 Found 148 hits with Last Name = 'narayanan' and Initial = 'a'
Found 148 hits with Last Name = 'narayanan' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50103072
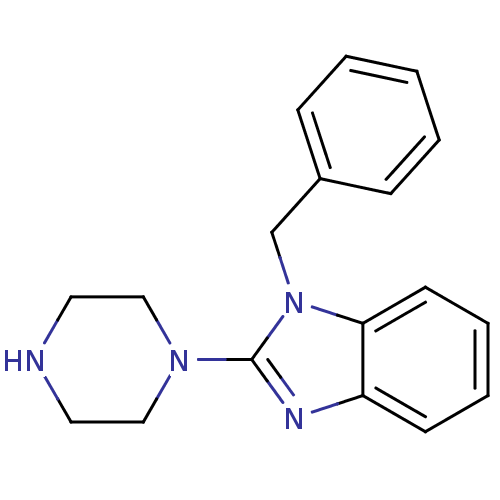
(1-Benzyl-2-piperazin-1-yl-1H-benzoimidazole | CHEM...)Show InChI InChI=1S/C18H20N4/c1-2-6-15(7-3-1)14-22-17-9-5-4-8-16(17)20-18(22)21-12-10-19-11-13-21/h1-9,19H,10-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Louisiana at Monroe
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against human 5-hydroxytryptamine 3A receptor |
Bioorg Med Chem Lett 11: 2133-6 (2001)
BindingDB Entry DOI: 10.7270/Q20G3JGG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50103070

(1-(4-Methoxy-benzyl)-2-piperazin-1-yl-1H-benzoimid...)Show InChI InChI=1S/C19H22N4O/c1-24-16-8-6-15(7-9-16)14-23-18-5-3-2-4-17(18)21-19(23)22-12-10-20-11-13-22/h2-9,20H,10-14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Louisiana at Monroe
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against human 5-hydroxytryptamine 3A receptor |
Bioorg Med Chem Lett 11: 2133-6 (2001)
BindingDB Entry DOI: 10.7270/Q20G3JGG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50103076
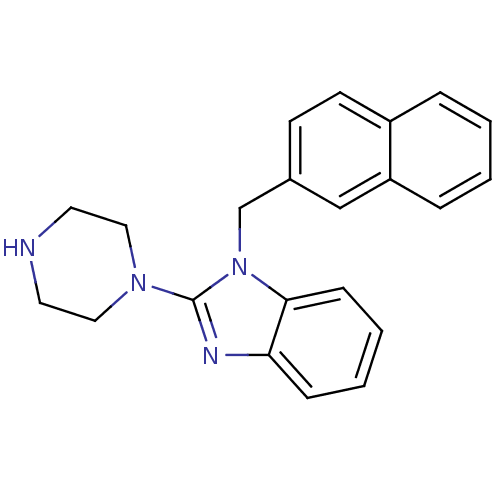
(1-Naphthalen-2-ylmethyl-2-piperazin-1-yl-1H-benzoi...)Show InChI InChI=1S/C22H22N4/c1-2-6-19-15-17(9-10-18(19)5-1)16-26-21-8-4-3-7-20(21)24-22(26)25-13-11-23-12-14-25/h1-10,15,23H,11-14,16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Louisiana at Monroe
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against human 5-hydroxytryptamine 3A receptor |
Bioorg Med Chem Lett 11: 2133-6 (2001)
BindingDB Entry DOI: 10.7270/Q20G3JGG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50103075
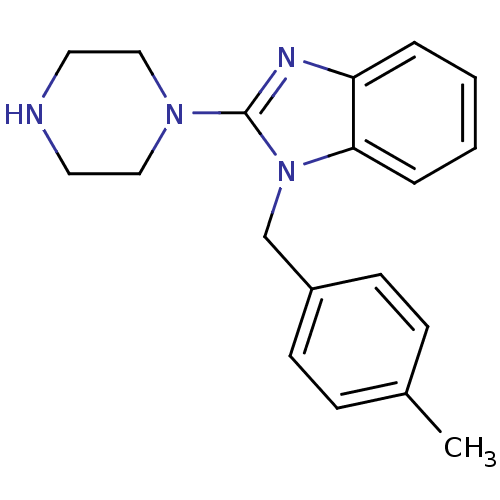
(1-(4-Methyl-benzyl)-2-piperazin-1-yl-1H-benzoimida...)Show InChI InChI=1S/C19H22N4/c1-15-6-8-16(9-7-15)14-23-18-5-3-2-4-17(18)21-19(23)22-12-10-20-11-13-22/h2-9,20H,10-14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Louisiana at Monroe
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against human 5-hydroxytryptamine 3A receptor |
Bioorg Med Chem Lett 11: 2133-6 (2001)
BindingDB Entry DOI: 10.7270/Q20G3JGG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50103069
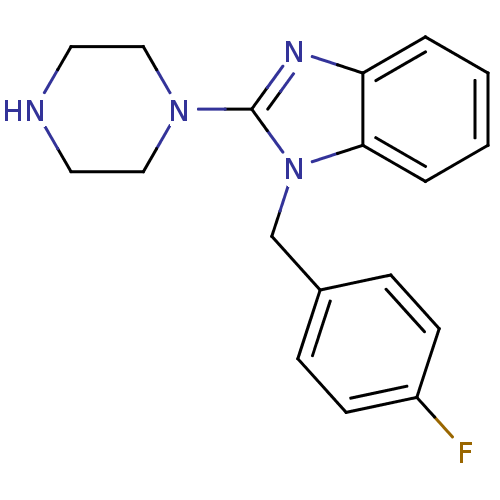
(1-(4-Fluoro-benzyl)-2-piperazin-1-yl-1H-benzoimida...)Show InChI InChI=1S/C18H19FN4/c19-15-7-5-14(6-8-15)13-23-17-4-2-1-3-16(17)21-18(23)22-11-9-20-10-12-22/h1-8,20H,9-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Louisiana at Monroe
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against human 5-hydroxytryptamine 3A receptor |
Bioorg Med Chem Lett 11: 2133-6 (2001)
BindingDB Entry DOI: 10.7270/Q20G3JGG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50103073
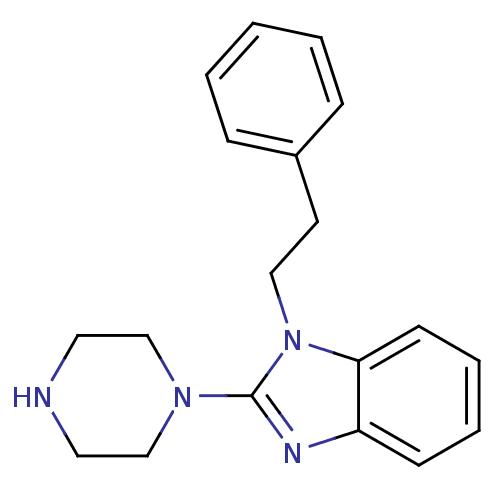
(1-Phenethyl-2-piperazin-1-yl-1H-benzoimidazole | C...)Show InChI InChI=1S/C19H22N4/c1-2-6-16(7-3-1)10-13-23-18-9-5-4-8-17(18)21-19(23)22-14-11-20-12-15-22/h1-9,20H,10-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Louisiana at Monroe
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against human 5-hydroxytryptamine 3A receptor |
Bioorg Med Chem Lett 11: 2133-6 (2001)
BindingDB Entry DOI: 10.7270/Q20G3JGG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50103071
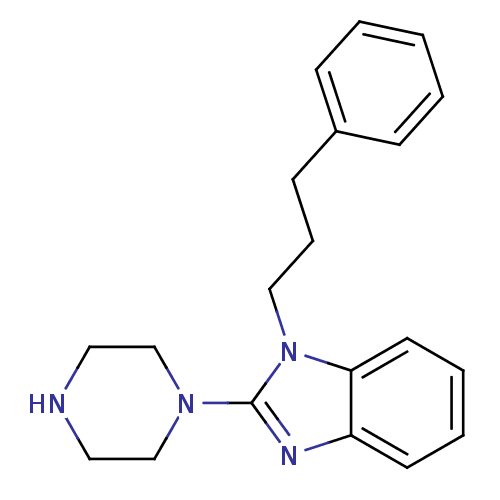
(1-(3-Phenyl-propyl)-2-piperazin-1-yl-1H-benzoimida...)Show InChI InChI=1S/C20H24N4/c1-2-7-17(8-3-1)9-6-14-24-19-11-5-4-10-18(19)22-20(24)23-15-12-21-13-16-23/h1-5,7-8,10-11,21H,6,9,12-16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Louisiana at Monroe
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against human 5-hydroxytryptamine 3A receptor |
Bioorg Med Chem Lett 11: 2133-6 (2001)
BindingDB Entry DOI: 10.7270/Q20G3JGG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50103077
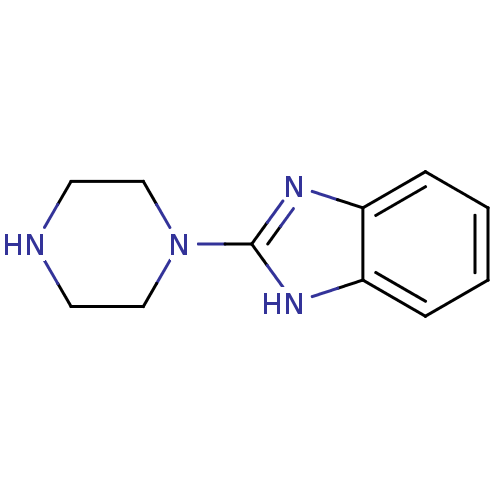
(2-Piperazin-1-yl-1H-benzoimidazole | CHEMBL292066)Show InChI InChI=1S/C11H14N4/c1-2-4-10-9(3-1)13-11(14-10)15-7-5-12-6-8-15/h1-4,12H,5-8H2,(H,13,14) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Louisiana at Monroe
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against human 5-hydroxytryptamine 3A receptor |
Bioorg Med Chem Lett 11: 2133-6 (2001)
BindingDB Entry DOI: 10.7270/Q20G3JGG |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50565931
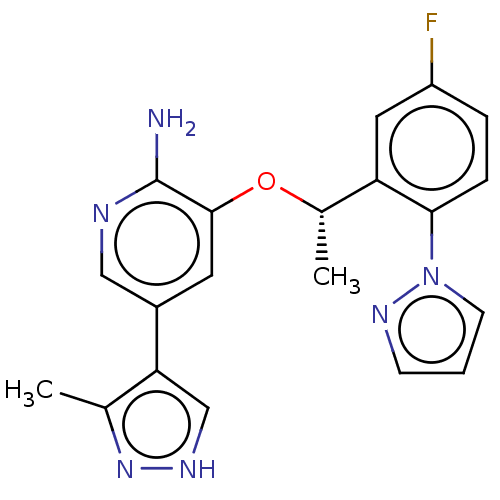
(CHEMBL4787096)Show SMILES C[C@H](Oc1cc(cnc1N)-c1c[nH]nc1C)c1cc(F)ccc1-n1cccn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human ERG by dofetilide fluorescence polarization binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01518
BindingDB Entry DOI: 10.7270/Q2QC079V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50565919
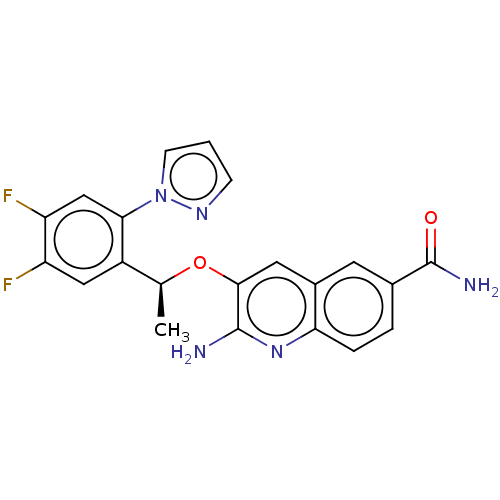
(CHEMBL4794362)Show SMILES C[C@H](Oc1cc2cc(ccc2nc1N)C(N)=O)c1cc(F)c(F)cc1-n1cccn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human ERG by dofetilide fluorescence polarization binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01518
BindingDB Entry DOI: 10.7270/Q2QC079V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50565920
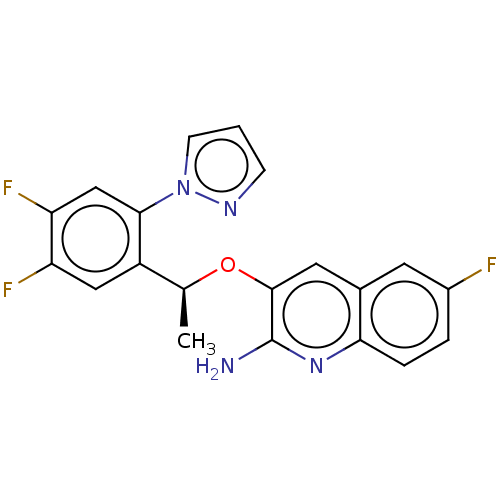
(CHEMBL4784517)Show SMILES C[C@H](Oc1cc2cc(F)ccc2nc1N)c1cc(F)c(F)cc1-n1cccn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human ERG by dofetilide fluorescence polarization binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01518
BindingDB Entry DOI: 10.7270/Q2QC079V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50565925
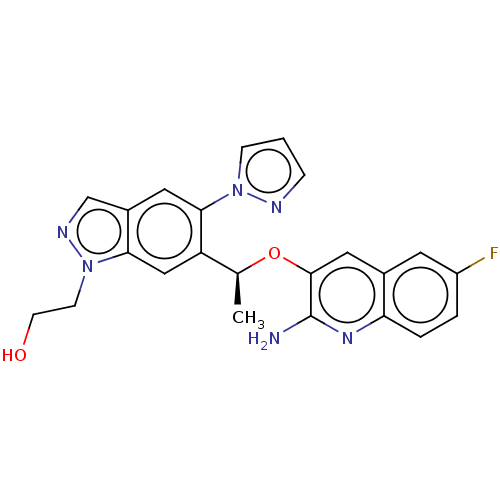
(CHEMBL4778780)Show SMILES C[C@H](Oc1cc2cc(F)ccc2nc1N)c1cc2n(CCO)ncc2cc1-n1cccn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human ERG by dofetilide fluorescence polarization binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01518
BindingDB Entry DOI: 10.7270/Q2QC079V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50565921
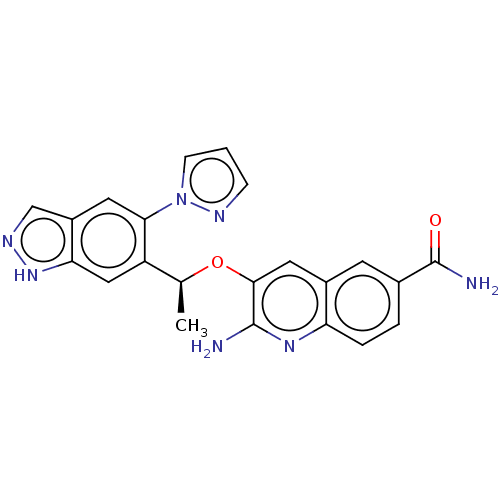
(CHEMBL4781765)Show SMILES C[C@H](Oc1cc2cc(ccc2nc1N)C(N)=O)c1cc2[nH]ncc2cc1-n1cccn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human ERG by dofetilide fluorescence polarization binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01518
BindingDB Entry DOI: 10.7270/Q2QC079V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50565918

(CHEMBL4778108)Show SMILES C[C@H](Oc1cc2cc(ccc2nc1N)C#N)c1cc(F)c(F)cc1-n1cccn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human ERG by dofetilide fluorescence polarization binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01518
BindingDB Entry DOI: 10.7270/Q2QC079V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50565917
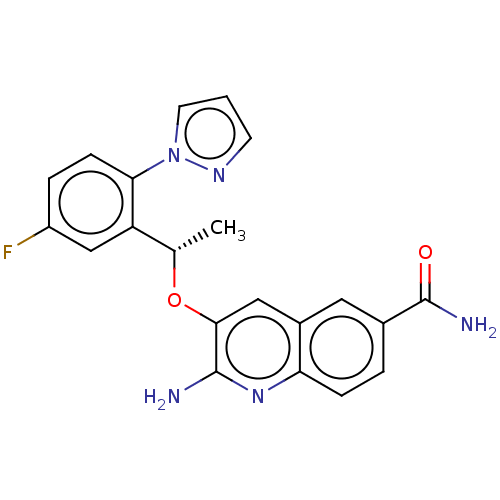
(CHEMBL4783261)Show SMILES C[C@H](Oc1cc2cc(ccc2nc1N)C(N)=O)c1cc(F)ccc1-n1cccn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human ERG by dofetilide fluorescence polarization binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01518
BindingDB Entry DOI: 10.7270/Q2QC079V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50565922

(CHEMBL4797664)Show SMILES C[C@H](Oc1cc2cc(ccc2nc1N)C(N)=O)c1ncc(F)cc1-n1cccn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human ERG by dofetilide fluorescence polarization binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01518
BindingDB Entry DOI: 10.7270/Q2QC079V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50565929

(Pf-07059013)Show SMILES C[C@H](Oc1cc2cc(F)ccc2nc1N)c1[nH]c(=O)ccc1-n1cccn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| >3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human ERG by dofetilide fluorescence polarization binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01518
BindingDB Entry DOI: 10.7270/Q2QC079V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50565928

(CHEMBL4785484)Show SMILES C[C@H](Oc1cc2cc(F)cc(F)c2nc1N)c1cc(ccc1-n1cccn1)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human ERG by dofetilide fluorescence polarization binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01518
BindingDB Entry DOI: 10.7270/Q2QC079V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50565927

(CHEMBL4779453)Show SMILES C[C@H](Oc1cc2cc(F)ccc2nc1N)c1cc(ccc1-n1cccn1)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human ERG by dofetilide fluorescence polarization binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01518
BindingDB Entry DOI: 10.7270/Q2QC079V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50565926

(CHEMBL4778770)Show SMILES C[C@H](Oc1cc2cc(F)ccc2nc1N)c1cc2n(CC(O)=O)ncc2cc1-n1cccn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human ERG by dofetilide fluorescence polarization binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01518
BindingDB Entry DOI: 10.7270/Q2QC079V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50565923

(CHEMBL4762748)Show SMILES C[C@H](Oc1cc2cc(cc(F)c2nc1N)C(N)=O)c1ncc(F)cc1-n1cccn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human ERG by dofetilide fluorescence polarization binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01518
BindingDB Entry DOI: 10.7270/Q2QC079V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50565916

(CHEMBL4777878)Show SMILES C[C@H](Oc1cc2cc(ccc2nc1N)C#N)c1cc(F)ccc1-n1cccn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human ERG by dofetilide fluorescence polarization binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01518
BindingDB Entry DOI: 10.7270/Q2QC079V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50565924

(CHEMBL4795396)Show SMILES C[C@H](Oc1cc2cc(ccc2nc1N)C(N)=O)c1cc2n(CCO)ncc2cc1-n1cccn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human ERG by dofetilide fluorescence polarization binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01518
BindingDB Entry DOI: 10.7270/Q2QC079V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50565930

(CHEMBL4796436)Show SMILES C[C@H](Oc1cc2cc(F)cc(F)c2nc1N)c1[nH]c(=O)ccc1-n1cccn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human ERG by dofetilide fluorescence polarization binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01518
BindingDB Entry DOI: 10.7270/Q2QC079V |
More data for this
Ligand-Target Pair | |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM50232534
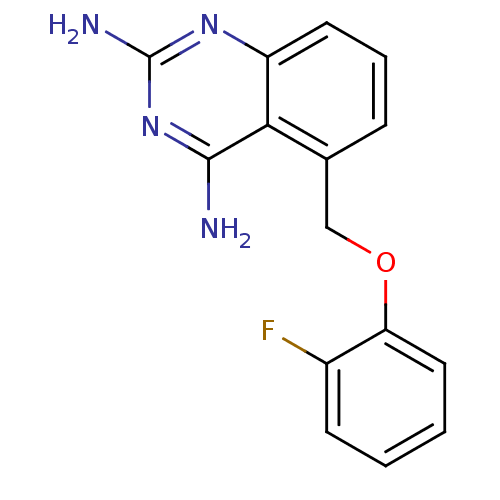
(5-((2-fluorophenoxy)methyl)quinazoline-2,4-diamine...)Show InChI InChI=1S/C15H13FN4O/c16-10-5-1-2-7-12(10)21-8-9-4-3-6-11-13(9)14(17)20-15(18)19-11/h1-7H,8H2,(H4,17,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DcpS assessed as increase in SMN2 promoter activity |
J Med Chem 60: 3094-3108 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00124
BindingDB Entry DOI: 10.7270/Q2251MG2 |
More data for this
Ligand-Target Pair | |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM50232526
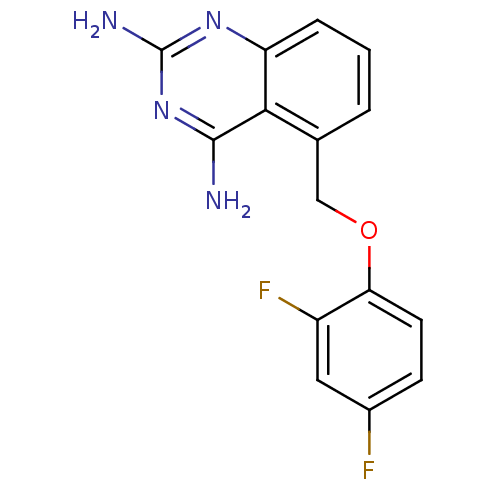
(5-((2,4-difluorophenoxy)methyl)quinazoline-2,4-dia...)Show InChI InChI=1S/C15H12F2N4O/c16-9-4-5-12(10(17)6-9)22-7-8-2-1-3-11-13(8)14(18)21-15(19)20-11/h1-6H,7H2,(H4,18,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DcpS assessed as increase in SMN2 promoter activity |
J Med Chem 60: 3094-3108 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00124
BindingDB Entry DOI: 10.7270/Q2251MG2 |
More data for this
Ligand-Target Pair | |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM50232538
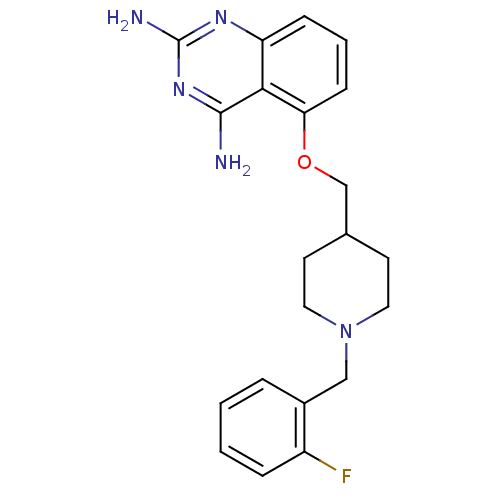
(5-((1-(2-fluorobenzyl)piperidin-4-yl)methoxy)quina...)Show InChI InChI=1S/C21H24FN5O/c22-16-5-2-1-4-15(16)12-27-10-8-14(9-11-27)13-28-18-7-3-6-17-19(18)20(23)26-21(24)25-17/h1-7,14H,8-13H2,(H4,23,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DcpS assessed as increase in SMN2 promoter activity |
J Med Chem 60: 3094-3108 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00124
BindingDB Entry DOI: 10.7270/Q2251MG2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM50237216
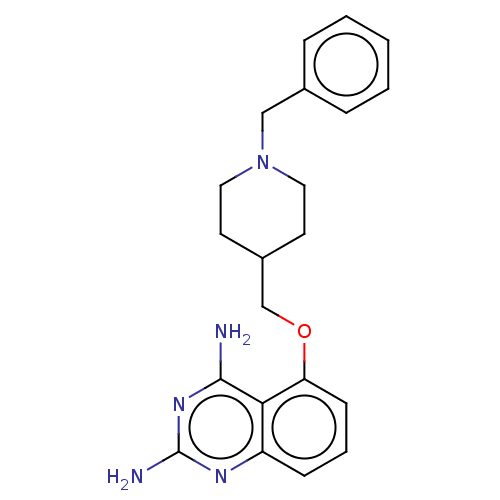
(CHEMBL4080254)Show InChI InChI=1S/C21H25N5O/c22-20-19-17(24-21(23)25-20)7-4-8-18(19)27-14-16-9-11-26(12-10-16)13-15-5-2-1-3-6-15/h1-8,16H,9-14H2,(H4,22,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DcpS assessed as increase in SMN2 promoter activity |
J Med Chem 60: 3094-3108 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00124
BindingDB Entry DOI: 10.7270/Q2251MG2 |
More data for this
Ligand-Target Pair | |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM50237201
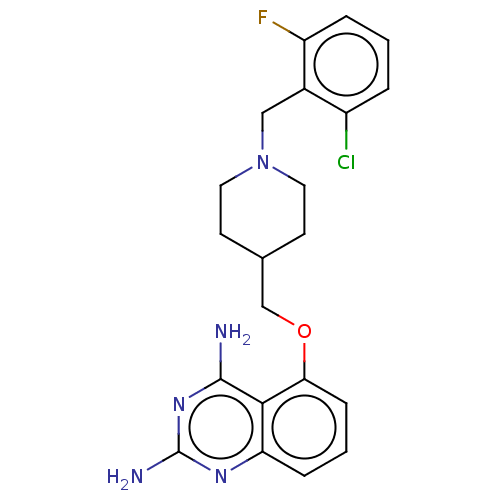
(CHEMBL4082618)Show SMILES Nc1nc(N)c2c(OCC3CCN(Cc4c(F)cccc4Cl)CC3)cccc2n1 Show InChI InChI=1S/C21H23ClFN5O/c22-15-3-1-4-16(23)14(15)11-28-9-7-13(8-10-28)12-29-18-6-2-5-17-19(18)20(24)27-21(25)26-17/h1-6,13H,7-12H2,(H4,24,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DcpS assessed as increase in SMN2 promoter activity |
J Med Chem 60: 3094-3108 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00124
BindingDB Entry DOI: 10.7270/Q2251MG2 |
More data for this
Ligand-Target Pair | |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM50232589
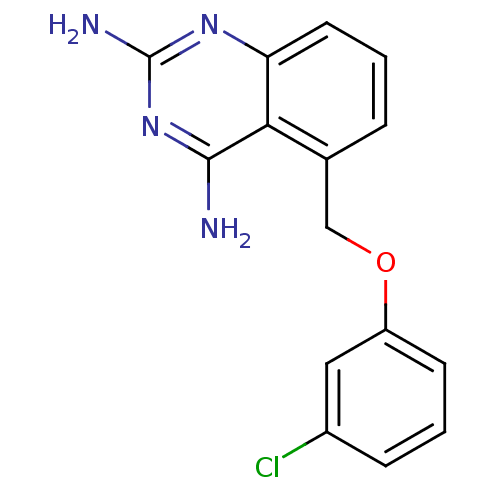
(5-((3-chlorophenoxy)methyl)quinazoline-2,4-diamine...)Show InChI InChI=1S/C15H13ClN4O/c16-10-4-2-5-11(7-10)21-8-9-3-1-6-12-13(9)14(17)20-15(18)19-12/h1-7H,8H2,(H4,17,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DcpS assessed as increase in SMN2 promoter activity |
J Med Chem 60: 3094-3108 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00124
BindingDB Entry DOI: 10.7270/Q2251MG2 |
More data for this
Ligand-Target Pair | |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM50237200
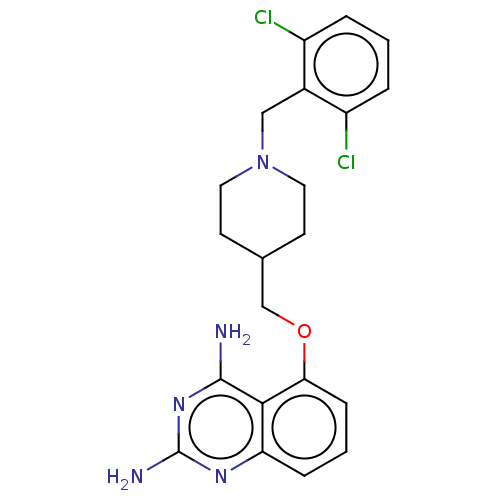
(CHEMBL4072132)Show SMILES Nc1nc(N)c2c(OCC3CCN(Cc4c(Cl)cccc4Cl)CC3)cccc2n1 Show InChI InChI=1S/C21H23Cl2N5O/c22-15-3-1-4-16(23)14(15)11-28-9-7-13(8-10-28)12-29-18-6-2-5-17-19(18)20(24)27-21(25)26-17/h1-6,13H,7-12H2,(H4,24,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0690 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DcpS assessed as increase in SMN2 promoter activity |
J Med Chem 60: 3094-3108 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00124
BindingDB Entry DOI: 10.7270/Q2251MG2 |
More data for this
Ligand-Target Pair | |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM36530
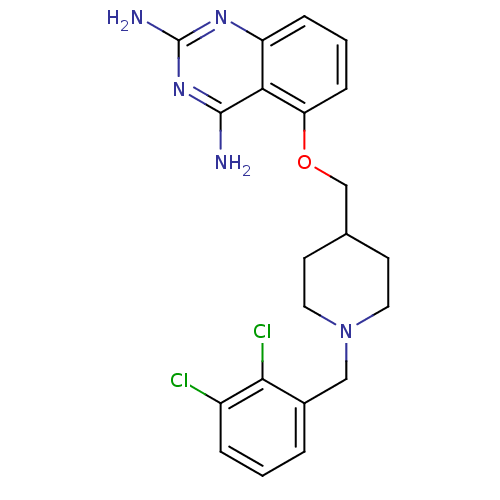
(D157493)Show SMILES Nc1nc(N)c2c(OCC3CCN(Cc4cccc(Cl)c4Cl)CC3)cccc2n1 Show InChI InChI=1S/C21H23Cl2N5O/c22-15-4-1-3-14(19(15)23)11-28-9-7-13(8-10-28)12-29-17-6-2-5-16-18(17)20(24)27-21(25)26-16/h1-6,13H,7-12H2,(H4,24,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards 5-HT3 receptor in rat was evaluated |
J Med Chem 60: 3094-3108 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00124
BindingDB Entry DOI: 10.7270/Q2251MG2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM50237203
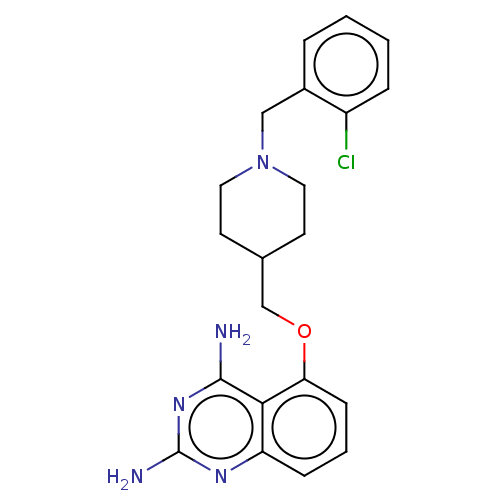
(CHEMBL250072)Show SMILES Nc1nc(N)c2c(OCC3CCN(Cc4ccccc4Cl)CC3)cccc2n1 Show InChI InChI=1S/C21H24ClN5O/c22-16-5-2-1-4-15(16)12-27-10-8-14(9-11-27)13-28-18-7-3-6-17-19(18)20(23)26-21(24)25-17/h1-7,14H,8-13H2,(H4,23,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DcpS assessed as increase in SMN2 promoter activity |
J Med Chem 60: 3094-3108 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00124
BindingDB Entry DOI: 10.7270/Q2251MG2 |
More data for this
Ligand-Target Pair | |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM50237199
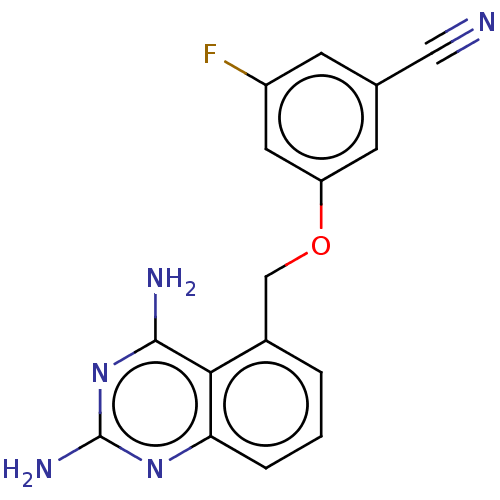
(CHEMBL4077061)Show InChI InChI=1S/C16H12FN5O/c17-11-4-9(7-18)5-12(6-11)23-8-10-2-1-3-13-14(10)15(19)22-16(20)21-13/h1-6H,8H2,(H4,19,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
In vitro reversal of vecuronium-induced block in isolated guinea pig hemi-diaphragm. |
J Med Chem 60: 3094-3108 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00124
BindingDB Entry DOI: 10.7270/Q2251MG2 |
More data for this
Ligand-Target Pair | |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM50237205
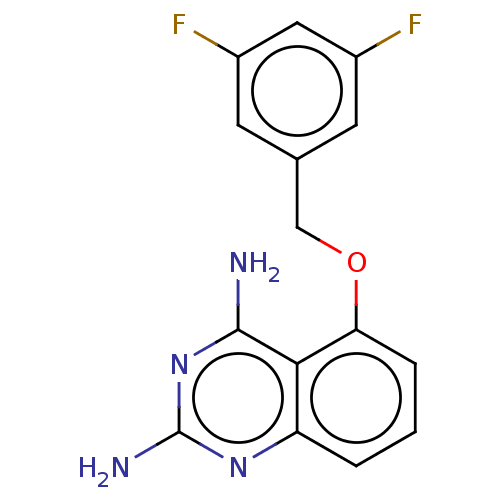
(CHEMBL398675)Show InChI InChI=1S/C15H12F2N4O/c16-9-4-8(5-10(17)6-9)7-22-12-3-1-2-11-13(12)14(18)21-15(19)20-11/h1-6H,7H2,(H4,18,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DcpS assessed as increase in SMN2 promoter activity |
J Med Chem 60: 3094-3108 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00124
BindingDB Entry DOI: 10.7270/Q2251MG2 |
More data for this
Ligand-Target Pair | |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM50237209
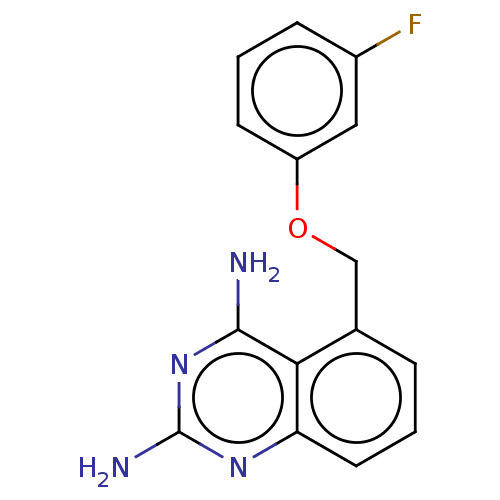
(CHEMBL4062544)Show InChI InChI=1S/C15H13FN4O/c16-10-4-2-5-11(7-10)21-8-9-3-1-6-12-13(9)14(17)20-15(18)19-12/h1-7H,8H2,(H4,17,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DcpS assessed as increase in SMN2 promoter activity |
J Med Chem 60: 3094-3108 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00124
BindingDB Entry DOI: 10.7270/Q2251MG2 |
More data for this
Ligand-Target Pair | |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM50237211
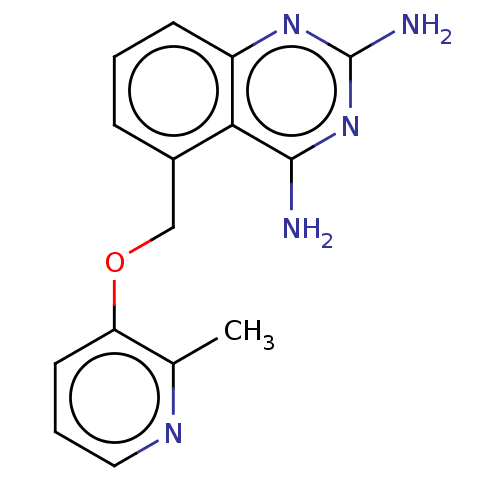
(CHEMBL4061457)Show InChI InChI=1S/C15H15N5O/c1-9-12(6-3-7-18-9)21-8-10-4-2-5-11-13(10)14(16)20-15(17)19-11/h2-7H,8H2,1H3,(H4,16,17,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DcpS assessed as increase in SMN2 promoter activity |
J Med Chem 60: 3094-3108 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00124
BindingDB Entry DOI: 10.7270/Q2251MG2 |
More data for this
Ligand-Target Pair | |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM50237210
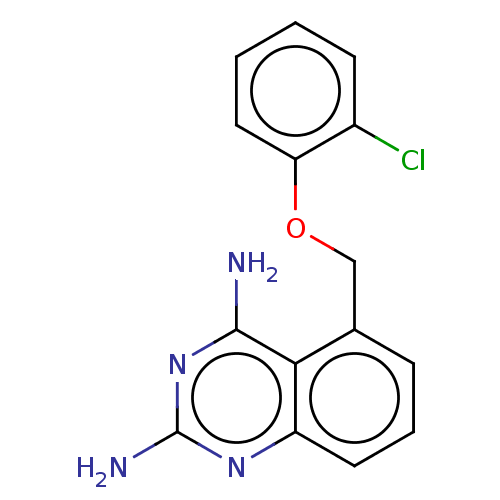
(CHEMBL399673)Show InChI InChI=1S/C15H13ClN4O/c16-10-5-1-2-7-12(10)21-8-9-4-3-6-11-13(9)14(17)20-15(18)19-11/h1-7H,8H2,(H4,17,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DcpS assessed as increase in SMN2 promoter activity |
J Med Chem 60: 3094-3108 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00124
BindingDB Entry DOI: 10.7270/Q2251MG2 |
More data for this
Ligand-Target Pair | |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM36534
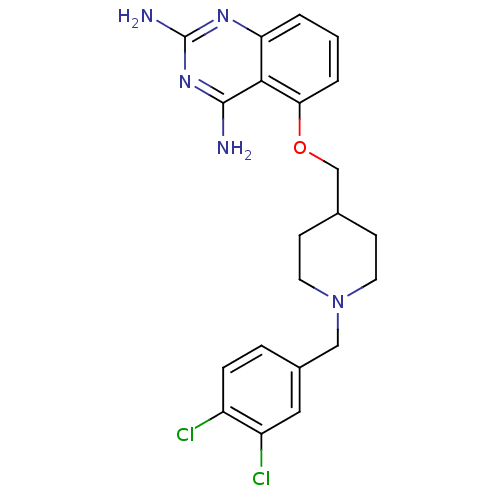
(D156095)Show SMILES Nc1nc(N)c2c(OCC3CCN(Cc4ccc(Cl)c(Cl)c4)CC3)cccc2n1 Show InChI InChI=1S/C21H23Cl2N5O/c22-15-5-4-14(10-16(15)23)11-28-8-6-13(7-9-28)12-29-18-3-1-2-17-19(18)20(24)27-21(25)26-17/h1-5,10,13H,6-9,11-12H2,(H4,24,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DcpS assessed as increase in SMN2 promoter activity |
J Med Chem 60: 3094-3108 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00124
BindingDB Entry DOI: 10.7270/Q2251MG2 |
More data for this
Ligand-Target Pair | |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM50237217
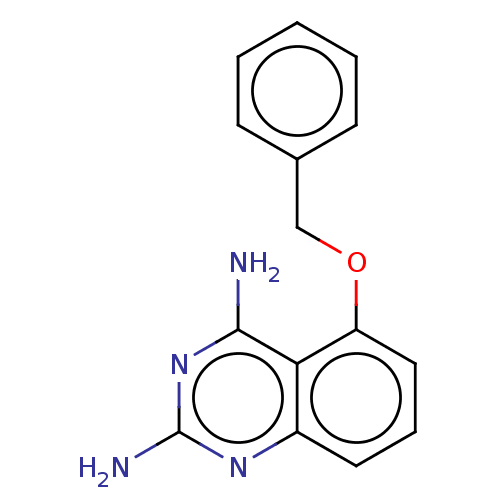
(CHEMBL342595)Show InChI InChI=1S/C15H14N4O/c16-14-13-11(18-15(17)19-14)7-4-8-12(13)20-9-10-5-2-1-3-6-10/h1-8H,9H2,(H4,16,17,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards rat 5-hydroxytryptamine 3 receptor was evaluated |
J Med Chem 60: 3094-3108 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00124
BindingDB Entry DOI: 10.7270/Q2251MG2 |
More data for this
Ligand-Target Pair | |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM50237202
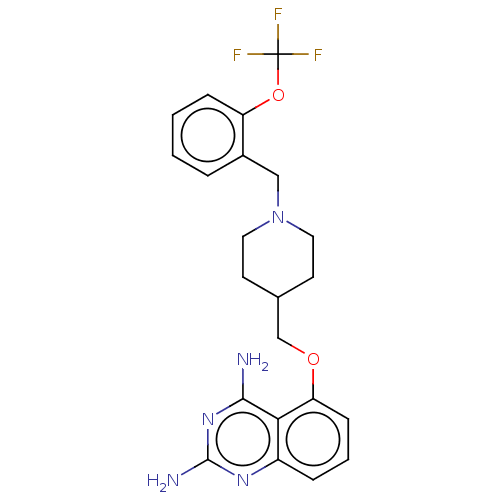
(CHEMBL4103454)Show SMILES Nc1nc(N)c2c(OCC3CCN(Cc4ccccc4OC(F)(F)F)CC3)cccc2n1 Show InChI InChI=1S/C22H24F3N5O2/c23-22(24,25)32-17-6-2-1-4-15(17)12-30-10-8-14(9-11-30)13-31-18-7-3-5-16-19(18)20(26)29-21(27)28-16/h1-7,14H,8-13H2,(H4,26,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DcpS assessed as increase in SMN2 promoter activity |
J Med Chem 60: 3094-3108 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00124
BindingDB Entry DOI: 10.7270/Q2251MG2 |
More data for this
Ligand-Target Pair | |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM50237198
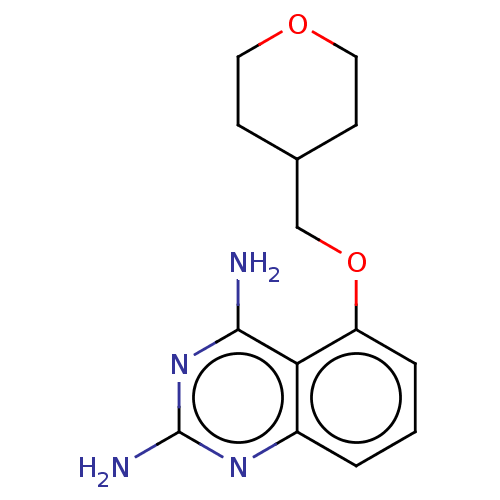
(CHEMBL4068466)Show InChI InChI=1S/C14H18N4O2/c15-13-12-10(17-14(16)18-13)2-1-3-11(12)20-8-9-4-6-19-7-5-9/h1-3,9H,4-8H2,(H4,15,16,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DcpS assessed as increase in SMN2 promoter activity |
J Med Chem 60: 3094-3108 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00124
BindingDB Entry DOI: 10.7270/Q2251MG2 |
More data for this
Ligand-Target Pair | |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM50237218

(CHEMBL4072481)Show InChI InChI=1S/C14H18N4O2/c15-13-12-9(8-20-10-4-6-19-7-5-10)2-1-3-11(12)17-14(16)18-13/h1-3,10H,4-8H2,(H4,15,16,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DcpS assessed as increase in SMN2 promoter activity |
J Med Chem 60: 3094-3108 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00124
BindingDB Entry DOI: 10.7270/Q2251MG2 |
More data for this
Ligand-Target Pair | |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM50232566
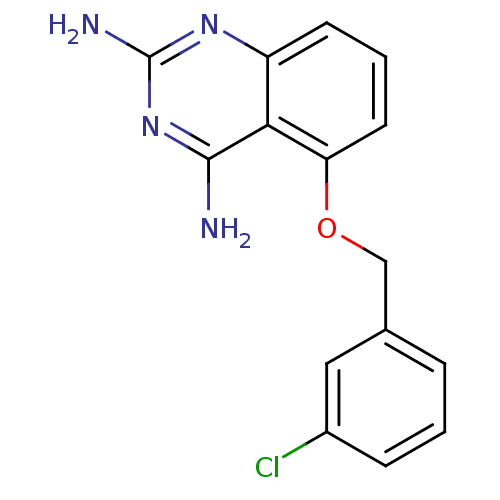
(5-(3-chlorobenzyloxy)quinazoline-2,4-diamine | CHE...)Show InChI InChI=1S/C15H13ClN4O/c16-10-4-1-3-9(7-10)8-21-12-6-2-5-11-13(12)14(17)20-15(18)19-11/h1-7H,8H2,(H4,17,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DcpS assessed as increase in SMN2 promoter activity |
J Med Chem 60: 3094-3108 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00124
BindingDB Entry DOI: 10.7270/Q2251MG2 |
More data for this
Ligand-Target Pair | |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM50237204

(CHEMBL343543)Show InChI InChI=1S/C9H10N4O/c1-14-6-4-2-3-5-7(6)8(10)13-9(11)12-5/h2-4H,1H3,(H4,10,11,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DcpS assessed as increase in SMN2 promoter activity |
J Med Chem 60: 3094-3108 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00124
BindingDB Entry DOI: 10.7270/Q2251MG2 |
More data for this
Ligand-Target Pair | |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM50237206
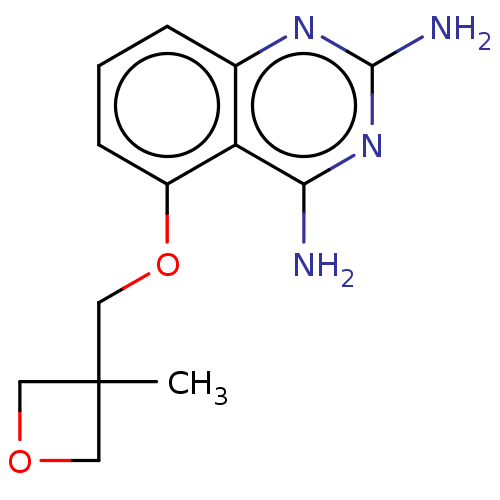
(CHEMBL4075292)Show InChI InChI=1S/C13H16N4O2/c1-13(5-18-6-13)7-19-9-4-2-3-8-10(9)11(14)17-12(15)16-8/h2-4H,5-7H2,1H3,(H4,14,15,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
In vitro reversal of vecuronium-induced block in isolated guinea pig hemi-diaphragm. |
J Med Chem 60: 3094-3108 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00124
BindingDB Entry DOI: 10.7270/Q2251MG2 |
More data for this
Ligand-Target Pair | |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM50237212
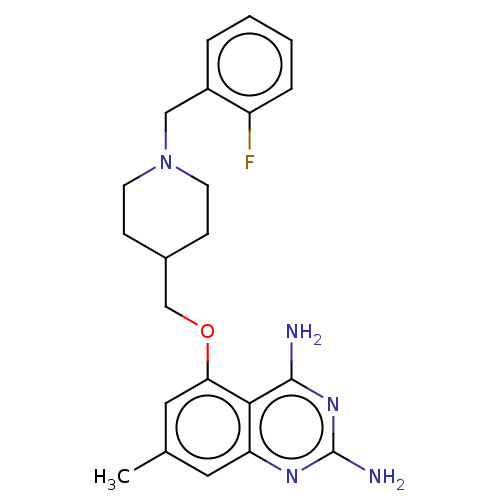
(CHEMBL4100442)Show SMILES Cc1cc(OCC2CCN(Cc3ccccc3F)CC2)c2c(N)nc(N)nc2c1 Show InChI InChI=1S/C22H26FN5O/c1-14-10-18-20(21(24)27-22(25)26-18)19(11-14)29-13-15-6-8-28(9-7-15)12-16-4-2-3-5-17(16)23/h2-5,10-11,15H,6-9,12-13H2,1H3,(H4,24,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DcpS assessed as increase in SMN2 promoter activity |
J Med Chem 60: 3094-3108 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00124
BindingDB Entry DOI: 10.7270/Q2251MG2 |
More data for this
Ligand-Target Pair | |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM50237214
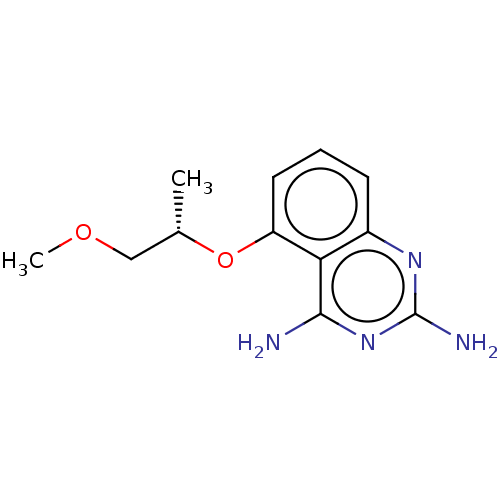
(CHEMBL4084161)Show InChI InChI=1S/C12H16N4O2/c1-7(6-17-2)18-9-5-3-4-8-10(9)11(13)16-12(14)15-8/h3-5,7H,6H2,1-2H3,(H4,13,14,15,16)/t7-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards 5-HT3 receptor in rat was evaluated |
J Med Chem 60: 3094-3108 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00124
BindingDB Entry DOI: 10.7270/Q2251MG2 |
More data for this
Ligand-Target Pair | |
m7GpppX diphosphatase
(Homo sapiens (Human)) | BDBM50237208
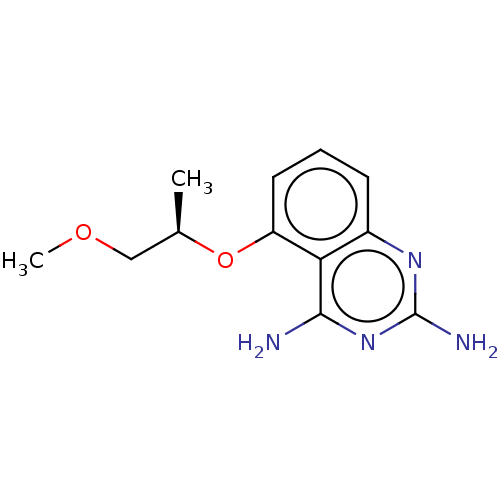
(CHEMBL4090641)Show InChI InChI=1S/C12H16N4O2/c1-7(6-17-2)18-9-5-3-4-8-10(9)11(13)16-12(14)15-8/h3-5,7H,6H2,1-2H3,(H4,13,14,15,16)/t7-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DcpS assessed as increase in SMN2 promoter activity |
J Med Chem 60: 3094-3108 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00124
BindingDB Entry DOI: 10.7270/Q2251MG2 |
More data for this
Ligand-Target Pair | |
Lysophosphatidic acid receptor 1
(Homo sapiens (Human)) | BDBM50398093
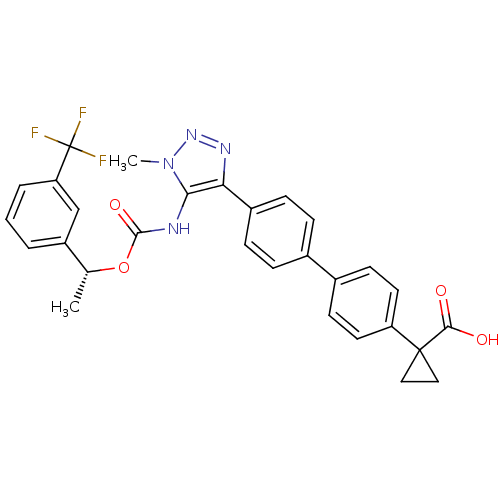
(CHEMBL2182046 | US9321738, 6)Show SMILES C[C@@H](OC(=O)Nc1c(nnn1C)-c1ccc(cc1)-c1ccc(cc1)C1(CC1)C(O)=O)c1cccc(c1)C(F)(F)F |r| Show InChI InChI=1S/C29H25F3N4O4/c1-17(21-4-3-5-23(16-21)29(30,31)32)40-27(39)33-25-24(34-35-36(25)2)20-8-6-18(7-9-20)19-10-12-22(13-11-19)28(14-15-28)26(37)38/h3-13,16-17H,14-15H2,1-2H3,(H,33,39)(H,37,38)/t17-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant LPA1 expressed in chem-1 cells assessed as inhibition of LPA-induced intracellular calcium mobilization incu... |
J Med Chem 55: 7920-39 (2012)
Article DOI: 10.1021/jm301022v
BindingDB Entry DOI: 10.7270/Q26974Q9 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data