 Found 5278 hits with Last Name = 'nargund' and Initial = 'r'
Found 5278 hits with Last Name = 'nargund' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598740
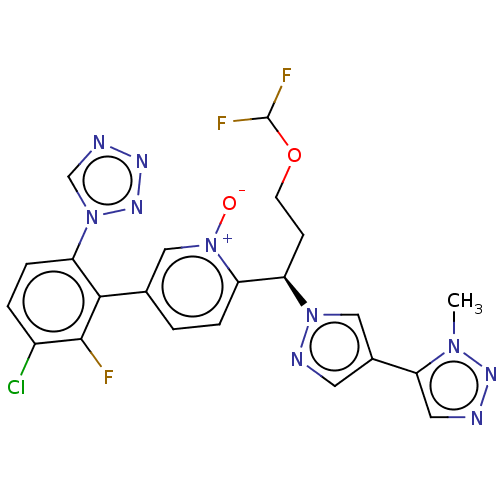
(CHEMBL5175227)Show SMILES Cn1nncc1-c1cnn(c1)[C@H](CCOC(F)F)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cnnn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598739

(CHEMBL5188215)Show SMILES COCC[C@H](c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cnnn1)n1cc(cn1)-c1cnnn1C |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598738
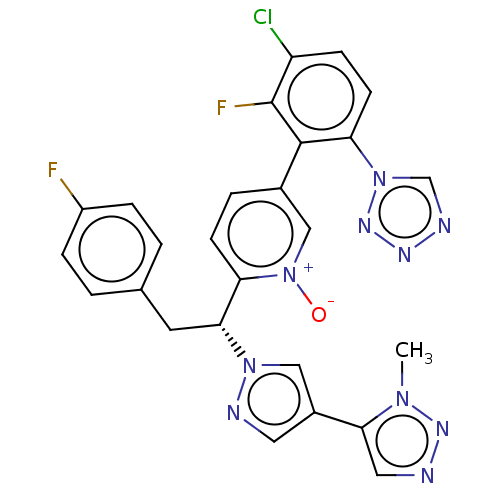
(CHEMBL5204065)Show SMILES Cn1nncc1-c1cnn(c1)[C@H](Cc1ccc(F)cc1)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cnnn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598724

(CHEMBL5170592)Show SMILES Cn1nncc1-c1cnn(c1)[C@H](CCOC(F)F)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cc(Cl)nn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598737
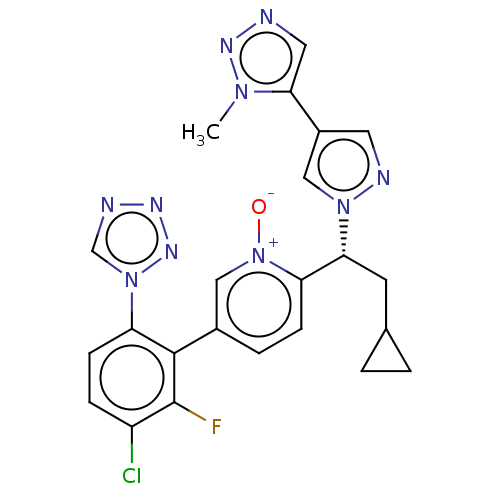
(CHEMBL5205631)Show SMILES Cn1nncc1-c1cnn(c1)[C@H](CC1CC1)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cnnn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598741

(CHEMBL5204894)Show SMILES Cn1nncc1-c1cnn(c1)[C@H](Cc1ccc(F)cc1)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cc(Cl)nn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM210759

(US9290454, 4.4)Show SMILES C[C@@H](NC(=O)c1ccc2c(c1)cc(CCCCC(O)=O)n(-c1ccc(F)cc1)c2=O)c1ccc(F)cc1 Show InChI InChI=1S/C29H26F2N2O4/c1-18(19-6-9-22(30)10-7-19)32-28(36)20-8-15-26-21(16-20)17-25(4-2-3-5-27(34)35)33(29(26)37)24-13-11-23(31)12-14-24/h6-18H,2-5H2,1H3,(H,32,36)(H,34,35)/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck
Curated by ChEMBL
| Assay Description
Antagonist activity at CRTh2 (unknown origin) assessed as inhibition of CD11b activation |
Bioorg Med Chem Lett 27: 5344-5348 (2017)
Article DOI: 10.1016/j.bmcl.2017.07.064
BindingDB Entry DOI: 10.7270/Q2HX1G7W |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50049478
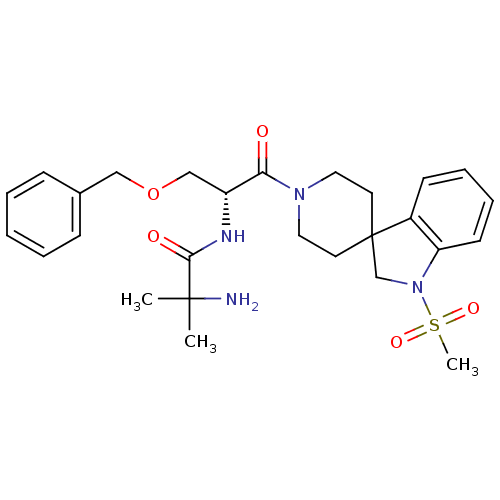
(1-{[(2R)-3-(benzyloxy)-1-{1-methanesulfonyl-1,2-di...)Show SMILES CC(C)(N)C(=O)N[C@H](COCc1ccccc1)C(=O)N1CCC2(CN(c3ccccc23)S(C)(=O)=O)CC1 Show InChI InChI=1S/C27H36N4O5S/c1-26(2,28)25(33)29-22(18-36-17-20-9-5-4-6-10-20)24(32)30-15-13-27(14-16-30)19-31(37(3,34)35)23-12-8-7-11-21(23)27/h4-12,22H,13-19,28H2,1-3H3,(H,29,33)/t22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding Affinity of the compound against Growth hormone secretagogue receptor of swine using [35S]-MK-0677 as radioligand |
J Med Chem 39: 1767-70 (1996)
Article DOI: 10.1021/jm960054c
BindingDB Entry DOI: 10.7270/Q2BK1BDG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598725

(CHEMBL5185397)Show SMILES COC(=O)Nc1ccc(cc1)-c1cnc([nH]1)C(CC1CC1)c1ccc(c[n+]1[O-])-c1cc(Cl)ccc1-n1cnnn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598743
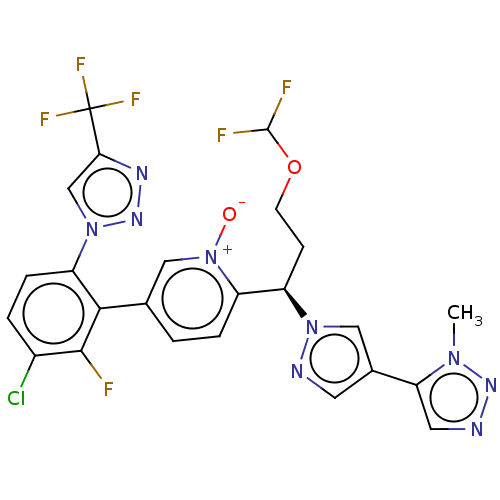
(CHEMBL5178223)Show SMILES Cn1nncc1-c1cnn(c1)[C@H](CCOC(F)F)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cc(nn1)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598734
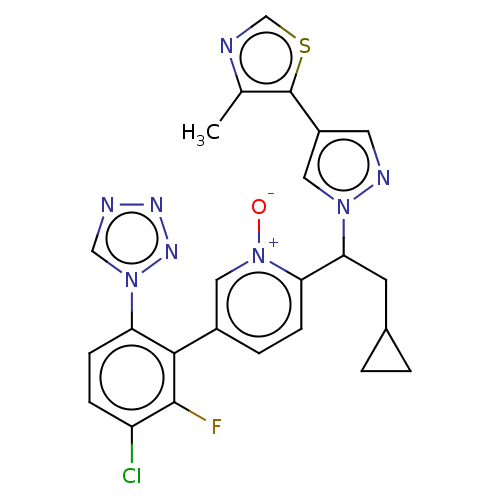
(CHEMBL5197480)Show SMILES Cc1ncsc1-c1cnn(c1)C(CC1CC1)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cnnn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598745
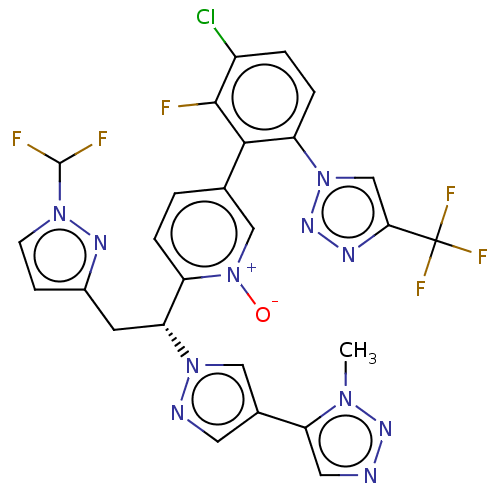
(CHEMBL5198823)Show SMILES Cn1nncc1-c1cnn(c1)[C@H](Cc1ccn(n1)C(F)F)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cc(nn1)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598736

(CHEMBL5208095)Show SMILES Cn1cncc1-c1cnn(c1)[C@H](CC1CC1)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cnnn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM210749
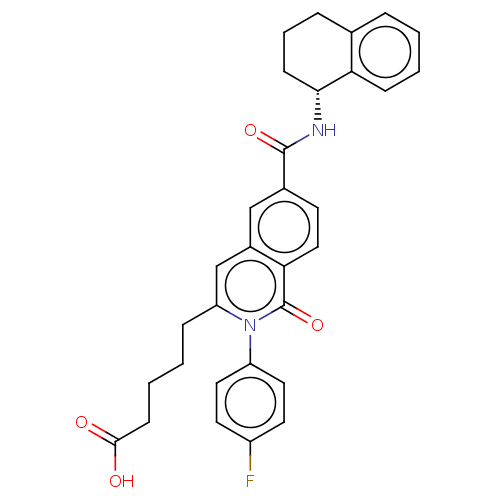
(US9290454, 3.1)Show SMILES OC(=O)CCCCc1cc2cc(ccc2c(=O)n1-c1ccc(F)cc1)C(=O)N[C@@H]1CCCc2ccccc12 Show InChI InChI=1S/C31H29FN2O4/c32-23-13-15-24(16-14-23)34-25(8-2-4-11-29(35)36)19-22-18-21(12-17-27(22)31(34)38)30(37)33-28-10-5-7-20-6-1-3-9-26(20)28/h1,3,6,9,12-19,28H,2,4-5,7-8,10-11H2,(H,33,37)(H,35,36)/t28-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck
Curated by ChEMBL
| Assay Description
Antagonist activity at CRTh2 (unknown origin) assessed as inhibition of CD11b activation |
Bioorg Med Chem Lett 27: 5344-5348 (2017)
Article DOI: 10.1016/j.bmcl.2017.07.064
BindingDB Entry DOI: 10.7270/Q2HX1G7W |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598744
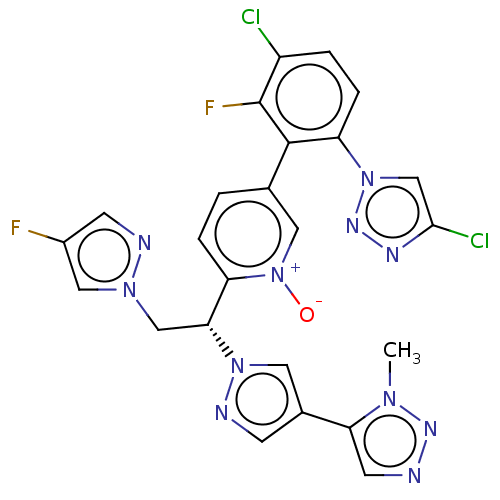
(CHEMBL5190323)Show SMILES Cn1nncc1-c1cnn(c1)[C@H](Cn1cc(F)cn1)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cc(Cl)nn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | |
Bombesin receptor subtype-3
(RAT) | BDBM50336889
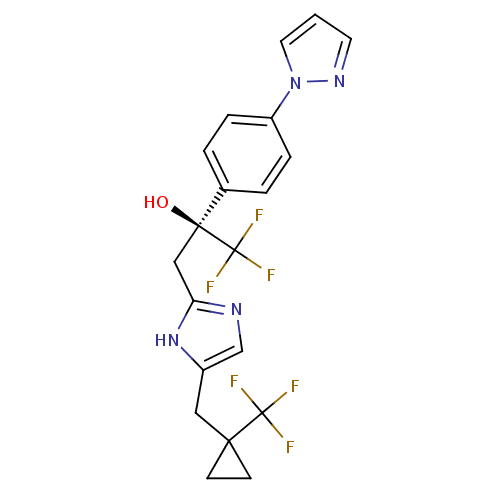
((2S)-1,1,1-trifluoro-2-[4-(1H-pyrazol-1-yl)phenyl]...)Show SMILES O[C@@](Cc1ncc(CC2(CC2)C(F)(F)F)[nH]1)(c1ccc(cc1)-n1cccn1)C(F)(F)F |r| Show InChI InChI=1S/C20H18F6N4O/c21-19(22,23)17(6-7-17)10-14-12-27-16(29-14)11-18(31,20(24,25)26)13-2-4-15(5-3-13)30-9-1-8-28-30/h1-5,8-9,12,31H,6-7,10-11H2,(H,27,29)/t18-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]Bag-3 from rat BRS-3 |
ACS Med Chem Lett 2: 43-47 (2011)
Article DOI: 10.1021/ml100196d
BindingDB Entry DOI: 10.7270/Q26D5T81 |
More data for this
Ligand-Target Pair | |
Bombesin receptor subtype-3
(RAT) | BDBM50336889

((2S)-1,1,1-trifluoro-2-[4-(1H-pyrazol-1-yl)phenyl]...)Show SMILES O[C@@](Cc1ncc(CC2(CC2)C(F)(F)F)[nH]1)(c1ccc(cc1)-n1cccn1)C(F)(F)F |r| Show InChI InChI=1S/C20H18F6N4O/c21-19(22,23)17(6-7-17)10-14-12-27-16(29-14)11-18(31,20(24,25)26)13-2-4-15(5-3-13)30-9-1-8-28-30/h1-5,8-9,12,31H,6-7,10-11H2,(H,27,29)/t18-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]Bag-3 from rat BRS-3 |
ACS Med Chem Lett 2: 43-47 (2011)
Article DOI: 10.1021/ml100196d
BindingDB Entry DOI: 10.7270/Q26D5T81 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50369890
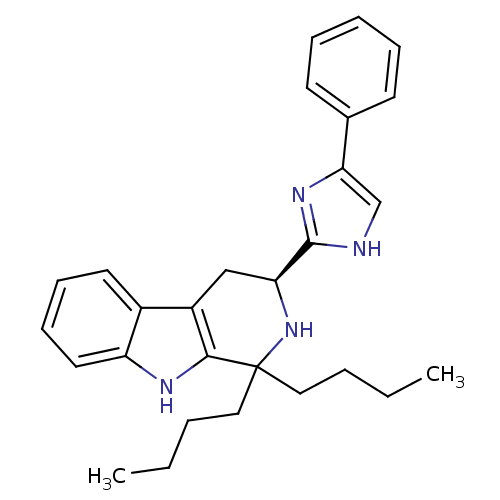
(CHEMBL1237140 | CHEMBL1788167)Show SMILES CCCCC1(CCCC)N[C@@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccccc1 |r| Show InChI InChI=1S/C28H34N4/c1-3-5-16-28(17-6-4-2)26-22(21-14-10-11-15-23(21)30-26)18-24(32-28)27-29-19-25(31-27)20-12-8-7-9-13-20/h7-15,19,24,30,32H,3-6,16-18H2,1-2H3,(H,29,31)/t24-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]SS14 from human SST3 expressed in CHO membrane after 60 to 90 mins by scintillation counting |
ACS Med Chem Lett 5: 690-5 (2014)
Article DOI: 10.1021/ml500079u
BindingDB Entry DOI: 10.7270/Q22N53V4 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50389603

(CHEMBL2069499)Show SMILES CCCCC1(CCCC)N[C@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccccc1 |r| Show InChI InChI=1S/C28H34N4/c1-3-5-16-28(17-6-4-2)26-22(21-14-10-11-15-23(21)30-26)18-24(32-28)27-29-19-25(31-27)20-12-8-7-9-13-20/h7-15,19,24,30,32H,3-6,16-18H2,1-2H3,(H,29,31)/t24-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125I]SS-14 from human SST3 receptor expressed in CHO cells after 60 mins by scintillation counting |
ACS Med Chem Lett 3: 289-293 (2012)
Article DOI: 10.1021/ml200272z
BindingDB Entry DOI: 10.7270/Q2K938MH |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598732
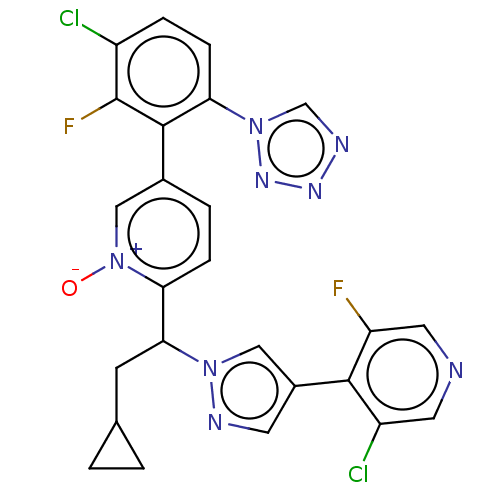
(CHEMBL5192284)Show SMILES [O-][n+]1cc(ccc1C(CC1CC1)n1cc(cn1)-c1c(F)cncc1Cl)-c1c(F)c(Cl)ccc1-n1cnnn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50021074

(CHEMBL3287628)Show SMILES Clc1ccc(CN[C@H]2CCCC[C@H]2NC(=O)c2ccc3ccccc3c2)cc1Cl |r| Show InChI InChI=1S/C24H24Cl2N2O/c25-20-12-9-16(13-21(20)26)15-27-22-7-3-4-8-23(22)28-24(29)19-11-10-17-5-1-2-6-18(17)14-19/h1-2,5-6,9-14,22-23,27H,3-4,7-8,15H2,(H,28,29)/t22-,23+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.815 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]SS14 from human SST3 expressed in CHO membrane after 60 to 90 mins by scintillation counting |
ACS Med Chem Lett 5: 690-5 (2014)
Article DOI: 10.1021/ml500079u
BindingDB Entry DOI: 10.7270/Q22N53V4 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598742
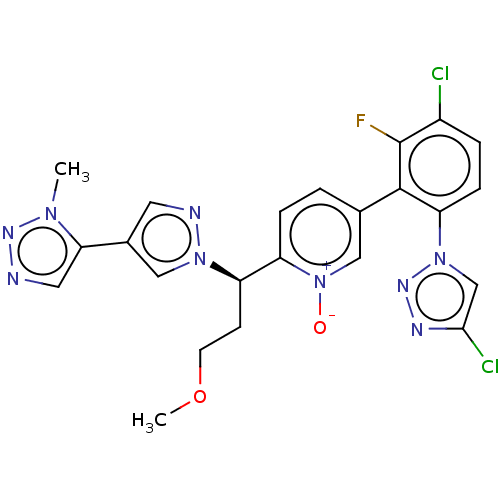
(CHEMBL5182855)Show SMILES COCC[C@H](c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cc(Cl)nn1)n1cc(cn1)-c1cnnn1C |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50110470

(CHEMBL3605798)Show SMILES Cc1ccc(cc1)-c1c[nH]c(n1)C1(CCCC1)NCCCc1ccccc1 Show InChI InChI=1S/C24H29N3/c1-19-11-13-21(14-12-19)22-18-25-23(27-22)24(15-5-6-16-24)26-17-7-10-20-8-3-2-4-9-20/h2-4,8-9,11-14,18,26H,5-7,10,15-17H2,1H3,(H,25,27) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]SS-14 from human SSTR3 expressed in CHO cells after 60 to 90 mins |
Bioorg Med Chem Lett 25: 3520-5 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.087
BindingDB Entry DOI: 10.7270/Q29W0H9M |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598729
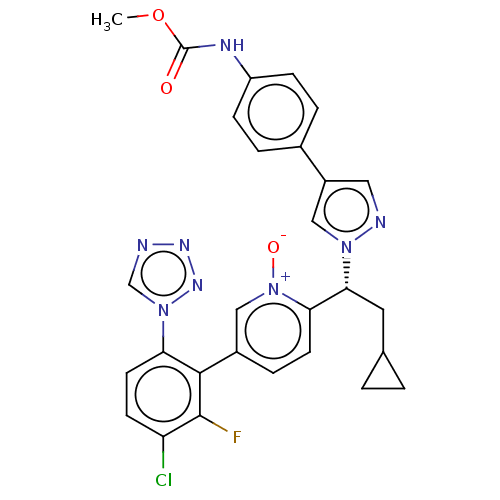
(CHEMBL5195600)Show SMILES COC(=O)Nc1ccc(cc1)-c1cnn(c1)[C@H](CC1CC1)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cnnn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM210750

(US9290454, 3.2)Show SMILES OC(=O)CCCCc1cc2cc(ccc2c(=O)n1-c1ccc(F)cc1)C(=O)N[C@@H]1CCCc2cc(F)ccc12 Show InChI InChI=1S/C31H28F2N2O4/c32-22-9-12-24(13-10-22)35-25(5-1-2-7-29(36)37)18-21-16-20(8-14-27(21)31(35)39)30(38)34-28-6-3-4-19-17-23(33)11-15-26(19)28/h8-18,28H,1-7H2,(H,34,38)(H,36,37)/t28-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck
Curated by ChEMBL
| Assay Description
Antagonist activity at CRTh2 (unknown origin) assessed as inhibition of CD11b activation |
Bioorg Med Chem Lett 27: 5344-5348 (2017)
Article DOI: 10.1016/j.bmcl.2017.07.064
BindingDB Entry DOI: 10.7270/Q2HX1G7W |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598727
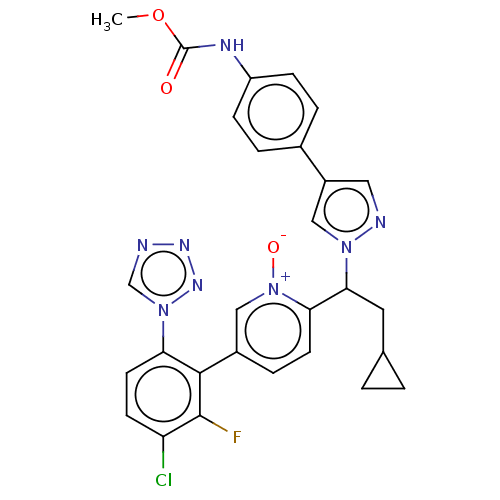
(CHEMBL5198338)Show SMILES COC(=O)Nc1ccc(cc1)-c1cnn(c1)C(CC1CC1)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cnnn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bombesin receptor subtype-3
(Mus musculus) | BDBM50336889

((2S)-1,1,1-trifluoro-2-[4-(1H-pyrazol-1-yl)phenyl]...)Show SMILES O[C@@](Cc1ncc(CC2(CC2)C(F)(F)F)[nH]1)(c1ccc(cc1)-n1cccn1)C(F)(F)F |r| Show InChI InChI=1S/C20H18F6N4O/c21-19(22,23)17(6-7-17)10-14-12-27-16(29-14)11-18(31,20(24,25)26)13-2-4-15(5-3-13)30-9-1-8-28-30/h1-5,8-9,12,31H,6-7,10-11H2,(H,27,29)/t18-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]Bag-3 from mouse BRS-3 |
ACS Med Chem Lett 2: 43-47 (2011)
Article DOI: 10.1021/ml100196d
BindingDB Entry DOI: 10.7270/Q26D5T81 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598735
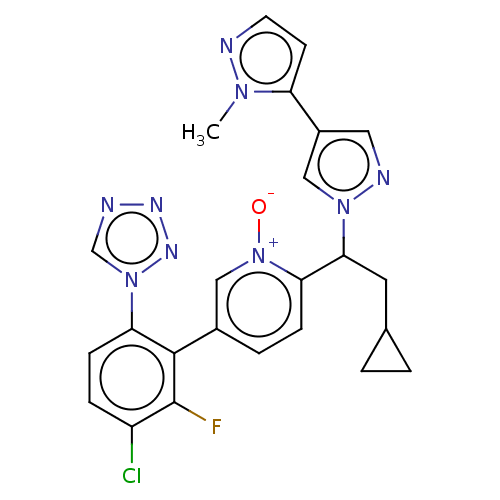
(CHEMBL5193267)Show SMILES Cn1nccc1-c1cnn(c1)C(CC1CC1)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cnnn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50389592
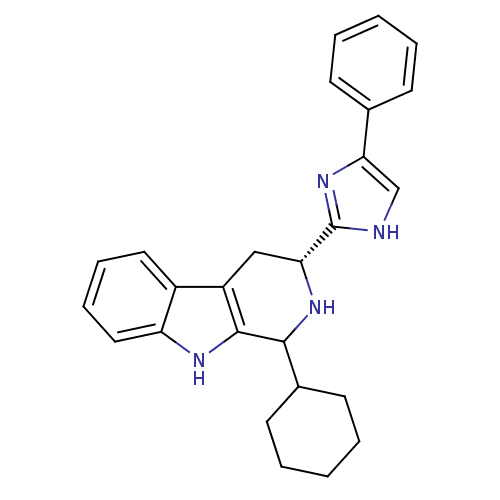
(CHEMBL2069500)Show SMILES C1CCC(CC1)C1N[C@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccccc1 |r| Show InChI InChI=1S/C26H28N4/c1-3-9-17(10-4-1)23-16-27-26(30-23)22-15-20-19-13-7-8-14-21(19)28-25(20)24(29-22)18-11-5-2-6-12-18/h1,3-4,7-10,13-14,16,18,22,24,28-29H,2,5-6,11-12,15H2,(H,27,30)/t22-,24?/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human SST3 receptor |
ACS Med Chem Lett 3: 289-293 (2012)
Article DOI: 10.1021/ml200272z
BindingDB Entry DOI: 10.7270/Q2K938MH |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50110461

(CHEMBL3605789)Show SMILES Fc1ccc(cc1)-c1c[nH]c(n1)C1(CCCC1)NCc1c[nH]c2ccccc12 Show InChI InChI=1S/C23H23FN4/c24-18-9-7-16(8-10-18)21-15-26-22(28-21)23(11-3-4-12-23)27-14-17-13-25-20-6-2-1-5-19(17)20/h1-2,5-10,13,15,25,27H,3-4,11-12,14H2,(H,26,28) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]SS-14 from human SSTR3 expressed in CHO cells after 60 to 90 mins |
Bioorg Med Chem Lett 25: 3520-5 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.087
BindingDB Entry DOI: 10.7270/Q29W0H9M |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50458670

(CHEMBL4212838)Show SMILES [H][C@@]12CCC[C@]1([H])N(C(=O)c1ccc(OC(F)(F)F)cc1)c1cc(Cl)ccc1[C@@H]2N(C1CC1)C(=O)CCC(O)=O |r| Show InChI InChI=1S/C27H26ClF3N2O5/c28-16-6-11-20-22(14-16)33(26(37)15-4-9-18(10-5-15)38-27(29,30)31)21-3-1-2-19(21)25(20)32(17-7-8-17)23(34)12-13-24(35)36/h4-6,9-11,14,17,19,21,25H,1-3,7-8,12-13H2,(H,35,36)/t19-,21+,25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGD2 from human CRTh2 expressed in HEK cell membranes after 60 mins by scintillation counting method |
ACS Med Chem Lett 9: 679-684 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00145
BindingDB Entry DOI: 10.7270/Q2F47RRN |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598730

(CHEMBL5204289)Show SMILES Cc1nc(N)ccc1-c1cnn(c1)C(CC1CC1)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cnnn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50458673

(CHEMBL4207731)Show SMILES [H][C@@]12CC[C@]1([H])N(C(=O)c1ccc(OC(F)(F)F)cc1)c1ccccc1[C@@H]2N(C1CC1)C(=O)OCC(O)=O |r| Show InChI InChI=1S/C25H23F3N2O6/c26-25(27,28)36-16-9-5-14(6-10-16)23(33)30-19-4-2-1-3-17(19)22(18-11-12-20(18)30)29(15-7-8-15)24(34)35-13-21(31)32/h1-6,9-10,15,18,20,22H,7-8,11-13H2,(H,31,32)/t18-,20+,22+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGD2 from human CRTh2 expressed in HEK cell membranes after 60 mins by scintillation counting method |
ACS Med Chem Lett 9: 679-684 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00145
BindingDB Entry DOI: 10.7270/Q2F47RRN |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50021075
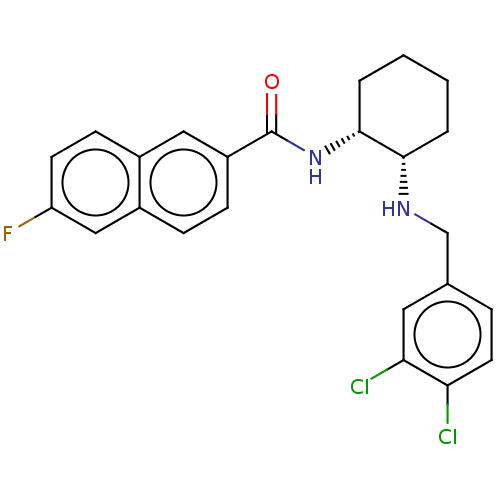
(CHEMBL3287629)Show SMILES Fc1ccc2cc(ccc2c1)C(=O)N[C@@H]1CCCC[C@@H]1NCc1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C24H23Cl2FN2O/c25-20-10-5-15(11-21(20)26)14-28-22-3-1-2-4-23(22)29-24(30)18-7-6-17-13-19(27)9-8-16(17)12-18/h5-13,22-23,28H,1-4,14H2,(H,29,30)/t22-,23+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]SS14 from human SST3 expressed in CHO membrane after 60 to 90 mins by scintillation counting |
ACS Med Chem Lett 5: 690-5 (2014)
Article DOI: 10.1021/ml500079u
BindingDB Entry DOI: 10.7270/Q22N53V4 |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50458666

(CHEMBL4204359)Show SMILES OC(=O)CCC(=O)N(C1CC1)C1C2CCCC2N(C(=O)OCc2ccccc2)c2ccccc12 Show InChI InChI=1S/C27H30N2O5/c30-24(15-16-25(31)32)28(19-13-14-19)26-20-9-4-5-11-22(20)29(23-12-6-10-21(23)26)27(33)34-17-18-7-2-1-3-8-18/h1-5,7-9,11,19,21,23,26H,6,10,12-17H2,(H,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGD2 from human CRTh2 expressed in HEK cell membranes after 60 mins by scintillation counting method |
ACS Med Chem Lett 9: 679-684 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00145
BindingDB Entry DOI: 10.7270/Q2F47RRN |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50458667

(CHEMBL4207935)Show SMILES [H][C@@]12CCC[C@]1([H])N(C(=O)c1ccc(OC(F)(F)F)cc1)c1cc(F)ccc1[C@@H]2N(C1CC1)C(=O)CCC(O)=O |r| Show InChI InChI=1S/C27H26F4N2O5/c28-16-6-11-20-22(14-16)33(26(37)15-4-9-18(10-5-15)38-27(29,30)31)21-3-1-2-19(21)25(20)32(17-7-8-17)23(34)12-13-24(35)36/h4-6,9-11,14,17,19,21,25H,1-3,7-8,12-13H2,(H,35,36)/t19-,21+,25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGD2 from human CRTh2 expressed in HEK cell membranes after 60 mins by scintillation counting method |
ACS Med Chem Lett 9: 679-684 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00145
BindingDB Entry DOI: 10.7270/Q2F47RRN |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598731
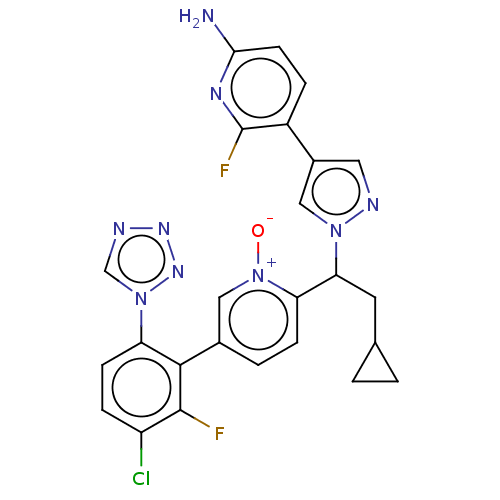
(CHEMBL5198972)Show SMILES Nc1ccc(-c2cnn(c2)C(CC2CC2)c2ccc(c[n+]2[O-])-c2c(F)c(Cl)ccc2-n2cnnn2)c(F)n1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50458672

(CHEMBL4203572)Show SMILES [H][C@@]12CCC[C@]1([H])N(C(=O)c1ccc(OC(F)(F)F)cc1)c1cc(Cl)c(F)cc1[C@@H]2N(C1CC1)C(=O)CCC(O)=O |r| Show InChI InChI=1S/C27H25ClF4N2O5/c28-19-13-22-18(12-20(19)29)25(33(15-6-7-15)23(35)10-11-24(36)37)17-2-1-3-21(17)34(22)26(38)14-4-8-16(9-5-14)39-27(30,31)32/h4-5,8-9,12-13,15,17,21,25H,1-3,6-7,10-11H2,(H,36,37)/t17-,21+,25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGD2 from human CRTh2 expressed in HEK cell membranes after 60 mins by scintillation counting method |
ACS Med Chem Lett 9: 679-684 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00145
BindingDB Entry DOI: 10.7270/Q2F47RRN |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50110440
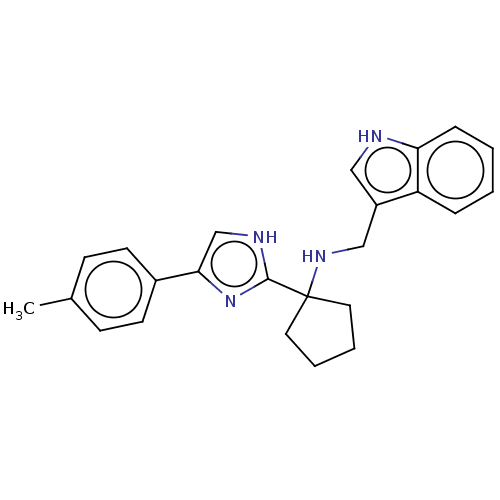
(CHEMBL3605785)Show SMILES Cc1ccc(cc1)-c1c[nH]c(n1)C1(CCCC1)NCc1c[nH]c2ccccc12 Show InChI InChI=1S/C24H26N4/c1-17-8-10-18(11-9-17)22-16-26-23(28-22)24(12-4-5-13-24)27-15-19-14-25-21-7-3-2-6-20(19)21/h2-3,6-11,14,16,25,27H,4-5,12-13,15H2,1H3,(H,26,28) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]SS-14 from human SSTR3 expressed in CHO cells after 60 to 90 mins |
Bioorg Med Chem Lett 25: 3520-5 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.087
BindingDB Entry DOI: 10.7270/Q29W0H9M |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50458652
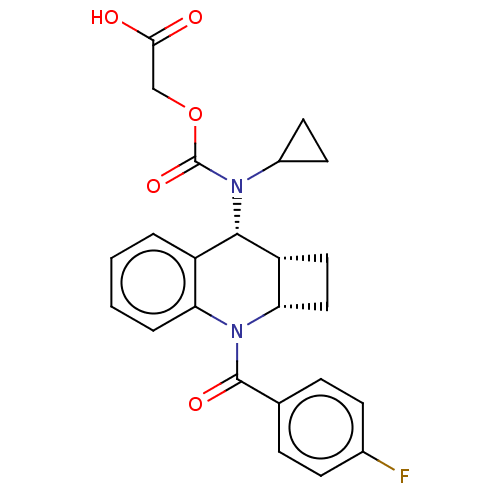
(CHEMBL4202859)Show SMILES [H][C@@]12CC[C@]1([H])N(C(=O)c1ccc(F)cc1)c1ccccc1[C@@H]2N(C1CC1)C(=O)OCC(O)=O |r| Show InChI InChI=1S/C24H23FN2O5/c25-15-7-5-14(6-8-15)23(30)27-19-4-2-1-3-17(19)22(18-11-12-20(18)27)26(16-9-10-16)24(31)32-13-21(28)29/h1-8,16,18,20,22H,9-13H2,(H,28,29)/t18-,20+,22+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGD2 from human CRTh2 expressed in HEK cell membranes after 60 mins by scintillation counting method |
ACS Med Chem Lett 9: 679-684 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00145
BindingDB Entry DOI: 10.7270/Q2F47RRN |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50021090
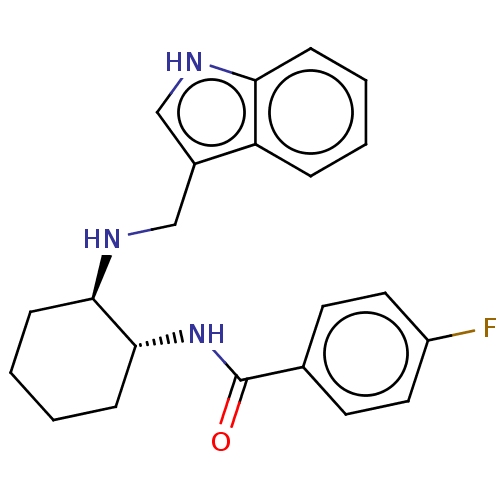
(CHEMBL3287632)Show SMILES Fc1ccc(cc1)C(=O)N[C@@H]1CCCC[C@H]1NCc1c[nH]c2ccccc12 |r| Show InChI InChI=1S/C22H24FN3O/c23-17-11-9-15(10-12-17)22(27)26-21-8-4-3-7-20(21)25-14-16-13-24-19-6-2-1-5-18(16)19/h1-2,5-6,9-13,20-21,24-25H,3-4,7-8,14H2,(H,26,27)/t20-,21-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]SS14 from human SST3 expressed in CHO membrane after 60 to 90 mins by scintillation counting |
ACS Med Chem Lett 5: 690-5 (2014)
Article DOI: 10.1021/ml500079u
BindingDB Entry DOI: 10.7270/Q22N53V4 |
More data for this
Ligand-Target Pair | |
Bombesin receptor subtype-3
(Homo sapiens (Human)) | BDBM50336889

((2S)-1,1,1-trifluoro-2-[4-(1H-pyrazol-1-yl)phenyl]...)Show SMILES O[C@@](Cc1ncc(CC2(CC2)C(F)(F)F)[nH]1)(c1ccc(cc1)-n1cccn1)C(F)(F)F |r| Show InChI InChI=1S/C20H18F6N4O/c21-19(22,23)17(6-7-17)10-14-12-27-16(29-14)11-18(31,20(24,25)26)13-2-4-15(5-3-13)30-9-1-8-28-30/h1-5,8-9,12,31H,6-7,10-11H2,(H,27,29)/t18-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125I]-[D-Tyr6,beta-Ala11,Phe13,Nle14]-Bombesin (6-14) from human BRS-3 |
ACS Med Chem Lett 2: 43-47 (2011)
Article DOI: 10.1021/ml100196d
BindingDB Entry DOI: 10.7270/Q26D5T81 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50021063
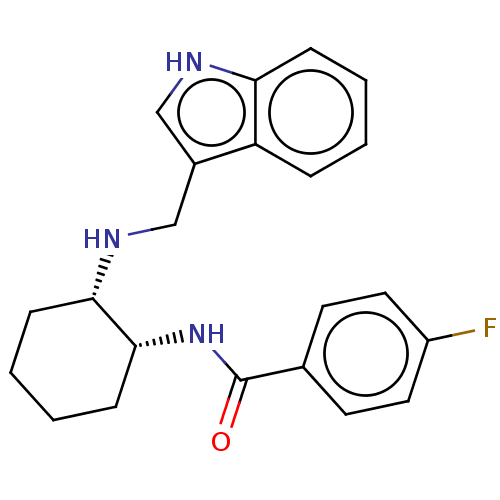
(CHEMBL3287613)Show SMILES Fc1ccc(cc1)C(=O)N[C@@H]1CCCC[C@@H]1NCc1c[nH]c2ccccc12 |r| Show InChI InChI=1S/C22H24FN3O/c23-17-11-9-15(10-12-17)22(27)26-21-8-4-3-7-20(21)25-14-16-13-24-19-6-2-1-5-18(16)19/h1-2,5-6,9-13,20-21,24-25H,3-4,7-8,14H2,(H,26,27)/t20-,21+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]SS14 from human SST3 expressed in CHO membrane after 60 to 90 mins by scintillation counting |
ACS Med Chem Lett 5: 690-5 (2014)
Article DOI: 10.1021/ml500079u
BindingDB Entry DOI: 10.7270/Q22N53V4 |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50458681

(CHEMBL4215576)Show SMILES [H][C@@]12CC[C@]1([H])N(C(=O)c1ccc(OC(F)(F)F)cc1)c1ccccc1[C@@H]2N(C1CC1)C(=O)CCC(O)=O |r| Show InChI InChI=1S/C26H25F3N2O5/c27-26(28,29)36-17-9-5-15(6-10-17)25(35)31-20-4-2-1-3-18(20)24(19-11-12-21(19)31)30(16-7-8-16)22(32)13-14-23(33)34/h1-6,9-10,16,19,21,24H,7-8,11-14H2,(H,33,34)/t19-,21+,24+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGD2 from human CRTh2 expressed in HEK cell membranes after 60 mins by scintillation counting method |
ACS Med Chem Lett 9: 679-684 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00145
BindingDB Entry DOI: 10.7270/Q2F47RRN |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50458677
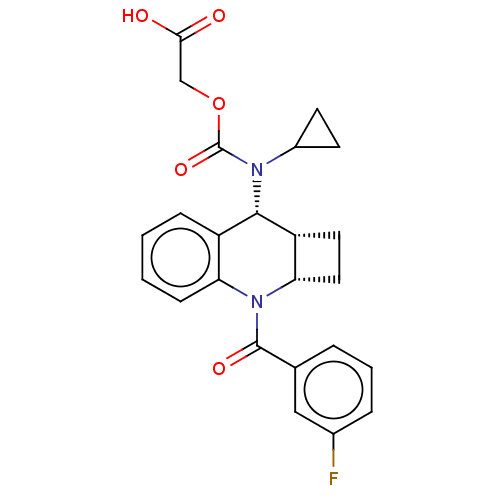
(CHEMBL4212084)Show SMILES [H][C@@]12CC[C@]1([H])N(C(=O)c1cccc(F)c1)c1ccccc1[C@@H]2N(C1CC1)C(=O)OCC(O)=O |r| Show InChI InChI=1S/C24H23FN2O5/c25-15-5-3-4-14(12-15)23(30)27-19-7-2-1-6-17(19)22(18-10-11-20(18)27)26(16-8-9-16)24(31)32-13-21(28)29/h1-7,12,16,18,20,22H,8-11,13H2,(H,28,29)/t18-,20+,22+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGD2 from human CRTh2 expressed in HEK cell membranes after 60 mins by scintillation counting method |
ACS Med Chem Lett 9: 679-684 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00145
BindingDB Entry DOI: 10.7270/Q2F47RRN |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50021109
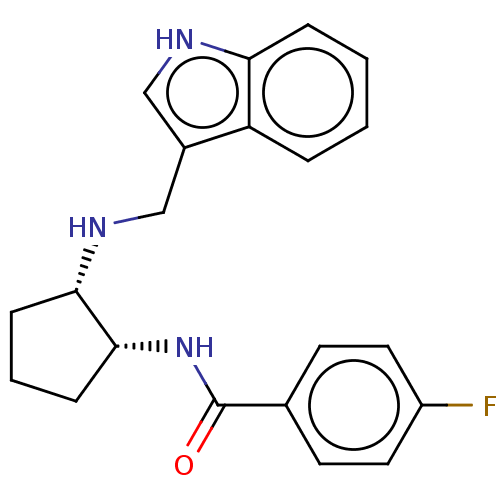
(CHEMBL3287633)Show SMILES Fc1ccc(cc1)C(=O)N[C@@H]1CCC[C@@H]1NCc1c[nH]c2ccccc12 |r| Show InChI InChI=1S/C21H22FN3O/c22-16-10-8-14(9-11-16)21(26)25-20-7-3-6-19(20)24-13-15-12-23-18-5-2-1-4-17(15)18/h1-2,4-5,8-12,19-20,23-24H,3,6-7,13H2,(H,25,26)/t19-,20+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]SS14 from human SST3 expressed in CHO membrane after 60 to 90 mins by scintillation counting |
ACS Med Chem Lett 5: 690-5 (2014)
Article DOI: 10.1021/ml500079u
BindingDB Entry DOI: 10.7270/Q22N53V4 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50021073
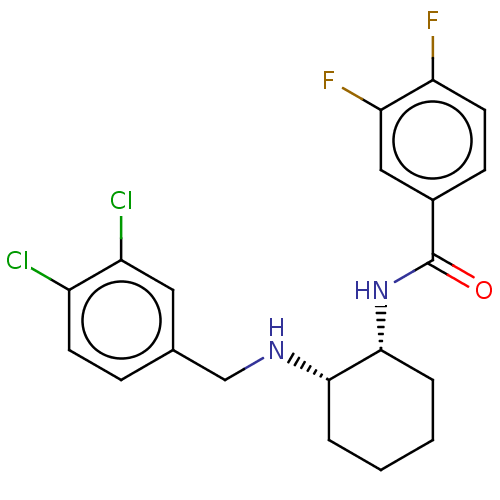
(CHEMBL3287627)Show SMILES Fc1ccc(cc1F)C(=O)N[C@@H]1CCCC[C@@H]1NCc1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C20H20Cl2F2N2O/c21-14-7-5-12(9-15(14)22)11-25-18-3-1-2-4-19(18)26-20(27)13-6-8-16(23)17(24)10-13/h5-10,18-19,25H,1-4,11H2,(H,26,27)/t18-,19+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]SS14 from human SST3 expressed in CHO membrane after 60 to 90 mins by scintillation counting |
ACS Med Chem Lett 5: 690-5 (2014)
Article DOI: 10.1021/ml500079u
BindingDB Entry DOI: 10.7270/Q22N53V4 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50110467

(CHEMBL3605795)Show SMILES Cc1ccc(cc1)-c1c[nH]c(n1)C1(CCCC1)NCc1cc2ccccc2[nH]1 Show InChI InChI=1S/C24H26N4/c1-17-8-10-18(11-9-17)22-16-25-23(28-22)24(12-4-5-13-24)26-15-20-14-19-6-2-3-7-21(19)27-20/h2-3,6-11,14,16,26-27H,4-5,12-13,15H2,1H3,(H,25,28) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]SS-14 from human SSTR3 expressed in CHO cells after 60 to 90 mins |
Bioorg Med Chem Lett 25: 3520-5 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.087
BindingDB Entry DOI: 10.7270/Q29W0H9M |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50458663

(CHEMBL4208457)Show SMILES [H][C@@]12CCC[C@]1([H])N(C(=O)c1ccc(OC(F)(F)F)cc1)c1ccc(F)cc1[C@@H]2N(C1CC1)C(=O)CCC(O)=O |r| Show InChI InChI=1S/C27H26F4N2O5/c28-16-6-11-22-20(14-16)25(32(17-7-8-17)23(34)12-13-24(35)36)19-2-1-3-21(19)33(22)26(37)15-4-9-18(10-5-15)38-27(29,30)31/h4-6,9-11,14,17,19,21,25H,1-3,7-8,12-13H2,(H,35,36)/t19-,21+,25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGD2 from human CRTh2 expressed in HEK cell membranes after 60 mins by scintillation counting method |
ACS Med Chem Lett 9: 679-684 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00145
BindingDB Entry DOI: 10.7270/Q2F47RRN |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50458669
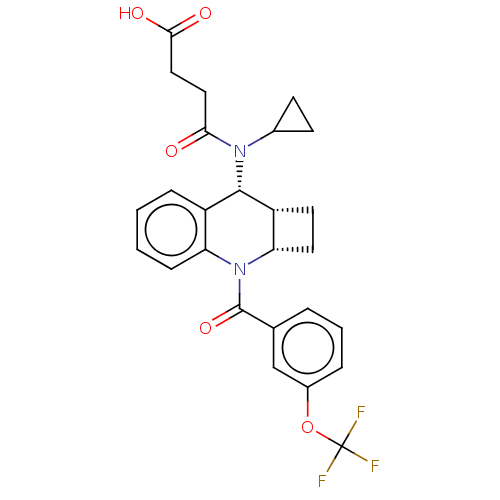
(CHEMBL4215049)Show SMILES [H][C@@]12CC[C@]1([H])N(C(=O)c1cccc(OC(F)(F)F)c1)c1ccccc1[C@@H]2N(C1CC1)C(=O)CCC(O)=O |r| Show InChI InChI=1S/C26H25F3N2O5/c27-26(28,29)36-17-5-3-4-15(14-17)25(35)31-20-7-2-1-6-18(20)24(19-10-11-21(19)31)30(16-8-9-16)22(32)12-13-23(33)34/h1-7,14,16,19,21,24H,8-13H2,(H,33,34)/t19-,21+,24+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGD2 from human CRTh2 expressed in HEK cell membranes after 60 mins by scintillation counting method |
ACS Med Chem Lett 9: 679-684 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00145
BindingDB Entry DOI: 10.7270/Q2F47RRN |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data