 Found 193 hits with Last Name = 'nambu' and Initial = 'md'
Found 193 hits with Last Name = 'nambu' and Initial = 'md' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50327904

(2-amino-4-methyl-6-(1H-pyrazol-3-yl)-8-(tetrahydro...)Show SMILES Cc1nc(N)nc2n(C3CCOCC3)c(=O)c(nc12)-c1cc[nH]n1 Show InChI InChI=1S/C15H17N7O2/c1-8-11-13(20-15(16)18-8)22(9-3-6-24-7-4-9)14(23)12(19-11)10-2-5-17-21-10/h2,5,9H,3-4,6-7H2,1H3,(H,17,21)(H2,16,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 6096-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.045
BindingDB Entry DOI: 10.7270/Q2ZK5GWH |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50327907
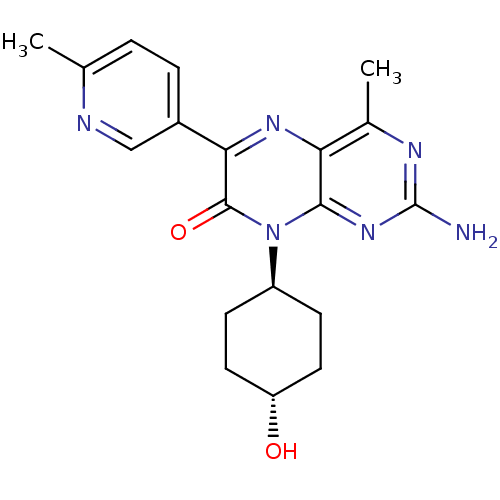
(CHEMBL1257295 | trans-2-amino-8-(4-hydroxycyclohex...)Show SMILES Cc1ccc(cn1)-c1nc2c(C)nc(N)nc2n([C@H]2CC[C@H](O)CC2)c1=O |r,wU:21.23,wD:18.19,(6.09,-29.48,;4.76,-30.26,;4.77,-31.8,;3.44,-32.58,;2.1,-31.81,;2.09,-30.28,;3.42,-29.5,;.78,-32.59,;-.55,-31.83,;-1.88,-32.6,;-3.22,-31.84,;-3.22,-30.3,;-4.54,-32.61,;-4.55,-34.15,;-5.88,-34.92,;-3.21,-34.92,;-1.88,-34.14,;-.54,-34.91,;-.54,-36.45,;-1.87,-37.22,;-1.87,-38.76,;-.54,-39.53,;-.54,-41.07,;.79,-38.76,;.8,-37.22,;.79,-34.14,;2.13,-34.9,)| Show InChI InChI=1S/C19H22N6O2/c1-10-3-4-12(9-21-10)16-18(27)25(13-5-7-14(26)8-6-13)17-15(23-16)11(2)22-19(20)24-17/h3-4,9,13-14,26H,5-8H2,1-2H3,(H2,20,22,24)/t13-,14- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 6096-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.045
BindingDB Entry DOI: 10.7270/Q2ZK5GWH |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50327894

(2-amino-8-isopropyl-6-(6-methoxypyridin-3-yl)-4-me...)Show InChI InChI=1S/C17H19N5O2/c1-9(2)22-15-12(10(3)20-17(18)21-15)7-13(16(22)23)11-5-6-14(24-4)19-8-11/h5-9H,1-4H3,(H2,18,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 6096-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.045
BindingDB Entry DOI: 10.7270/Q2ZK5GWH |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50327906
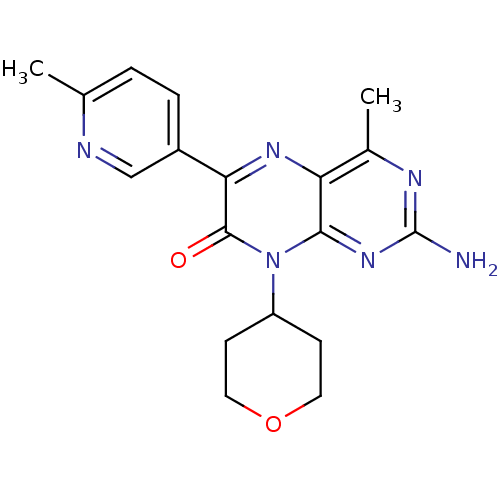
(2-amino-4-methyl-6-(6-methylpyridin-3-yl)-8-(tetra...)Show SMILES Cc1ccc(cn1)-c1nc2c(C)nc(N)nc2n(C2CCOCC2)c1=O Show InChI InChI=1S/C18H20N6O2/c1-10-3-4-12(9-20-10)15-17(25)24(13-5-7-26-8-6-13)16-14(22-15)11(2)21-18(19)23-16/h3-4,9,13H,5-8H2,1-2H3,(H2,19,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 6096-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.045
BindingDB Entry DOI: 10.7270/Q2ZK5GWH |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50327903
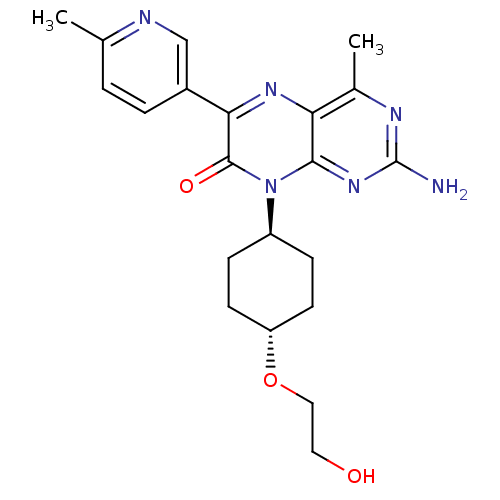
(CHEMBL1258910 | trans-2-amino-8-(4-(2-hydroxyethox...)Show SMILES Cc1ccc(cn1)-c1nc2c(C)nc(N)nc2n([C@H]2CC[C@@H](CC2)OCCO)c1=O |r,wU:18.19,wD:21.26,(6.04,1.56,;4.72,.78,;4.73,-.76,;3.4,-1.53,;2.06,-.77,;2.05,.76,;3.37,1.55,;.74,-1.54,;-.6,-.78,;-1.93,-1.56,;-3.26,-.79,;-3.27,.75,;-4.59,-1.56,;-4.59,-3.11,;-5.93,-3.88,;-3.26,-3.88,;-1.93,-3.1,;-.59,-3.87,;-.59,-5.41,;.75,-6.17,;.75,-7.72,;-.59,-8.49,;-1.92,-7.71,;-1.92,-6.18,;-.59,-10.03,;-1.93,-10.8,;-1.93,-12.34,;-3.26,-13.1,;.74,-3.09,;2.08,-3.86,)| Show InChI InChI=1S/C21H26N6O3/c1-12-3-4-14(11-23-12)18-20(29)27(15-5-7-16(8-6-15)30-10-9-28)19-17(25-18)13(2)24-21(22)26-19/h3-4,11,15-16,28H,5-10H2,1-2H3,(H2,22,24,26)/t15-,16- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 6096-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.045
BindingDB Entry DOI: 10.7270/Q2ZK5GWH |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50327897
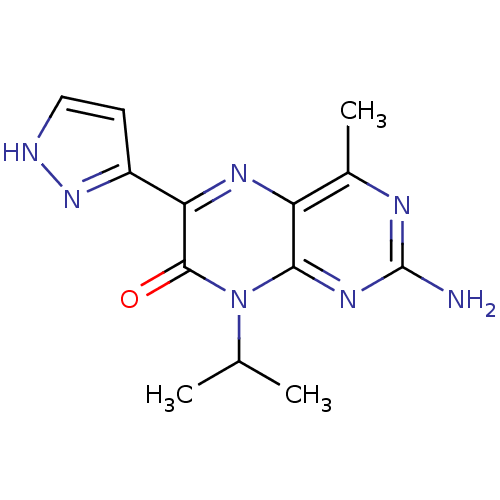
(2-amino-8-isopropyl-4-methyl-6-(1H-pyrazol-5-yl)pt...)Show InChI InChI=1S/C13H15N7O/c1-6(2)20-11-9(7(3)16-13(14)18-11)17-10(12(20)21)8-4-5-15-19-8/h4-6H,1-3H3,(H,15,19)(H2,14,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 6096-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.045
BindingDB Entry DOI: 10.7270/Q2ZK5GWH |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50327905
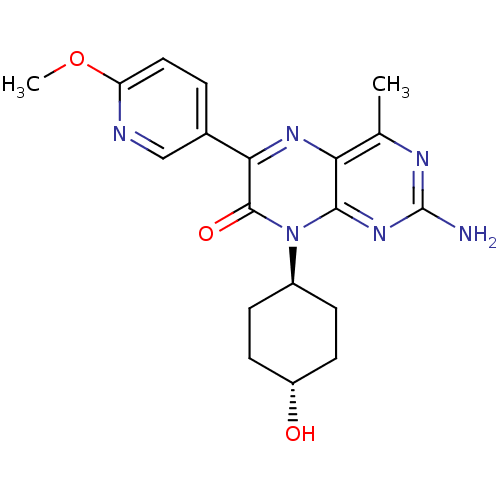
(CHEMBL1257177 | trans-2-amino-8-((1r,4r)-4-hydroxy...)Show SMILES COc1ccc(cn1)-c1nc2c(C)nc(N)nc2n([C@H]2CC[C@H](O)CC2)c1=O |r,wU:19.20,wD:22.24,(6.1,-15.1,;4.76,-14.34,;3.43,-15.12,;3.44,-16.66,;2.11,-17.44,;.77,-16.67,;.76,-15.14,;2.09,-14.36,;-.55,-17.45,;-1.88,-16.69,;-3.21,-17.46,;-4.55,-16.7,;-4.55,-15.16,;-5.87,-17.47,;-5.88,-19.01,;-7.21,-19.78,;-4.54,-19.78,;-3.21,-19,;-1.87,-19.77,;-1.87,-21.31,;-.53,-22.08,;-.54,-23.62,;-1.87,-24.39,;-1.87,-25.93,;-3.2,-23.62,;-3.2,-22.08,;-.54,-19,;.8,-19.76,)| Show InChI InChI=1S/C19H22N6O3/c1-10-15-17(24-19(20)22-10)25(12-4-6-13(26)7-5-12)18(27)16(23-15)11-3-8-14(28-2)21-9-11/h3,8-9,12-13,26H,4-7H2,1-2H3,(H2,20,22,24)/t12-,13- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 6096-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.045
BindingDB Entry DOI: 10.7270/Q2ZK5GWH |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50327896
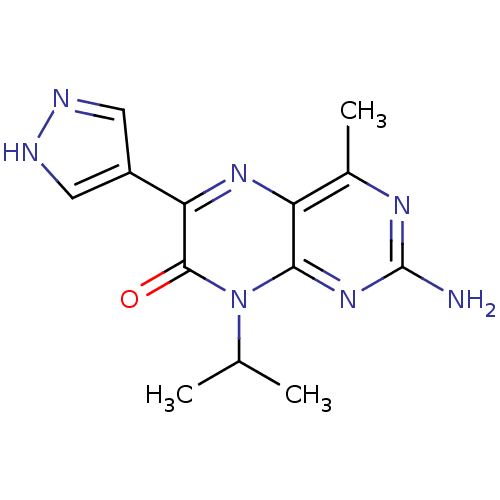
(2-amino-4-methyl-8-(1-methylethyl)-6-(1H-pyrazol-4...)Show InChI InChI=1S/C13H15N7O/c1-6(2)20-11-9(7(3)17-13(14)19-11)18-10(12(20)21)8-4-15-16-5-8/h4-6H,1-3H3,(H,15,16)(H2,14,17,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 6096-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.045
BindingDB Entry DOI: 10.7270/Q2ZK5GWH |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50327901
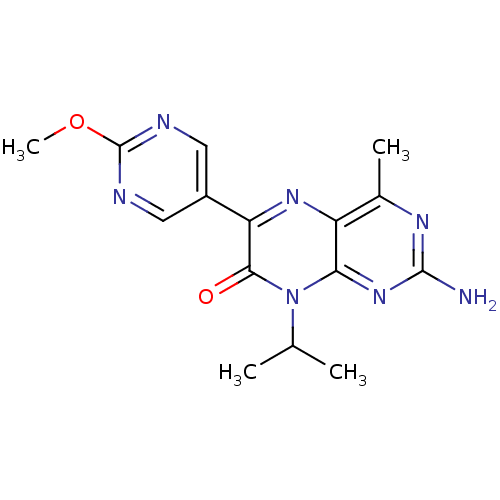
(2-amino-8-isopropyl-6-(2-methoxypyrimidin-5-yl)-4-...)Show InChI InChI=1S/C15H17N7O2/c1-7(2)22-12-10(8(3)19-14(16)21-12)20-11(13(22)23)9-5-17-15(24-4)18-6-9/h5-7H,1-4H3,(H2,16,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 6096-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.045
BindingDB Entry DOI: 10.7270/Q2ZK5GWH |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50327900
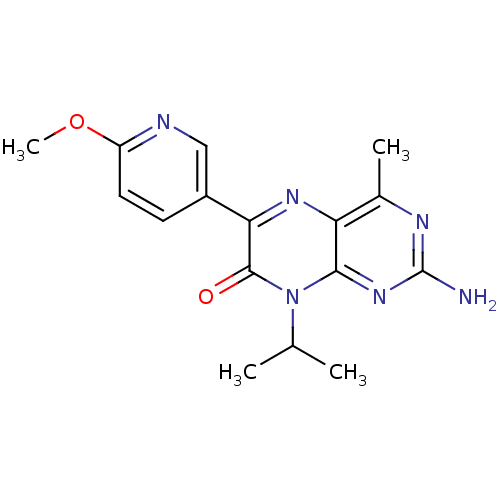
(2-amino-8-isopropyl-6-(6-methoxypyridin-3-yl)-4-me...)Show InChI InChI=1S/C16H18N6O2/c1-8(2)22-14-12(9(3)19-16(17)21-14)20-13(15(22)23)10-5-6-11(24-4)18-7-10/h5-8H,1-4H3,(H2,17,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 6096-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.045
BindingDB Entry DOI: 10.7270/Q2ZK5GWH |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50327898
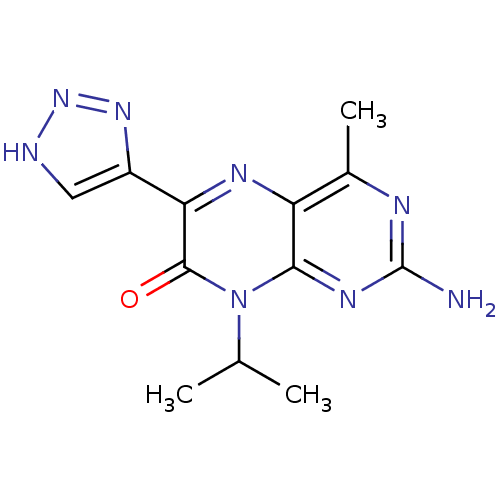
(2-amino-8-isopropyl-4-methyl-6-(1H-1,2,3-triazol-5...)Show InChI InChI=1S/C12H14N8O/c1-5(2)20-10-8(6(3)15-12(13)17-10)16-9(11(20)21)7-4-14-19-18-7/h4-5H,1-3H3,(H2,13,15,17)(H,14,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 113 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 6096-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.045
BindingDB Entry DOI: 10.7270/Q2ZK5GWH |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50327902
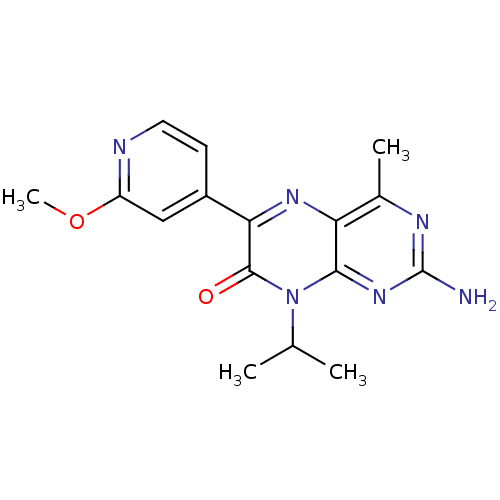
(2-amino-8-isopropyl-6-(2-methoxypyridin-4-yl)-4-me...)Show InChI InChI=1S/C16H18N6O2/c1-8(2)22-14-12(9(3)19-16(17)21-14)20-13(15(22)23)10-5-6-18-11(7-10)24-4/h5-8H,1-4H3,(H2,17,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 947 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 6096-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.045
BindingDB Entry DOI: 10.7270/Q2ZK5GWH |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50327899
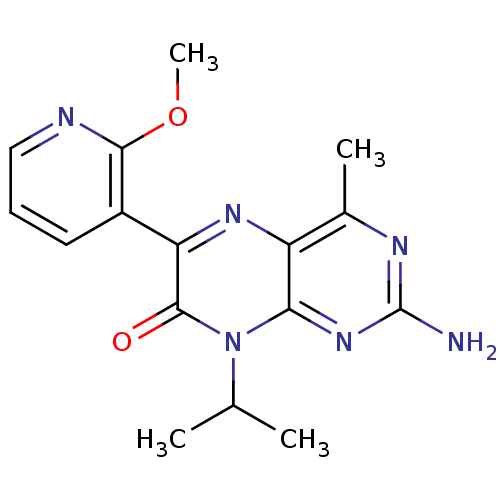
(2-amino-8-isopropyl-6-(2-methoxypyridin-3-yl)-4-me...)Show InChI InChI=1S/C16H18N6O2/c1-8(2)22-13-11(9(3)19-16(17)21-13)20-12(15(22)23)10-6-5-7-18-14(10)24-4/h5-8H,1-4H3,(H2,17,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 6096-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.045
BindingDB Entry DOI: 10.7270/Q2ZK5GWH |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50327895

(2-amino-8-isopropyl-4-methoxy-6-(6-methoxypyridin-...)Show SMILES COc1ccc(cn1)-c1cc2c(OC)nc(N)nc2n(C(C)C)c1=O Show InChI InChI=1S/C17H19N5O3/c1-9(2)22-14-12(15(25-4)21-17(18)20-14)7-11(16(22)23)10-5-6-13(24-3)19-8-10/h5-9H,1-4H3,(H2,18,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 6096-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.045
BindingDB Entry DOI: 10.7270/Q2ZK5GWH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM113428
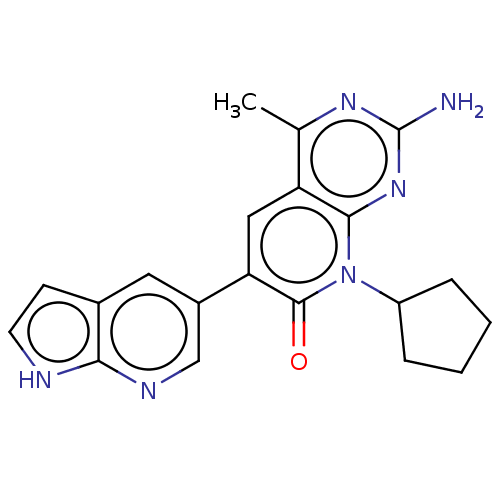
(US8633204, 127)Show SMILES Cc1nc(N)nc2n(C3CCCC3)c(=O)c(cc12)-c1cnc2[nH]ccc2c1 Show InChI InChI=1S/C20H20N6O/c1-11-15-9-16(13-8-12-6-7-22-17(12)23-10-13)19(27)26(14-4-2-3-5-14)18(15)25-20(21)24-11/h6-10,14H,2-5H2,1H3,(H,22,23)(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50433038
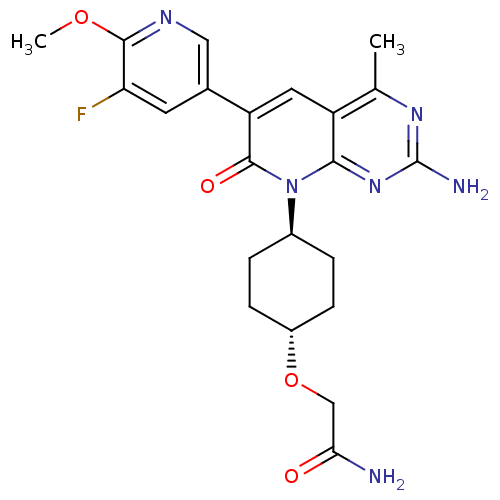
(CHEMBL2375957 | US8633204, 299)Show SMILES COc1ncc(cc1F)-c1cc2c(C)nc(N)nc2n([C@H]2CC[C@@H](CC2)OCC(N)=O)c1=O |r,wU:23.28,wD:20.21,(27.28,-44.28,;25.95,-43.51,;24.61,-44.28,;24.61,-45.82,;23.27,-46.58,;21.94,-45.81,;21.94,-44.27,;23.27,-43.5,;23.27,-41.96,;20.6,-46.56,;19.26,-45.78,;17.92,-46.56,;16.58,-45.8,;16.58,-44.26,;15.25,-46.57,;15.25,-48.11,;13.92,-48.88,;16.58,-48.88,;17.91,-48.12,;19.25,-48.89,;19.24,-50.43,;17.9,-51.19,;17.9,-52.72,;19.22,-53.51,;20.56,-52.74,;20.57,-51.2,;19.21,-55.04,;17.88,-55.81,;16.55,-55.03,;15.21,-55.79,;16.56,-53.49,;20.6,-48.12,;21.93,-48.89,)| Show InChI InChI=1S/C22H25FN6O4/c1-11-15-8-16(12-7-17(23)20(32-2)26-9-12)21(31)29(19(15)28-22(25)27-11)13-3-5-14(6-4-13)33-10-18(24)30/h7-9,13-14H,3-6,10H2,1-2H3,(H2,24,30)(H2,25,27,28)/t13-,14- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.686 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM113443
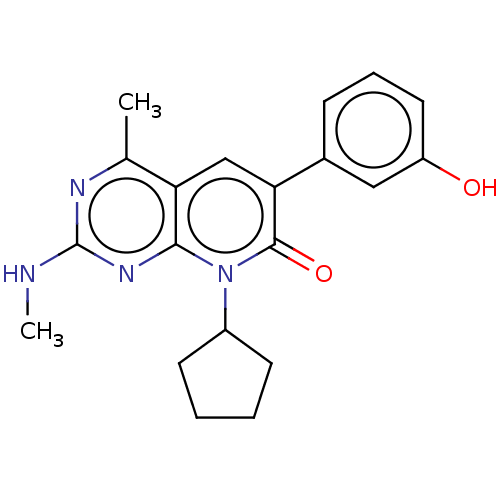
(US8633204, 146)Show SMILES CNc1nc(C)c2cc(-c3cccc(O)c3)c(=O)n(C3CCCC3)c2n1 Show InChI InChI=1S/C20H22N4O2/c1-12-16-11-17(13-6-5-9-15(25)10-13)19(26)24(14-7-3-4-8-14)18(16)23-20(21-2)22-12/h5-6,9-11,14,25H,3-4,7-8H2,1-2H3,(H,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.731 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM113420
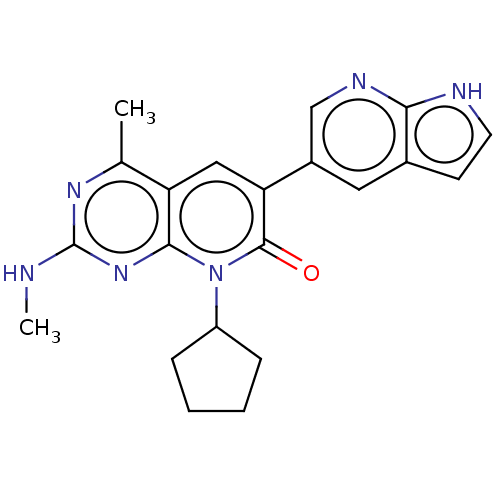
(US8633204, 115)Show SMILES CNc1nc(C)c2cc(-c3cnc4[nH]ccc4c3)c(=O)n(C3CCCC3)c2n1 Show InChI InChI=1S/C21H22N6O/c1-12-16-10-17(14-9-13-7-8-23-18(13)24-11-14)20(28)27(15-5-3-4-6-15)19(16)26-21(22-2)25-12/h7-11,15H,3-6H2,1-2H3,(H,23,24)(H,22,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM113542

(US8633204, 276)Show SMILES CCNc1nc(C)c2cc(-c3cnc(OC)c(F)c3)c(=O)n([C@H]3CC[C@H](O)CC3)c2n1 |r,wU:22.22,wD:25.26,(-7.96,.31,;-6.47,.7,;-5.38,-.39,;-4.05,.39,;-4.05,1.92,;-2.71,2.69,;-2.71,4.24,;-1.38,1.92,;-.05,2.69,;1.29,1.92,;2.62,2.69,;3.96,1.92,;5.29,2.69,;5.29,4.24,;6.62,5,;7.96,4.24,;3.96,5,;3.96,6.54,;2.62,4.24,;1.29,.39,;2.62,-.39,;-.05,-.39,;-.05,-1.92,;1.29,-2.69,;1.29,-4.24,;-.05,-5,;-.05,-6.54,;-1.38,-4.24,;-1.38,-2.69,;-1.38,.39,;-2.71,-.39,)| Show InChI InChI=1S/C22H26FN5O3/c1-4-24-22-26-12(2)16-10-17(13-9-18(23)20(31-3)25-11-13)21(30)28(19(16)27-22)14-5-7-15(29)8-6-14/h9-11,14-15,29H,4-8H2,1-3H3,(H,24,26,27)/t14-,15- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.788 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM113434

(US8633204, 137)Show SMILES COc1cc(ccc1O)-c1cc2c(C)nc(N)nc2n(C2CCCC2)c1=O Show InChI InChI=1S/C20H22N4O3/c1-11-14-10-15(12-7-8-16(25)17(9-12)27-2)19(26)24(13-5-3-4-6-13)18(14)23-20(21)22-11/h7-10,13,25H,3-6H2,1-2H3,(H2,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.989 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM113441
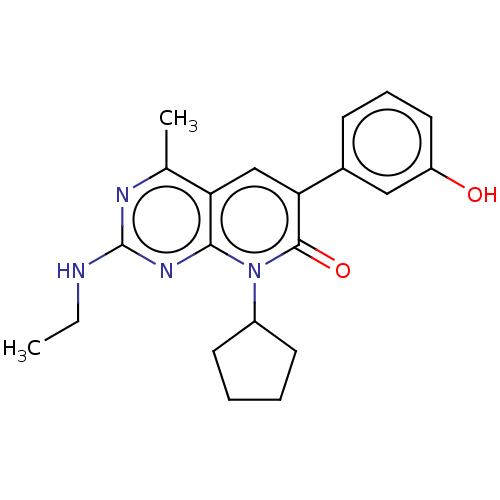
(US8633204, 144)Show SMILES CCNc1nc(C)c2cc(-c3cccc(O)c3)c(=O)n(C3CCCC3)c2n1 Show InChI InChI=1S/C21H24N4O2/c1-3-22-21-23-13(2)17-12-18(14-7-6-10-16(26)11-14)20(27)25(19(17)24-21)15-8-4-5-9-15/h6-7,10-12,15,26H,3-5,8-9H2,1-2H3,(H,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.01 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM113437
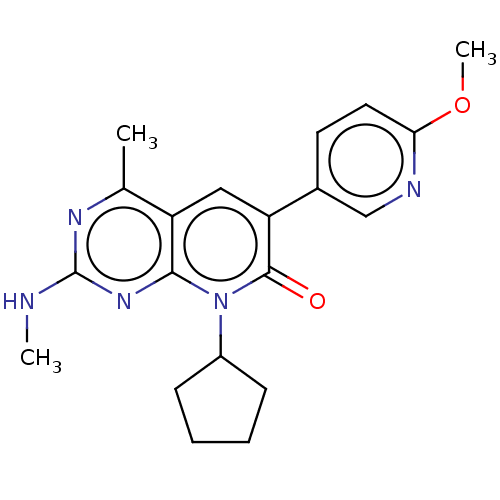
(US8633204, 140)Show SMILES CNc1nc(C)c2cc(-c3ccc(OC)nc3)c(=O)n(C3CCCC3)c2n1 Show InChI InChI=1S/C20H23N5O2/c1-12-15-10-16(13-8-9-17(27-3)22-11-13)19(26)25(14-6-4-5-7-14)18(15)24-20(21-2)23-12/h8-11,14H,4-7H2,1-3H3,(H,21,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.03 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM113479

(US8633204, 191)Show SMILES COc1ncc(cc1F)-c1cc2c(C)nc(N)nc2n([C@H]2CC[C@H](O)CC2)c1=O |r,wU:20.21,wD:23.25,(6.67,5,;5.33,5.77,;4,5,;2.67,5.77,;1.33,5,;1.33,3.46,;2.67,2.69,;4,3.46,;5.33,2.69,;,2.69,;-1.33,3.46,;-2.67,2.69,;-4,3.46,;-4,5,;-5.33,2.69,;-5.33,1.15,;-6.67,.39,;-4,.39,;-2.67,1.15,;-1.33,.39,;-1.33,-1.15,;,-1.92,;,-3.46,;-1.33,-4.23,;-1.33,-5.77,;-2.67,-3.46,;-2.67,-1.92,;,1.15,;1.33,.39,)| Show InChI InChI=1S/C20H22FN5O3/c1-10-14-8-15(11-7-16(21)18(29-2)23-9-11)19(28)26(17(14)25-20(22)24-10)12-3-5-13(27)6-4-12/h7-9,12-13,27H,3-6H2,1-2H3,(H2,22,24,25)/t12-,13- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM113453
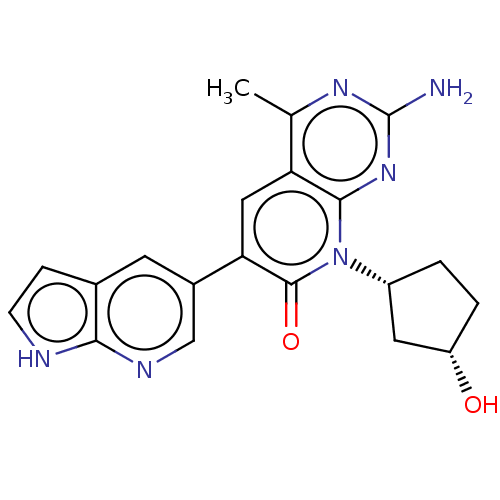
(US8633204, 163)Show SMILES Cc1nc(N)nc2n([C@@H]3CC[C@H](O)C3)c(=O)c(cc12)-c1cnc2[nH]ccc2c1 |r| Show InChI InChI=1S/C20H20N6O2/c1-10-15-8-16(12-6-11-4-5-22-17(11)23-9-12)19(28)26(13-2-3-14(27)7-13)18(15)25-20(21)24-10/h4-6,8-9,13-14,27H,2-3,7H2,1H3,(H,22,23)(H2,21,24,25)/t13-,14+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM113455
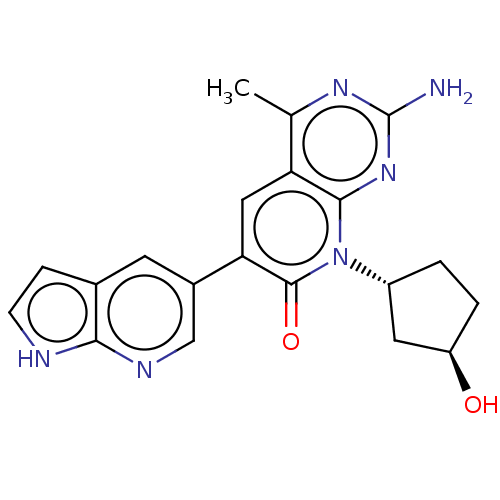
(US8633204, 165)Show SMILES Cc1nc(N)nc2n([C@@H]3CC[C@@H](O)C3)c(=O)c(cc12)-c1cnc2[nH]ccc2c1 |r| Show InChI InChI=1S/C20H20N6O2/c1-10-15-8-16(12-6-11-4-5-22-17(11)23-9-12)19(28)26(13-2-3-14(27)7-13)18(15)25-20(21)24-10/h4-6,8-9,13-14,27H,2-3,7H2,1H3,(H,22,23)(H2,21,24,25)/t13-,14-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM113448
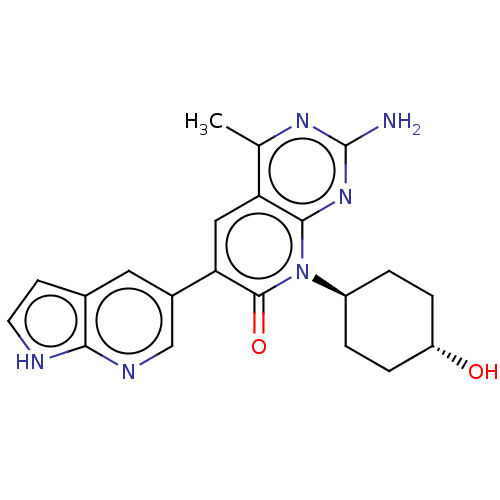
(US8633204, 158)Show SMILES Cc1nc(N)nc2n([C@H]3CC[C@H](O)CC3)c(=O)c(cc12)-c1cnc2[nH]ccc2c1 |r,wU:8.7,wD:11.11,(-3.85,4.49,;-3.85,2.95,;-5.19,2.18,;-5.19,.64,;-6.52,-.13,;-3.85,-.13,;-2.52,.64,;-1.18,-.13,;-1.18,-1.67,;.15,-2.44,;.15,-3.98,;-1.18,-4.75,;-1.18,-6.29,;-2.52,-3.98,;-2.52,-2.44,;.15,.64,;1.48,-.13,;.15,2.18,;-1.18,2.95,;-2.52,2.18,;1.48,2.95,;1.48,4.49,;2.82,5.26,;4.15,4.49,;5.61,4.96,;6.52,3.72,;5.61,2.47,;4.15,2.95,;2.82,2.18,)| Show InChI InChI=1S/C21H22N6O2/c1-11-16-9-17(13-8-12-6-7-23-18(12)24-10-13)20(29)27(19(16)26-21(22)25-11)14-2-4-15(28)5-3-14/h6-10,14-15,28H,2-5H2,1H3,(H,23,24)(H2,22,25,26)/t14-,15- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM113544
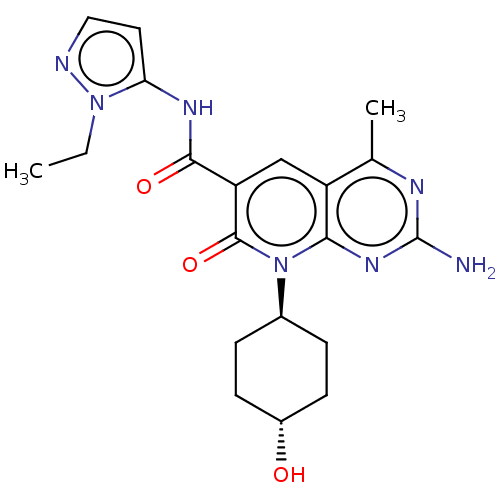
(US8633204, 278)Show SMILES CCn1nccc1NC(=O)c1cc2c(C)nc(N)nc2n([C@H]2CC[C@H](O)CC2)c1=O |r,wU:21.22,wD:24.26,(2.82,6.54,;2.82,5,;4.15,4.23,;5.61,4.71,;6.52,3.47,;5.61,2.22,;4.15,2.69,;2.82,1.93,;1.48,2.69,;1.48,4.23,;.15,1.93,;-1.18,2.69,;-2.52,1.93,;-3.85,2.69,;-3.85,4.23,;-5.19,1.93,;-5.19,.38,;-6.52,-.38,;-3.85,-.38,;-2.52,.38,;-1.18,-.38,;-1.18,-1.93,;.15,-2.69,;.15,-4.23,;-1.18,-5,;-1.18,-6.54,;-2.52,-4.23,;-2.52,-2.69,;.15,.38,;1.48,-.38,)| Show InChI InChI=1S/C20H25N7O3/c1-3-26-16(8-9-22-26)24-18(29)15-10-14-11(2)23-20(21)25-17(14)27(19(15)30)12-4-6-13(28)7-5-12/h8-10,12-13,28H,3-7H2,1-2H3,(H,24,29)(H2,21,23,25)/t12-,13- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
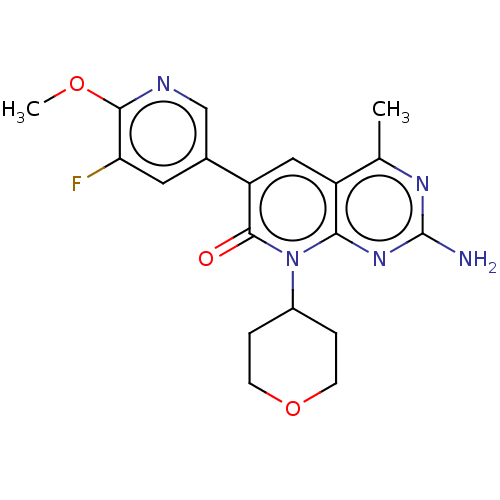
(Homo sapiens (Human)) | BDBM113535
(US8633204, 265)Show SMILES COc1ncc(cc1F)-c1cc2c(C)nc(N)nc2n(C2CCOCC2)c1=O Show InChI InChI=1S/C19H20FN5O3/c1-10-13-8-14(11-7-15(20)17(27-2)22-9-11)18(26)25(12-3-5-28-6-4-12)16(13)24-19(21)23-10/h7-9,12H,3-6H2,1-2H3,(H2,21,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.43 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
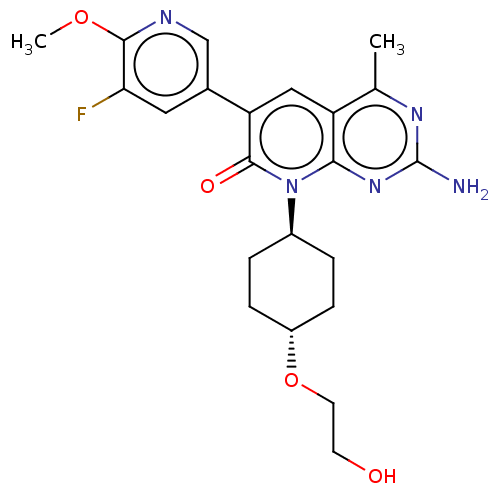
(Homo sapiens (Human)) | BDBM113551
(US8633204, 288)Show SMILES COc1ncc(cc1F)-c1cc2c(C)nc(N)nc2n([C@H]2CC[C@@H](CC2)OCCO)c1=O |r,wU:20.21,wD:23.28,(6,6.93,;6,5.39,;4.67,4.62,;3.33,5.39,;2,4.62,;2,3.08,;3.33,2.31,;4.67,3.08,;6,2.31,;.67,2.31,;-.67,3.08,;-2,2.31,;-3.33,3.08,;-3.33,4.62,;-4.67,2.31,;-4.67,.77,;-6,,;-3.33,,;-2,.77,;-.67,,;-.67,-1.54,;.67,-2.31,;.67,-3.85,;-.67,-4.62,;-2,-3.85,;-2,-2.31,;-.67,-6.16,;.67,-6.93,;2,-6.16,;3.33,-6.93,;.67,.77,;2,,)| Show InChI InChI=1S/C22H26FN5O4/c1-12-16-10-17(13-9-18(23)20(31-2)25-11-13)21(30)28(19(16)27-22(24)26-12)14-3-5-15(6-4-14)32-8-7-29/h9-11,14-15,29H,3-8H2,1-2H3,(H2,24,26,27)/t14-,15- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.44 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
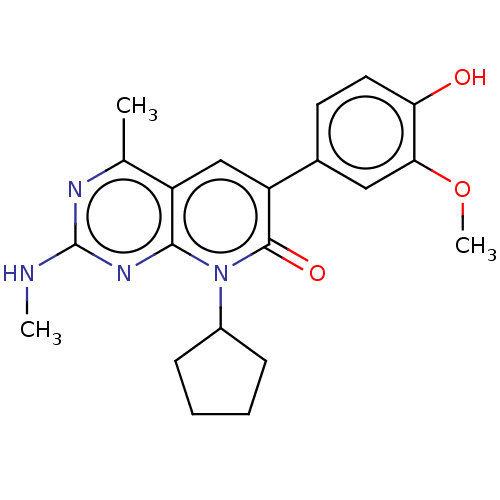
(Homo sapiens (Human)) | BDBM113417
(US8633204, 108)Show SMILES CNc1nc(C)c2cc(-c3ccc(O)c(OC)c3)c(=O)n(C3CCCC3)c2n1 Show InChI InChI=1S/C21H24N4O3/c1-12-15-11-16(13-8-9-17(26)18(10-13)28-3)20(27)25(14-6-4-5-7-14)19(15)24-21(22-2)23-12/h8-11,14,26H,4-7H2,1-3H3,(H,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.57 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50433044
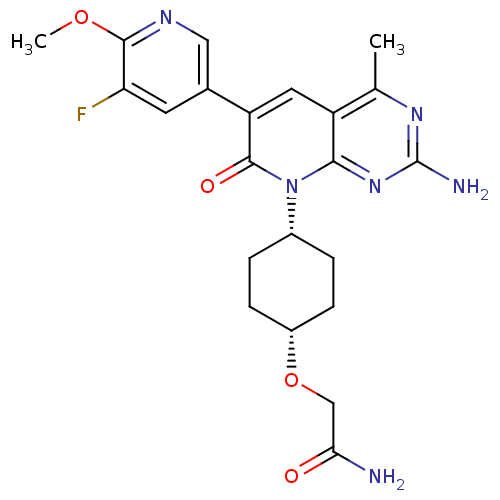
(CHEMBL2375963 | US8633204, 304)Show SMILES COc1ncc(cc1F)-c1cc2c(C)nc(N)nc2n([C@@H]2CC[C@@H](CC2)OCC(N)=O)c1=O |r,wD:20.21,23.28,(27.43,-30.66,;26.1,-29.89,;24.76,-30.66,;24.76,-32.2,;23.42,-32.96,;22.09,-32.19,;22.09,-30.65,;23.42,-29.88,;23.42,-28.34,;20.75,-32.94,;19.4,-32.16,;18.06,-32.94,;16.73,-32.18,;16.73,-30.64,;15.4,-32.95,;15.4,-34.49,;14.07,-35.26,;16.73,-35.26,;18.06,-34.49,;19.4,-35.27,;19.39,-36.81,;20.72,-37.58,;20.71,-39.12,;19.37,-39.88,;18.05,-39.1,;18.05,-37.57,;19.36,-41.42,;18.02,-42.18,;16.7,-41.41,;15.36,-42.17,;16.71,-39.87,;20.75,-34.5,;22.08,-35.27,)| Show InChI InChI=1S/C22H25FN6O4/c1-11-15-8-16(12-7-17(23)20(32-2)26-9-12)21(31)29(19(15)28-22(25)27-11)13-3-5-14(6-4-13)33-10-18(24)30/h7-9,13-14H,3-6,10H2,1-2H3,(H2,24,30)(H2,25,27,28)/t13-,14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.58 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM113543

(US8633204, 277)Show SMILES CCNc1nc(C)c2cc(-c3cnc(OC)nc3)c(=O)n([C@H]3CC[C@H](O)CC3)c2n1 |r,wU:21.21,wD:24.25,(-7.96,1.08,;-6.47,1.47,;-5.38,.39,;-4.05,1.15,;-4.05,2.69,;-2.71,3.46,;-2.71,5,;-1.38,2.69,;-.05,3.46,;1.29,2.69,;2.62,3.46,;2.62,5,;3.96,5.77,;5.29,5,;6.62,5.77,;7.96,5,;5.29,3.46,;3.96,2.69,;1.29,1.15,;2.62,.39,;-.05,.39,;-.05,-1.15,;1.29,-1.92,;1.29,-3.46,;-.05,-4.23,;-.05,-5.77,;-1.38,-3.46,;-1.38,-1.92,;-1.38,1.15,;-2.71,.39,)| Show InChI InChI=1S/C21H26N6O3/c1-4-22-20-25-12(2)16-9-17(13-10-23-21(30-3)24-11-13)19(29)27(18(16)26-20)14-5-7-15(28)8-6-14/h9-11,14-15,28H,4-8H2,1-3H3,(H,22,25,26)/t14-,15- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.59 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM113469

(US8633204, 180)Show SMILES CO[C@H]1CC[C@@H](CC1)n1c2nc(N)nc(C)c2cc(-c2cnc(OC)c(F)c2)c1=O |r,wU:5.8,wD:2.1,(,-6.16,;-1.33,-5.39,;-1.33,-3.85,;,-3.08,;,-1.54,;-1.33,-.77,;-2.67,-1.54,;-2.67,-3.08,;-1.33,.77,;-2.67,1.54,;-4,.77,;-5.33,1.54,;-6.67,.77,;-5.33,3.08,;-4,3.85,;-4,5.39,;-2.67,3.08,;-1.33,3.85,;,3.08,;1.33,3.85,;1.33,5.39,;2.67,6.16,;4,5.39,;5.33,6.16,;6.67,5.39,;4,3.85,;5.33,3.08,;2.67,3.08,;,1.54,;1.33,.77,)| Show InChI InChI=1S/C21H24FN5O3/c1-11-15-9-16(12-8-17(22)19(30-3)24-10-12)20(28)27(18(15)26-21(23)25-11)13-4-6-14(29-2)7-5-13/h8-10,13-14H,4-7H2,1-3H3,(H2,23,25,26)/t13-,14- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |
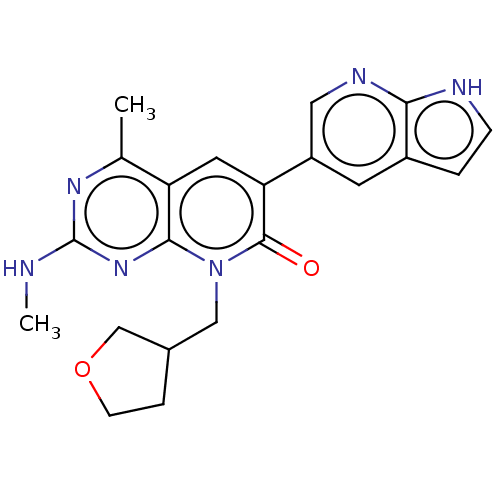
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM113495
(US8633204, 217)Show SMILES CNc1nc(C)c2cc(-c3cnc4[nH]ccc4c3)c(=O)n(CC3CCOC3)c2n1 Show InChI InChI=1S/C21H22N6O2/c1-12-16-8-17(15-7-14-3-5-23-18(14)24-9-15)20(28)27(10-13-4-6-29-11-13)19(16)26-21(22-2)25-12/h3,5,7-9,13H,4,6,10-11H2,1-2H3,(H,23,24)(H,22,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM113461
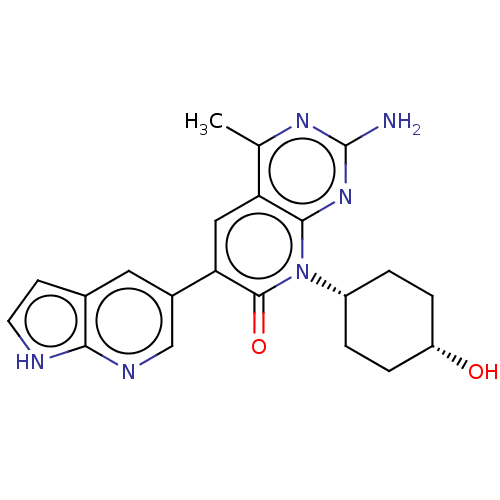
(US8633204, 171)Show SMILES Cc1nc(N)nc2n([C@@H]3CC[C@H](O)CC3)c(=O)c(cc12)-c1cnc2[nH]ccc2c1 |r,wU:8.7,11.11,(-3.85,4.49,;-3.85,2.95,;-5.19,2.18,;-5.19,.64,;-6.52,-.13,;-3.85,-.13,;-2.52,.64,;-1.18,-.13,;-1.18,-1.67,;-2.52,-2.44,;-2.52,-3.98,;-1.18,-4.75,;-1.18,-6.29,;.15,-3.98,;.15,-2.44,;.15,.64,;1.48,-.13,;.15,2.18,;-1.18,2.95,;-2.52,2.18,;1.48,2.95,;1.48,4.49,;2.82,5.26,;4.15,4.49,;5.61,4.96,;6.52,3.72,;5.61,2.47,;4.15,2.95,;2.82,2.18,)| Show InChI InChI=1S/C21H22N6O2/c1-11-16-9-17(13-8-12-6-7-23-18(12)24-10-13)20(29)27(19(16)26-21(22)25-11)14-2-4-15(28)5-3-14/h6-10,14-15,28H,2-5H2,1H3,(H,23,24)(H2,22,25,26)/t14-,15+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |
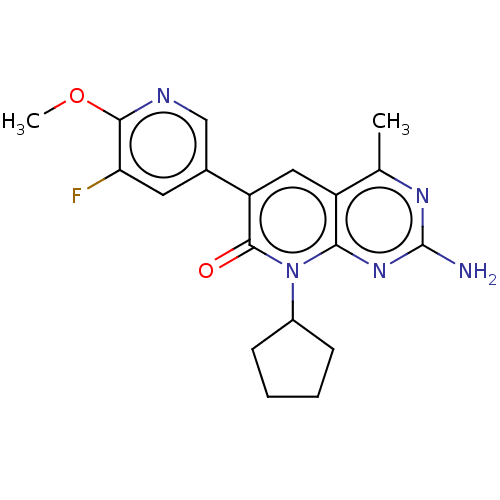
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM113526
(US8633204, 254)Show SMILES COc1ncc(cc1F)-c1cc2c(C)nc(N)nc2n(C2CCCC2)c1=O Show InChI InChI=1S/C19H20FN5O2/c1-10-13-8-14(11-7-15(20)17(27-2)22-9-11)18(26)25(12-5-3-4-6-12)16(13)24-19(21)23-10/h7-9,12H,3-6H2,1-2H3,(H2,21,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM113432

(US8633204, 135)Show SMILES CCNc1nc(C)c2cc(-c3cnc4[nH]ccc4c3)c(=O)n(C3CCCC3)c2n1 Show InChI InChI=1S/C22H24N6O/c1-3-23-22-26-13(2)17-11-18(15-10-14-8-9-24-19(14)25-12-15)21(29)28(20(17)27-22)16-6-4-5-7-16/h8-12,16H,3-7H2,1-2H3,(H,24,25)(H,23,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.77 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM113541

(US8633204, 274)Show SMILES CNc1nc(C)c2cc(-c3ccc(OC)nc3)c(=O)n([C@H]3CC[C@H](O)CC3)c2n1 |r,wU:20.20,wD:23.24,(-7.34,1.16,;-6,.39,;-4.67,1.16,;-4.67,2.7,;-3.33,3.47,;-3.33,5.01,;-2,2.7,;-.67,3.47,;.67,2.7,;2,3.47,;2,5.01,;3.33,5.78,;4.67,5.01,;6,5.78,;7.34,5.01,;4.67,3.47,;3.33,2.7,;.67,1.16,;2,.39,;-.67,.39,;-.67,-1.16,;.67,-1.93,;.67,-3.47,;-.67,-4.24,;-.67,-5.78,;-2,-3.47,;-2,-1.93,;-2,1.16,;-3.33,.39,)| Show InChI InChI=1S/C21H25N5O3/c1-12-16-10-17(13-4-9-18(29-3)23-11-13)20(28)26(14-5-7-15(27)8-6-14)19(16)25-21(22-2)24-12/h4,9-11,14-15,27H,5-8H2,1-3H3,(H,22,24,25)/t14-,15- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM113527
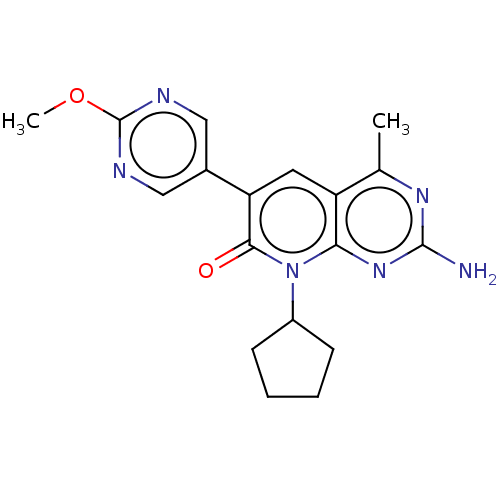
(US8633204, 255)Show SMILES COc1ncc(cn1)-c1cc2c(C)nc(N)nc2n(C2CCCC2)c1=O Show InChI InChI=1S/C18H20N6O2/c1-10-13-7-14(11-8-20-18(26-2)21-9-11)16(25)24(12-5-3-4-6-12)15(13)23-17(19)22-10/h7-9,12H,3-6H2,1-2H3,(H2,19,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM113489
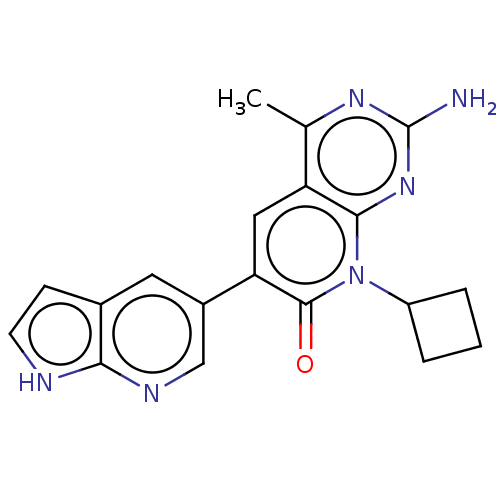
(US8633204, 207)Show SMILES Cc1nc(N)nc2n(C3CCC3)c(=O)c(cc12)-c1cnc2[nH]ccc2c1 Show InChI InChI=1S/C19H18N6O/c1-10-14-8-15(12-7-11-5-6-21-16(11)22-9-12)18(26)25(13-3-2-4-13)17(14)24-19(20)23-10/h5-9,13H,2-4H2,1H3,(H,21,22)(H2,20,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM113571

(US8633204, 318)Show SMILES COc1ncc(cc1F)-c1cc2c(C)nc(N)nc2n([C@H]2C[C@@H](C2)OCC(N)=O)c1=O |r,wU:20.21,wD:22.26,(6,6.48,;6,4.94,;4.67,4.17,;3.33,4.94,;2,4.17,;2,2.63,;3.33,1.86,;4.67,2.63,;6,1.86,;.67,1.86,;-.67,2.63,;-2,1.86,;-3.33,2.63,;-3.33,4.17,;-4.67,1.86,;-4.67,.32,;-6,-.45,;-3.33,-.45,;-2,.32,;-.67,-.45,;-.67,-1.99,;.42,-3.08,;-.67,-4.17,;-1.76,-3.08,;-.67,-5.71,;.67,-6.48,;2,-5.71,;3.33,-6.48,;2,-4.17,;.67,.32,;2,-.45,)| Show InChI InChI=1S/C20H21FN6O4/c1-9-13-6-14(10-3-15(21)18(30-2)24-7-10)19(29)27(17(13)26-20(23)25-9)11-4-12(5-11)31-8-16(22)28/h3,6-7,11-12H,4-5,8H2,1-2H3,(H2,22,28)(H2,23,25,26)/t11-,12- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2.14 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM113536

(US8633204, 266)Show InChI InChI=1S/C16H18N6O2/c1-8(2)22-13-11(9(3)20-15(17)21-13)5-12(14(22)23)10-6-18-16(24-4)19-7-10/h5-8H,1-4H3,(H2,17,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM113550
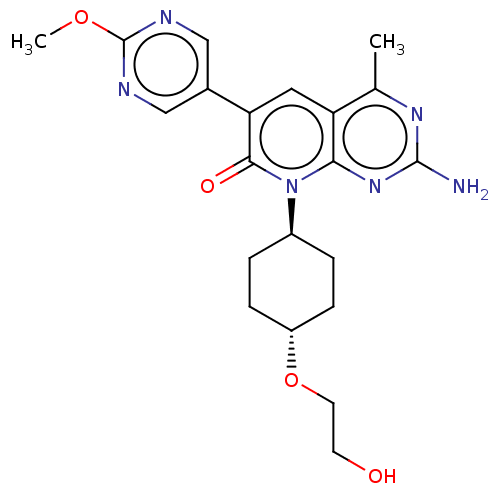
(US8633204, 287)Show SMILES COc1ncc(cn1)-c1cc2c(C)nc(N)nc2n([C@H]2CC[C@@H](CC2)OCCO)c1=O |r,wU:19.20,wD:22.27,(6,6.93,;6,5.39,;4.67,4.62,;4.67,3.08,;3.33,2.31,;2,3.08,;2,4.62,;3.33,5.39,;.67,2.31,;-.67,3.08,;-2,2.31,;-3.33,3.08,;-3.33,4.62,;-4.67,2.31,;-4.67,.77,;-6,,;-3.33,,;-2,.77,;-.67,,;-.67,-1.54,;.67,-2.31,;.67,-3.85,;-.67,-4.62,;-2,-3.85,;-2,-2.31,;-.67,-6.16,;.67,-6.93,;2,-6.16,;3.33,-6.93,;.67,.77,;2,,)| Show InChI InChI=1S/C21H26N6O4/c1-12-16-9-17(13-10-23-21(30-2)24-11-13)19(29)27(18(16)26-20(22)25-12)14-3-5-15(6-4-14)31-8-7-28/h9-11,14-15,28H,3-8H2,1-2H3,(H2,22,25,26)/t14-,15- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM113490

(US8633204, 210)Show SMILES CC(C)n1c2nc(N)nc(C)c2cc(-c2cnc3[nH]ccc3c2)c1=O Show InChI InChI=1S/C18H18N6O/c1-9(2)24-16-13(10(3)22-18(19)23-16)7-14(17(24)25)12-6-11-4-5-20-15(11)21-8-12/h4-9H,1-3H3,(H,20,21)(H2,19,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM113540

(US8633204, 273)Show SMILES COc1ncc(cn1)-c1cc2c(C)nc(N)nc2n([C@H]2CC[C@H](O)CC2)c1=O |r,wU:19.20,wD:22.24,(6.67,5,;5.33,5.77,;4,5,;2.67,5.77,;1.33,5,;1.33,3.46,;2.67,2.69,;4,3.46,;,2.69,;-1.33,3.46,;-2.67,2.69,;-4,3.46,;-4,5,;-5.33,2.69,;-5.33,1.15,;-6.67,.39,;-4,.39,;-2.67,1.15,;-1.33,.39,;-1.33,-1.15,;,-1.92,;,-3.46,;-1.33,-4.23,;-1.33,-5.77,;-2.67,-3.46,;-2.67,-1.92,;,1.15,;1.33,.39,)| Show InChI InChI=1S/C19H22N6O3/c1-10-14-7-15(11-8-21-19(28-2)22-9-11)17(27)25(16(14)24-18(20)23-10)12-3-5-13(26)6-4-12/h7-9,12-13,26H,3-6H2,1-2H3,(H2,20,23,24)/t12-,13- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM113467

(US8633204, 177)Show SMILES COc1ncc(cc1F)-c1cc2c(C)nc(N)nc2n([C@@H]2CC[C@H](O)CC2)c1=O |r,wU:20.21,23.25,(6,6.54,;6,5,;4.67,4.23,;3.33,5,;2,4.23,;2,2.69,;3.33,1.93,;4.67,2.69,;6,1.93,;.67,1.93,;-.67,2.69,;-2,1.93,;-3.33,2.69,;-3.33,4.23,;-4.67,1.93,;-4.67,.38,;-6,-.38,;-3.33,-.38,;-2,.38,;-.67,-.38,;-.67,-1.93,;-2,-2.69,;-2,-4.23,;-.67,-5,;-.67,-6.54,;.67,-4.23,;.67,-2.69,;.67,.38,;2,-.38,)| Show InChI InChI=1S/C20H22FN5O3/c1-10-14-8-15(11-7-16(21)18(29-2)23-9-11)19(28)26(17(14)25-20(22)24-10)12-3-5-13(27)6-4-12/h7-9,12-13,27H,3-6H2,1-2H3,(H2,22,24,25)/t12-,13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM113557

(US8633204, 294)Show SMILES COc1ncc(cc1F)-c1cc2c(C)nc(N)nc2n([C@@H]2CC[C@@H](CC2)OCCO)c1=O |r,wU:20.21,23.28,(6,6.93,;6,5.39,;4.67,4.62,;3.33,5.39,;2,4.62,;2,3.08,;3.33,2.31,;4.67,3.08,;6,2.31,;.67,2.31,;-.67,3.08,;-2,2.31,;-3.33,3.08,;-3.33,4.62,;-4.67,2.31,;-4.67,.77,;-6,,;-3.33,,;-2,.77,;-.67,,;-.67,-1.54,;-2,-2.31,;-2,-3.85,;-.67,-4.62,;.67,-3.85,;.67,-2.31,;-.67,-6.16,;.67,-6.93,;2,-6.16,;3.33,-6.93,;.67,.77,;2,,)| Show InChI InChI=1S/C22H26FN5O4/c1-12-16-10-17(13-9-18(23)20(31-2)25-11-13)21(30)28(19(16)27-22(24)26-12)14-3-5-15(6-4-14)32-8-7-29/h9-11,14-15,29H,3-8H2,1-2H3,(H2,24,26,27)/t14-,15+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM113477

(US8633204, 189)Show SMILES CCOc1ccc(cn1)-c1cc2c(C)nc(N)nc2n([C@H]2CC[C@H](O)CC2)c1=O |r,wU:20.21,wD:23.25,(7.34,5.77,;6,5,;4.67,5.77,;3.33,5,;3.33,3.46,;2,2.69,;.67,3.46,;.67,5,;2,5.77,;-.67,2.69,;-2,3.46,;-3.33,2.69,;-4.67,3.46,;-4.67,5,;-6,2.69,;-6,1.15,;-7.34,.39,;-4.67,.39,;-3.33,1.15,;-2,.39,;-2,-1.15,;-.67,-1.92,;-.67,-3.46,;-2,-4.23,;-2,-5.77,;-3.33,-3.46,;-3.33,-1.92,;-.67,1.15,;.67,.39,)| Show InChI InChI=1S/C21H25N5O3/c1-3-29-18-9-4-13(11-23-18)17-10-16-12(2)24-21(22)25-19(16)26(20(17)28)14-5-7-15(27)8-6-14/h4,9-11,14-15,27H,3,5-8H2,1-2H3,(H2,22,24,25)/t14-,15- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM113499
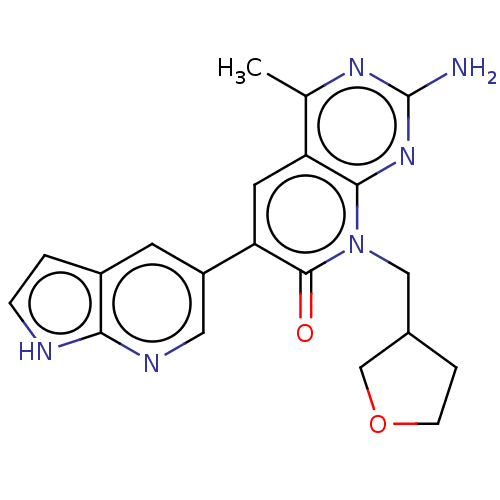
(US8633204, 221)Show SMILES Cc1nc(N)nc2n(CC3CCOC3)c(=O)c(cc12)-c1cnc2[nH]ccc2c1 Show InChI InChI=1S/C20H20N6O2/c1-11-15-7-16(14-6-13-2-4-22-17(13)23-8-14)19(27)26(9-12-3-5-28-10-12)18(15)25-20(21)24-11/h2,4,6-8,12H,3,5,9-10H2,1H3,(H,22,23)(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM113545
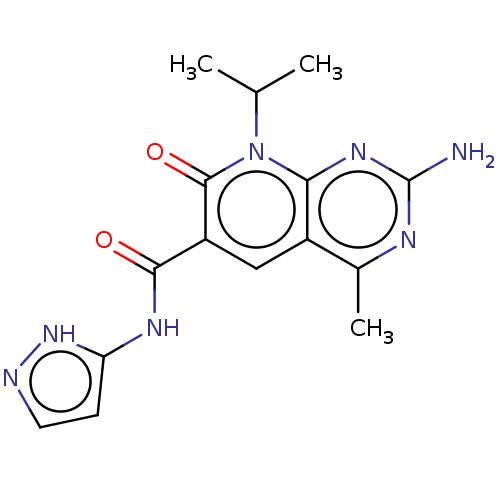
(US8633204, 279)Show SMILES CC(C)n1c2nc(N)nc(C)c2cc(C(=O)Nc2ccn[nH]2)c1=O Show InChI InChI=1S/C15H17N7O2/c1-7(2)22-12-9(8(3)18-15(16)20-12)6-10(14(22)24)13(23)19-11-4-5-17-21-11/h4-7H,1-3H3,(H2,16,18,20)(H2,17,19,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data