

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM82551 (C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | PubMed | 1.09 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Ability to displace [3H]U-69593 from Opioid receptor kappa 1 of guinea pig brain | J Med Chem 45: 3524-30 (2002) BindingDB Entry DOI: 10.7270/Q2959J8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50116621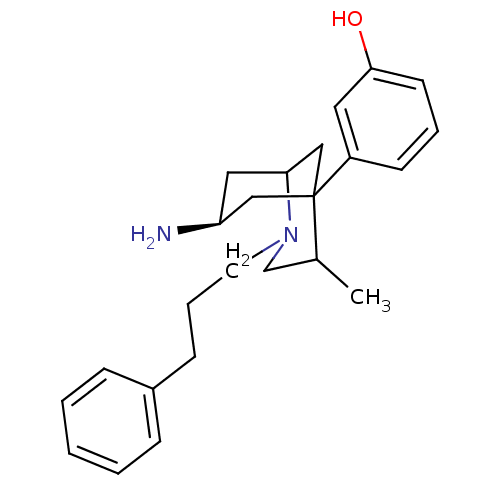 (3-[7-Amino-4-methyl-2-(3-phenyl-propyl)-2-aza-bicy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Ability to displace [3H]DAMGO from Opioid receptor mu 1 of rat brain | J Med Chem 45: 3524-30 (2002) BindingDB Entry DOI: 10.7270/Q2959J8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50116622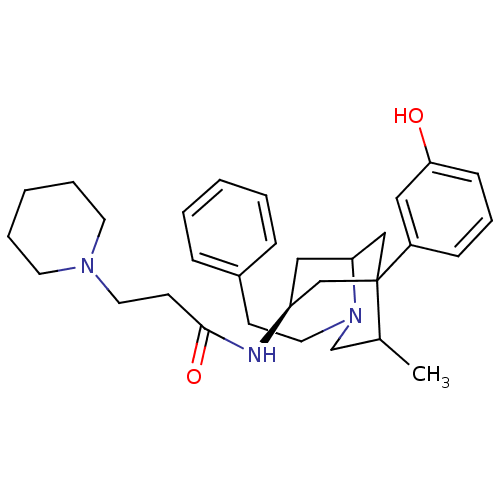 (CHEMBL117457 | N-[5-(3-Hydroxy-phenyl)-4-methyl-2-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Ability to displace [3H]U-69593 from Opioid receptor kappa 1 of guinea pig brain | J Med Chem 45: 3524-30 (2002) BindingDB Entry DOI: 10.7270/Q2959J8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50116624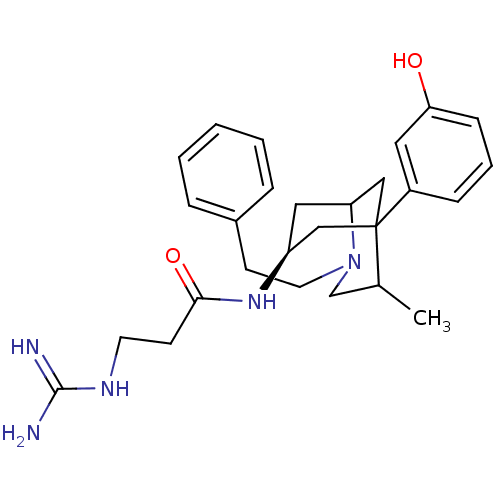 (3-Guanidino-N-[5-(3-hydroxy-phenyl)-4-methyl-2-phe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Ability to displace [3H]DAMGO from Opioid receptor mu 1 of rat brain | J Med Chem 45: 3524-30 (2002) BindingDB Entry DOI: 10.7270/Q2959J8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50116623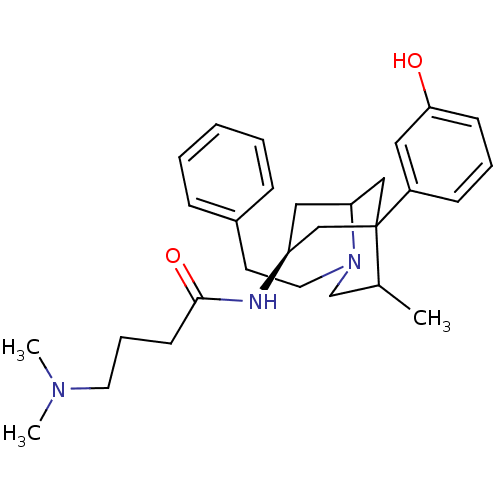 (4-Dimethylamino-N-[5-(3-hydroxy-phenyl)-4-methyl-2...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Ability to displace [3H]U-69593 from Opioid receptor kappa 1 of guinea pig brain | J Med Chem 45: 3524-30 (2002) BindingDB Entry DOI: 10.7270/Q2959J8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50116620 (4-Guanidino-N-[5-(3-hydroxy-phenyl)-4-methyl-2-phe...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 13.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Ability to displace [3H]U-69593 from Opioid receptor kappa 1 of guinea pig brain | J Med Chem 45: 3524-30 (2002) BindingDB Entry DOI: 10.7270/Q2959J8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50116621 (3-[7-Amino-4-methyl-2-(3-phenyl-propyl)-2-aza-bicy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 14.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Ability to displace [3H]DADLE from Opioid receptor delta 1 of rat brain | J Med Chem 45: 3524-30 (2002) BindingDB Entry DOI: 10.7270/Q2959J8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50116624 (3-Guanidino-N-[5-(3-hydroxy-phenyl)-4-methyl-2-phe...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 23.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Ability to displace [3H]U-69593 from Opioid receptor kappa 1 of guinea pig brain | J Med Chem 45: 3524-30 (2002) BindingDB Entry DOI: 10.7270/Q2959J8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50116622 (CHEMBL117457 | N-[5-(3-Hydroxy-phenyl)-4-methyl-2-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Ability to displace [3H]U-69593 from Opioid receptor kappa 1 of guinea pig brain | J Med Chem 45: 3524-30 (2002) BindingDB Entry DOI: 10.7270/Q2959J8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50116624 (3-Guanidino-N-[5-(3-hydroxy-phenyl)-4-methyl-2-phe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 56.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Ability to displace [3H]DADLE from Opioid receptor delta 1 of rat brain | J Med Chem 45: 3524-30 (2002) BindingDB Entry DOI: 10.7270/Q2959J8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50116623 (4-Dimethylamino-N-[5-(3-hydroxy-phenyl)-4-methyl-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Ability to displace [3H]DAMGO from Opioid receptor mu 1 of rat brain | J Med Chem 45: 3524-30 (2002) BindingDB Entry DOI: 10.7270/Q2959J8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM82551 (C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | PubMed | 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Ability to displace [3H]DAMGO from Opioid receptor mu 1 of rat brain | J Med Chem 45: 3524-30 (2002) BindingDB Entry DOI: 10.7270/Q2959J8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM82551 (C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | PubMed | 86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Ability to displace [3H]DADLE from Opioid receptor delta 1 of rat brain | J Med Chem 45: 3524-30 (2002) BindingDB Entry DOI: 10.7270/Q2959J8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50116620 (4-Guanidino-N-[5-(3-hydroxy-phenyl)-4-methyl-2-phe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 87 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Ability to displace [3H]DAMGO from Opioid receptor mu 1 of rat brain | J Med Chem 45: 3524-30 (2002) BindingDB Entry DOI: 10.7270/Q2959J8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50116621 (3-[7-Amino-4-methyl-2-(3-phenyl-propyl)-2-aza-bicy...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 122 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Ability to displace [3H]U-69593 from Opioid receptor kappa 1 of guinea pig brain | J Med Chem 45: 3524-30 (2002) BindingDB Entry DOI: 10.7270/Q2959J8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50116622 (CHEMBL117457 | N-[5-(3-Hydroxy-phenyl)-4-methyl-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 147 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Ability to displace [3H]DAMGO from Opioid receptor mu 1 of rat brain | J Med Chem 45: 3524-30 (2002) BindingDB Entry DOI: 10.7270/Q2959J8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50116621 (3-[7-Amino-4-methyl-2-(3-phenyl-propyl)-2-aza-bicy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 207 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Ability to displace [3H]DAMGO from Opioid receptor mu 1 of rat brain | J Med Chem 45: 3524-30 (2002) BindingDB Entry DOI: 10.7270/Q2959J8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50116621 (3-[7-Amino-4-methyl-2-(3-phenyl-propyl)-2-aza-bicy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 246 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Ability to displace [3H]DADLE from Opioid receptor delta 1 of rat brain | J Med Chem 45: 3524-30 (2002) BindingDB Entry DOI: 10.7270/Q2959J8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50116622 (CHEMBL117457 | N-[5-(3-Hydroxy-phenyl)-4-methyl-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 775 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Ability to displace [3H]DAMGO from Opioid receptor mu 1 of rat brain | J Med Chem 45: 3524-30 (2002) BindingDB Entry DOI: 10.7270/Q2959J8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50116621 (3-[7-Amino-4-methyl-2-(3-phenyl-propyl)-2-aza-bicy...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 893 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Ability to displace [3H]U-69593 from Opioid receptor kappa 1 of guinea pig brain | J Med Chem 45: 3524-30 (2002) BindingDB Entry DOI: 10.7270/Q2959J8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50116623 (4-Dimethylamino-N-[5-(3-hydroxy-phenyl)-4-methyl-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Ability to displace [3H]DADLE from Opioid receptor delta 1 of rat brain | J Med Chem 45: 3524-30 (2002) BindingDB Entry DOI: 10.7270/Q2959J8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50116620 (4-Guanidino-N-[5-(3-hydroxy-phenyl)-4-methyl-2-phe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Ability to displace [3H]DADLE from Opioid receptor delta 1 of rat brain | J Med Chem 45: 3524-30 (2002) BindingDB Entry DOI: 10.7270/Q2959J8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50116622 (CHEMBL117457 | N-[5-(3-Hydroxy-phenyl)-4-methyl-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Ability to displace [3H]DADLE from Opioid receptor delta 1 of rat brain | J Med Chem 45: 3524-30 (2002) BindingDB Entry DOI: 10.7270/Q2959J8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50116622 (CHEMBL117457 | N-[5-(3-Hydroxy-phenyl)-4-methyl-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Ability to displace [3H]DADLE from Opioid receptor delta 1 of rat brain | J Med Chem 45: 3524-30 (2002) BindingDB Entry DOI: 10.7270/Q2959J8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM108434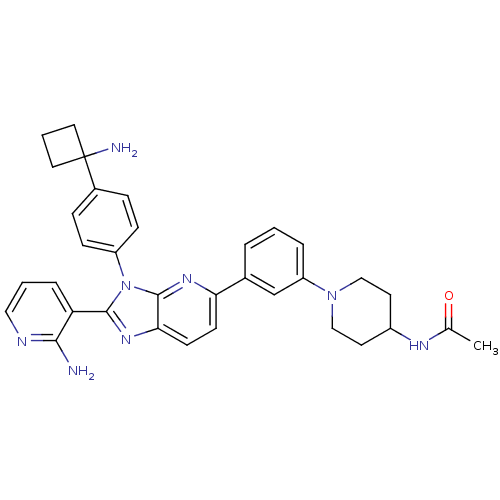 (US8609688, 12) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.44 | n/a | n/a | n/a | n/a | 8.0 | 25 |
ArQule, Inc. US Patent | Assay Description AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen™ (Amplified Lum... | US Patent US8609688 (2013) BindingDB Entry DOI: 10.7270/Q2H41Q2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM108434 (US8609688, 12) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.44 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen (Amplified Luminesce... | US Patent US8962619 (2015) BindingDB Entry DOI: 10.7270/Q2W957W7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM108430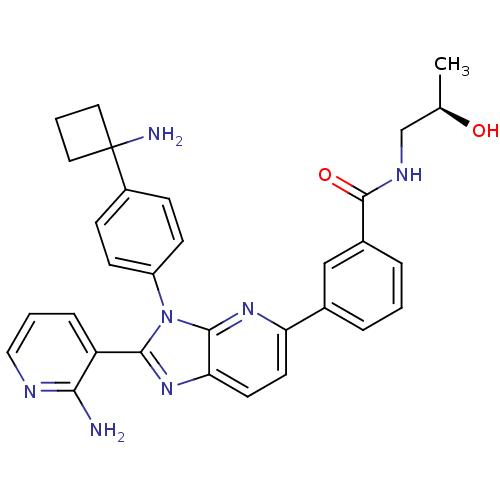 (US8609688, 8) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | 8.0 | 25 |
ArQule, Inc. US Patent | Assay Description AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen™ (Amplified Lum... | US Patent US8609688 (2013) BindingDB Entry DOI: 10.7270/Q2H41Q2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM108430 (US8609688, 8) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen (Amplified Luminesce... | US Patent US8962619 (2015) BindingDB Entry DOI: 10.7270/Q2W957W7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM108428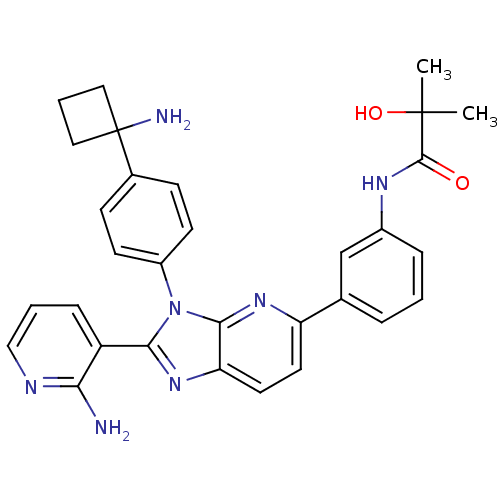 (US8609688, 6) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.63 | n/a | n/a | n/a | n/a | 8.0 | 25 |
ArQule, Inc. US Patent | Assay Description AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen™ (Amplified Lum... | US Patent US8609688 (2013) BindingDB Entry DOI: 10.7270/Q2H41Q2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM108428 (US8609688, 6) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.63 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen (Amplified Luminesce... | US Patent US8962619 (2015) BindingDB Entry DOI: 10.7270/Q2W957W7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM130225 (US8815854, 14) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.69 | n/a | n/a | n/a | n/a | 8.0 | n/a |
ArQule, Inc. US Patent | Assay Description AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen (Amplified Luminesce... | US Patent US8815854 (2014) BindingDB Entry DOI: 10.7270/Q2J67FMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM108426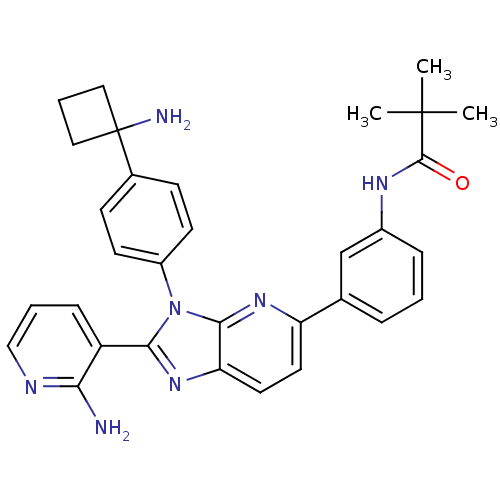 (US8609688, 4) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | 8.0 | 25 |
ArQule, Inc. US Patent | Assay Description AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen™ (Amplified Lum... | US Patent US8609688 (2013) BindingDB Entry DOI: 10.7270/Q2H41Q2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM108426 (US8609688, 4) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen (Amplified Luminesce... | US Patent US8962619 (2015) BindingDB Entry DOI: 10.7270/Q2W957W7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM108440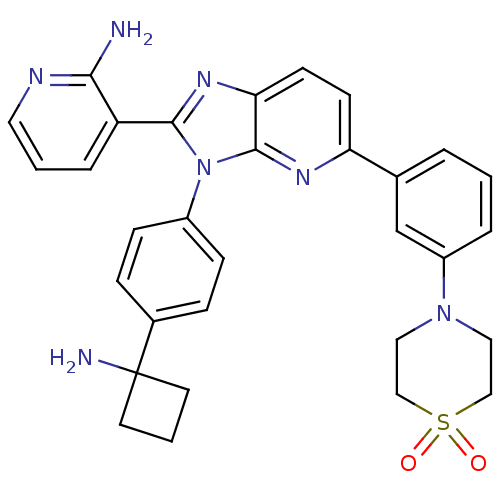 (US8609688, 18) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.79 | n/a | n/a | n/a | n/a | 8.0 | 25 |
ArQule, Inc. US Patent | Assay Description AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen™ (Amplified Lum... | US Patent US8609688 (2013) BindingDB Entry DOI: 10.7270/Q2H41Q2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM108440 (US8609688, 18) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.79 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen (Amplified Luminesce... | US Patent US8962619 (2015) BindingDB Entry DOI: 10.7270/Q2W957W7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM108436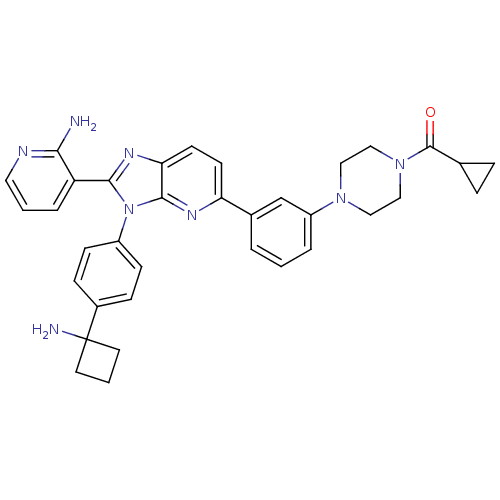 (US8609688, 14) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.84 | n/a | n/a | n/a | n/a | 8.0 | 25 |
ArQule, Inc. US Patent | Assay Description AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen™ (Amplified Lum... | US Patent US8609688 (2013) BindingDB Entry DOI: 10.7270/Q2H41Q2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM108436 (US8609688, 14) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.84 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen (Amplified Luminesce... | US Patent US8962619 (2015) BindingDB Entry DOI: 10.7270/Q2W957W7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50536168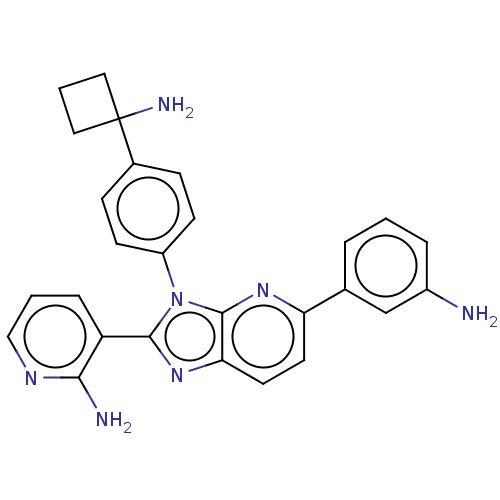 (CHEMBL4574111) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc. Curated by ChEMBL | Assay Description Inhibition of full length unphosphorylated AKT1 (1 to 480 residues) (unknown origin) expressed in baculovirus infected Sf9 insect cells using biotin-... | J Med Chem 59: 6455-69 (2016) Article DOI: 10.1021/acs.jmedchem.6b00619 BindingDB Entry DOI: 10.7270/Q2RN3CCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM130222 (US8815854, 11) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.99 | n/a | n/a | n/a | n/a | 8.0 | n/a |
ArQule, Inc. US Patent | Assay Description AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen (Amplified Luminesce... | US Patent US8815854 (2014) BindingDB Entry DOI: 10.7270/Q2J67FMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM108435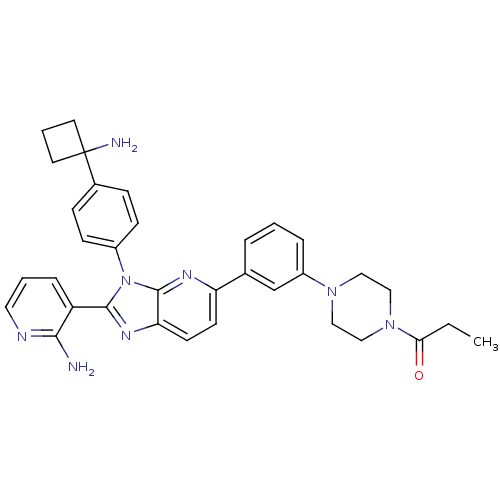 (US8609688, 13) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.15 | n/a | n/a | n/a | n/a | 8.0 | 25 |
ArQule, Inc. US Patent | Assay Description AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen™ (Amplified Lum... | US Patent US8609688 (2013) BindingDB Entry DOI: 10.7270/Q2H41Q2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM108435 (US8609688, 13) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.15 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen (Amplified Luminesce... | US Patent US8962619 (2015) BindingDB Entry DOI: 10.7270/Q2W957W7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50536169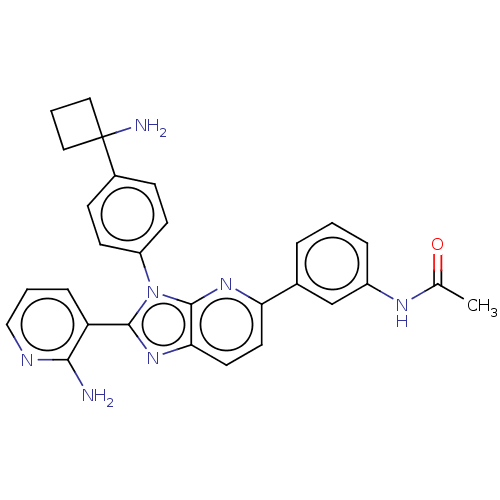 (CHEMBL4519558) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc. Curated by ChEMBL | Assay Description Inhibition of full length active AKT1 (1 to 480 residues) (unknown origin) expressed in baculovirus infected Sf9 insect cells using biotin-GRPRTSSFAE... | J Med Chem 59: 6455-69 (2016) Article DOI: 10.1021/acs.jmedchem.6b00619 BindingDB Entry DOI: 10.7270/Q2RN3CCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM108429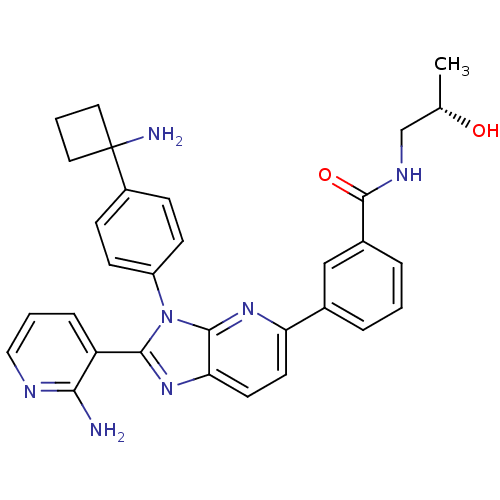 (US8609688, 7) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.45 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen (Amplified Luminesce... | US Patent US8962619 (2015) BindingDB Entry DOI: 10.7270/Q2W957W7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM108429 (US8609688, 7) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.45 | n/a | n/a | n/a | n/a | 8.0 | 25 |
ArQule, Inc. US Patent | Assay Description AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen™ (Amplified Lum... | US Patent US8609688 (2013) BindingDB Entry DOI: 10.7270/Q2H41Q2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50536170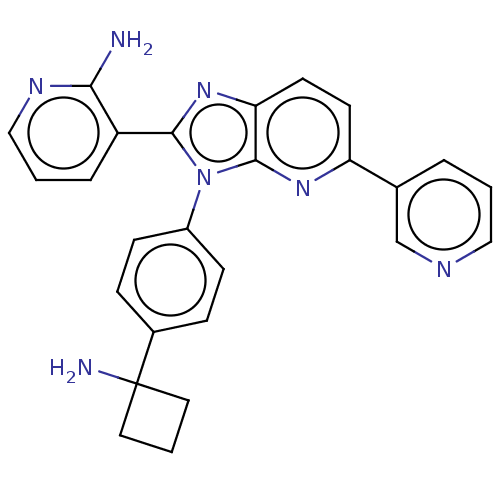 (CHEMBL4544109) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc. Curated by ChEMBL | Assay Description Inhibition of full length unphosphorylated AKT2 (1 to 481 residues) (unknown origin) expressed in baculovirus infected Sf9 insect cells using biotin-... | J Med Chem 59: 6455-69 (2016) Article DOI: 10.1021/acs.jmedchem.6b00619 BindingDB Entry DOI: 10.7270/Q2RN3CCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM108431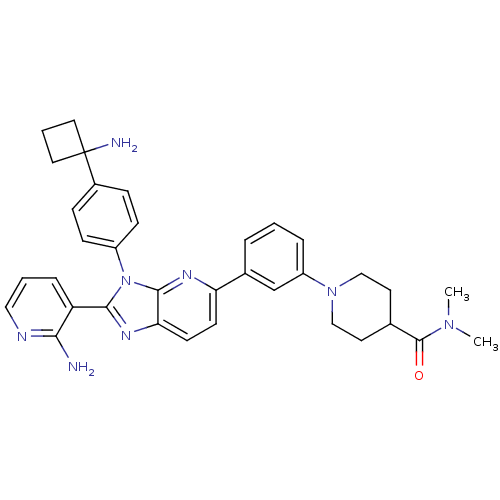 (US8609688, 9) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.51 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen (Amplified Luminesce... | US Patent US8962619 (2015) BindingDB Entry DOI: 10.7270/Q2W957W7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM108431 (US8609688, 9) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.51 | n/a | n/a | n/a | n/a | 8.0 | 25 |
ArQule, Inc. US Patent | Assay Description AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen™ (Amplified Lum... | US Patent US8609688 (2013) BindingDB Entry DOI: 10.7270/Q2H41Q2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50536168 (CHEMBL4574111) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc. Curated by ChEMBL | Assay Description Inhibition of full length active AKT1 (1 to 480 residues) (unknown origin) expressed in baculovirus infected Sf9 insect cells using biotin-GRPRTSSFAE... | J Med Chem 59: 6455-69 (2016) Article DOI: 10.1021/acs.jmedchem.6b00619 BindingDB Entry DOI: 10.7270/Q2RN3CCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50536167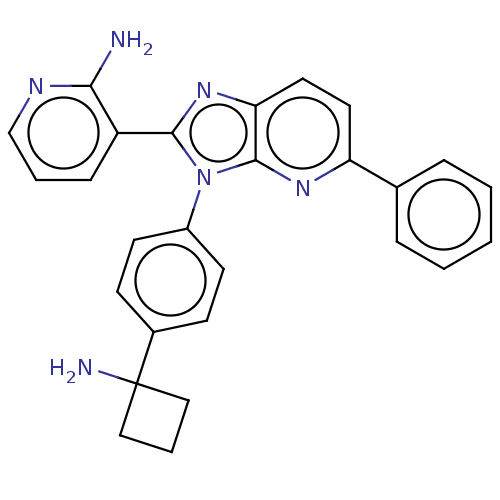 (CHEMBL4523032) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc. Curated by ChEMBL | Assay Description Inhibition of full length unphosphorylated AKT1 (1 to 480 residues) (unknown origin) expressed in baculovirus infected Sf9 insect cells using biotin-... | J Med Chem 59: 6455-69 (2016) Article DOI: 10.1021/acs.jmedchem.6b00619 BindingDB Entry DOI: 10.7270/Q2RN3CCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50536169 (CHEMBL4519558) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc. Curated by ChEMBL | Assay Description Inhibition of full length unphosphorylated AKT1 (1 to 480 residues) (unknown origin) expressed in baculovirus infected Sf9 insect cells using biotin-... | J Med Chem 59: 6455-69 (2016) Article DOI: 10.1021/acs.jmedchem.6b00619 BindingDB Entry DOI: 10.7270/Q2RN3CCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 406 total ) | Next | Last >> |