDBM85615

(L-Phe-L-Val-L-Pro-L-Ile-L-Phe-L-Thr-L-Tyr-Gly-L-Gl...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccccc1)-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#8])-[#6](-[#8])=O Show InChI InChI=1S/C125H197N35O37/c1-10-67(8)101(158-119(192)90-32-23-54-160(90)122(195)100(66(6)7)157-103(176)73(128)58-69-24-13-11-14-25-69)120(193)156-88(59-70-26-15-12-16-27-70)118(191)159-102(68(9)162)121(194)155-87(60-71-33-35-72(163)36-34-71)105(178)140-63-96(169)142-79(40-46-97(170)171)110(183)153-86(57-65(4)5)116(189)149-80(38-44-92(130)165)111(184)145-76(30-21-52-137-124(133)134)107(180)152-85(56-64(2)3)115(188)150-81(39-45-93(131)166)112(185)148-83(42-48-99(174)175)113(186)144-75(29-18-20-51-127)106(179)147-82(41-47-98(172)173)114(187)146-77(31-22-53-138-125(135)136)108(181)154-89(61-94(132)167)117(190)143-74(28-17-19-50-126)104(177)139-62-95(168)141-78(37-43-91(129)164)109(182)151-84(49-55-161)123(196)197/h11-16,24-27,33-36,64-68,73-90,100-102,161-163H,10,17-23,28-32,37-63,126-128H2,1-9H3,(H2,129,164)(H2,130,165)(H2,131,166)(H2,132,167)(H,139,177)(H,140,178)(H,141,168)(H,142,169)(H,143,190)(H,144,186)(H,145,184)(H,146,187)(H,147,179)(H,148,185)(H,149,189)(H,150,188)(H,151,182)(H,152,180)(H,153,183)(H,154,181)(H,155,194)(H,156,193)(H,157,176)(H,158,192)(H,159,191)(H,170,171)(H,172,173)(H,174,175)(H,196,197)(H4,133,134,137)(H4,135,136,138)/t67-,68+,73-,74-,75-,76-,77-,78-,79-,80-,81-,82-,83-,84-,85-,86-,87-,88-,89-,90-,100-,101-,102-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanwa Kagaku Kenkyusho Co., Ltd.
Curated by PDSP Ki Database
| |
Pharmacology 60: 128-35 (2000)
Article DOI: 28357
BindingDB Entry DOI: 10.7270/Q2RV0M7N |
More data for this
Ligand-Target Pair | |
Promotilin
(Homo sapiens (Human)) | BDBM85614

([Leu13]motilin | [leu13]pMOT(1-22))Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccccc1)-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](-[#8])=O |r| Show InChI InChI=1S/C121H190N34O35/c1-10-65(8)98(153-115(185)87-32-23-53-155(87)118(188)97(64(6)7)152-100(170)71(124)56-67-24-13-11-14-25-67)116(186)151-85(57-68-26-15-12-16-27-68)114(184)154-99(66(9)156)117(187)150-84(58-69-33-35-70(157)36-34-69)102(172)136-60-92(162)137-76(40-46-94(164)165)106(176)148-83(55-63(4)5)112(182)145-77(37-43-88(125)158)107(177)141-74(30-21-51-133-120(129)130)104(174)147-82(54-62(2)3)111(181)146-78(38-44-89(126)159)108(178)144-80(42-48-96(168)169)109(179)140-73(29-18-20-50-123)103(173)143-79(41-47-95(166)167)110(180)142-75(31-22-52-134-121(131)132)105(175)149-86(59-91(128)161)113(183)139-72(28-17-19-49-122)101(171)135-61-93(163)138-81(119(189)190)39-45-90(127)160/h11-16,24-27,33-36,62-66,71-87,97-99,156-157H,10,17-23,28-32,37-61,122-124H2,1-9H3,(H2,125,158)(H2,126,159)(H2,127,160)(H2,128,161)(H,135,171)(H,136,172)(H,137,162)(H,138,163)(H,139,183)(H,140,179)(H,141,177)(H,142,180)(H,143,173)(H,144,178)(H,145,182)(H,146,181)(H,147,174)(H,148,176)(H,149,175)(H,150,187)(H,151,186)(H,152,170)(H,153,185)(H,154,184)(H,164,165)(H,166,167)(H,168,169)(H,189,190)(H4,129,130,133)(H4,131,132,134)/t65-,66+,71-,72-,73-,74-,75-,76-,77-,78-,79-,80-,81-,82-,83-,84-,85-,86-,87-,97-,98-,99-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanwa Kagaku Kenkyusho Co., Ltd.
Curated by PDSP Ki Database
| |
Pharmacology 60: 128-35 (2000)
Article DOI: 28357
BindingDB Entry DOI: 10.7270/Q2RV0M7N |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Mus musculus (Mouse)) | BDBM50010545

(2-{5-Guanidino-2-[(5-guanidino-2-{2-[2-(2-{2-[3-(4...)Show SMILES CCNC(=O)C(CC(C)C)NC(=O)C(CCC[N-]C(N)=[NH2+])N(C)C(=O)C(CCC[N-]C(N)=[NH2+])NC(=O)C(CC(C)C)NC(=O)C(Cc1ccc(cc1)[N+]([O-])=O)NC(=O)CNC(=O)CNC(=O)C(Cc1ccc(O)cc1)NC Show InChI InChI=1S/C50H80N16O11/c1-8-56-44(71)37(23-29(2)3)64-47(74)40(12-10-22-58-50(53)54)65(7)48(75)35(11-9-21-57-49(51)52)62-45(72)38(24-30(4)5)63-46(73)39(26-31-13-17-33(18-14-31)66(76)77)61-42(69)28-59-41(68)27-60-43(70)36(55-6)25-32-15-19-34(67)20-16-32/h13-20,29-30,35-40,55H,8-12,21-28H2,1-7H3,(H16,51,52,53,54,56,57,58,59,60,61,62,63,64,67,68,69,70,71,72,73,74) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinities against Opioid receptor kappa 1 of guinea pig brain membrane using [3H]U69,593 as the radioligand using competition binding assays... |
J Med Chem 33: 206-12 (1990)
BindingDB Entry DOI: 10.7270/Q2QV3KG6 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(GUINEA PIG) | BDBM50010534

(2-[2-(2-{2-[3-(4-Hydroxy-phenyl)-2-methylamino-pro...)Show SMILES CCNC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCNC(N)=N)N(C)C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@H](Cc1ccc(O)cc1)NC Show InChI InChI=1S/C50H81N15O9/c1-8-56-44(70)37(24-30(2)3)64-47(73)40(17-13-23-58-50(53)54)65(7)48(74)35(16-12-22-57-49(51)52)62-45(71)38(25-31(4)5)63-46(72)39(27-32-14-10-9-11-15-32)61-42(68)29-59-41(67)28-60-43(69)36(55-6)26-33-18-20-34(66)21-19-33/h9-11,14-15,18-21,30-31,35-40,55,66H,8,12-13,16-17,22-29H2,1-7H3,(H,56,70)(H,59,67)(H,60,69)(H,61,68)(H,62,71)(H,63,72)(H,64,73)(H4,51,52,57)(H4,53,54,58)/t35-,36-,37-,38-,39+,40-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against Opioid receptor mu 1 of guinea pig ileum (GPI) |
J Med Chem 33: 206-12 (1990)
BindingDB Entry DOI: 10.7270/Q2QV3KG6 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(GUINEA PIG) | BDBM50010538

(2-[2-(2-{2-[2-(2-{2-[2-Amino-3-(4-hydroxy-phenyl)-...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(N)=O Show InChI InChI=1S/C46H73N15O9/c1-5-27(4)38(39(48)65)61-42(68)33(14-10-20-54-46(51)52)58-41(67)32(13-9-19-53-45(49)50)59-43(69)34(21-26(2)3)60-44(70)35(23-28-11-7-6-8-12-28)57-37(64)25-55-36(63)24-56-40(66)31(47)22-29-15-17-30(62)18-16-29/h6-8,11-12,15-18,26-27,31-35,38,62H,5,9-10,13-14,19-25,47H2,1-4H3,(H2,48,65)(H,55,63)(H,56,66)(H,57,64)(H,58,67)(H,59,69)(H,60,70)(H,61,68)(H4,49,50,53)(H4,51,52,54)/t27-,31-,32-,33-,34-,35+,38-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinities against Opioid receptor mu 1 of guinea pig brain membrane using [3H]-DAGO as the radioligand using competition binding assays. |
J Med Chem 33: 206-12 (1990)
BindingDB Entry DOI: 10.7270/Q2QV3KG6 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(GUINEA PIG) | BDBM50000092

((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...)Show SMILES CN1CC[C@@]23[C@H]4Oc5c2c(C[C@@H]1[C@@H]3C=C[C@@H]4O)ccc5O |r,c:16,TLB:13:12:8.9.10:3.2.1| Show InChI InChI=1S/C17H19NO3/c1-18-7-6-17-10-3-5-13(20)16(17)21-15-12(19)4-2-9(14(15)17)8-11(10)18/h2-5,10-11,13,16,19-20H,6-8H2,1H3/t10-,11+,13-,16-,17-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinities against Opioid receptor mu 1 of guinea pig brain membrane using [3H]-DAGO as the radioligand using competition binding assays. |
J Med Chem 33: 206-12 (1990)
BindingDB Entry DOI: 10.7270/Q2QV3KG6 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Mus musculus (Mouse)) | BDBM50010537

(2-[5-Guanidino-2-(5-guanidino-2-{2-[2-(2-{2-[3-(4-...)Show SMILES CN[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](C)C(=O)NCC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(N)=O Show InChI InChI=1S/C48H77N15O9/c1-27(2)22-35(40(49)66)62-43(69)34(15-11-21-56-48(52)53)60-42(68)33(14-10-20-55-47(50)51)61-45(71)37(23-28(3)4)63-46(72)38(25-30-12-8-7-9-13-30)59-39(65)26-57-41(67)29(5)58-44(70)36(54-6)24-31-16-18-32(64)19-17-31/h7-9,12-13,16-19,27-29,33-38,54,64H,10-11,14-15,20-26H2,1-6H3,(H2,49,66)(H,57,67)(H,58,70)(H,59,65)(H,60,68)(H,61,71)(H,62,69)(H,63,72)(H4,50,51,55)(H4,52,53,56)/t29-,33-,34-,35-,36-,37-,38-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 9.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinities against Opioid receptor kappa 1 of guinea pig brain membrane using [3H]U69,593 as the radioligand using competition binding assays... |
J Med Chem 33: 206-12 (1990)
BindingDB Entry DOI: 10.7270/Q2QV3KG6 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50010545

(2-{5-Guanidino-2-[(5-guanidino-2-{2-[2-(2-{2-[3-(4...)Show SMILES CCNC(=O)C(CC(C)C)NC(=O)C(CCC[N-]C(N)=[NH2+])N(C)C(=O)C(CCC[N-]C(N)=[NH2+])NC(=O)C(CC(C)C)NC(=O)C(Cc1ccc(cc1)[N+]([O-])=O)NC(=O)CNC(=O)CNC(=O)C(Cc1ccc(O)cc1)NC Show InChI InChI=1S/C50H80N16O11/c1-8-56-44(71)37(23-29(2)3)64-47(74)40(12-10-22-58-50(53)54)65(7)48(75)35(11-9-21-57-49(51)52)62-45(72)38(24-30(4)5)63-46(73)39(26-31-13-17-33(18-14-31)66(76)77)61-42(69)28-59-41(68)27-60-43(70)36(55-6)25-32-15-19-34(67)20-16-32/h13-20,29-30,35-40,55H,8-12,21-28H2,1-7H3,(H16,51,52,53,54,56,57,58,59,60,61,62,63,64,67,68,69,70,71,72,73,74) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinities against Opioid receptor delta 1 of guinea pig brain membrane using [3H]-DPDPE as the radioligand using competition binding assays. |
J Med Chem 33: 206-12 (1990)
BindingDB Entry DOI: 10.7270/Q2QV3KG6 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50010538

(2-[2-(2-{2-[2-(2-{2-[2-Amino-3-(4-hydroxy-phenyl)-...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(N)=O Show InChI InChI=1S/C46H73N15O9/c1-5-27(4)38(39(48)65)61-42(68)33(14-10-20-54-46(51)52)58-41(67)32(13-9-19-53-45(49)50)59-43(69)34(21-26(2)3)60-44(70)35(23-28-11-7-6-8-12-28)57-37(64)25-55-36(63)24-56-40(66)31(47)22-29-15-17-30(62)18-16-29/h6-8,11-12,15-18,26-27,31-35,38,62H,5,9-10,13-14,19-25,47H2,1-4H3,(H2,48,65)(H,55,63)(H,56,66)(H,57,64)(H,58,67)(H,59,69)(H,60,70)(H,61,68)(H4,49,50,53)(H4,51,52,54)/t27-,31-,32-,33-,34-,35+,38-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinities against Opioid receptor delta 1 of guinea pig brain membrane using [3H]-DPDPE as the radioligand using competition binding assays. |
J Med Chem 33: 206-12 (1990)
BindingDB Entry DOI: 10.7270/Q2QV3KG6 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50010534

(2-[2-(2-{2-[3-(4-Hydroxy-phenyl)-2-methylamino-pro...)Show SMILES CCNC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCNC(N)=N)N(C)C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@H](Cc1ccc(O)cc1)NC Show InChI InChI=1S/C50H81N15O9/c1-8-56-44(70)37(24-30(2)3)64-47(73)40(17-13-23-58-50(53)54)65(7)48(74)35(16-12-22-57-49(51)52)62-45(71)38(25-31(4)5)63-46(72)39(27-32-14-10-9-11-15-32)61-42(68)29-59-41(67)28-60-43(69)36(55-6)26-33-18-20-34(66)21-19-33/h9-11,14-15,18-21,30-31,35-40,55,66H,8,12-13,16-17,22-29H2,1-7H3,(H,56,70)(H,59,67)(H,60,69)(H,61,68)(H,62,71)(H,63,72)(H,64,73)(H4,51,52,57)(H4,53,54,58)/t35-,36-,37-,38-,39+,40-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinities against Opioid receptor delta 1 of guinea pig brain membrane using [3H]-DPDPE as the radioligand using competition binding assays. |
J Med Chem 33: 206-12 (1990)
BindingDB Entry DOI: 10.7270/Q2QV3KG6 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50010542

(2-[5-Guanidino-2-(5-guanidino-2-{2-[2-(2-{2-[3-(4-...)Show SMILES CNC(Cc1ccc(O)cc1)C(=O)NCC(=O)NCC(=O)NC(Cc1ccc(cc1)[N+]([O-])=O)C(=O)NC(CC(C)C)C(=O)NC(CCC[N-]C(N)=[NH2+])C(=O)NC(CCC[N-]C(N)=[NH2+])C(=O)NC(CC(C)C)C(N)=O Show InChI InChI=1S/C47H74N16O11/c1-26(2)20-34(40(48)67)61-43(70)33(9-7-19-55-47(51)52)59-42(69)32(8-6-18-54-46(49)50)60-44(71)36(21-27(3)4)62-45(72)37(23-28-10-14-30(15-11-28)63(73)74)58-39(66)25-56-38(65)24-57-41(68)35(53-5)22-29-12-16-31(64)17-13-29/h10-17,26-27,32-37,53H,6-9,18-25H2,1-5H3,(H18,48,49,50,51,52,54,55,56,57,58,59,60,61,62,64,65,66,67,68,69,70,71,72) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinities against Opioid receptor delta 1 of guinea pig brain membrane using [3H]-DPDPE as the radioligand using competition binding assays. |
J Med Chem 33: 206-12 (1990)
BindingDB Entry DOI: 10.7270/Q2QV3KG6 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50010537

(2-[5-Guanidino-2-(5-guanidino-2-{2-[2-(2-{2-[3-(4-...)Show SMILES CN[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](C)C(=O)NCC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(N)=O Show InChI InChI=1S/C48H77N15O9/c1-27(2)22-35(40(49)66)62-43(69)34(15-11-21-56-48(52)53)60-42(68)33(14-10-20-55-47(50)51)61-45(71)37(23-28(3)4)63-46(72)38(25-30-12-8-7-9-13-30)59-39(65)26-57-41(67)29(5)58-44(70)36(54-6)24-31-16-18-32(64)19-17-31/h7-9,12-13,16-19,27-29,33-38,54,64H,10-11,14-15,20-26H2,1-6H3,(H2,49,66)(H,57,67)(H,58,70)(H,59,65)(H,60,68)(H,61,71)(H,62,69)(H,63,72)(H4,50,51,55)(H4,52,53,56)/t29-,33-,34-,35-,36-,37-,38-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinities against Opioid receptor delta 1 of guinea pig brain membrane using [3H]-DPDPE as the radioligand using competition binding assays. |
J Med Chem 33: 206-12 (1990)
BindingDB Entry DOI: 10.7270/Q2QV3KG6 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50010536

(2-[5-Guanidino-2-(5-guanidino-2-{2-[2-(2-{2-[3-(4-...)Show SMILES CN[C@@H](Cc1ccc(O)cc1)C(=O)NCC(=O)NCC(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(N)=O Show InChI InChI=1S/C47H75N15O9/c1-27(2)21-34(40(48)66)61-43(69)33(14-10-20-55-47(51)52)59-42(68)32(13-9-19-54-46(49)50)60-44(70)36(22-28(3)4)62-45(71)37(24-29-11-7-6-8-12-29)58-39(65)26-56-38(64)25-57-41(67)35(53-5)23-30-15-17-31(63)18-16-30/h6-8,11-12,15-18,27-28,32-37,53,63H,9-10,13-14,19-26H2,1-5H3,(H2,48,66)(H,56,64)(H,57,67)(H,58,65)(H,59,68)(H,60,70)(H,61,69)(H,62,71)(H4,49,50,54)(H4,51,52,55)/t32-,33-,34-,35-,36-,37+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinities against Opioid receptor delta 1 of guinea pig brain membrane using [3H]-DPDPE as the radioligand using competition binding assays. |
J Med Chem 33: 206-12 (1990)
BindingDB Entry DOI: 10.7270/Q2QV3KG6 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50010535

(2-{5-Guanidino-2-[(5-guanidino-2-{2-[2-(2-{2-[3-(4...)Show SMILES CN[C@@H](Cc1ccc(O)cc1)C(=O)NCC(=O)NCC(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N(C)[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(N)=O Show InChI InChI=1S/C48H77N15O9/c1-28(2)22-34(41(49)67)61-45(71)38(15-11-21-56-48(52)53)63(6)46(72)33(14-10-20-55-47(50)51)60-43(69)36(23-29(3)4)62-44(70)37(25-30-12-8-7-9-13-30)59-40(66)27-57-39(65)26-58-42(68)35(54-5)24-31-16-18-32(64)19-17-31/h7-9,12-13,16-19,28-29,33-38,54,64H,10-11,14-15,20-27H2,1-6H3,(H2,49,67)(H,57,65)(H,58,68)(H,59,66)(H,60,69)(H,61,71)(H,62,70)(H4,50,51,55)(H4,52,53,56)/t33-,34-,35-,36-,37+,38-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinities against Opioid receptor delta 1 of guinea pig brain membrane using [3H]-DPDPE as the radioligand using competition binding assays. |
J Med Chem 33: 206-12 (1990)
BindingDB Entry DOI: 10.7270/Q2QV3KG6 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50000092

((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...)Show SMILES CN1CC[C@@]23[C@H]4Oc5c2c(C[C@@H]1[C@@H]3C=C[C@@H]4O)ccc5O |r,c:16,TLB:13:12:8.9.10:3.2.1| Show InChI InChI=1S/C17H19NO3/c1-18-7-6-17-10-3-5-13(20)16(17)21-15-12(19)4-2-9(14(15)17)8-11(10)18/h2-5,10-11,13,16,19-20H,6-8H2,1H3/t10-,11+,13-,16-,17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| 630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinities against Opioid receptor delta 1 of guinea pig brain membrane using [3H]-DPDPE as the radioligand using competition binding assays. |
J Med Chem 33: 206-12 (1990)
BindingDB Entry DOI: 10.7270/Q2QV3KG6 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Mus musculus (Mouse)) | BDBM50000092

((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...)Show SMILES CN1CC[C@@]23[C@H]4Oc5c2c(C[C@@H]1[C@@H]3C=C[C@@H]4O)ccc5O |r,c:16,TLB:13:12:8.9.10:3.2.1| Show InChI InChI=1S/C17H19NO3/c1-18-7-6-17-10-3-5-13(20)16(17)21-15-12(19)4-2-9(14(15)17)8-11(10)18/h2-5,10-11,13,16,19-20H,6-8H2,1H3/t10-,11+,13-,16-,17-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 1.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinities against Opioid receptor kappa 1 of guinea pig brain membrane using [3H]U69,593 as the radioligand using competition binding assays... |
J Med Chem 33: 206-12 (1990)
BindingDB Entry DOI: 10.7270/Q2QV3KG6 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(GUINEA PIG) | BDBM50010532

(13-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]-7...)Show SMILES CCNC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCNC(N)=N)N(C)C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H]1CSSC[C@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@H](Cc2ccccc2)C(=O)N1 Show InChI InChI=1S/C47H73N15O9S2/c1-5-53-40(66)33(21-27(2)3)59-44(70)37(14-10-20-55-47(51)52)62(4)45(71)32(13-9-19-54-46(49)50)58-43(69)36-26-73-72-25-35(60-39(65)31(48)22-29-15-17-30(63)18-16-29)41(67)56-24-38(64)57-34(42(68)61-36)23-28-11-7-6-8-12-28/h6-8,11-12,15-18,27,31-37,63H,5,9-10,13-14,19-26,48H2,1-4H3,(H,53,66)(H,56,67)(H,57,64)(H,58,69)(H,59,70)(H,60,65)(H,61,68)(H4,49,50,54)(H4,51,52,55)/t31-,32-,33-,34+,35-,36+,37-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinities against Opioid receptor mu 1 of guinea pig brain membrane using [3H]-DAGO as the radioligand using competition binding assays. |
J Med Chem 33: 206-12 (1990)
BindingDB Entry DOI: 10.7270/Q2QV3KG6 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50010532

(13-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]-7...)Show SMILES CCNC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCNC(N)=N)N(C)C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H]1CSSC[C@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@H](Cc2ccccc2)C(=O)N1 Show InChI InChI=1S/C47H73N15O9S2/c1-5-53-40(66)33(21-27(2)3)59-44(70)37(14-10-20-55-47(51)52)62(4)45(71)32(13-9-19-54-46(49)50)58-43(69)36-26-73-72-25-35(60-39(65)31(48)22-29-15-17-30(63)18-16-29)41(67)56-24-38(64)57-34(42(68)61-36)23-28-11-7-6-8-12-28/h6-8,11-12,15-18,27,31-37,63H,5,9-10,13-14,19-26,48H2,1-4H3,(H,53,66)(H,56,67)(H,57,64)(H,58,69)(H,59,70)(H,60,65)(H,61,68)(H4,49,50,54)(H4,51,52,55)/t31-,32-,33-,34+,35-,36+,37-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against Opioid receptor kappa 1 of rabbit vas deferens (RVD) |
J Med Chem 33: 206-12 (1990)
BindingDB Entry DOI: 10.7270/Q2QV3KG6 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50010532

(13-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]-7...)Show SMILES CCNC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCNC(N)=N)N(C)C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H]1CSSC[C@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@H](Cc2ccccc2)C(=O)N1 Show InChI InChI=1S/C47H73N15O9S2/c1-5-53-40(66)33(21-27(2)3)59-44(70)37(14-10-20-55-47(51)52)62(4)45(71)32(13-9-19-54-46(49)50)58-43(69)36-26-73-72-25-35(60-39(65)31(48)22-29-15-17-30(63)18-16-29)41(67)56-24-38(64)57-34(42(68)61-36)23-28-11-7-6-8-12-28/h6-8,11-12,15-18,27,31-37,63H,5,9-10,13-14,19-26,48H2,1-4H3,(H,53,66)(H,56,67)(H,57,64)(H,58,69)(H,59,70)(H,60,65)(H,61,68)(H4,49,50,54)(H4,51,52,55)/t31-,32-,33-,34+,35-,36+,37-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against Opioid receptor delta 1 of mouse vas deferens (MVD). |
J Med Chem 33: 206-12 (1990)
BindingDB Entry DOI: 10.7270/Q2QV3KG6 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(GUINEA PIG) | BDBM50010538

(2-[2-(2-{2-[2-(2-{2-[2-Amino-3-(4-hydroxy-phenyl)-...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(N)=O Show InChI InChI=1S/C46H73N15O9/c1-5-27(4)38(39(48)65)61-42(68)33(14-10-20-54-46(51)52)58-41(67)32(13-9-19-53-45(49)50)59-43(69)34(21-26(2)3)60-44(70)35(23-28-11-7-6-8-12-28)57-37(64)25-55-36(63)24-56-40(66)31(47)22-29-15-17-30(62)18-16-29/h6-8,11-12,15-18,26-27,31-35,38,62H,5,9-10,13-14,19-25,47H2,1-4H3,(H2,48,65)(H,55,63)(H,56,66)(H,57,64)(H,58,67)(H,59,69)(H,60,70)(H,61,68)(H4,49,50,53)(H4,51,52,54)/t27-,31-,32-,33-,34-,35+,38-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against Opioid receptor mu 1 of guinea pig ileum (GPI) |
J Med Chem 33: 206-12 (1990)
BindingDB Entry DOI: 10.7270/Q2QV3KG6 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(GUINEA PIG) | BDBM50010534

(2-[2-(2-{2-[3-(4-Hydroxy-phenyl)-2-methylamino-pro...)Show SMILES CCNC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCNC(N)=N)N(C)C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@H](Cc1ccc(O)cc1)NC Show InChI InChI=1S/C50H81N15O9/c1-8-56-44(70)37(24-30(2)3)64-47(73)40(17-13-23-58-50(53)54)65(7)48(74)35(16-12-22-57-49(51)52)62-45(71)38(25-31(4)5)63-46(72)39(27-32-14-10-9-11-15-32)61-42(68)29-59-41(67)28-60-43(69)36(55-6)26-33-18-20-34(66)21-19-33/h9-11,14-15,18-21,30-31,35-40,55,66H,8,12-13,16-17,22-29H2,1-7H3,(H,56,70)(H,59,67)(H,60,69)(H,61,68)(H,62,71)(H,63,72)(H,64,73)(H4,51,52,57)(H4,53,54,58)/t35-,36-,37-,38-,39+,40-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against Opioid receptor mu 1 of guinea pig ileum (GPI) |
J Med Chem 33: 206-12 (1990)
BindingDB Entry DOI: 10.7270/Q2QV3KG6 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(GUINEA PIG) | BDBM50010545

(2-{5-Guanidino-2-[(5-guanidino-2-{2-[2-(2-{2-[3-(4...)Show SMILES CCNC(=O)C(CC(C)C)NC(=O)C(CCC[N-]C(N)=[NH2+])N(C)C(=O)C(CCC[N-]C(N)=[NH2+])NC(=O)C(CC(C)C)NC(=O)C(Cc1ccc(cc1)[N+]([O-])=O)NC(=O)CNC(=O)CNC(=O)C(Cc1ccc(O)cc1)NC Show InChI InChI=1S/C50H80N16O11/c1-8-56-44(71)37(23-29(2)3)64-47(74)40(12-10-22-58-50(53)54)65(7)48(75)35(11-9-21-57-49(51)52)62-45(72)38(24-30(4)5)63-46(73)39(26-31-13-17-33(18-14-31)66(76)77)61-42(69)28-59-41(68)27-60-43(70)36(55-6)25-32-15-19-34(67)20-16-32/h13-20,29-30,35-40,55H,8-12,21-28H2,1-7H3,(H16,51,52,53,54,56,57,58,59,60,61,62,63,64,67,68,69,70,71,72,73,74) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against Opioid receptor mu 1 of guinea pig ileum (GPI) |
J Med Chem 33: 206-12 (1990)
BindingDB Entry DOI: 10.7270/Q2QV3KG6 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(GUINEA PIG) | BDBM50010535

(2-{5-Guanidino-2-[(5-guanidino-2-{2-[2-(2-{2-[3-(4...)Show SMILES CN[C@@H](Cc1ccc(O)cc1)C(=O)NCC(=O)NCC(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N(C)[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(N)=O Show InChI InChI=1S/C48H77N15O9/c1-28(2)22-34(41(49)67)61-45(71)38(15-11-21-56-48(52)53)63(6)46(72)33(14-10-20-55-47(50)51)60-43(69)36(23-29(3)4)62-44(70)37(25-30-12-8-7-9-13-30)59-40(66)27-57-39(65)26-58-42(68)35(54-5)24-31-16-18-32(64)19-17-31/h7-9,12-13,16-19,28-29,33-38,54,64H,10-11,14-15,20-27H2,1-6H3,(H2,49,67)(H,57,65)(H,58,68)(H,59,66)(H,60,69)(H,61,71)(H,62,70)(H4,50,51,55)(H4,52,53,56)/t33-,34-,35-,36-,37+,38-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against Opioid receptor mu 1 of guinea pig ileum (GPI) |
J Med Chem 33: 206-12 (1990)
BindingDB Entry DOI: 10.7270/Q2QV3KG6 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(GUINEA PIG) | BDBM50010540
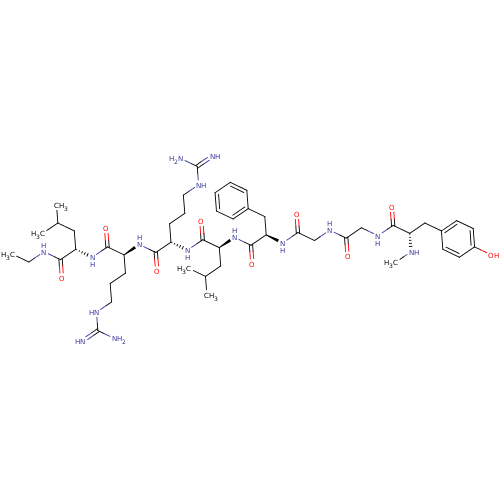
(2-[2-(2-{2-[3-(4-Hydroxy-phenyl)-2-methylamino-pro...)Show SMILES CCNC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@H](Cc1ccc(O)cc1)NC Show InChI InChI=1S/C49H79N15O9/c1-7-55-43(69)37(23-29(2)3)63-45(71)35(16-12-22-57-49(52)53)61-44(70)34(15-11-21-56-48(50)51)62-46(72)38(24-30(4)5)64-47(73)39(26-31-13-9-8-10-14-31)60-41(67)28-58-40(66)27-59-42(68)36(54-6)25-32-17-19-33(65)20-18-32/h8-10,13-14,17-20,29-30,34-39,54,65H,7,11-12,15-16,21-28H2,1-6H3,(H,55,69)(H,58,66)(H,59,68)(H,60,67)(H,61,70)(H,62,72)(H,63,71)(H,64,73)(H4,50,51,56)(H4,52,53,57)/t34-,35-,36-,37-,38-,39+/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against Opioid receptor mu 1 of guinea pig ileum (GPI) |
J Med Chem 33: 206-12 (1990)
BindingDB Entry DOI: 10.7270/Q2QV3KG6 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50010546
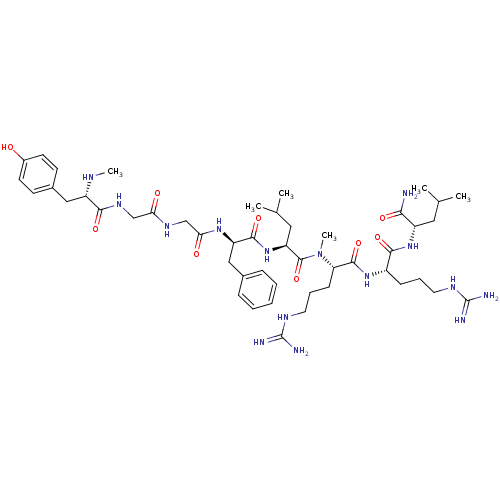
(2-{5-Guanidino-2-[5-guanidino-2-({2-[2-(2-{2-[3-(4...)Show SMILES CN[C@@H](Cc1ccc(O)cc1)C(=O)NCC(=O)NCC(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CC(C)C)C(=O)N(C)[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(N)=O Show InChI InChI=1S/C48H77N15O9/c1-28(2)22-34(41(49)67)61-43(69)33(14-10-20-55-47(50)51)60-45(71)38(15-11-21-56-48(52)53)63(6)46(72)37(23-29(3)4)62-44(70)36(25-30-12-8-7-9-13-30)59-40(66)27-57-39(65)26-58-42(68)35(54-5)24-31-16-18-32(64)19-17-31/h7-9,12-13,16-19,28-29,33-38,54,64H,10-11,14-15,20-27H2,1-6H3,(H2,49,67)(H,57,65)(H,58,68)(H,59,66)(H,60,71)(H,61,69)(H,62,70)(H4,50,51,55)(H4,52,53,56)/t33-,34-,35-,36+,37-,38-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against Opioid receptor kappa 1 of rabbit vas deferens (RVD) |
J Med Chem 33: 206-12 (1990)
BindingDB Entry DOI: 10.7270/Q2QV3KG6 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 2
(Homo sapiens (Human)) | BDBM50559408
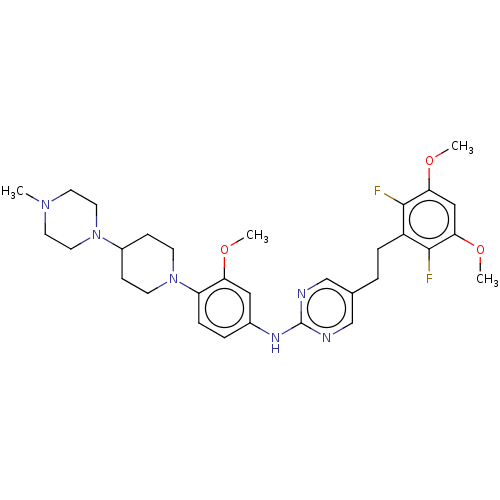
(CHEMBL4763773)Show SMILES COc1cc(OC)c(F)c(CCc2cnc(Nc3ccc(N4CCC(CC4)N4CCN(C)CC4)c(OC)c3)nc2)c1F | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human N-terminal GST-tagged FGFR2 (399 to end residues) expressed in baculovirus expression system using CSKtide as substrate incubated... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116019
BindingDB Entry DOI: 10.7270/Q2QN6BGC |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 3
(Homo sapiens (Human)) | BDBM50559408

(CHEMBL4763773)Show SMILES COc1cc(OC)c(F)c(CCc2cnc(Nc3ccc(N4CCC(CC4)N4CCN(C)CC4)c(OC)c3)nc2)c1F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human N-terminal GST-tagged FGFR3 (436 to end residues) expressed in baculovirus expression system using CSKtide as substrate incubated... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116019
BindingDB Entry DOI: 10.7270/Q2QN6BGC |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(GUINEA PIG) | BDBM50010542

(2-[5-Guanidino-2-(5-guanidino-2-{2-[2-(2-{2-[3-(4-...)Show SMILES CNC(Cc1ccc(O)cc1)C(=O)NCC(=O)NCC(=O)NC(Cc1ccc(cc1)[N+]([O-])=O)C(=O)NC(CC(C)C)C(=O)NC(CCC[N-]C(N)=[NH2+])C(=O)NC(CCC[N-]C(N)=[NH2+])C(=O)NC(CC(C)C)C(N)=O Show InChI InChI=1S/C47H74N16O11/c1-26(2)20-34(40(48)67)61-43(70)33(9-7-19-55-47(51)52)59-42(69)32(8-6-18-54-46(49)50)60-44(71)36(21-27(3)4)62-45(72)37(23-28-10-14-30(15-11-28)63(73)74)58-39(66)25-56-38(65)24-57-41(68)35(53-5)22-29-12-16-31(64)17-13-29/h10-17,26-27,32-37,53H,6-9,18-25H2,1-5H3,(H18,48,49,50,51,52,54,55,56,57,58,59,60,61,62,64,65,66,67,68,69,70,71,72) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against Opioid receptor mu 1 of guinea pig ileum (GPI) |
J Med Chem 33: 206-12 (1990)
BindingDB Entry DOI: 10.7270/Q2QV3KG6 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(GUINEA PIG) | BDBM50010536

(2-[5-Guanidino-2-(5-guanidino-2-{2-[2-(2-{2-[3-(4-...)Show SMILES CN[C@@H](Cc1ccc(O)cc1)C(=O)NCC(=O)NCC(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(N)=O Show InChI InChI=1S/C47H75N15O9/c1-27(2)21-34(40(48)66)61-43(69)33(14-10-20-55-47(51)52)59-42(68)32(13-9-19-54-46(49)50)60-44(70)36(22-28(3)4)62-45(71)37(24-29-11-7-6-8-12-29)58-39(65)26-56-38(64)25-57-41(67)35(53-5)23-30-15-17-31(63)18-16-30/h6-8,11-12,15-18,27-28,32-37,53,63H,9-10,13-14,19-26H2,1-5H3,(H2,48,66)(H,56,64)(H,57,67)(H,58,65)(H,59,68)(H,60,70)(H,61,69)(H,62,71)(H4,49,50,54)(H4,51,52,55)/t32-,33-,34-,35-,36-,37+/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinities against Opioid receptor mu 1 of guinea pig brain membrane using [3H]-DAGO as the radioligand using competition binding assays. |
J Med Chem 33: 206-12 (1990)
BindingDB Entry DOI: 10.7270/Q2QV3KG6 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 3
(Homo sapiens (Human)) | BDBM50559406
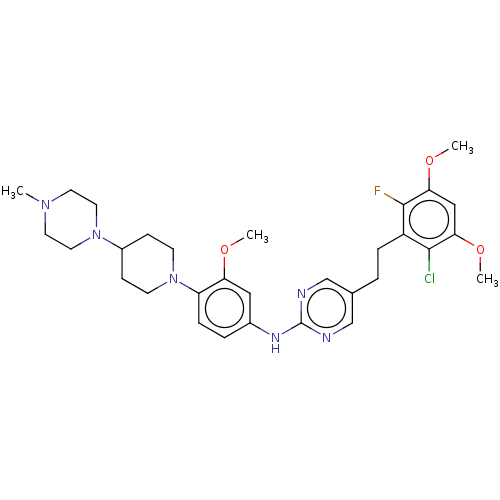
(CHEMBL4746045)Show SMILES COc1cc(OC)c(Cl)c(CCc2cnc(Nc3ccc(N4CCC(CC4)N4CCN(C)CC4)c(OC)c3)nc2)c1F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human N-terminal GST-tagged FGFR3 (436 to end residues) expressed in baculovirus expression system using CSKtide as substrate incubated... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116019
BindingDB Entry DOI: 10.7270/Q2QN6BGC |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50559408

(CHEMBL4763773)Show SMILES COc1cc(OC)c(F)c(CCc2cnc(Nc3ccc(N4CCC(CC4)N4CCN(C)CC4)c(OC)c3)nc2)c1F | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human N-terminal GST-tagged FGFR1 (398 to end residues) expressed in baculovirus expression system using CSKtide as substrate incubated... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116019
BindingDB Entry DOI: 10.7270/Q2QN6BGC |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50010535

(2-{5-Guanidino-2-[(5-guanidino-2-{2-[2-(2-{2-[3-(4...)Show SMILES CN[C@@H](Cc1ccc(O)cc1)C(=O)NCC(=O)NCC(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N(C)[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(N)=O Show InChI InChI=1S/C48H77N15O9/c1-28(2)22-34(41(49)67)61-45(71)38(15-11-21-56-48(52)53)63(6)46(72)33(14-10-20-55-47(50)51)60-43(69)36(23-29(3)4)62-44(70)37(25-30-12-8-7-9-13-30)59-40(66)27-57-39(65)26-58-42(68)35(54-5)24-31-16-18-32(64)19-17-31/h7-9,12-13,16-19,28-29,33-38,54,64H,10-11,14-15,20-27H2,1-6H3,(H2,49,67)(H,57,65)(H,58,68)(H,59,66)(H,60,69)(H,61,71)(H,62,70)(H4,50,51,55)(H4,52,53,56)/t33-,34-,35-,36-,37+,38-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against Opioid receptor kappa 1 of rabbit vas deferens (RVD) |
J Med Chem 33: 206-12 (1990)
BindingDB Entry DOI: 10.7270/Q2QV3KG6 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50010534

(2-[2-(2-{2-[3-(4-Hydroxy-phenyl)-2-methylamino-pro...)Show SMILES CCNC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCNC(N)=N)N(C)C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@H](Cc1ccc(O)cc1)NC Show InChI InChI=1S/C50H81N15O9/c1-8-56-44(70)37(24-30(2)3)64-47(73)40(17-13-23-58-50(53)54)65(7)48(74)35(16-12-22-57-49(51)52)62-45(71)38(25-31(4)5)63-46(72)39(27-32-14-10-9-11-15-32)61-42(68)29-59-41(67)28-60-43(69)36(55-6)26-33-18-20-34(66)21-19-33/h9-11,14-15,18-21,30-31,35-40,55,66H,8,12-13,16-17,22-29H2,1-7H3,(H,56,70)(H,59,67)(H,60,69)(H,61,68)(H,62,71)(H,63,72)(H,64,73)(H4,51,52,57)(H4,53,54,58)/t35-,36-,37-,38-,39+,40-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against Opioid receptor kappa 1 of rabbit vas deferens (RVD) |
J Med Chem 33: 206-12 (1990)
BindingDB Entry DOI: 10.7270/Q2QV3KG6 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(GUINEA PIG) | BDBM50010543
(6-Guanidino-2-{2-[2-(2-{2-[3-(4-hydroxy-phenyl)-2-...)Show SMILES CN[C@@H](Cc1ccc(O)cc1)C(=O)NCC(=O)NCC(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(N)=O Show InChI InChI=1S/C48H77N15O9/c1-28(2)22-35(41(49)67)62-44(70)34(15-11-21-56-48(52)53)60-43(69)33(14-9-10-20-55-47(50)51)61-45(71)37(23-29(3)4)63-46(72)38(25-30-12-7-6-8-13-30)59-40(66)27-57-39(65)26-58-42(68)36(54-5)24-31-16-18-32(64)19-17-31/h6-8,12-13,16-19,28-29,33-38,54,64H,9-11,14-15,20-27H2,1-5H3,(H2,49,67)(H,57,65)(H,58,68)(H,59,66)(H,60,69)(H,61,71)(H,62,70)(H,63,72)(H4,50,51,55)(H4,52,53,56)/t33-,34-,35-,36-,37-,38+/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against Opioid receptor mu 1 of guinea pig ileum (GPI) |
J Med Chem 33: 206-12 (1990)
BindingDB Entry DOI: 10.7270/Q2QV3KG6 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

















































