

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor VII/Tissue factor (Homo sapiens (Human)) | BDBM50205841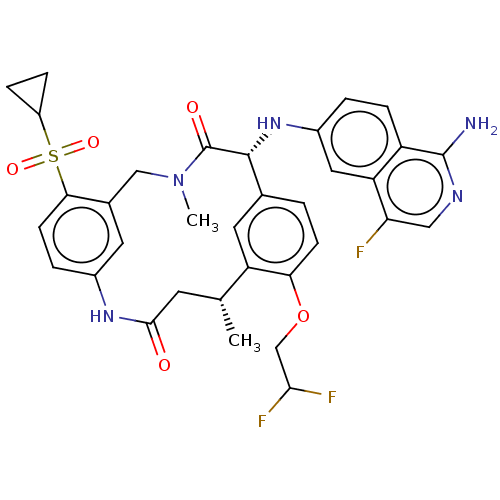 (CHEMBL3898956) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of recombinant human factor-7a/TF using S2288 as substrate measured after 60 mins at 37 degC | ACS Med Chem Lett 8: 67-72 (2017) Article DOI: 10.1021/acsmedchemlett.6b00375 BindingDB Entry DOI: 10.7270/Q20P120Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18699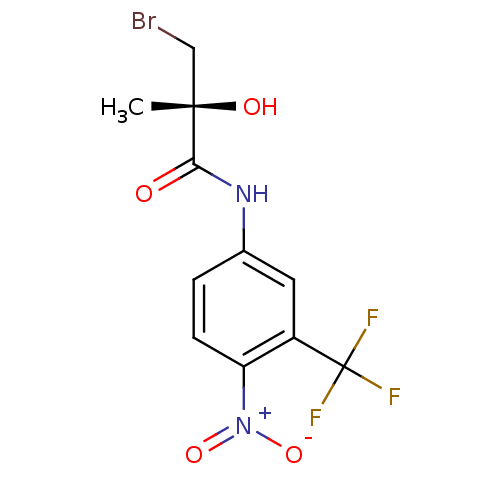 ((2R)-3-bromo-2-hydroxy-2-methyl-N-[4-nitro-3-(trif...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GTx, Inc. Curated by ChEMBL | Assay Description Agonist activity at androgen receptor (unknown origin) | J Med Chem 52: 3597-617 (2009) Article DOI: 10.1021/jm900280m BindingDB Entry DOI: 10.7270/Q2GH9HWD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50063581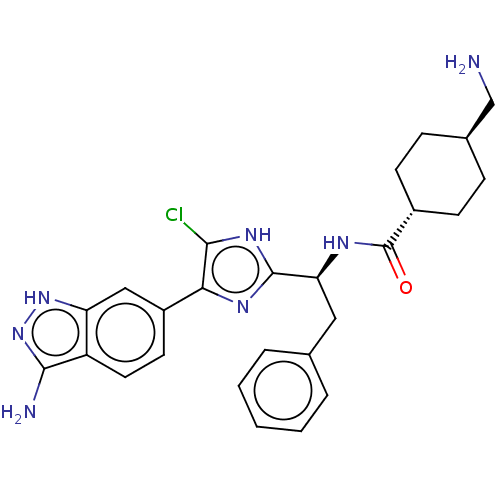 (CHEMBL3398612) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Competitive inhibition of human coagulation factor 11a assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten eq... | Bioorg Med Chem 24: 2257-72 (2016) Article DOI: 10.1016/j.bmc.2016.03.062 BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50136575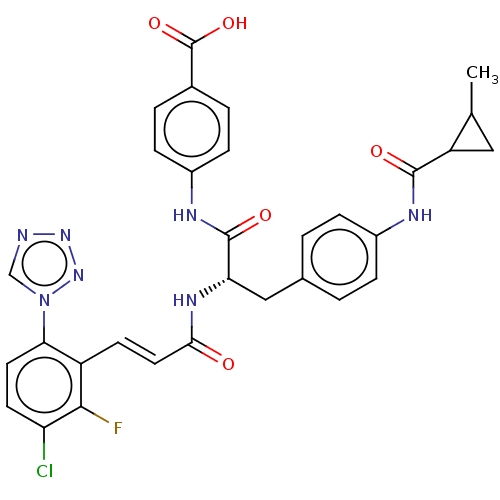 (CHEMBL3752610) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 11a assessed as substrate hydrolysis to p-nitroaniline incubated for 10 to 120 mins by spectrophotometry analy... | Bioorg Med Chem Lett 26: 472-8 (2016) Article DOI: 10.1016/j.bmcl.2015.11.089 BindingDB Entry DOI: 10.7270/Q27H1MFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50215713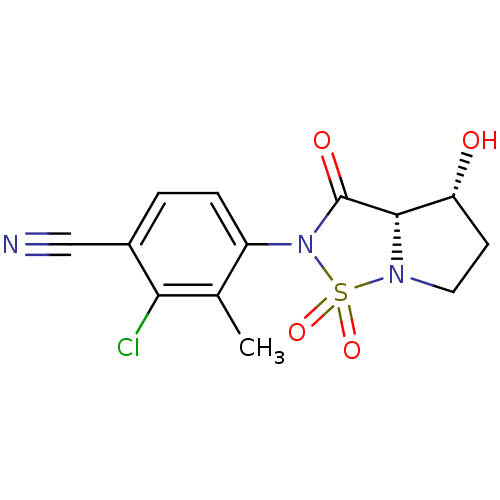 (2-Chloro-4-((3aS,4R)-4-hydroxy-1,1,3-trioxo-tetrah...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GTx, Inc. Curated by ChEMBL | Assay Description Agonist activity at androgen receptor (unknown origin) | J Med Chem 52: 3597-617 (2009) Article DOI: 10.1021/jm900280m BindingDB Entry DOI: 10.7270/Q2GH9HWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR2 (Homo sapiens (Human)) | BDBM312140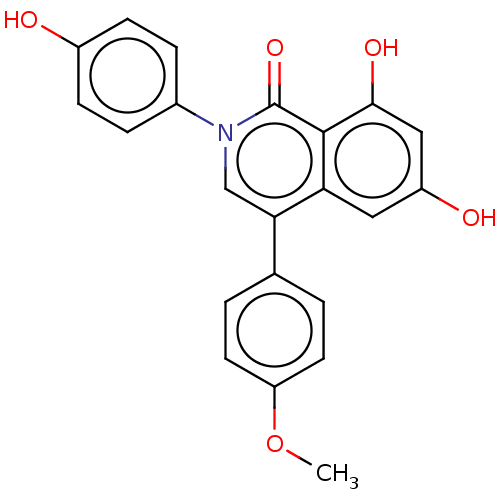 (6,8-dihydroxy-2-(4-hydroxyphenyl)-4-(4-methoxyphen...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GTx, Inc.; THE UNITED STATES OF AMERICA AS REPRESENTED BY THE DEPARTMENT OF VETERANS AFFAIRS US Patent | Assay Description Estrogen receptor (ERβ) binding affinity of the NRBAs was also determined using an in vitro competitive radioligand-binding assay with [3H]-estr... | US Patent US9604931 (2017) BindingDB Entry DOI: 10.7270/Q23N25FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM312140 (6,8-dihydroxy-2-(4-hydroxyphenyl)-4-(4-methoxyphen...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GTX, Inc. US Patent | Assay Description Recombinant ER-α or ER-β ligand binding domain (LBD) was combined with [3H]E2 (PerkinElmer, Waltham, Mass.) in buffer A (10 mM Tris, pH 7.4... | US Patent US9623021 (2017) BindingDB Entry DOI: 10.7270/Q20C4XV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18177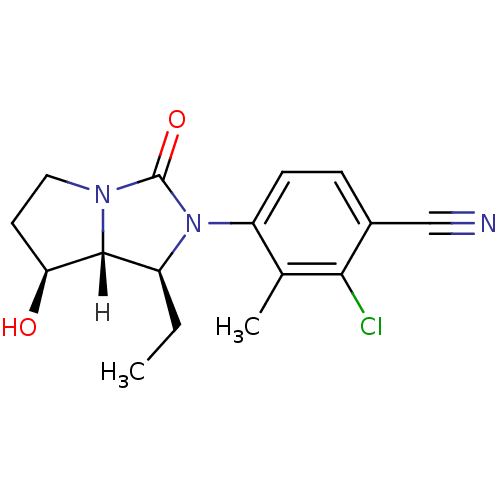 (4-[(1S,7S,7aR)-1-ethyl-7-hydroxy-3-oxo-hexahydro-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GTx, Inc. Curated by ChEMBL | Assay Description Agonist activity at androgen receptor (unknown origin) | J Med Chem 52: 3597-617 (2009) Article DOI: 10.1021/jm900280m BindingDB Entry DOI: 10.7270/Q2GH9HWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50258751 ((R)-2-(5,6-dichloro-1H-benzo[d]imidazol-2-yl)-1,1,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GTx, Inc. Curated by ChEMBL | Assay Description Agonist activity at androgen receptor (unknown origin) | J Med Chem 52: 3597-617 (2009) Article DOI: 10.1021/jm900280m BindingDB Entry DOI: 10.7270/Q2GH9HWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50136614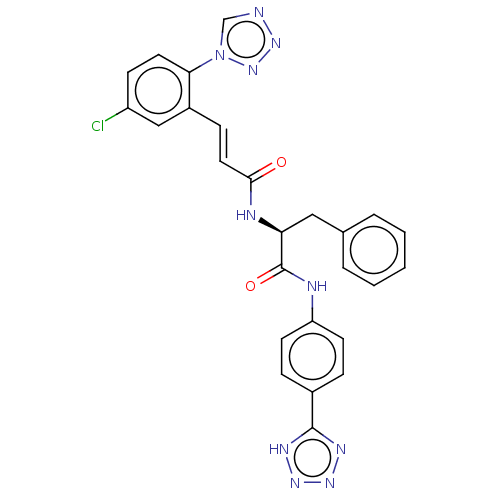 (CHEMBL3753020) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | PDB Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 11a assessed as substrate hydrolysis to p-nitroaniline incubated for 10 to 120 mins by spectrophotometry analy... | Bioorg Med Chem Lett 26: 472-8 (2016) Article DOI: 10.1016/j.bmcl.2015.11.089 BindingDB Entry DOI: 10.7270/Q27H1MFM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18524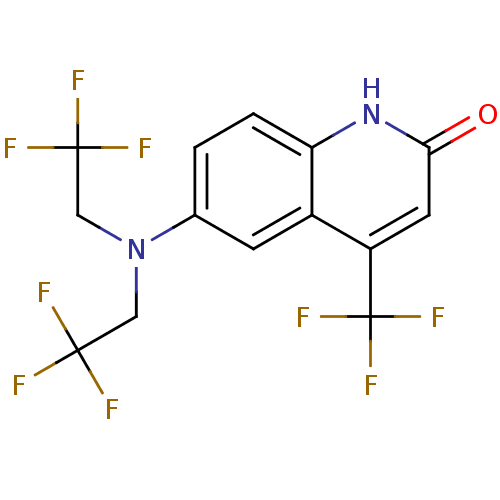 (6-[bis(2,2,2-trifluoroethyl)amino]-4-(trifluoromet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GTx, Inc. Curated by ChEMBL | Assay Description Agonist activity at androgen receptor (unknown origin) | J Med Chem 52: 3597-617 (2009) Article DOI: 10.1021/jm900280m BindingDB Entry DOI: 10.7270/Q2GH9HWD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18522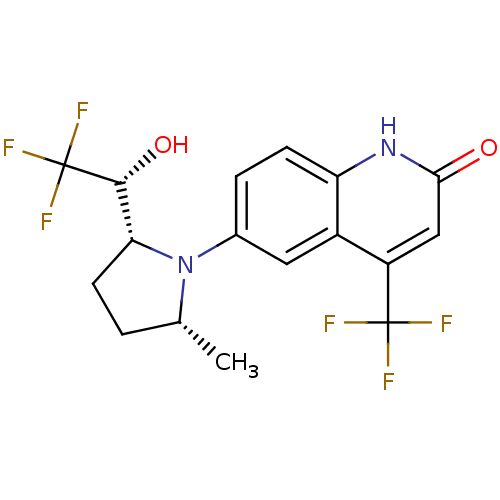 (6-(1-Pyrrolidine)quinolin-2(1H)-one, 6a | 6-[(2R,5...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GTx, Inc. Curated by ChEMBL | Assay Description Agonist activity at androgen receptor (unknown origin) | J Med Chem 52: 3597-617 (2009) Article DOI: 10.1021/jm900280m BindingDB Entry DOI: 10.7270/Q2GH9HWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50136579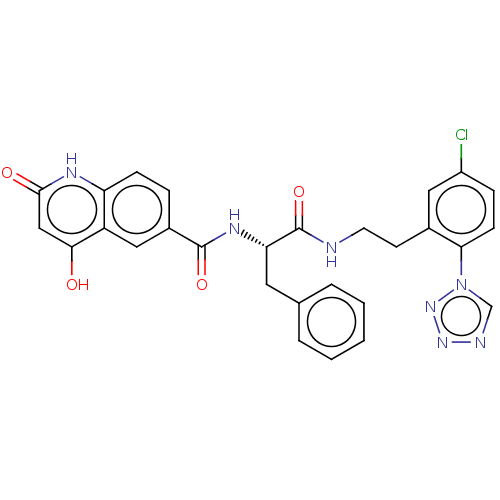 (CHEMBL3754781) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 11a assessed as substrate hydrolysis to p-nitroaniline incubated for 10 to 120 mins by spectrophotometry analy... | Bioorg Med Chem Lett 26: 472-8 (2016) Article DOI: 10.1016/j.bmcl.2015.11.089 BindingDB Entry DOI: 10.7270/Q27H1MFM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18685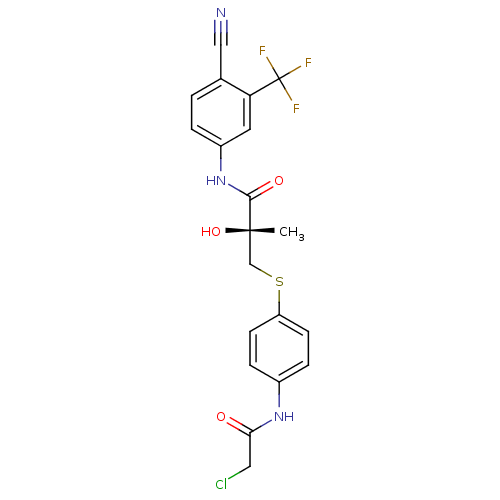 ((2R)-3-{[4-(2-chloroacetamido)phenyl]sulfanyl}-N-[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GTx, Inc. Curated by ChEMBL | Assay Description Agonist activity at androgen receptor (unknown origin) | J Med Chem 52: 3597-617 (2009) Article DOI: 10.1021/jm900280m BindingDB Entry DOI: 10.7270/Q2GH9HWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM312151 ((E)-6,8-dihydroxy-2-(4-hydroxyphenyl)-4-(prop-1-en...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GTX, Inc. US Patent | Assay Description Recombinant ER-α or ER-β ligand binding domain (LBD) was combined with [3H]E2 (PerkinElmer, Waltham, Mass.) in buffer A (10 mM Tris, pH 7.4... | US Patent US9623021 (2017) BindingDB Entry DOI: 10.7270/Q20C4XV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR2 (Homo sapiens (Human)) | BDBM312151 ((E)-6,8-dihydroxy-2-(4-hydroxyphenyl)-4-(prop-1-en...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GTx, Inc.; THE UNITED STATES OF AMERICA AS REPRESENTED BY THE DEPARTMENT OF VETERANS AFFAIRS US Patent | Assay Description Estrogen receptor (ERβ) binding affinity of the NRBAs was also determined using an in vitro competitive radioligand-binding assay with [3H]-estr... | US Patent US9604931 (2017) BindingDB Entry DOI: 10.7270/Q23N25FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM26261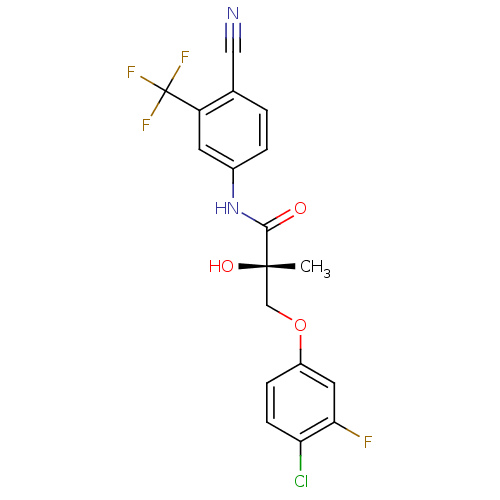 ((2S)-3-(4-chloro-3-fluorophenoxy)-N-[4-cyano-3-(tr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GTx, Inc. Curated by ChEMBL | Assay Description Agonist activity at androgen receptor (unknown origin) | J Med Chem 52: 3597-617 (2009) Article DOI: 10.1021/jm900280m BindingDB Entry DOI: 10.7270/Q2GH9HWD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50136570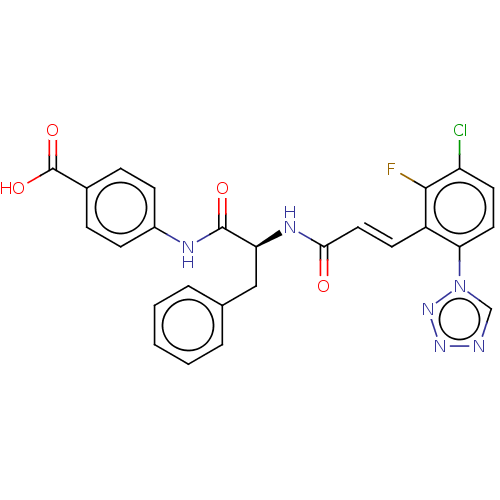 (CHEMBL3752669) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 11a assessed as substrate hydrolysis to p-nitroaniline incubated for 10 to 120 mins by spectrophotometry analy... | Bioorg Med Chem Lett 26: 472-8 (2016) Article DOI: 10.1016/j.bmcl.2015.11.089 BindingDB Entry DOI: 10.7270/Q27H1MFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50136574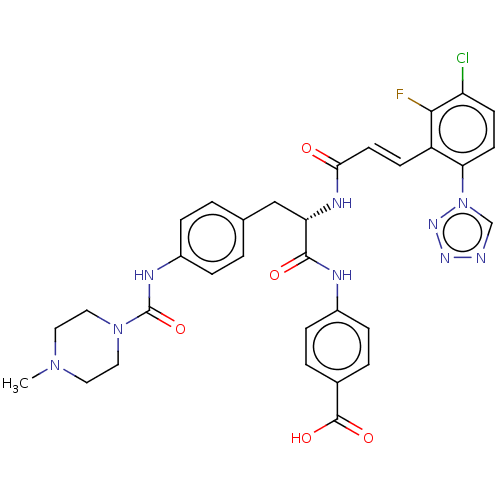 (CHEMBL3752266) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 11a assessed as substrate hydrolysis to p-nitroaniline incubated for 10 to 120 mins by spectrophotometry analy... | Bioorg Med Chem Lett 26: 472-8 (2016) Article DOI: 10.1016/j.bmcl.2015.11.089 BindingDB Entry DOI: 10.7270/Q27H1MFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50136608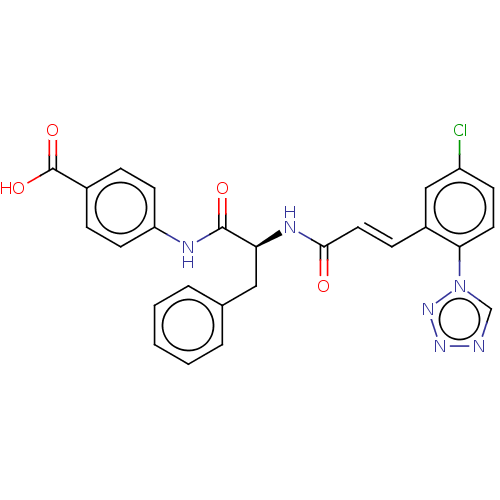 (CHEMBL3754069) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | PDB Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 11a assessed as substrate hydrolysis to p-nitroaniline incubated for 10 to 120 mins by spectrophotometry analy... | Bioorg Med Chem Lett 26: 472-8 (2016) Article DOI: 10.1016/j.bmcl.2015.11.089 BindingDB Entry DOI: 10.7270/Q27H1MFM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50136618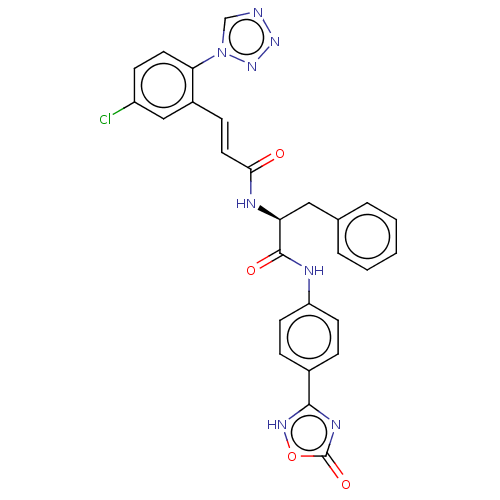 (CHEMBL3752251) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 11a assessed as substrate hydrolysis to p-nitroaniline incubated for 10 to 120 mins by spectrophotometry analy... | Bioorg Med Chem Lett 26: 472-8 (2016) Article DOI: 10.1016/j.bmcl.2015.11.089 BindingDB Entry DOI: 10.7270/Q27H1MFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50136573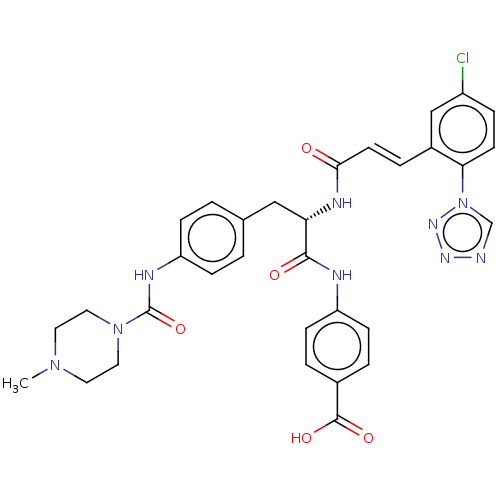 (CHEMBL3752047) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 11a assessed as substrate hydrolysis to p-nitroaniline incubated for 10 to 120 mins by spectrophotometry analy... | Bioorg Med Chem Lett 26: 472-8 (2016) Article DOI: 10.1016/j.bmcl.2015.11.089 BindingDB Entry DOI: 10.7270/Q27H1MFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18173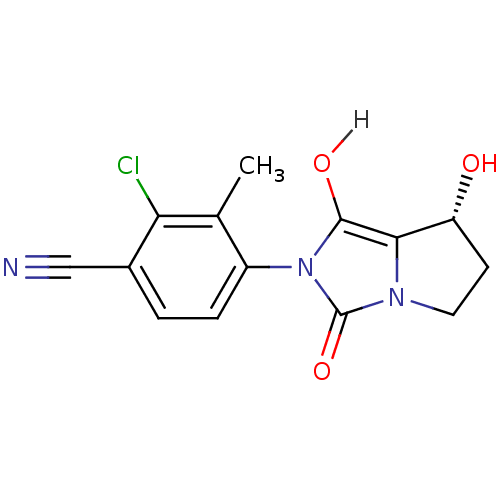 (4-[(7R,7aS)-7-hydroxy-1,3-dioxo-hexahydro-1H-pyrro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GTx, Inc. Curated by ChEMBL | Assay Description Agonist activity at androgen receptor (unknown origin) | J Med Chem 52: 3597-617 (2009) Article DOI: 10.1021/jm900280m BindingDB Entry DOI: 10.7270/Q2GH9HWD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50153001 (CHEMBL3781742) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Competitive inhibition of human coagulation factor 11a assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten eq... | Bioorg Med Chem 24: 2257-72 (2016) Article DOI: 10.1016/j.bmc.2016.03.062 BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50153005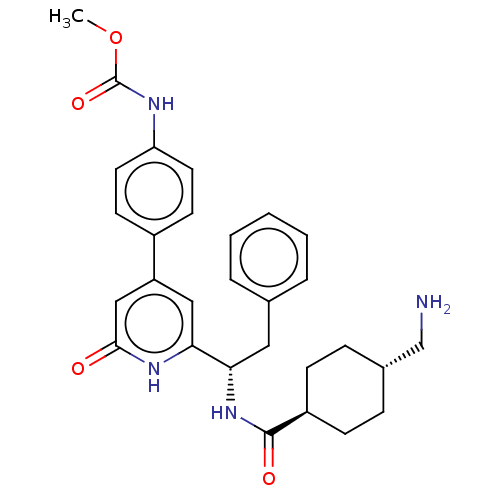 (CHEMBL3780342) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Competitive inhibition of human coagulation factor 11a assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten eq... | Bioorg Med Chem 24: 2257-72 (2016) Article DOI: 10.1016/j.bmc.2016.03.062 BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50309343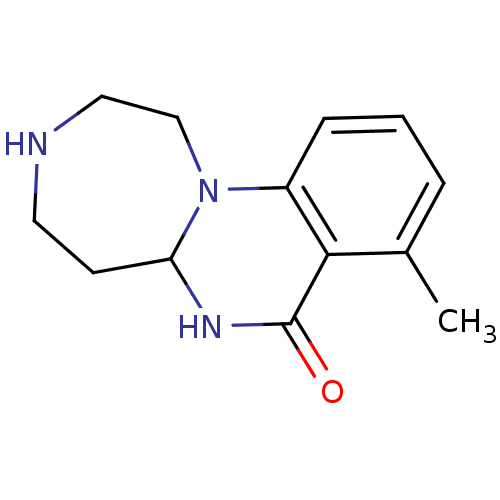 (8-methyl-2,3,4,5,5a,6-hexahydro-[1,4]diazepino[1,7...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co Curated by ChEMBL | Assay Description Displacement of [125I]DOI from human recombinant 5HT2C receptor expressed in HEK293E cells | Bioorg Med Chem Lett 20: 1128-33 (2010) Article DOI: 10.1016/j.bmcl.2009.12.014 BindingDB Entry DOI: 10.7270/Q2K074DK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM312096 (4-bromo-6,8-dihydroxy-2-(4-hydroxyphenyl)isoquinol...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GTX, Inc. US Patent | Assay Description Recombinant ER-α or ER-β ligand binding domain (LBD) was combined with [3H]E2 (PerkinElmer, Waltham, Mass.) in buffer A (10 mM Tris, pH 7.4... | US Patent US9623021 (2017) BindingDB Entry DOI: 10.7270/Q20C4XV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM317457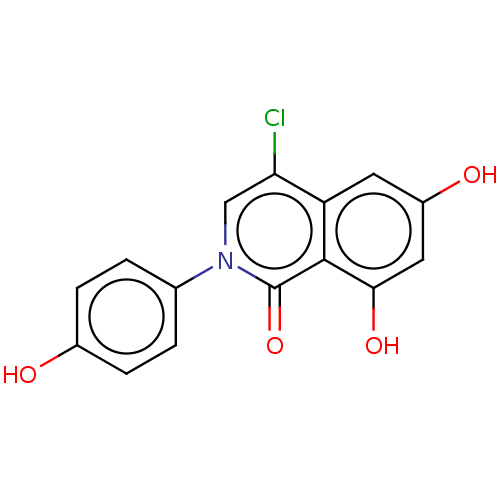 (4-chloro-6,8-dihydroxy-2-(4-hydroxyphenyl)isoquino...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GTX, Inc. US Patent | Assay Description Recombinant ER-α or ER-β ligand binding domain (LBD) was combined with [3H]E2 (PerkinElmer, Waltham, Mass.) in buffer A (10 mM Tris, pH 7.4... | US Patent US9623021 (2017) BindingDB Entry DOI: 10.7270/Q20C4XV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR2 (Homo sapiens (Human)) | BDBM312133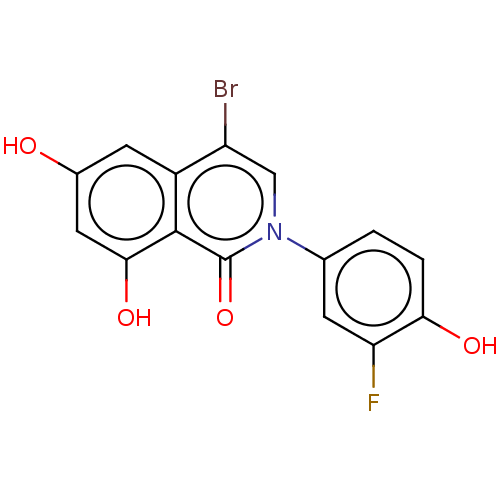 (US9604931, 12z) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GTx, Inc.; THE UNITED STATES OF AMERICA AS REPRESENTED BY THE DEPARTMENT OF VETERANS AFFAIRS US Patent | Assay Description Estrogen receptor (ERβ) binding affinity of the NRBAs was also determined using an in vitro competitive radioligand-binding assay with [3H]-estr... | US Patent US9604931 (2017) BindingDB Entry DOI: 10.7270/Q23N25FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR2 (Homo sapiens (Human)) | BDBM312096 (4-bromo-6,8-dihydroxy-2-(4-hydroxyphenyl)isoquinol...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GTx, Inc.; THE UNITED STATES OF AMERICA AS REPRESENTED BY THE DEPARTMENT OF VETERANS AFFAIRS US Patent | Assay Description Estrogen receptor (ERβ) binding affinity of the NRBAs was also determined using an in vitro competitive radioligand-binding assay with [3H]-estr... | US Patent US9604931 (2017) BindingDB Entry DOI: 10.7270/Q23N25FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR2 (Homo sapiens (Human)) | BDBM312149 (6,8-dihydroxy-2-(4-hydroxyphenyl)-4-(4-methoxyphen...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3.03 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GTx, Inc.; THE UNITED STATES OF AMERICA AS REPRESENTED BY THE DEPARTMENT OF VETERANS AFFAIRS US Patent | Assay Description Estrogen receptor (ERβ) binding affinity of the NRBAs was also determined using an in vitro competitive radioligand-binding assay with [3H]-estr... | US Patent US9604931 (2017) BindingDB Entry DOI: 10.7270/Q23N25FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM312149 (6,8-dihydroxy-2-(4-hydroxyphenyl)-4-(4-methoxyphen...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3.03 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GTX, Inc. US Patent | Assay Description Recombinant ER-α or ER-β ligand binding domain (LBD) was combined with [3H]E2 (PerkinElmer, Waltham, Mass.) in buffer A (10 mM Tris, pH 7.4... | US Patent US9623021 (2017) BindingDB Entry DOI: 10.7270/Q20C4XV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50153067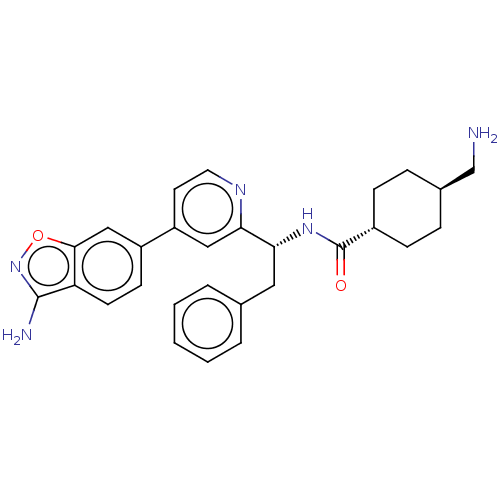 (CHEMBL3781202) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Competitive inhibition of human coagulation factor 11a assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten eq... | Bioorg Med Chem 24: 2257-72 (2016) Article DOI: 10.1016/j.bmc.2016.03.062 BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50136578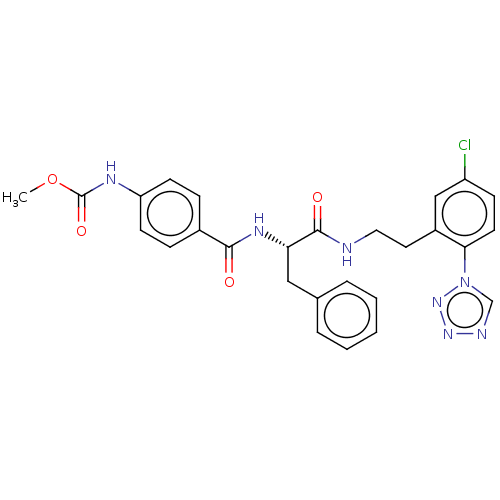 (CHEMBL3752066) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 11a assessed as substrate hydrolysis to p-nitroaniline incubated for 10 to 120 mins by spectrophotometry analy... | Bioorg Med Chem Lett 26: 472-8 (2016) Article DOI: 10.1016/j.bmcl.2015.11.089 BindingDB Entry DOI: 10.7270/Q27H1MFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50153057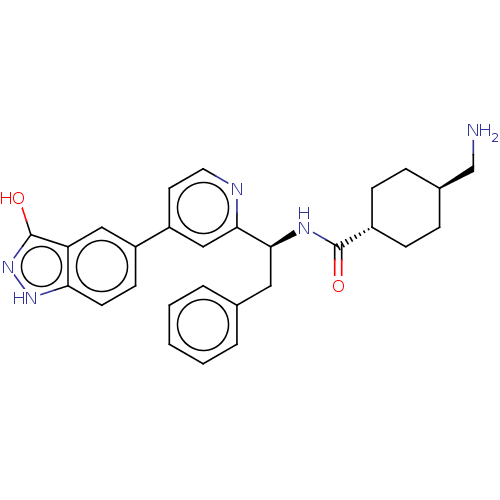 (CHEMBL3780324) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Competitive inhibition of human coagulation factor 11a assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten eq... | Bioorg Med Chem 24: 2257-72 (2016) Article DOI: 10.1016/j.bmc.2016.03.062 BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50529668 (Enobosarm | Gtx-024 | MK-2866 | Ostarine | US10806...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Science Center Curated by ChEMBL | Assay Description Displacement of [3H]-mibolerone from recombinant wild-type GST-tagged androgen receptor LBD (unknown origin) after 16 hrs by scintillation counting a... | J Med Chem 62: 491-511 (2019) Article DOI: 10.1021/acs.jmedchem.8b00973 BindingDB Entry DOI: 10.7270/Q2GF0XZ5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50529668 (Enobosarm | Gtx-024 | MK-2866 | Ostarine | US10806...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Science Center Curated by ChEMBL | Assay Description Displacement of [3H]-mibolerone from recombinant wild-type GST-tagged androgen receptor LBD (unknown origin) after 16 hrs by scintillation counting a... | J Med Chem 62: 491-511 (2019) Article DOI: 10.1021/acs.jmedchem.8b00973 BindingDB Entry DOI: 10.7270/Q2GF0XZ5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor VII/Tissue factor (Homo sapiens (Human)) | BDBM50205843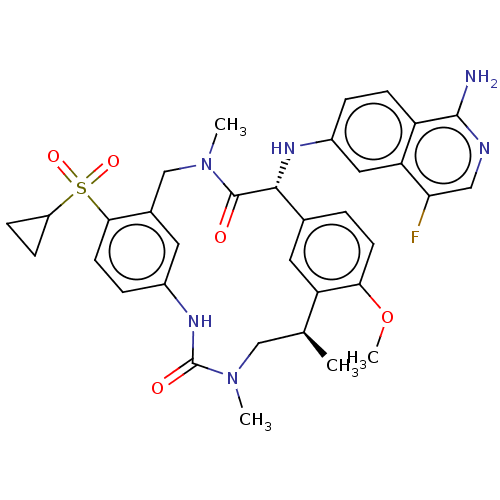 (CHEMBL3978216) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of recombinant human factor-7a/TF using S2288 as substrate measured after 60 mins at 37 degC | ACS Med Chem Lett 8: 67-72 (2017) Article DOI: 10.1021/acsmedchemlett.6b00375 BindingDB Entry DOI: 10.7270/Q20P120Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50006222 ((1S,2R,5R)-5-(6-Amino-purin-9-yl)-3-hydroxymethyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Time course of inactivation of bovine liver S-adenosyl-homocysteine hydrolase | J Med Chem 31: 500-3 (1988) BindingDB Entry DOI: 10.7270/Q28P61Q3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50153063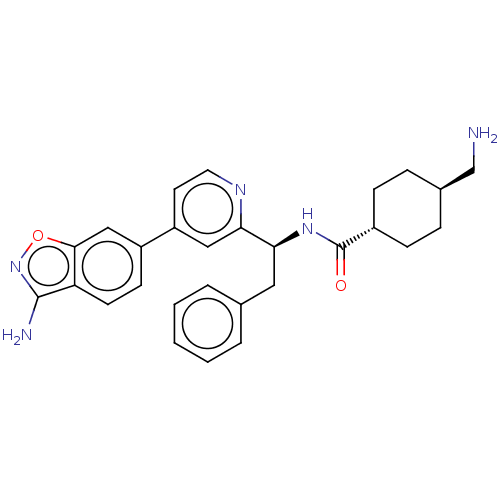 (CHEMBL3780922) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Competitive inhibition of human coagulation factor 11a assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten eq... | Bioorg Med Chem 24: 2257-72 (2016) Article DOI: 10.1016/j.bmc.2016.03.062 BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR2 (Homo sapiens (Human)) | BDBM312139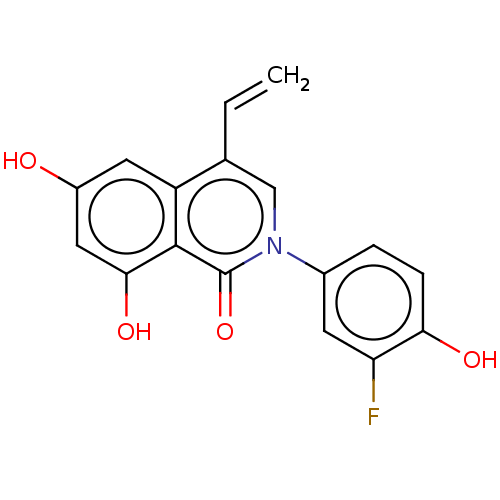 (2-(3-fluoro-4-hydroxyphenyl)-6,8-dihydroxy-4-vinyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3.96 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GTx, Inc.; THE UNITED STATES OF AMERICA AS REPRESENTED BY THE DEPARTMENT OF VETERANS AFFAIRS US Patent | Assay Description Estrogen receptor (ERβ) binding affinity of the NRBAs was also determined using an in vitro competitive radioligand-binding assay with [3H]-estr... | US Patent US9604931 (2017) BindingDB Entry DOI: 10.7270/Q23N25FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM312139 (2-(3-fluoro-4-hydroxyphenyl)-6,8-dihydroxy-4-vinyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3.96 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GTX, Inc. US Patent | Assay Description Recombinant ER-α or ER-β ligand binding domain (LBD) was combined with [3H]E2 (PerkinElmer, Waltham, Mass.) in buffer A (10 mM Tris, pH 7.4... | US Patent US9623021 (2017) BindingDB Entry DOI: 10.7270/Q20C4XV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18665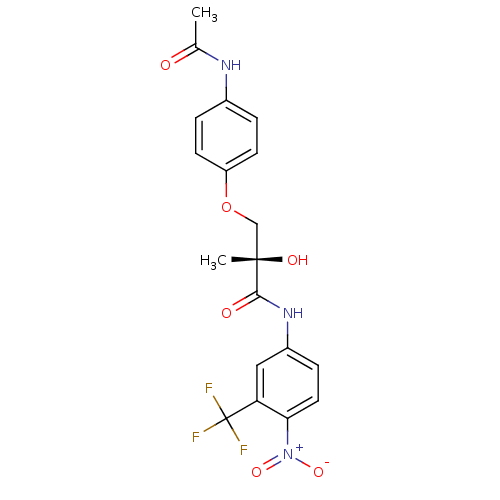 ((2S)-3-(4-acetamidophenoxy)-2-hydroxy-2-methyl-N-[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GTx, Inc. Curated by ChEMBL | Assay Description Agonist activity at androgen receptor (unknown origin) | J Med Chem 52: 3597-617 (2009) Article DOI: 10.1021/jm900280m BindingDB Entry DOI: 10.7270/Q2GH9HWD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor VII/Tissue factor (Homo sapiens (Human)) | BDBM50205817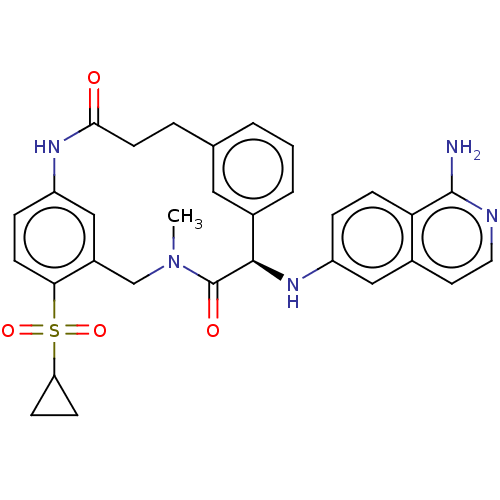 (CHEMBL3905354) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | <5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of recombinant human factor-7a/TF using S2288 as substrate measured after 60 mins at 25 degC | ACS Med Chem Lett 8: 67-72 (2017) Article DOI: 10.1021/acsmedchemlett.6b00375 BindingDB Entry DOI: 10.7270/Q20P120Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor VII/Tissue factor (Homo sapiens (Human)) | BDBM50205843 (CHEMBL3978216) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | <5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of recombinant human factor-7a/TF using S2288 as substrate measured after 60 mins at 25 degC | ACS Med Chem Lett 8: 67-72 (2017) Article DOI: 10.1021/acsmedchemlett.6b00375 BindingDB Entry DOI: 10.7270/Q20P120Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta/Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50204085 (CHEMBL3960606) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Displacement of [3H]-24,25-epoxycholesterol from human LXRbeta/RXRalpha expressed in baculovirus infected Sf9 cells by scintillation proximity analys... | ACS Med Chem Lett 7: 1207-1212 (2016) Article DOI: 10.1021/acsmedchemlett.6b00234 BindingDB Entry DOI: 10.7270/Q2V98B13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50258791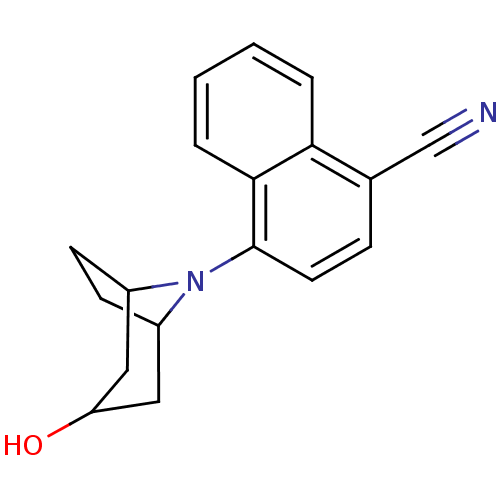 (4-(3-exo-Hydroxy-8-azabicyclo[3.2.1]oct-8-yl)napht...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GTx, Inc. Curated by ChEMBL | Assay Description Agonist activity at androgen receptor (unknown origin) | J Med Chem 52: 3597-617 (2009) Article DOI: 10.1021/jm900280m BindingDB Entry DOI: 10.7270/Q2GH9HWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50153054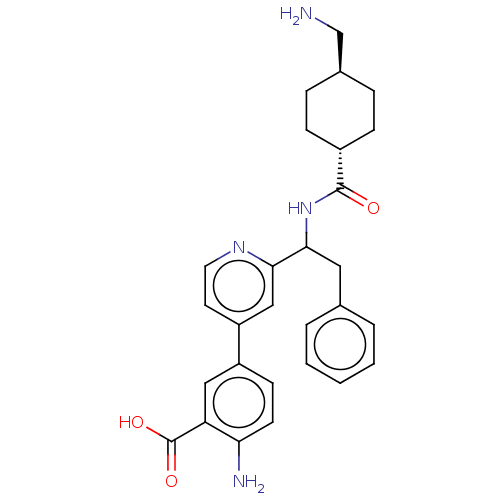 (CHEMBL3781553) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Competitive inhibition of human coagulation factor 11a assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten eq... | Bioorg Med Chem 24: 2257-72 (2016) Article DOI: 10.1016/j.bmc.2016.03.062 BindingDB Entry DOI: 10.7270/Q2DN46X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50136616 (CHEMBL3752951) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 11a assessed as substrate hydrolysis to p-nitroaniline incubated for 10 to 120 mins by spectrophotometry analy... | Bioorg Med Chem Lett 26: 472-8 (2016) Article DOI: 10.1016/j.bmcl.2015.11.089 BindingDB Entry DOI: 10.7270/Q27H1MFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50136619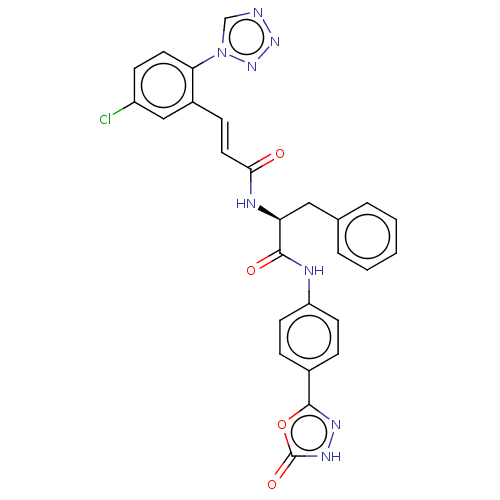 (CHEMBL3754487) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 11a assessed as substrate hydrolysis to p-nitroaniline incubated for 10 to 120 mins by spectrophotometry analy... | Bioorg Med Chem Lett 26: 472-8 (2016) Article DOI: 10.1016/j.bmcl.2015.11.089 BindingDB Entry DOI: 10.7270/Q27H1MFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1293 total ) | Next | Last >> |