 Found 59 hits with Last Name = 'natsume' and Initial = 'k'
Found 59 hits with Last Name = 'natsume' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM22904

((2R)-1-(1H-imidazol-5-yl)propan-2-amine | (R)-alph...)Show InChI InChI=1S/C6H11N3/c1-5(7)2-6-3-8-4-9-6/h3-5H,2,7H2,1H3,(H,8,9)/t5-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of Histamine H3 receptor |
J Med Chem 46: 1980-8 (2003)
Article DOI: 10.1021/jm020415q
BindingDB Entry DOI: 10.7270/Q2XG9QG6 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50127610
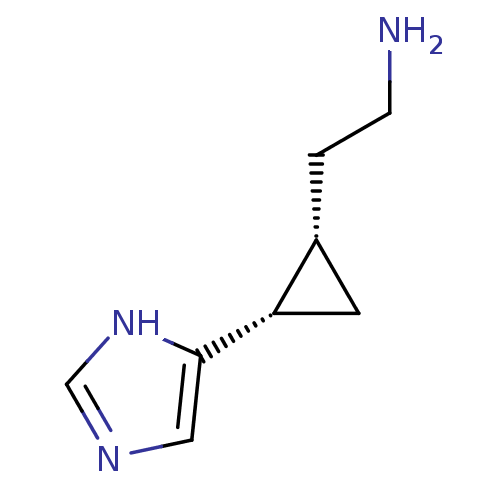
((1S,2S)-2-(2-aminoethyl)-1-(1H-imidazol-4-yl)cyclo...)Show InChI InChI=1S/C8H13N3/c9-2-1-6-3-7(6)8-4-10-5-11-8/h4-7H,1-3,9H2,(H,10,11)/t6-,7+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Affinity against rat Histamine H3 receptor using [3H]-N-alpha-methyl histamine |
J Med Chem 46: 1980-8 (2003)
Article DOI: 10.1021/jm020415q
BindingDB Entry DOI: 10.7270/Q2XG9QG6 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50127604
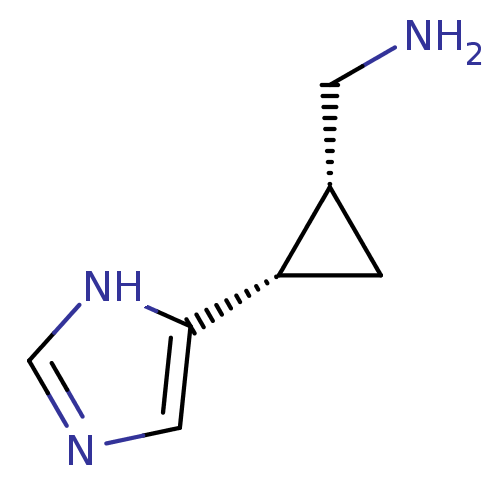
(C-[2-(1H-Imidazol-4-yl)-cyclopropyl]-methylamine |...)Show InChI InChI=1S/C7H11N3/c8-2-5-1-6(5)7-3-9-4-10-7/h3-6H,1-2,8H2,(H,9,10)/t5-,6-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Affinity against rat Histamine H3 receptor using [3H]-N-alpha-methyl histamine |
J Med Chem 46: 1980-8 (2003)
Article DOI: 10.1021/jm020415q
BindingDB Entry DOI: 10.7270/Q2XG9QG6 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM22914

(CHEMBL260374 | N-cyclohexyl-4-(1H-imidazol-5-yl)pi...)Show InChI InChI=1S/C15H24N4S/c20-15(18-13-4-2-1-3-5-13)19-8-6-12(7-9-19)14-10-16-11-17-14/h10-13H,1-9H2,(H,16,17)(H,18,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of rat Histamine H3 receptor using [3H]-N-alpha-methyl histamine |
J Med Chem 46: 1980-8 (2003)
Article DOI: 10.1021/jm020415q
BindingDB Entry DOI: 10.7270/Q2XG9QG6 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50127605
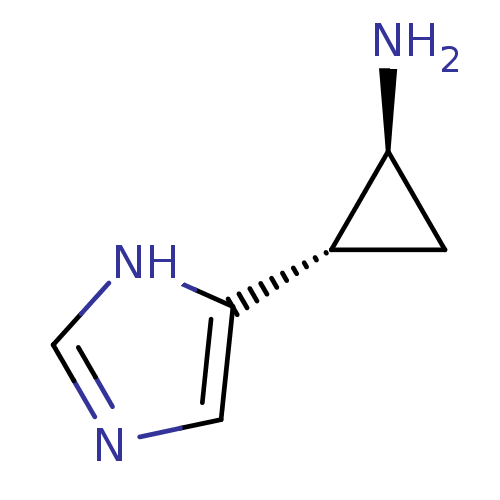
((1S,2S)-2-(1H-Imidazol-4-yl)-cyclopropylamine | (1...)Show InChI InChI=1S/C6H9N3/c7-5-1-4(5)6-2-8-3-9-6/h2-5H,1,7H2,(H,8,9)/t4-,5-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Affinity against rat Histamine H3 receptor using [3H]-N-alpha-methyl histamine |
J Med Chem 46: 1980-8 (2003)
Article DOI: 10.1021/jm020415q
BindingDB Entry DOI: 10.7270/Q2XG9QG6 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50127608
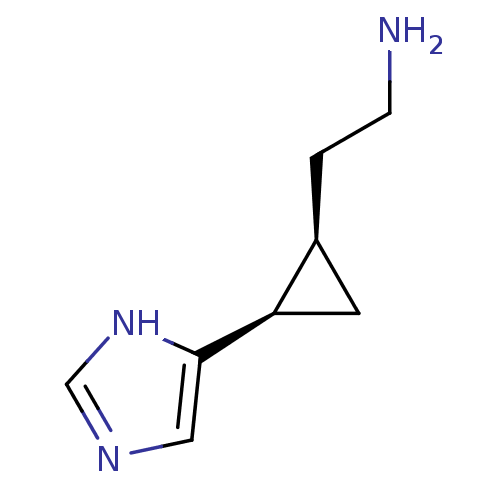
(2-[2-(1H-Imidazol-4-yl)-cyclopropyl]-ethylamine | ...)Show InChI InChI=1S/C8H13N3/c9-2-1-6-3-7(6)8-4-10-5-11-8/h4-7H,1-3,9H2,(H,10,11)/t6-,7+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Affinity against rat Histamine H3 receptor using [3H]-N-alpha-methyl histamine |
J Med Chem 46: 1980-8 (2003)
Article DOI: 10.1021/jm020415q
BindingDB Entry DOI: 10.7270/Q2XG9QG6 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50127603
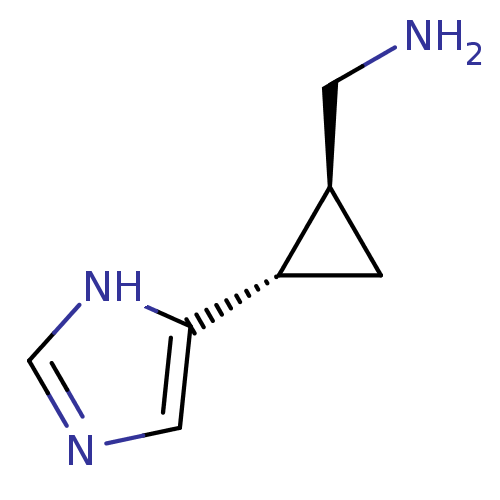
(C-[2-(1H-Imidazol-4-yl)-cyclopropyl]-methylamine |...)Show InChI InChI=1S/C7H11N3/c8-2-5-1-6(5)7-3-9-4-10-7/h3-6H,1-2,8H2,(H,9,10)/t5-,6+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Affinity against rat Histamine H3 receptor using [3H]-N-alpha-methyl histamine |
J Med Chem 46: 1980-8 (2003)
Article DOI: 10.1021/jm020415q
BindingDB Entry DOI: 10.7270/Q2XG9QG6 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50127609
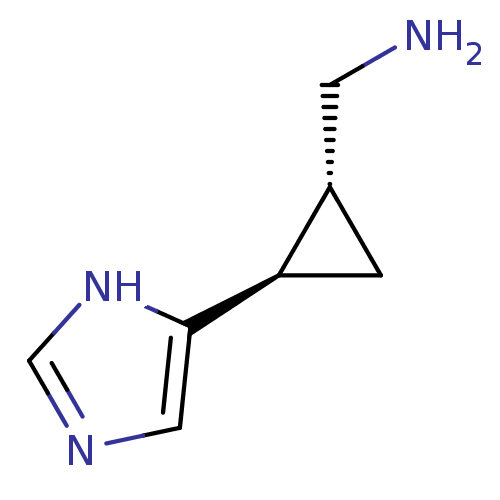
(C-[2-(1H-Imidazol-4-yl)-cyclopropyl]-methylamine |...)Show InChI InChI=1S/C7H11N3/c8-2-5-1-6(5)7-3-9-4-10-7/h3-6H,1-2,8H2,(H,9,10)/t5-,6+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Affinity against rat Histamine H3 receptor using [3H]-N-alpha-methyl histamine |
J Med Chem 46: 1980-8 (2003)
Article DOI: 10.1021/jm020415q
BindingDB Entry DOI: 10.7270/Q2XG9QG6 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50127607
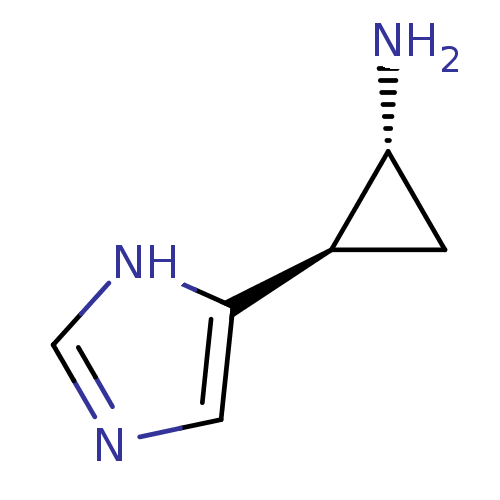
((1R,2R)-2-(1H-Imidazol-4-yl)-cyclopropylamine | (1...)Show InChI InChI=1S/C6H9N3/c7-5-1-4(5)6-2-8-3-9-6/h2-5H,1,7H2,(H,8,9)/t4-,5-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Affinity against rat Histamine H3 receptor using [3H]-N-alpha-methyl histamine |
J Med Chem 46: 1980-8 (2003)
Article DOI: 10.1021/jm020415q
BindingDB Entry DOI: 10.7270/Q2XG9QG6 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50127602

(C-[2-(1H-Imidazol-4-yl)-cyclopropyl]-methylamine |...)Show InChI InChI=1S/C7H11N3/c8-2-5-1-6(5)7-3-9-4-10-7/h3-6H,1-2,8H2,(H,9,10)/t5-,6-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Affinity against rat Histamine H3 receptor using [3H]-N-alpha-methyl histamine |
J Med Chem 46: 1980-8 (2003)
Article DOI: 10.1021/jm020415q
BindingDB Entry DOI: 10.7270/Q2XG9QG6 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50127601
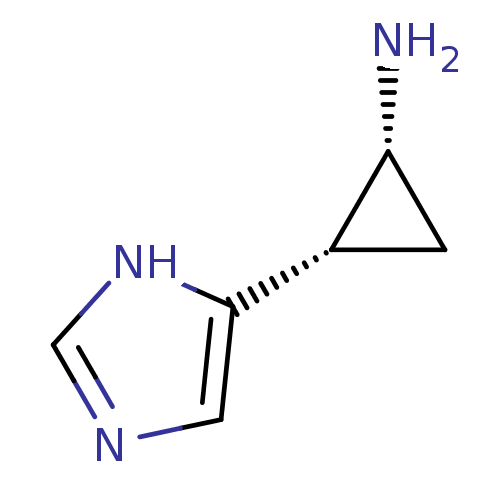
(2-(1H-Imidazol-4-yl)-cyclopropylamine | CHEMBL5938...)Show InChI InChI=1S/C6H9N3/c7-5-1-4(5)6-2-8-3-9-6/h2-5H,1,7H2,(H,8,9)/t4-,5+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 91 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Affinity against rat Histamine H3 receptor using [3H]-N-alpha-methyl histamine |
J Med Chem 46: 1980-8 (2003)
Article DOI: 10.1021/jm020415q
BindingDB Entry DOI: 10.7270/Q2XG9QG6 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50127606
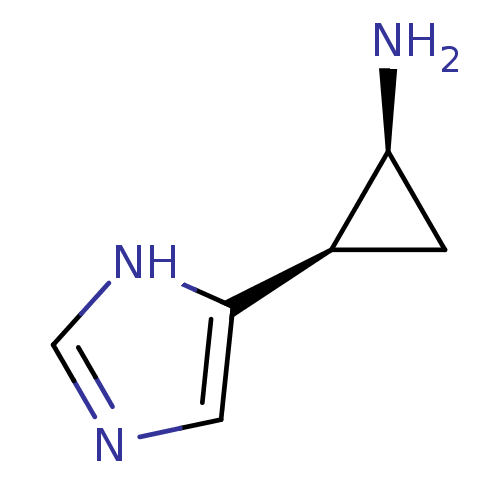
(2-(1H-Imidazol-4-yl)-cyclopropylamine | CHEMBL2940...)Show InChI InChI=1S/C6H9N3/c7-5-1-4(5)6-2-8-3-9-6/h2-5H,1,7H2,(H,8,9)/t4-,5+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Affinity against rat Histamine H3 receptor using [3H]-N-alpha-methyl histamine |
J Med Chem 46: 1980-8 (2003)
Article DOI: 10.1021/jm020415q
BindingDB Entry DOI: 10.7270/Q2XG9QG6 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50245094
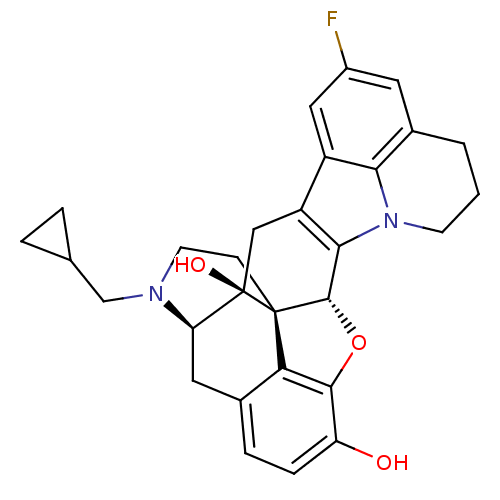
((5R,9R,13S,14S)-17-cyclopropylmethyl-6,7-didehydro...)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)c1c(C[C@@]35O)c2cc(F)cc3CCCn1c23 |r| Show InChI InChI=1S/C29H29FN2O3/c30-18-10-17-2-1-8-32-24(17)19(12-18)20-13-29(34)22-11-16-5-6-21(33)26-23(16)28(29,27(35-26)25(20)32)7-9-31(22)14-15-3-4-15/h5-6,10,12,15,22,27,33-34H,1-4,7-9,11,13-14H2/t22-,27+,28+,29-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at delta opioid receptor in ddy mouse vas deferens assessed as inhibition of DPDPE-induced electrically-stimulated contraction |
Bioorg Med Chem 16: 7956-67 (2008)
Article DOI: 10.1016/j.bmc.2008.07.065
BindingDB Entry DOI: 10.7270/Q2PN95G4 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50127609

(C-[2-(1H-Imidazol-4-yl)-cyclopropyl]-methylamine |...)Show InChI InChI=1S/C7H11N3/c8-2-5-1-6(5)7-3-9-4-10-7/h3-6H,1-2,8H2,(H,9,10)/t5-,6+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >0.000100 | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of human Histamine H1 receptor using [3H]-pyrilamine |
J Med Chem 46: 1980-8 (2003)
Article DOI: 10.1021/jm020415q
BindingDB Entry DOI: 10.7270/Q2XG9QG6 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50127608

(2-[2-(1H-Imidazol-4-yl)-cyclopropyl]-ethylamine | ...)Show InChI InChI=1S/C8H13N3/c9-2-1-6-3-7(6)8-4-10-5-11-8/h4-7H,1-3,9H2,(H,10,11)/t6-,7+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >0.000100 | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of human Histamine H1 receptor using [3H]-pyrilamine |
J Med Chem 46: 1980-8 (2003)
Article DOI: 10.1021/jm020415q
BindingDB Entry DOI: 10.7270/Q2XG9QG6 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50127610

((1S,2S)-2-(2-aminoethyl)-1-(1H-imidazol-4-yl)cyclo...)Show InChI InChI=1S/C8H13N3/c9-2-1-6-3-7(6)8-4-10-5-11-8/h4-7H,1-3,9H2,(H,10,11)/t6-,7+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >0.000100 | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of human Histamine H1 receptor using [3H]-pyrilamine |
J Med Chem 46: 1980-8 (2003)
Article DOI: 10.1021/jm020415q
BindingDB Entry DOI: 10.7270/Q2XG9QG6 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50127606

(2-(1H-Imidazol-4-yl)-cyclopropylamine | CHEMBL2940...)Show InChI InChI=1S/C6H9N3/c7-5-1-4(5)6-2-8-3-9-6/h2-5H,1,7H2,(H,8,9)/t4-,5+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >0.000100 | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of human Histamine H1 receptor using [3H]-pyrilamine |
J Med Chem 46: 1980-8 (2003)
Article DOI: 10.1021/jm020415q
BindingDB Entry DOI: 10.7270/Q2XG9QG6 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50127602

(C-[2-(1H-Imidazol-4-yl)-cyclopropyl]-methylamine |...)Show InChI InChI=1S/C7H11N3/c8-2-5-1-6(5)7-3-9-4-10-7/h3-6H,1-2,8H2,(H,9,10)/t5-,6-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >0.000100 | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of human Histamine H4 receptor |
J Med Chem 46: 1980-8 (2003)
Article DOI: 10.1021/jm020415q
BindingDB Entry DOI: 10.7270/Q2XG9QG6 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50127609

(C-[2-(1H-Imidazol-4-yl)-cyclopropyl]-methylamine |...)Show InChI InChI=1S/C7H11N3/c8-2-5-1-6(5)7-3-9-4-10-7/h3-6H,1-2,8H2,(H,9,10)/t5-,6+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >0.000100 | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of human Histamine H4 receptor |
J Med Chem 46: 1980-8 (2003)
Article DOI: 10.1021/jm020415q
BindingDB Entry DOI: 10.7270/Q2XG9QG6 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50127603

(C-[2-(1H-Imidazol-4-yl)-cyclopropyl]-methylamine |...)Show InChI InChI=1S/C7H11N3/c8-2-5-1-6(5)7-3-9-4-10-7/h3-6H,1-2,8H2,(H,9,10)/t5-,6+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >0.000100 | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of human Histamine H4 receptor |
J Med Chem 46: 1980-8 (2003)
Article DOI: 10.1021/jm020415q
BindingDB Entry DOI: 10.7270/Q2XG9QG6 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50127610

((1S,2S)-2-(2-aminoethyl)-1-(1H-imidazol-4-yl)cyclo...)Show InChI InChI=1S/C8H13N3/c9-2-1-6-3-7(6)8-4-10-5-11-8/h4-7H,1-3,9H2,(H,10,11)/t6-,7+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >0.000100 | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of human Histamine H4 receptor |
J Med Chem 46: 1980-8 (2003)
Article DOI: 10.1021/jm020415q
BindingDB Entry DOI: 10.7270/Q2XG9QG6 |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50127604

(C-[2-(1H-Imidazol-4-yl)-cyclopropyl]-methylamine |...)Show InChI InChI=1S/C7H11N3/c8-2-5-1-6(5)7-3-9-4-10-7/h3-6H,1-2,8H2,(H,9,10)/t5-,6-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >0.000100 | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of human Histamine H2 receptor using [3H]-tiotidine |
J Med Chem 46: 1980-8 (2003)
Article DOI: 10.1021/jm020415q
BindingDB Entry DOI: 10.7270/Q2XG9QG6 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50127607

((1R,2R)-2-(1H-Imidazol-4-yl)-cyclopropylamine | (1...)Show InChI InChI=1S/C6H9N3/c7-5-1-4(5)6-2-8-3-9-6/h2-5H,1,7H2,(H,8,9)/t4-,5-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >0.000100 | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of human Histamine H1 receptor using [3H]-pyrilamine |
J Med Chem 46: 1980-8 (2003)
Article DOI: 10.1021/jm020415q
BindingDB Entry DOI: 10.7270/Q2XG9QG6 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50127601

(2-(1H-Imidazol-4-yl)-cyclopropylamine | CHEMBL5938...)Show InChI InChI=1S/C6H9N3/c7-5-1-4(5)6-2-8-3-9-6/h2-5H,1,7H2,(H,8,9)/t4-,5+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >0.000100 | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of human Histamine H1 receptor using [3H]-pyrilamine |
J Med Chem 46: 1980-8 (2003)
Article DOI: 10.1021/jm020415q
BindingDB Entry DOI: 10.7270/Q2XG9QG6 |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50127606

(2-(1H-Imidazol-4-yl)-cyclopropylamine | CHEMBL2940...)Show InChI InChI=1S/C6H9N3/c7-5-1-4(5)6-2-8-3-9-6/h2-5H,1,7H2,(H,8,9)/t4-,5+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >0.000100 | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of human Histamine H2 receptor using [3H]-tiotidine |
J Med Chem 46: 1980-8 (2003)
Article DOI: 10.1021/jm020415q
BindingDB Entry DOI: 10.7270/Q2XG9QG6 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50127602

(C-[2-(1H-Imidazol-4-yl)-cyclopropyl]-methylamine |...)Show InChI InChI=1S/C7H11N3/c8-2-5-1-6(5)7-3-9-4-10-7/h3-6H,1-2,8H2,(H,9,10)/t5-,6-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >0.000100 | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of human Histamine H3 receptor using [3H]-N-alpha-methyl histamine |
J Med Chem 46: 1980-8 (2003)
Article DOI: 10.1021/jm020415q
BindingDB Entry DOI: 10.7270/Q2XG9QG6 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50127606

(2-(1H-Imidazol-4-yl)-cyclopropylamine | CHEMBL2940...)Show InChI InChI=1S/C6H9N3/c7-5-1-4(5)6-2-8-3-9-6/h2-5H,1,7H2,(H,8,9)/t4-,5+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >0.000100 | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of human Histamine H4 receptor |
J Med Chem 46: 1980-8 (2003)
Article DOI: 10.1021/jm020415q
BindingDB Entry DOI: 10.7270/Q2XG9QG6 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50127604

(C-[2-(1H-Imidazol-4-yl)-cyclopropyl]-methylamine |...)Show InChI InChI=1S/C7H11N3/c8-2-5-1-6(5)7-3-9-4-10-7/h3-6H,1-2,8H2,(H,9,10)/t5-,6-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >0.000100 | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of human Histamine H1 receptor using [3H]-pyrilamine |
J Med Chem 46: 1980-8 (2003)
Article DOI: 10.1021/jm020415q
BindingDB Entry DOI: 10.7270/Q2XG9QG6 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50127603

(C-[2-(1H-Imidazol-4-yl)-cyclopropyl]-methylamine |...)Show InChI InChI=1S/C7H11N3/c8-2-5-1-6(5)7-3-9-4-10-7/h3-6H,1-2,8H2,(H,9,10)/t5-,6+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >0.000100 | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of human Histamine H3 receptor using [3H]-N-alpha-methyl histamine |
J Med Chem 46: 1980-8 (2003)
Article DOI: 10.1021/jm020415q
BindingDB Entry DOI: 10.7270/Q2XG9QG6 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50127601

(2-(1H-Imidazol-4-yl)-cyclopropylamine | CHEMBL5938...)Show InChI InChI=1S/C6H9N3/c7-5-1-4(5)6-2-8-3-9-6/h2-5H,1,7H2,(H,8,9)/t4-,5+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.33E+3 | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of human H3-receptor using [3H]-N-alpha-methyl histamine |
J Med Chem 46: 1980-8 (2003)
Article DOI: 10.1021/jm020415q
BindingDB Entry DOI: 10.7270/Q2XG9QG6 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50127604

(C-[2-(1H-Imidazol-4-yl)-cyclopropyl]-methylamine |...)Show InChI InChI=1S/C7H11N3/c8-2-5-1-6(5)7-3-9-4-10-7/h3-6H,1-2,8H2,(H,9,10)/t5-,6-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 96 | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of human Histamine H3 receptor using [3H]-N-alpha-methyl histamine |
J Med Chem 46: 1980-8 (2003)
Article DOI: 10.1021/jm020415q
BindingDB Entry DOI: 10.7270/Q2XG9QG6 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM7966

(2-(1H-imidazol-4-yl)ethan-1-amine | CHEMBL544208 |...)Show InChI InChI=1S/C5H9N3/c6-2-1-5-3-7-4-8-5/h3-4H,1-2,6H2,(H,7,8) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | n/a | n/a | 77 | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of human Histamine H3 receptor using [3H]-N-alpha-methyl histamine |
J Med Chem 46: 1980-8 (2003)
Article DOI: 10.1021/jm020415q
BindingDB Entry DOI: 10.7270/Q2XG9QG6 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50127605

((1S,2S)-2-(1H-Imidazol-4-yl)-cyclopropylamine | (1...)Show InChI InChI=1S/C6H9N3/c7-5-1-4(5)6-2-8-3-9-6/h2-5H,1,7H2,(H,8,9)/t4-,5-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 42 | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of human Histamine H3 receptor using [3H]-N-alpha-methyl histamine |
J Med Chem 46: 1980-8 (2003)
Article DOI: 10.1021/jm020415q
BindingDB Entry DOI: 10.7270/Q2XG9QG6 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50127607

((1R,2R)-2-(1H-Imidazol-4-yl)-cyclopropylamine | (1...)Show InChI InChI=1S/C6H9N3/c7-5-1-4(5)6-2-8-3-9-6/h2-5H,1,7H2,(H,8,9)/t4-,5-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 724 | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of human Histamine H3 receptor using [3H]-N-alpha-methyl histamine |
J Med Chem 46: 1980-8 (2003)
Article DOI: 10.1021/jm020415q
BindingDB Entry DOI: 10.7270/Q2XG9QG6 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM7966

(2-(1H-imidazol-4-yl)ethan-1-amine | CHEMBL544208 |...)Show InChI InChI=1S/C5H9N3/c6-2-1-5-3-7-4-8-5/h3-4H,1-2,6H2,(H,7,8) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | n/a | n/a | 75 | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of human Histamine H4 receptor |
J Med Chem 46: 1980-8 (2003)
Article DOI: 10.1021/jm020415q
BindingDB Entry DOI: 10.7270/Q2XG9QG6 |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50127607

((1R,2R)-2-(1H-Imidazol-4-yl)-cyclopropylamine | (1...)Show InChI InChI=1S/C6H9N3/c7-5-1-4(5)6-2-8-3-9-6/h2-5H,1,7H2,(H,8,9)/t4-,5-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of human Histamine H2 receptor using [3H]-tiotidine |
J Med Chem 46: 1980-8 (2003)
Article DOI: 10.1021/jm020415q
BindingDB Entry DOI: 10.7270/Q2XG9QG6 |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50127608

(2-[2-(1H-Imidazol-4-yl)-cyclopropyl]-ethylamine | ...)Show InChI InChI=1S/C8H13N3/c9-2-1-6-3-7(6)8-4-10-5-11-8/h4-7H,1-3,9H2,(H,10,11)/t6-,7+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 93 | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of human Histamine H2 receptor using [3H]-tiotidine |
J Med Chem 46: 1980-8 (2003)
Article DOI: 10.1021/jm020415q
BindingDB Entry DOI: 10.7270/Q2XG9QG6 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM22911
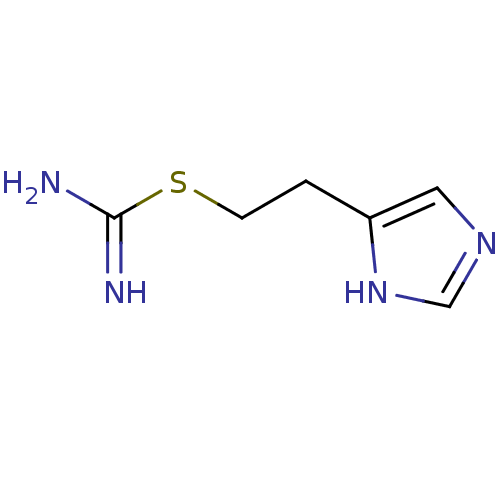
(2-(3H-imidazol-4-yl)ethylsulfanylmethanimidamide |...)Show InChI InChI=1S/C6H10N4S/c7-6(8)11-2-1-5-3-9-4-10-5/h3-4H,1-2H2,(H3,7,8)(H,9,10) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of human Histamine H3 receptor using [3H]-N-alpha-methyl histamine |
J Med Chem 46: 1980-8 (2003)
Article DOI: 10.1021/jm020415q
BindingDB Entry DOI: 10.7270/Q2XG9QG6 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM22911

(2-(3H-imidazol-4-yl)ethylsulfanylmethanimidamide |...)Show InChI InChI=1S/C6H10N4S/c7-6(8)11-2-1-5-3-9-4-10-5/h3-4H,1-2H2,(H3,7,8)(H,9,10) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 51 | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of human Histamine H4 receptor |
J Med Chem 46: 1980-8 (2003)
Article DOI: 10.1021/jm020415q
BindingDB Entry DOI: 10.7270/Q2XG9QG6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50127608

(2-[2-(1H-Imidazol-4-yl)-cyclopropyl]-ethylamine | ...)Show InChI InChI=1S/C8H13N3/c9-2-1-6-3-7(6)8-4-10-5-11-8/h4-7H,1-3,9H2,(H,10,11)/t6-,7+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.41E+3 | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of human Histamine H3 receptor using [3H]-N-alpha-methyl histamine |
J Med Chem 46: 1980-8 (2003)
Article DOI: 10.1021/jm020415q
BindingDB Entry DOI: 10.7270/Q2XG9QG6 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM7966

(2-(1H-imidazol-4-yl)ethan-1-amine | CHEMBL544208 |...)Show InChI InChI=1S/C5H9N3/c6-2-1-5-3-7-4-8-5/h3-4H,1-2,6H2,(H,7,8) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | n/a | n/a | 13 | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of human Histamine H1 receptor using [3H]-pyrilamine |
J Med Chem 46: 1980-8 (2003)
Article DOI: 10.1021/jm020415q
BindingDB Entry DOI: 10.7270/Q2XG9QG6 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50127605

((1S,2S)-2-(1H-Imidazol-4-yl)-cyclopropylamine | (1...)Show InChI InChI=1S/C6H9N3/c7-5-1-4(5)6-2-8-3-9-6/h2-5H,1,7H2,(H,8,9)/t4-,5-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 864 | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of human Histamine H1 receptor using [3H]-pyrilamine |
J Med Chem 46: 1980-8 (2003)
Article DOI: 10.1021/jm020415q
BindingDB Entry DOI: 10.7270/Q2XG9QG6 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50127610

((1S,2S)-2-(2-aminoethyl)-1-(1H-imidazol-4-yl)cyclo...)Show InChI InChI=1S/C8H13N3/c9-2-1-6-3-7(6)8-4-10-5-11-8/h4-7H,1-3,9H2,(H,10,11)/t6-,7+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of human Histamine H3 receptor using [3H]-N-alpha-methyl histamine |
J Med Chem 46: 1980-8 (2003)
Article DOI: 10.1021/jm020415q
BindingDB Entry DOI: 10.7270/Q2XG9QG6 |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50127605

((1S,2S)-2-(1H-Imidazol-4-yl)-cyclopropylamine | (1...)Show InChI InChI=1S/C6H9N3/c7-5-1-4(5)6-2-8-3-9-6/h2-5H,1,7H2,(H,8,9)/t4-,5-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 696 | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of human Histamine H2 receptor using [3H]-tiotidine |
J Med Chem 46: 1980-8 (2003)
Article DOI: 10.1021/jm020415q
BindingDB Entry DOI: 10.7270/Q2XG9QG6 |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM7966

(2-(1H-imidazol-4-yl)ethan-1-amine | CHEMBL544208 |...)Show InChI InChI=1S/C5H9N3/c6-2-1-5-3-7-4-8-5/h3-4H,1-2,6H2,(H,7,8) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | n/a | n/a | 463 | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of human Histamine H2 receptor using [3H]-tiotidine |
J Med Chem 46: 1980-8 (2003)
Article DOI: 10.1021/jm020415q
BindingDB Entry DOI: 10.7270/Q2XG9QG6 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50127601

(2-(1H-Imidazol-4-yl)-cyclopropylamine | CHEMBL5938...)Show InChI InChI=1S/C6H9N3/c7-5-1-4(5)6-2-8-3-9-6/h2-5H,1,7H2,(H,8,9)/t4-,5+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >0.000100 | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of human Histamine H4 receptor |
J Med Chem 46: 1980-8 (2003)
Article DOI: 10.1021/jm020415q
BindingDB Entry DOI: 10.7270/Q2XG9QG6 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50127602

(C-[2-(1H-Imidazol-4-yl)-cyclopropyl]-methylamine |...)Show InChI InChI=1S/C7H11N3/c8-2-5-1-6(5)7-3-9-4-10-7/h3-6H,1-2,8H2,(H,9,10)/t5-,6-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >0.000100 | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of human Histamine H1 receptor using [3H]-pyrilamine |
J Med Chem 46: 1980-8 (2003)
Article DOI: 10.1021/jm020415q
BindingDB Entry DOI: 10.7270/Q2XG9QG6 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50127603

(C-[2-(1H-Imidazol-4-yl)-cyclopropyl]-methylamine |...)Show InChI InChI=1S/C7H11N3/c8-2-5-1-6(5)7-3-9-4-10-7/h3-6H,1-2,8H2,(H,9,10)/t5-,6+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >0.000100 | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of human Histamine H1 receptor using [3H]-pyrilamine |
J Med Chem 46: 1980-8 (2003)
Article DOI: 10.1021/jm020415q
BindingDB Entry DOI: 10.7270/Q2XG9QG6 |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50127609

(C-[2-(1H-Imidazol-4-yl)-cyclopropyl]-methylamine |...)Show InChI InChI=1S/C7H11N3/c8-2-5-1-6(5)7-3-9-4-10-7/h3-6H,1-2,8H2,(H,9,10)/t5-,6+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >0.000100 | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of human Histamine H2 receptor using [3H]-tiotidine |
J Med Chem 46: 1980-8 (2003)
Article DOI: 10.1021/jm020415q
BindingDB Entry DOI: 10.7270/Q2XG9QG6 |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50127610

((1S,2S)-2-(2-aminoethyl)-1-(1H-imidazol-4-yl)cyclo...)Show InChI InChI=1S/C8H13N3/c9-2-1-6-3-7(6)8-4-10-5-11-8/h4-7H,1-3,9H2,(H,10,11)/t6-,7+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >0.000100 | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of human Histamine H2 receptor using [3H]-tiotidine |
J Med Chem 46: 1980-8 (2003)
Article DOI: 10.1021/jm020415q
BindingDB Entry DOI: 10.7270/Q2XG9QG6 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data