

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Translocator protein (Rattus norvegicus (rat)) | BDBM50122294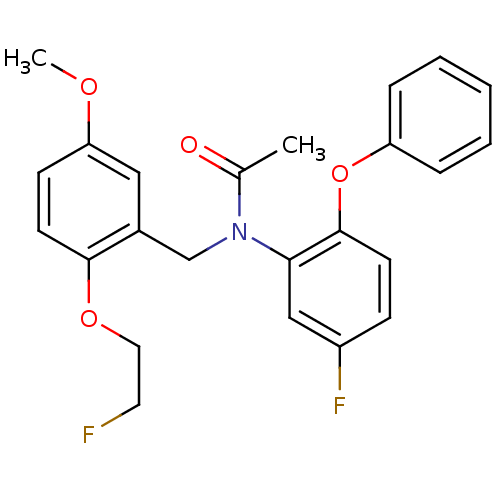 (CHEMBL292092 | N-(5-fluoro-2-phenoxyphenyl)-N-(2-(...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Radiological Sciences Curated by ChEMBL | Assay Description Displacement of [11C]DAA1106 from PBR receptor in rat brain membrane | Bioorg Med Chem Lett 19: 1707-10 (2009) Article DOI: 10.1016/j.bmcl.2009.01.093 BindingDB Entry DOI: 10.7270/Q2C82B6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50111728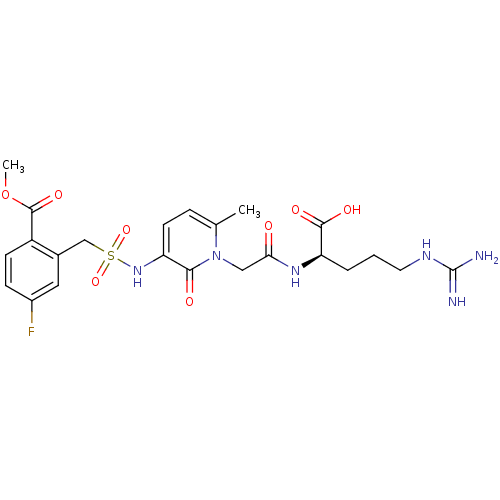 (4-Fluoro-2-({1-[((R)-1-formyl-4-guanidino-butylcar...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory constant against human thrombin (FIIa). | Bioorg Med Chem Lett 12: 1203-8 (2002) BindingDB Entry DOI: 10.7270/Q2PK0FGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50111741 (CHEMBL19666 | N-(5-Carbamimidoyl-thiophen-2-ylmeth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory constant against human thrombin (FIIa). | Bioorg Med Chem Lett 12: 1203-8 (2002) BindingDB Entry DOI: 10.7270/Q2PK0FGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50111729 (CHEMBL277695 | N-(5-Carbamimidoyl-thiophen-2-ylmet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory constant against human thrombin (FIIa). | Bioorg Med Chem Lett 12: 1203-8 (2002) BindingDB Entry DOI: 10.7270/Q2PK0FGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50111730 (2-[3-(2-Fluoro-phenylmethanesulfonylamino)-6-methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory constant against human thrombin (FIIa). | Bioorg Med Chem Lett 12: 1203-8 (2002) BindingDB Entry DOI: 10.7270/Q2PK0FGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50266889 (CHEMBL513922 | N-benzyl-N-ethyl-2-(7-methyl-8-oxo-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Radiological Sciences Curated by ChEMBL | Assay Description Displacement of [11C]PK11195 from PBR receptor in rat brain membrane | Bioorg Med Chem Lett 19: 1707-10 (2009) Article DOI: 10.1016/j.bmcl.2009.01.093 BindingDB Entry DOI: 10.7270/Q2C82B6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM82247 (8-Chloro-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-b...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York Curated by ChEMBL | Assay Description Antagonist activity at dopamine D1 receptor (unknown origin) | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127305 BindingDB Entry DOI: 10.7270/Q2377D85 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Rattus norvegicus (Rat)) | BDBM50312840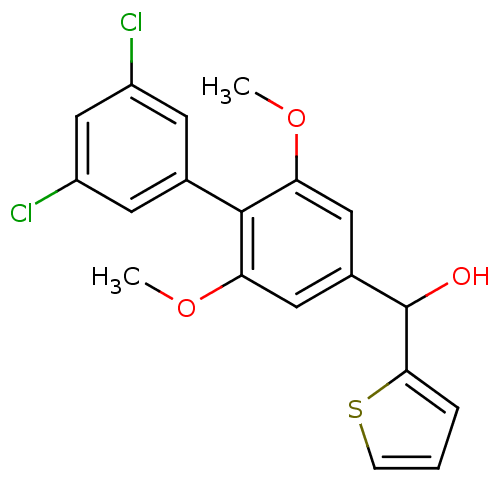 (CHEMBL1076680 | US9139546, 16 | [11C](3',5'-dichlo...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Radiological Sciences Curated by ChEMBL | Assay Description Binding affinity to rat brain CB2 receptor | Bioorg Med Chem Lett 20: 1565-8 (2010) Article DOI: 10.1016/j.bmcl.2010.01.074 BindingDB Entry DOI: 10.7270/Q2N29X34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1B) dopamine receptor (Homo sapiens (Human)) | BDBM82247 (8-Chloro-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-b...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York Curated by ChEMBL | Assay Description Antagonist activity at dopamine D5 receptor (unknown origin) | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127305 BindingDB Entry DOI: 10.7270/Q2377D85 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM22032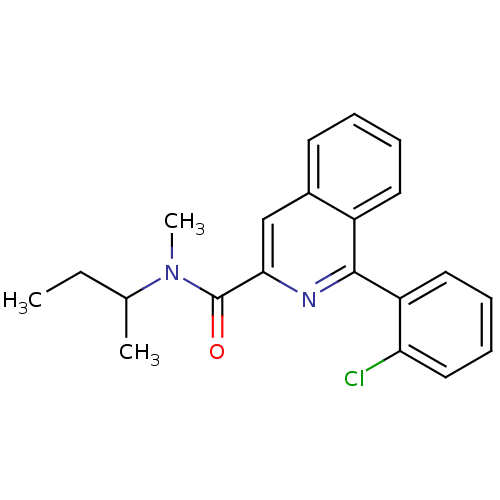 (1-(2-chlorophenyl)-N-methyl-N-(1-methylpropyl)isoq...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Radiological Sciences Curated by ChEMBL | Assay Description Displacement of [11C]PK11195 from PBR receptor in rat brain membrane | Bioorg Med Chem Lett 19: 1707-10 (2009) Article DOI: 10.1016/j.bmcl.2009.01.093 BindingDB Entry DOI: 10.7270/Q2C82B6C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50316621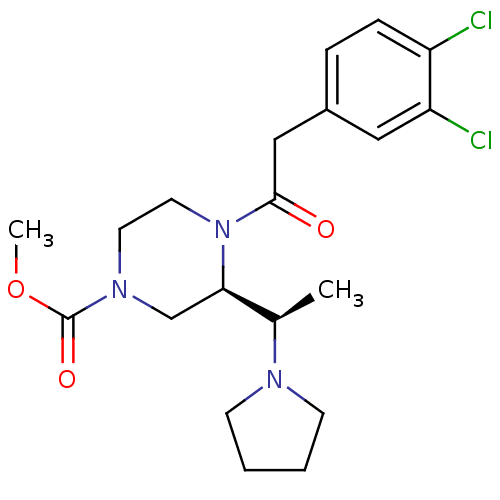 ((R)-methyl 4-(2-(3,4-dichlorophenyl)acetyl)-3-((R)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Pharmazeutische und Medizinische Chemie der Universit£t M£nster Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from kappa opioid receptor | Eur J Med Chem 46: 1972-82 (2011) Article DOI: 10.1016/j.ejmech.2011.01.064 BindingDB Entry DOI: 10.7270/Q2PV6KQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (Homo sapiens (Human)) | BDBM50163592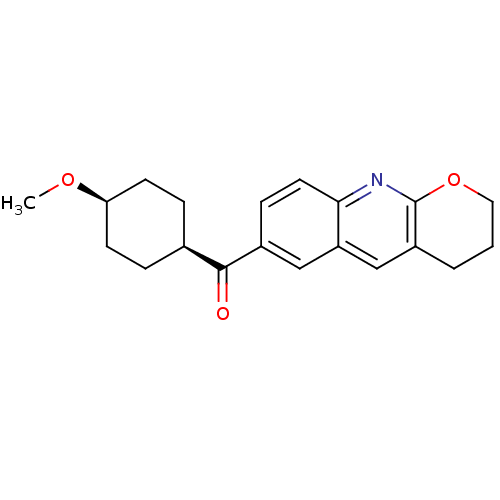 ((3,4-Dihydro-2H-1-oxa-9-aza-anthracen-6-yl)-(4-met...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Radiological Sciences Curated by ChEMBL | Assay Description Binding affinity to mGluR1 | Bioorg Med Chem 19: 102-10 (2011) Article DOI: 10.1016/j.bmc.2010.11.048 BindingDB Entry DOI: 10.7270/Q21R6QSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50111724 (CHEMBL19731 | N-(4-Carbamimidoyl-benzyl)-2-(6-meth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory constant against human thrombin (FIIa). | Bioorg Med Chem Lett 12: 1203-8 (2002) BindingDB Entry DOI: 10.7270/Q2PK0FGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (Homo sapiens (Human)) | BDBM50301822 (5-(1-(2-fluoropyridin-3-yl)-5-methyl-1H-1,2,3-tria...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Radiological Sciences Curated by ChEMBL | Assay Description Binding affinity to mGluR1 | Bioorg Med Chem 19: 102-10 (2011) Article DOI: 10.1016/j.bmc.2010.11.048 BindingDB Entry DOI: 10.7270/Q21R6QSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50346474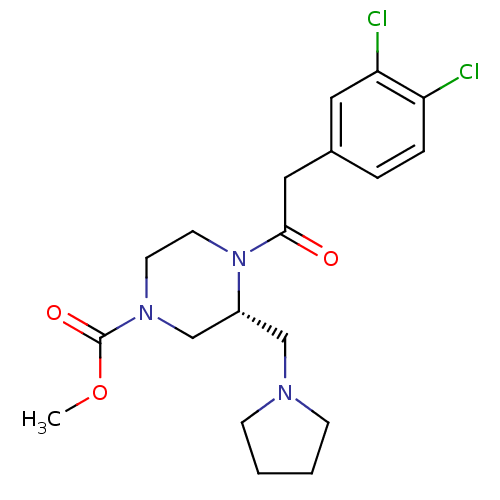 ((R)-4-[2-(3,4-Dichloro-phenyl)-acetyl]-3-pyrrolidi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Pharmazeutische und Medizinische Chemie der Universit£t M£nster Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from kappa opioid receptor | Eur J Med Chem 46: 1972-82 (2011) Article DOI: 10.1016/j.ejmech.2011.01.064 BindingDB Entry DOI: 10.7270/Q2PV6KQ5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50266861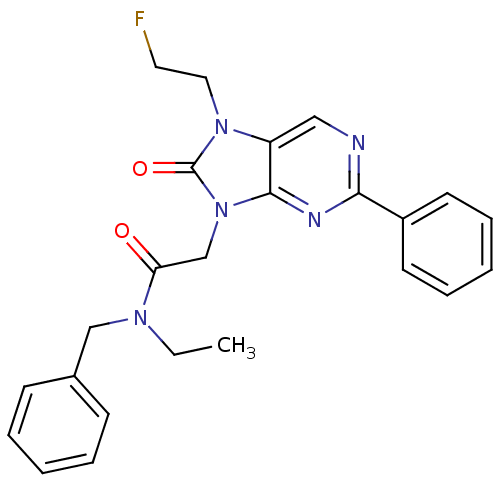 (CHEMBL515622 | N-benzyl-N-ethyl-2-(7-(2-fluoroethy...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Radiological Sciences Curated by ChEMBL | Assay Description Displacement of [11C]PK11195 from PBR receptor in rat brain membrane | Bioorg Med Chem Lett 19: 1707-10 (2009) Article DOI: 10.1016/j.bmcl.2009.01.093 BindingDB Entry DOI: 10.7270/Q2C82B6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1B) dopamine receptor (Homo sapiens (Human)) | BDBM50004822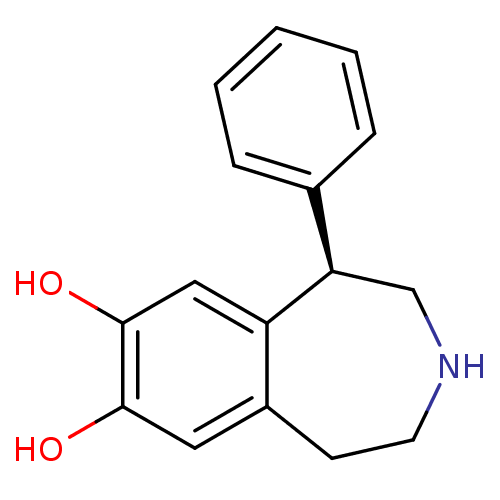 ((R)-1-phenyl-2,3,4,5-tetrahydro-1H-benzo[d]azepine...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York Curated by ChEMBL | Assay Description Agonist activity at dopamine D5 receptor (unknown origin) | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127305 BindingDB Entry DOI: 10.7270/Q2377D85 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50266863 (CHEMBL476811 | N-benzyl-N-(2-fluoroethyl)-2-(7-met...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Radiological Sciences Curated by ChEMBL | Assay Description Displacement of [11C]PK11195 from PBR receptor in rat brain membrane | Bioorg Med Chem Lett 19: 1707-10 (2009) Article DOI: 10.1016/j.bmcl.2009.01.093 BindingDB Entry DOI: 10.7270/Q2C82B6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50111717 (2-[3-(2-Fluoro-phenylmethanesulfonylamino)-2-oxo-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory constant against human thrombin (FIIa). | Bioorg Med Chem Lett 12: 1203-8 (2002) BindingDB Entry DOI: 10.7270/Q2PK0FGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50111726 (CHEMBL19359 | N-(4-Carbamimidoyl-benzyl)-2-(6-meth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory constant against human thrombin (FIIa). | Bioorg Med Chem Lett 12: 1203-8 (2002) BindingDB Entry DOI: 10.7270/Q2PK0FGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50266889 (CHEMBL513922 | N-benzyl-N-ethyl-2-(7-methyl-8-oxo-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Radiological Sciences Curated by ChEMBL | Assay Description Displacement of [11C]-PK-11195 from TPSO in Sprague-Dawley rat brain homogenate after 30 mins by gamma counting | J Med Chem 54: 6040-9 (2011) Article DOI: 10.1021/jm200516a BindingDB Entry DOI: 10.7270/Q2C53N16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50067796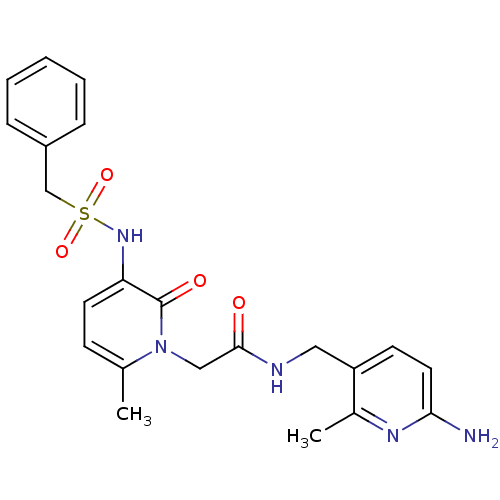 (CHEMBL11157 | L-374087 | N-((6-amino-2-methylpyrid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory constant against human thrombin (FIIa). | Bioorg Med Chem Lett 12: 1203-8 (2002) BindingDB Entry DOI: 10.7270/Q2PK0FGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50054139 ((R)1-(2-Chloro-phenyl)-isoquinoline-3-carboxylic a...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Radiological Sciences Curated by ChEMBL | Assay Description Displacement of [11C]-PK-11195 from TPSO in Sprague-Dawley rat brain homogenate after 30 mins by gamma counting | J Med Chem 54: 6040-9 (2011) Article DOI: 10.1021/jm200516a BindingDB Entry DOI: 10.7270/Q2C53N16 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50067796 (CHEMBL11157 | L-374087 | N-((6-amino-2-methylpyrid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against human Thrombin (FIIa) cleavage of the chromogenic substrate | Bioorg Med Chem Lett 12: 2925-30 (2002) BindingDB Entry DOI: 10.7270/Q27946WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50487259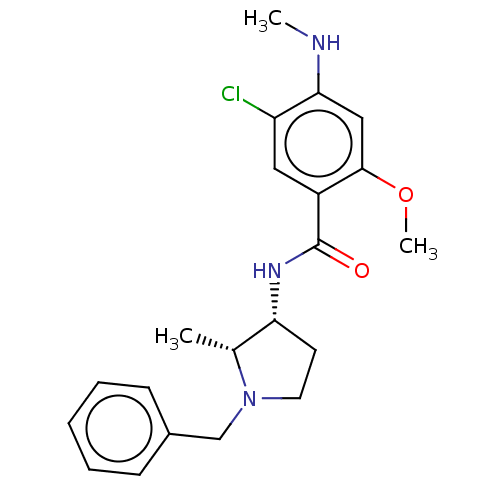 (CHEBI:64219 | [3H]NEMONAPRIDE) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem | Article PubMed | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-methylspiperone from dopamine D4 receptor (unknown origin) | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128047 BindingDB Entry DOI: 10.7270/Q2CJ8J8S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM21393 (7-(dipropylamino)-5,6,7,8-tetrahydronaphthalen-1-o...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York Curated by ChEMBL | Assay Description Displacement of [3H]-WAY100635 from human 5-HT1A receptor expresssed in stable CHO cell membrane incubated for 90 mins by microbeta counting method | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115578 BindingDB Entry DOI: 10.7270/Q22B92KJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens) | BDBM50001775 ((ritanserin)6-(2-{4-[Bis-(4-fluoro-phenyl)-methyle...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jnatprod.2c00365 BindingDB Entry DOI: 10.7270/Q2GX4GN3 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM21393 (7-(dipropylamino)-5,6,7,8-tetrahydronaphthalen-1-o...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York Curated by ChEMBL | Assay Description Binding affinity to human 5HT1A receptor | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127053 BindingDB Entry DOI: 10.7270/Q2H135KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50487259 (CHEBI:64219 | [3H]NEMONAPRIDE) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem | Article PubMed | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York Curated by ChEMBL | Assay Description Displacement of [3H]-N-Methylspiperone from human dopamine D4 receptor expressed in stable HEK cells incubated for 90 mins by microbeta counting meth... | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115578 BindingDB Entry DOI: 10.7270/Q22B92KJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens) | BDBM50487259 (CHEBI:64219 | [3H]NEMONAPRIDE) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem | Article PubMed | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-methylspiperone from dopamine D3 receptor (unknown origin) | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128047 BindingDB Entry DOI: 10.7270/Q2CJ8J8S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (Homo sapiens (Human)) | BDBM50333368 (CHEMBL1645348 | [11C]-cis-(3-ethyl-2-methylquinoli...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Radiological Sciences Curated by ChEMBL | Assay Description Binding affinity to mGluR1 | Bioorg Med Chem 19: 102-10 (2011) Article DOI: 10.1016/j.bmc.2010.11.048 BindingDB Entry DOI: 10.7270/Q21R6QSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50067797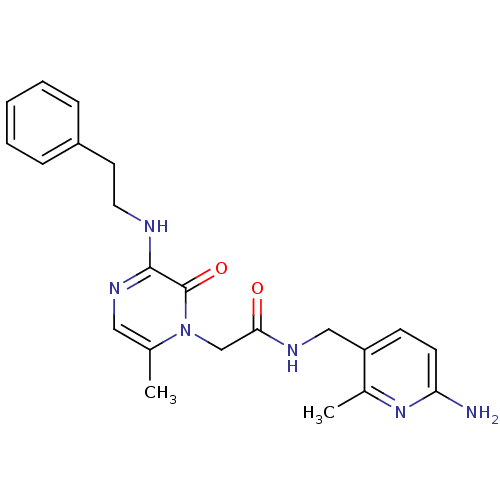 (CHEMBL19080 | L-37378 | N-(6-Amino-2-methyl-pyridi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against human Thrombin (FIIa) cleavage of the chromogenic substrate | Bioorg Med Chem Lett 12: 2925-30 (2002) BindingDB Entry DOI: 10.7270/Q27946WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50081157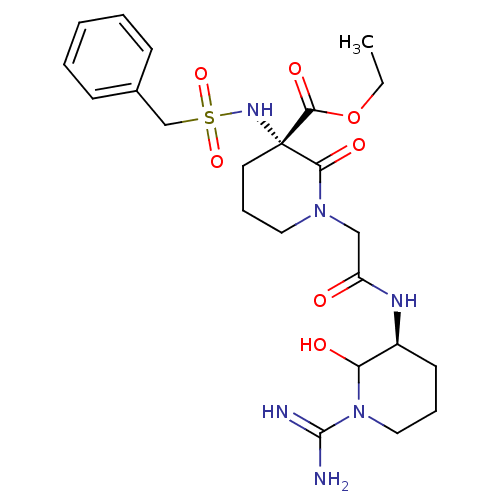 ((S)-1-[((S)-1-Carbamimidoyl-2-hydroxy-piperidin-3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description Evaluation of inhibition of transition state thrombin | Bioorg Med Chem Lett 9: 2625-8 (1999) BindingDB Entry DOI: 10.7270/Q2JW8D3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50071693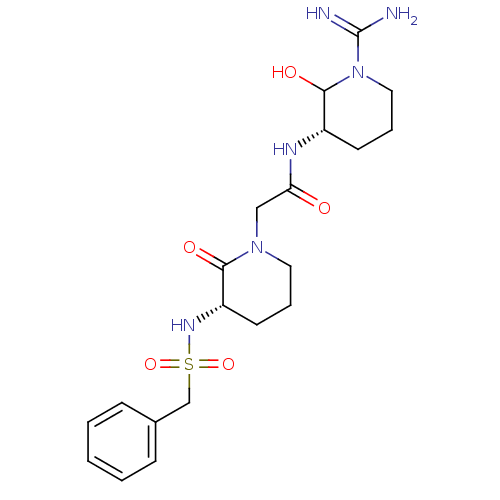 (CHEMBL38927 | CVS-1578 | N-((S)-1-Carbamimidoyl-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description Evaluation of inhibition of transition state thrombin | Bioorg Med Chem Lett 9: 2625-8 (1999) BindingDB Entry DOI: 10.7270/Q2JW8D3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50081158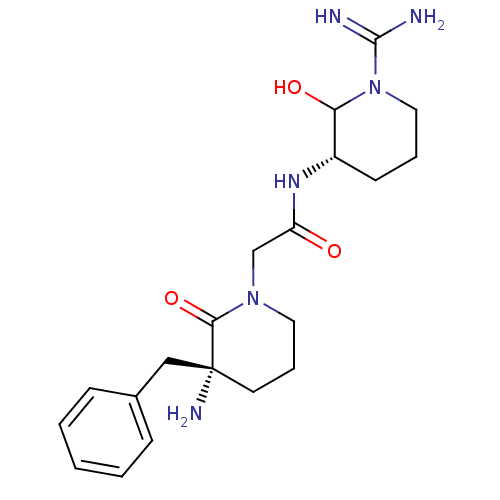 (2-((R)-3-Amino-3-benzyl-2-oxo-piperidin-1-yl)-N-((...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description Evaluation of inhibition of transition state thrombin | Bioorg Med Chem Lett 9: 2625-8 (1999) BindingDB Entry DOI: 10.7270/Q2JW8D3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50067797 (CHEMBL19080 | L-37378 | N-(6-Amino-2-methyl-pyridi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory constant against human thrombin (FIIa). | Bioorg Med Chem Lett 12: 1203-8 (2002) BindingDB Entry DOI: 10.7270/Q2PK0FGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50111739 (CHEMBL19811 | N-((S)-2-Amino-1,4,5,6-tetrahydro-py...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory constant against human thrombin (FIIa). | Bioorg Med Chem Lett 12: 1203-8 (2002) BindingDB Entry DOI: 10.7270/Q2PK0FGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50004822 ((R)-1-phenyl-2,3,4,5-tetrahydro-1H-benzo[d]azepine...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York Curated by ChEMBL | Assay Description Agonist activity at dopamine D1 receptor (unknown origin) | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127305 BindingDB Entry DOI: 10.7270/Q2377D85 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50054502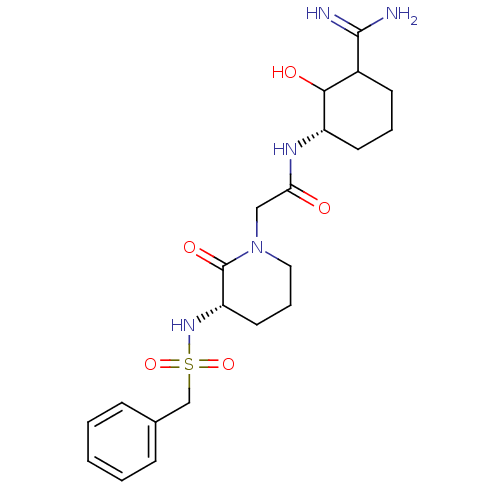 (CHEMBL140494 | N-((S)-3-Carbamimidoyl-2-hydroxy-cy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description compound was tested in vitro for inhibition of serine protease thrombin(FIIa). | J Med Chem 39: 4531-6 (1996) Article DOI: 10.1021/jm960572n BindingDB Entry DOI: 10.7270/Q28051QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50111738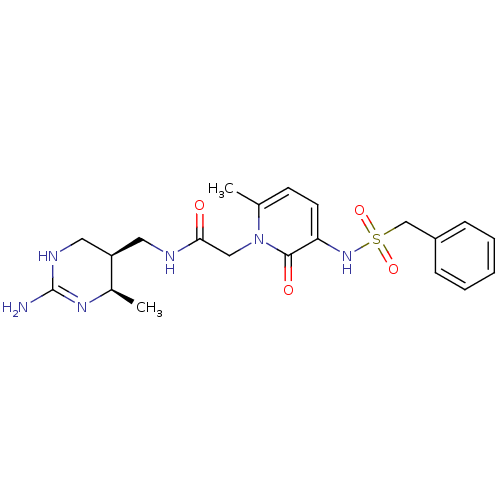 (CHEMBL274968 | N-((4R,5S)-2-Amino-4-methyl-1,4,5,6...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory constant against human thrombin (FIIa). | Bioorg Med Chem Lett 12: 1203-8 (2002) BindingDB Entry DOI: 10.7270/Q2PK0FGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50087033 ((1R,5aR)-1-methoxy-1,6-dimethyl-1,5,5a,6,7,8-hexah...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York Curated by ChEMBL | Assay Description Displacement of [3H]-LSD from human 5-HT7 receptor expresssed in stable HEK cell membrane incubated for 90 mins by microbeta counting method | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115578 BindingDB Entry DOI: 10.7270/Q22B92KJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens) | BDBM50570808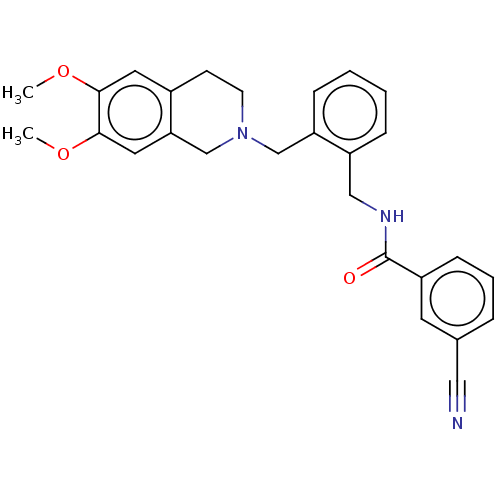 (CHEMBL4846574) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-methylspiperone from dopamine D3 receptor (unknown origin) | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128047 BindingDB Entry DOI: 10.7270/Q2CJ8J8S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50487259 (CHEBI:64219 | [3H]NEMONAPRIDE) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York Curated by ChEMBL | Assay Description Displacement of [3H]-N-Methylspiperone from dopamine D3 receptor (unknown origin) incubated for 90 mins by microbeta counting method | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115578 BindingDB Entry DOI: 10.7270/Q22B92KJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50266862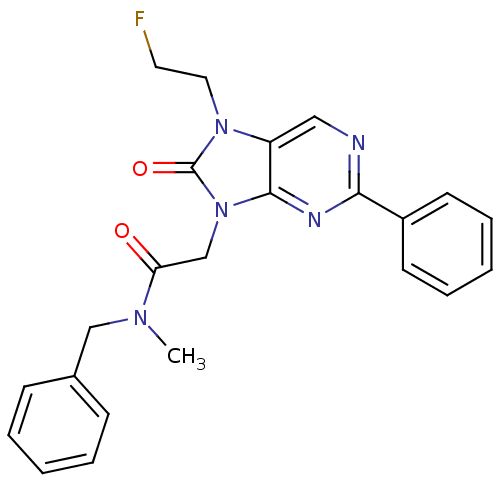 (CHEMBL476607 | N-benzyl-2-(7-(2-fluoroethyl)-8-oxo...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Radiological Sciences Curated by ChEMBL | Assay Description Displacement of [11C]PK11195 from PBR receptor in rat brain membrane | Bioorg Med Chem Lett 19: 1707-10 (2009) Article DOI: 10.1016/j.bmcl.2009.01.093 BindingDB Entry DOI: 10.7270/Q2C82B6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50111714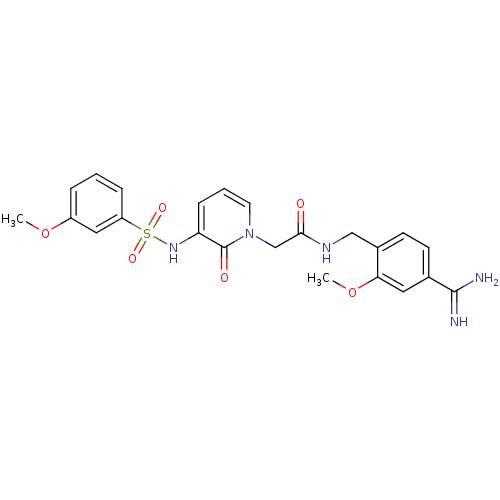 (CHEMBL416912 | N-(4-Carbamimidoyl-2-methoxy-benzyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory constant against human thrombin (FIIa). | Bioorg Med Chem Lett 12: 1203-8 (2002) BindingDB Entry DOI: 10.7270/Q2PK0FGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1B) dopamine receptor (Homo sapiens (Human)) | BDBM50010301 (8-Bromo-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-be...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]SCH23390 from dopamine D5 receptor (unknown origin) | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128047 BindingDB Entry DOI: 10.7270/Q2CJ8J8S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50000092 ((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Pharmazeutische und Medizinische Chemie der Universit£t M£nster Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from delta opioid receptor in rat brain membranes | Eur J Med Chem 46: 1972-82 (2011) Article DOI: 10.1016/j.ejmech.2011.01.064 BindingDB Entry DOI: 10.7270/Q2PV6KQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50111742 (CHEMBL417635 | N-((S)-2-Amino-1,4,5,6-tetrahydro-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory constant against human thrombin (FIIa). | Bioorg Med Chem Lett 12: 1203-8 (2002) BindingDB Entry DOI: 10.7270/Q2PK0FGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens) | BDBM50570806 (CHEMBL4876260) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-methylspiperone from dopamine D3 receptor (unknown origin) | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128047 BindingDB Entry DOI: 10.7270/Q2CJ8J8S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50008735 ((+)-3-(tert-butyl)-(3S,4aS,13bS)-2,3,4,4a,8,9,13b,...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York Curated by ChEMBL | Assay Description Binding affinity to human D1 receptor | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127053 BindingDB Entry DOI: 10.7270/Q2H135KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 3478 total ) | Next | Last >> |