

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) assessed as inhibition of acetylcholine hydrolysis preincubated for 15 mins by Ellman method | J Nat Prod 77: 2716-9 (2014) Article DOI: 10.1021/np500558b BindingDB Entry DOI: 10.7270/Q2Z32183 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50176956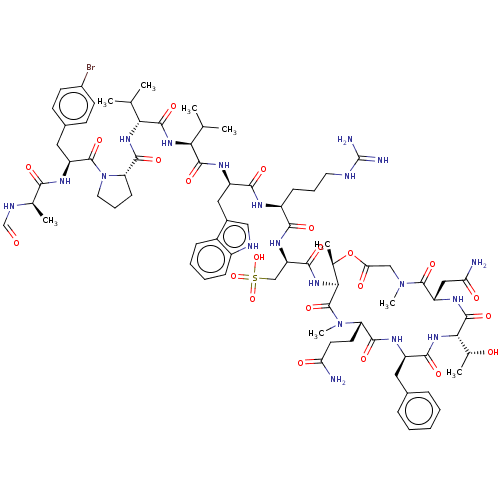 (CHEMBL3815164) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Daegu-Gyeongbuk Medical Innovation Foundation Curated by ChEMBL | Assay Description Antagonist activity at human FXR expressed in CV-1 cells assessed as inhibition of CDCA-induced receptor transactivation after 24 hrs by luciferase r... | J Nat Prod 79: 499-506 (2016) Article DOI: 10.1021/acs.jnatprod.5b00871 BindingDB Entry DOI: 10.7270/Q2KD20V0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily B member 1 (Homo sapiens (Human)) | BDBM50088573 (CHEMBL3577208) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition of Kv2.1 channel in human INS1 cells assessed as inhibition of outward K+ current by electrophysiology/patch clamp technique | J Nat Prod 78: 363-7 (2015) Article DOI: 10.1021/np5007586 BindingDB Entry DOI: 10.7270/Q20P11R5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily B member 1 (Homo sapiens (Human)) | BDBM50088572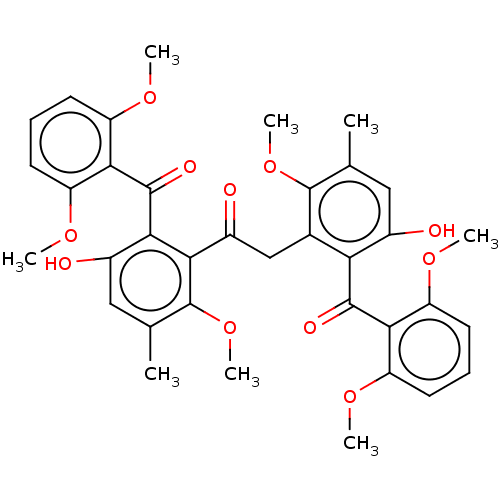 (CHEMBL3577209) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition of Kv2.1 channel in human INS1 cells assessed as inhibition of outward K+ current by electrophysiology/patch clamp technique | J Nat Prod 78: 363-7 (2015) Article DOI: 10.1021/np5007586 BindingDB Entry DOI: 10.7270/Q20P11R5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50335908 (CHEMBL1668774 | tuberatolide B) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at human FXR expressed in CV-1 cells assessed as inhibition of CDCA-induced transactivation after 24 hrs by luciferase reporter g... | J Nat Prod 74: 90-4 (2011) Article DOI: 10.1021/np100489u BindingDB Entry DOI: 10.7270/Q2WH2QZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50226460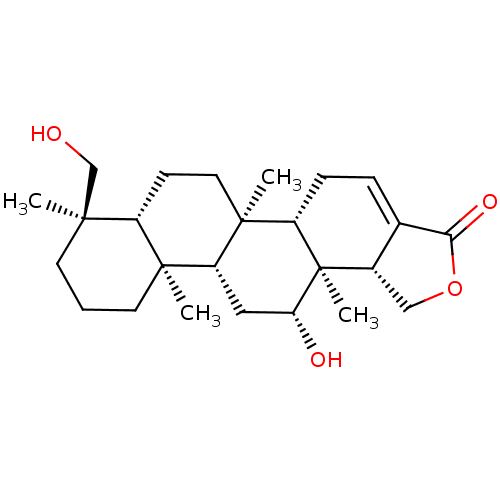 (12-O-deacetyl-12-epi-19-deoxy-21-hydroxyscalarin |...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at human FXR expressed in monkey CV1 cells after 24 hrs by ecdysone receptor response element-driven luciferase reporter assay | J Nat Prod 70: 1691-5 (2007) Article DOI: 10.1021/np070024k BindingDB Entry DOI: 10.7270/Q269739H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50335909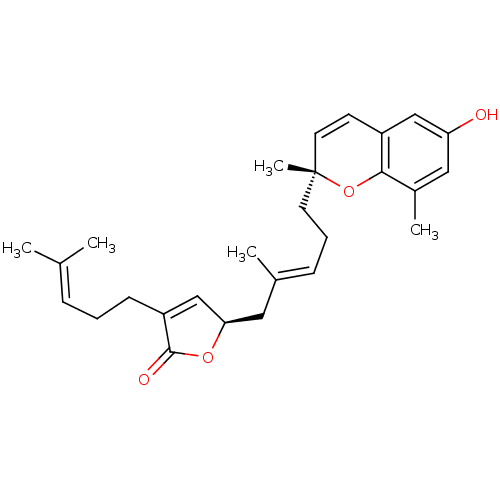 (2'-epi-Tuberatolide B | CHEMBL1668775) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at human FXR expressed in CV-1 cells assessed as inhibition of CDCA-induced transactivation after 24 hrs by luciferase reporter g... | J Nat Prod 74: 90-4 (2011) Article DOI: 10.1021/np100489u BindingDB Entry DOI: 10.7270/Q2WH2QZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50335907 (CHEMBL1668773 | tuberatolide A) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at human FXR expressed in CV-1 cells assessed as inhibition of CDCA-induced transactivation after 24 hrs by luciferase reporter g... | J Nat Prod 74: 90-4 (2011) Article DOI: 10.1021/np100489u BindingDB Entry DOI: 10.7270/Q2WH2QZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50226465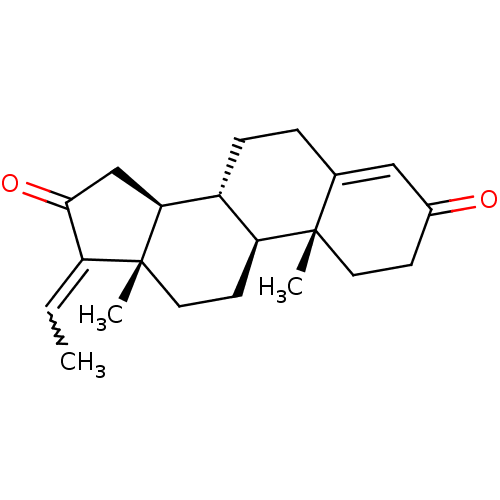 (CHEMBL402063 | E-guggulsterone | pregna-4,17(20)-c...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at human FXR expressed in monkey CV1 cells after 24 hrs by ecdysone receptor response element-driven luciferase reporter assay | J Nat Prod 70: 1691-5 (2007) Article DOI: 10.1021/np070024k BindingDB Entry DOI: 10.7270/Q269739H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50176910 (CHEMBL3814608) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Daegu-Gyeongbuk Medical Innovation Foundation Curated by ChEMBL | Assay Description Antagonist activity at human FXR expressed in CV-1 cells assessed as inhibition of CDCA-induced receptor transactivation after 24 hrs by luciferase r... | J Nat Prod 79: 499-506 (2016) Article DOI: 10.1021/acs.jnatprod.5b00871 BindingDB Entry DOI: 10.7270/Q2KD20V0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50335910 (CHEMBL1668776 | yezoquinolide) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at human FXR expressed in CV-1 cells assessed as inhibition of CDCA-induced transactivation after 24 hrs by luciferase reporter g... | J Nat Prod 74: 90-4 (2011) Article DOI: 10.1021/np100489u BindingDB Entry DOI: 10.7270/Q2WH2QZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50176963 (CHEMBL3814453) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Daegu-Gyeongbuk Medical Innovation Foundation Curated by ChEMBL | Assay Description Antagonist activity at human FXR expressed in CV-1 cells assessed as inhibition of CDCA-induced receptor transactivation after 24 hrs by luciferase r... | J Nat Prod 79: 499-506 (2016) Article DOI: 10.1021/acs.jnatprod.5b00871 BindingDB Entry DOI: 10.7270/Q2KD20V0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50192927 (CHEMBL373765 | acetic acid (1R,5bS,11aS,13R,13aS,1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity against FXR assessed as transactivation of luciferase reporter gene in CV1 cells | Bioorg Med Chem Lett 16: 5398-402 (2006) Article DOI: 10.1016/j.bmcl.2006.07.079 BindingDB Entry DOI: 10.7270/Q2H41S84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50335911 ((R)-sargachromenol | CHEMBL1668777) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at human FXR expressed in CV-1 cells assessed as inhibition of CDCA-induced transactivation after 24 hrs by luciferase reporter g... | J Nat Prod 74: 90-4 (2011) Article DOI: 10.1021/np100489u BindingDB Entry DOI: 10.7270/Q2WH2QZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM21725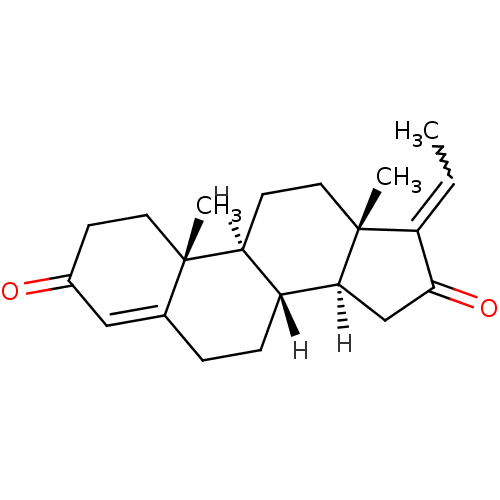 ((1S,2R,10R,11S,14Z,15S)-14-ethylidene-2,15-dimethy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity against FXR assessed as transactivation of luciferase reporter gene in CV1 cells | Bioorg Med Chem Lett 16: 5398-402 (2006) Article DOI: 10.1016/j.bmcl.2006.07.079 BindingDB Entry DOI: 10.7270/Q2H41S84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50335912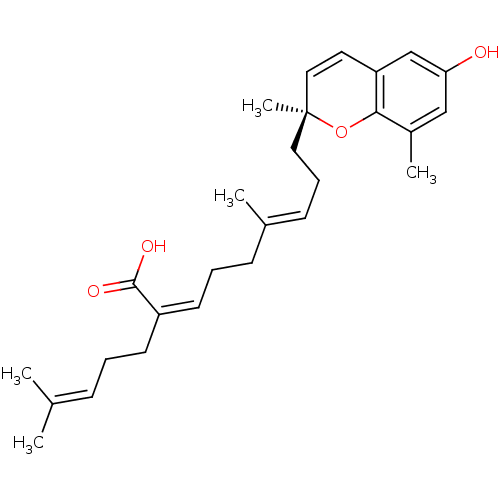 ((S)-sargachromenol | CHEMBL1668778) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at human FXR expressed in CV-1 cells assessed as inhibition of CDCA-induced transactivation after 24 hrs by luciferase reporter g... | J Nat Prod 74: 90-4 (2011) Article DOI: 10.1021/np100489u BindingDB Entry DOI: 10.7270/Q2WH2QZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50226461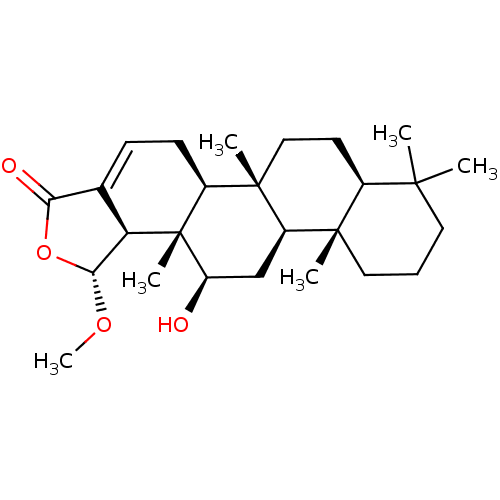 (12-O-deacetyl-12-epi-19-O-methylscalarin | CHEMBL2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at human FXR expressed in monkey CV1 cells after 24 hrs by ecdysone receptor response element-driven luciferase reporter assay | J Nat Prod 70: 1691-5 (2007) Article DOI: 10.1021/np070024k BindingDB Entry DOI: 10.7270/Q269739H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50192928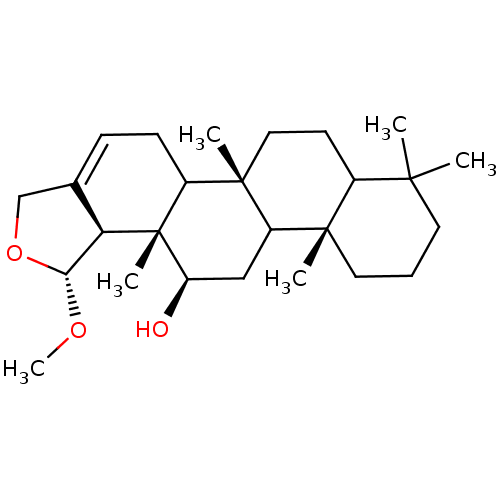 ((1R,5bR,11aS,13R,13aS,13bS)-1-methoxy-5b,8,8,11a,1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.48E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity against FXR assessed as transactivation of luciferase reporter gene in CV1 cells | Bioorg Med Chem Lett 16: 5398-402 (2006) Article DOI: 10.1016/j.bmcl.2006.07.079 BindingDB Entry DOI: 10.7270/Q2H41S84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50192925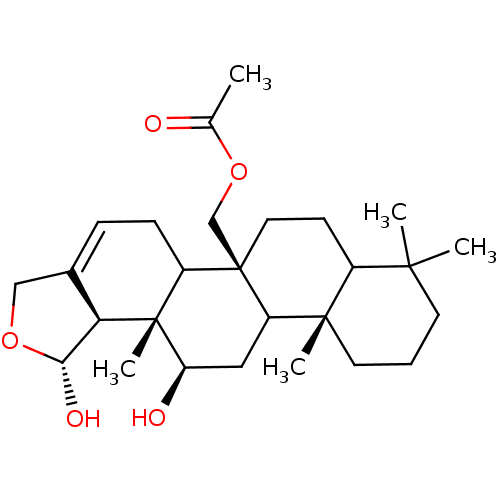 (((1R,5bS,11aS,13R,13aS,13bS)-1,13-dihydroxy-8,8,11...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.53E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity against FXR assessed as transactivation of luciferase reporter gene in CV1 cells | Bioorg Med Chem Lett 16: 5398-402 (2006) Article DOI: 10.1016/j.bmcl.2006.07.079 BindingDB Entry DOI: 10.7270/Q2H41S84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50226463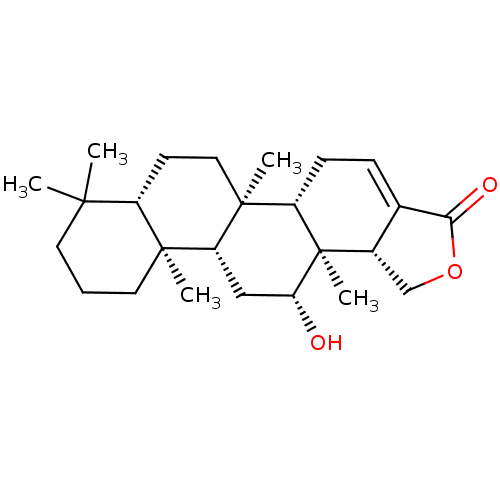 ((5aS,5bR,7aS,11aS,11bR,13R,13aS,13bR)-13-hydroxy-5...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at human FXR expressed in monkey CV1 cells after 24 hrs by ecdysone receptor response element-driven luciferase reporter assay | J Nat Prod 70: 1691-5 (2007) Article DOI: 10.1021/np070024k BindingDB Entry DOI: 10.7270/Q269739H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50226465 (CHEMBL402063 | E-guggulsterone | pregna-4,17(20)-c...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Daegu-Gyeongbuk Medical Innovation Foundation Curated by ChEMBL | Assay Description Antagonist activity at human FXR expressed in HEK293 cells assessed as inhibition of GW4064-induced transactivation after 24 hrs by luciferase report... | J Nat Prod 79: 499-506 (2016) Article DOI: 10.1021/acs.jnatprod.5b00871 BindingDB Entry DOI: 10.7270/Q2KD20V0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50226465 (CHEMBL402063 | E-guggulsterone | pregna-4,17(20)-c...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at human FXR expressed in CV-1 cells assessed as inhibition of CDCA-induced transactivation after 24 hrs by luciferase reporter g... | J Nat Prod 74: 90-4 (2011) Article DOI: 10.1021/np100489u BindingDB Entry DOI: 10.7270/Q2WH2QZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50042944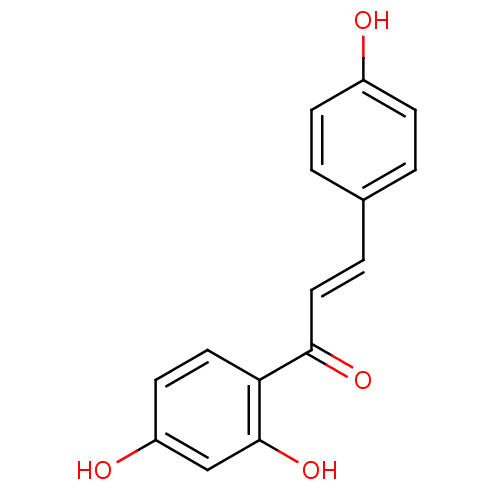 ((E)-1-(2,4-Dihydroxy-phenyl)-3-(4-hydroxy-phenyl)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Ability to displace [3H]quipazine binding to 5-hydroxytryptamine 3 receptor sites in NG 108-15. | Bioorg Med Chem Lett 27: 3123-3126 (2017) Article DOI: 10.1016/j.bmcl.2017.05.035 BindingDB Entry DOI: 10.7270/Q2QR50K7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50036515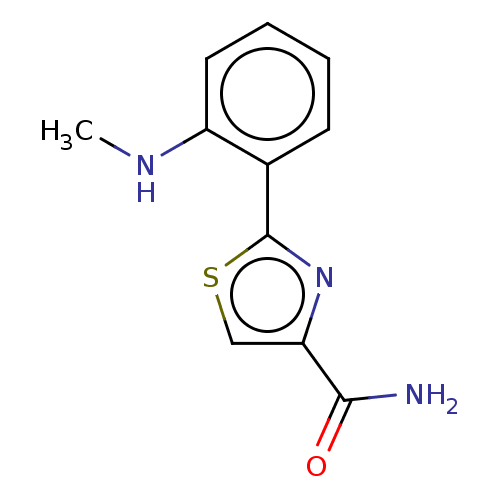 (CHEMBL3353567) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) assessed as inhibition of acetylcholine hydrolysis preincubated for 15 mins by Ellman method | J Nat Prod 77: 2716-9 (2014) Article DOI: 10.1021/np500558b BindingDB Entry DOI: 10.7270/Q2Z32183 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50226462 (12-Episcalarin | 12-epi-scalarin | CHEMBL269105) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at human FXR expressed in monkey CV1 cells after 24 hrs by ecdysone receptor response element-driven luciferase reporter assay | J Nat Prod 70: 1691-5 (2007) Article DOI: 10.1021/np070024k BindingDB Entry DOI: 10.7270/Q269739H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50036514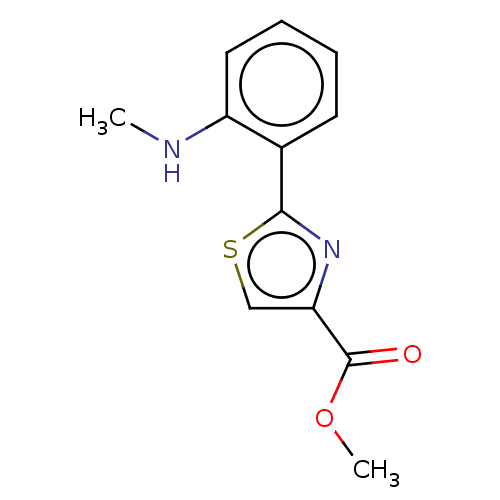 (CHEMBL3353566) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) assessed as inhibition of acetylcholine hydrolysis preincubated for 15 mins by Ellman method | J Nat Prod 77: 2716-9 (2014) Article DOI: 10.1021/np500558b BindingDB Entry DOI: 10.7270/Q2Z32183 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50192926 ((1R,5bS,11aS,13R,13aS,13bS)-5b-(hydroxymethyl)-8,8...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity against FXR assessed as transactivation of luciferase reporter gene in CV1 cells | Bioorg Med Chem Lett 16: 5398-402 (2006) Article DOI: 10.1016/j.bmcl.2006.07.079 BindingDB Entry DOI: 10.7270/Q2H41S84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50036516 (CHEMBL3353568) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) assessed as inhibition of acetylcholine hydrolysis preincubated for 15 mins by Ellman method | J Nat Prod 77: 2716-9 (2014) Article DOI: 10.1021/np500558b BindingDB Entry DOI: 10.7270/Q2Z32183 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50226459 (12-O-deacetyl-12-epi-scalarin | CHEMBL399743) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at human FXR expressed in monkey CV1 cells after 24 hrs by ecdysone receptor response element-driven luciferase reporter assay | J Nat Prod 70: 1691-5 (2007) Article DOI: 10.1021/np070024k BindingDB Entry DOI: 10.7270/Q269739H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50192924 ((1R,5bR,11aS,13R,13aS,13bS)-1-hydroxy-5b,8,8,11a,1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity against FXR assessed as transactivation of luciferase reporter gene in CV1 cells | Bioorg Med Chem Lett 16: 5398-402 (2006) Article DOI: 10.1016/j.bmcl.2006.07.079 BindingDB Entry DOI: 10.7270/Q2H41S84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50226464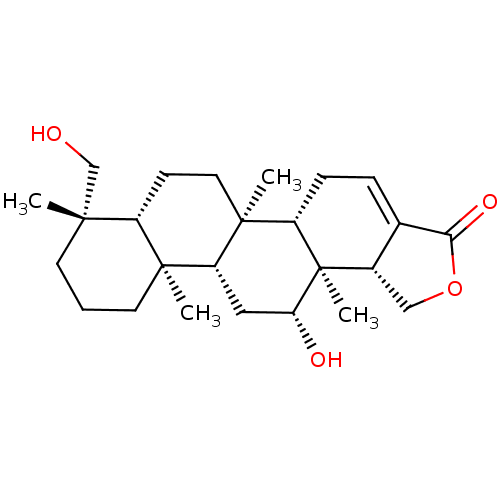 (12-O-deacetyl-12-epi-19-deoxy-22-hydroxyscalarin |...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at human FXR expressed in monkey CV1 cells after 24 hrs by ecdysone receptor response element-driven luciferase reporter assay | J Nat Prod 70: 1691-5 (2007) Article DOI: 10.1021/np070024k BindingDB Entry DOI: 10.7270/Q269739H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50332462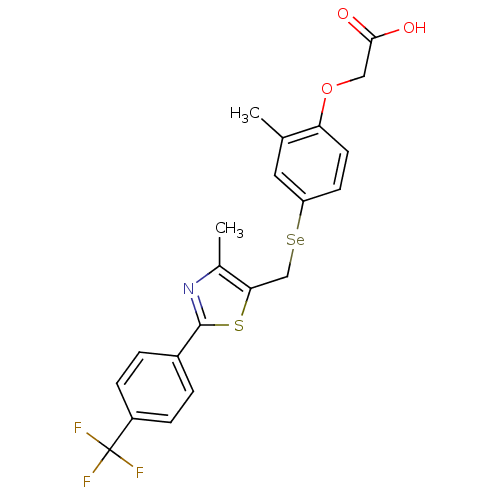 (2-(2-methyl-4-((4-methyl-2-(4-(trifluoromethyl)phe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Agonist activity at human PPAR-alpha expressed in african green monkey CV-1 cells after 24 hrs by luciferase reporter gene assay | Bioorg Med Chem Lett 20: 7239-42 (2010) Article DOI: 10.1016/j.bmcl.2010.10.103 BindingDB Entry DOI: 10.7270/Q2SN0975 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50332463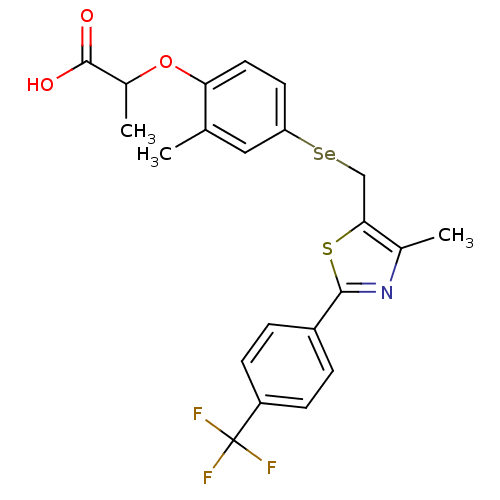 (2-(2-methyl-4-((4-methyl-2-(4-(trifluoromethyl)phe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 120 | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Agonist activity at human PPAR-alpha expressed in african green monkey CV-1 cells after 24 hrs by luciferase reporter gene assay | Bioorg Med Chem Lett 20: 7239-42 (2010) Article DOI: 10.1016/j.bmcl.2010.10.103 BindingDB Entry DOI: 10.7270/Q2SN0975 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50332464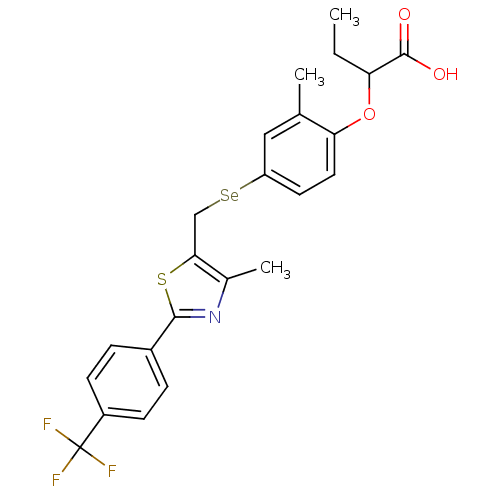 (2-(2-methyl-4-((4-methyl-2-(4-(trifluoromethyl)phe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 83.1 | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Agonist activity at human PPAR-alpha expressed in african green monkey CV-1 cells after 24 hrs by luciferase reporter gene assay | Bioorg Med Chem Lett 20: 7239-42 (2010) Article DOI: 10.1016/j.bmcl.2010.10.103 BindingDB Entry DOI: 10.7270/Q2SN0975 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50332465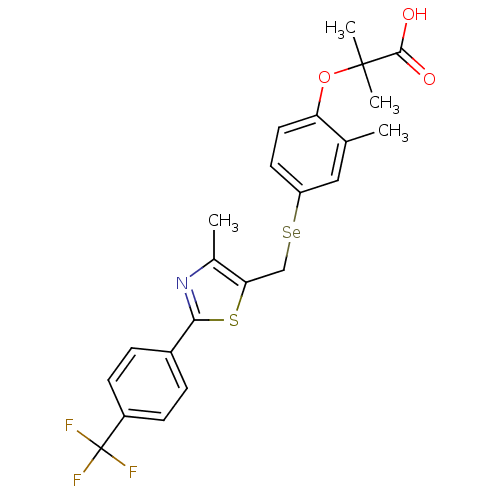 (2-methyl-2-(2-methyl-4-((4-methyl-2-(4-(trifluorom...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 15.2 | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Agonist activity at human PPAR-alpha expressed in african green monkey CV-1 cells after 24 hrs by luciferase reporter gene assay | Bioorg Med Chem Lett 20: 7239-42 (2010) Article DOI: 10.1016/j.bmcl.2010.10.103 BindingDB Entry DOI: 10.7270/Q2SN0975 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50332466 (2-(4-((2-(3-fluoro-4-(trifluoromethyl)phenyl)-4-me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 350 | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Agonist activity at human PPAR-alpha expressed in african green monkey CV-1 cells after 24 hrs by luciferase reporter gene assay | Bioorg Med Chem Lett 20: 7239-42 (2010) Article DOI: 10.1016/j.bmcl.2010.10.103 BindingDB Entry DOI: 10.7270/Q2SN0975 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50332467 (2-(4-((2-(3-fluoro-4-(trifluoromethyl)phenyl)-4-me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 39.4 | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Agonist activity at human PPAR-alpha expressed in african green monkey CV-1 cells after 24 hrs by luciferase reporter gene assay | Bioorg Med Chem Lett 20: 7239-42 (2010) Article DOI: 10.1016/j.bmcl.2010.10.103 BindingDB Entry DOI: 10.7270/Q2SN0975 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50332468 (2-(4-((2-(3-fluoro-4-(trifluoromethyl)phenyl)-4-me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Agonist activity at human PPAR-alpha expressed in african green monkey CV-1 cells after 24 hrs by luciferase reporter gene assay | Bioorg Med Chem Lett 20: 7239-42 (2010) Article DOI: 10.1016/j.bmcl.2010.10.103 BindingDB Entry DOI: 10.7270/Q2SN0975 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50332469 (2-(4-((2-(3-fluoro-4-(trifluoromethyl)phenyl)-4-me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 24.6 | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Agonist activity at human PPAR-alpha expressed in african green monkey CV-1 cells after 24 hrs by luciferase reporter gene assay | Bioorg Med Chem Lett 20: 7239-42 (2010) Article DOI: 10.1016/j.bmcl.2010.10.103 BindingDB Entry DOI: 10.7270/Q2SN0975 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50332462 (2-(2-methyl-4-((4-methyl-2-(4-(trifluoromethyl)phe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Agonist activity at human PPAR-delta expressed in african green monkey CV-1 cells after 24 hrs by luciferase reporter gene assay | Bioorg Med Chem Lett 20: 7239-42 (2010) Article DOI: 10.1016/j.bmcl.2010.10.103 BindingDB Entry DOI: 10.7270/Q2SN0975 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50332463 (2-(2-methyl-4-((4-methyl-2-(4-(trifluoromethyl)phe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Agonist activity at human PPAR-delta expressed in african green monkey CV-1 cells after 24 hrs by luciferase reporter gene assay | Bioorg Med Chem Lett 20: 7239-42 (2010) Article DOI: 10.1016/j.bmcl.2010.10.103 BindingDB Entry DOI: 10.7270/Q2SN0975 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50332464 (2-(2-methyl-4-((4-methyl-2-(4-(trifluoromethyl)phe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Agonist activity at human PPAR-delta expressed in african green monkey CV-1 cells after 24 hrs by luciferase reporter gene assay | Bioorg Med Chem Lett 20: 7239-42 (2010) Article DOI: 10.1016/j.bmcl.2010.10.103 BindingDB Entry DOI: 10.7270/Q2SN0975 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50332465 (2-methyl-2-(2-methyl-4-((4-methyl-2-(4-(trifluorom...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Agonist activity at human PPAR-delta expressed in african green monkey CV-1 cells after 24 hrs by luciferase reporter gene assay | Bioorg Med Chem Lett 20: 7239-42 (2010) Article DOI: 10.1016/j.bmcl.2010.10.103 BindingDB Entry DOI: 10.7270/Q2SN0975 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50332466 (2-(4-((2-(3-fluoro-4-(trifluoromethyl)phenyl)-4-me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Agonist activity at human PPAR-delta expressed in african green monkey CV-1 cells after 24 hrs by luciferase reporter gene assay | Bioorg Med Chem Lett 20: 7239-42 (2010) Article DOI: 10.1016/j.bmcl.2010.10.103 BindingDB Entry DOI: 10.7270/Q2SN0975 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50332467 (2-(4-((2-(3-fluoro-4-(trifluoromethyl)phenyl)-4-me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 8.5 | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Agonist activity at human PPAR-delta expressed in african green monkey CV-1 cells after 24 hrs by luciferase reporter gene assay | Bioorg Med Chem Lett 20: 7239-42 (2010) Article DOI: 10.1016/j.bmcl.2010.10.103 BindingDB Entry DOI: 10.7270/Q2SN0975 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50332468 (2-(4-((2-(3-fluoro-4-(trifluoromethyl)phenyl)-4-me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 7 | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Agonist activity at human PPAR-delta expressed in african green monkey CV-1 cells after 24 hrs by luciferase reporter gene assay | Bioorg Med Chem Lett 20: 7239-42 (2010) Article DOI: 10.1016/j.bmcl.2010.10.103 BindingDB Entry DOI: 10.7270/Q2SN0975 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50332469 (2-(4-((2-(3-fluoro-4-(trifluoromethyl)phenyl)-4-me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Agonist activity at human PPAR-delta expressed in african green monkey CV-1 cells after 24 hrs by luciferase reporter gene assay | Bioorg Med Chem Lett 20: 7239-42 (2010) Article DOI: 10.1016/j.bmcl.2010.10.103 BindingDB Entry DOI: 10.7270/Q2SN0975 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50332462 (2-(2-methyl-4-((4-methyl-2-(4-(trifluoromethyl)phe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Agonist activity at human PPAR-gamma expressed in african green monkey CV-1 cells after 24 hrs by luciferase reporter gene assay | Bioorg Med Chem Lett 20: 7239-42 (2010) Article DOI: 10.1016/j.bmcl.2010.10.103 BindingDB Entry DOI: 10.7270/Q2SN0975 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50332463 (2-(2-methyl-4-((4-methyl-2-(4-(trifluoromethyl)phe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Agonist activity at human PPAR-gamma expressed in african green monkey CV-1 cells after 24 hrs by luciferase reporter gene assay | Bioorg Med Chem Lett 20: 7239-42 (2010) Article DOI: 10.1016/j.bmcl.2010.10.103 BindingDB Entry DOI: 10.7270/Q2SN0975 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50332464 (2-(2-methyl-4-((4-methyl-2-(4-(trifluoromethyl)phe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Agonist activity at human PPAR-gamma expressed in african green monkey CV-1 cells after 24 hrs by luciferase reporter gene assay | Bioorg Med Chem Lett 20: 7239-42 (2010) Article DOI: 10.1016/j.bmcl.2010.10.103 BindingDB Entry DOI: 10.7270/Q2SN0975 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 143 total ) | Next | Last >> |