

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cathepsin K (Homo sapiens (Human)) | BDBM50113662 (1-(2,3-Dichloro-phenyl)-1H-[1,2,4]triazole-3-carbo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Eq. constant (Ki) for inhibition of Cathepsin K was determined by progress curves, following hydrolysis of Z-Phe-Arg- Amc in the absence and presence... | J Med Chem 45: 2352-4 (2002) BindingDB Entry DOI: 10.7270/Q2DV1J73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50113667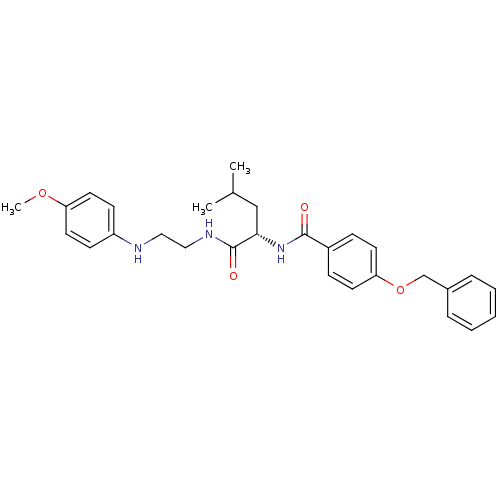 (4-Benzyloxy-N-{1-[2-(4-methoxy-phenylamino)-ethylc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Eq. constant (Ki) for inhibition of Cathepsin K was determined by progress curves, following hydrolysis of Z-Phe-Arg- Amc in the absence and presence... | J Med Chem 45: 2352-4 (2002) BindingDB Entry DOI: 10.7270/Q2DV1J73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Homo sapiens (Human)) | BDBM50308093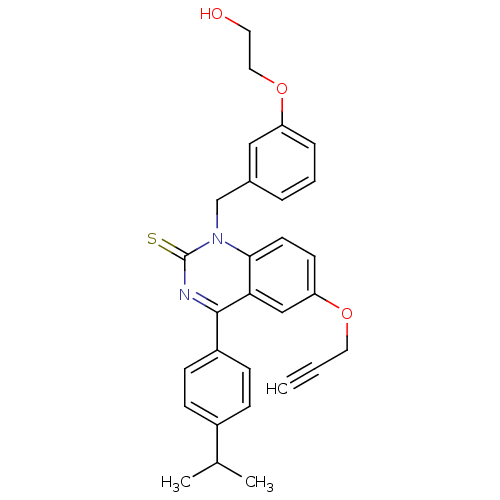 (1-[3-(2-Hydroxy-ethoxy)-benzyl]-4-(4-isopropyl-phe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at human CaSR expressed in CCL39 cells assessed as inhibition of extracellular calcium-induced intracellular calcium transient by... | J Med Chem 53: 2250-63 (2010) Article DOI: 10.1021/jm901811v BindingDB Entry DOI: 10.7270/Q2Q240BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM176622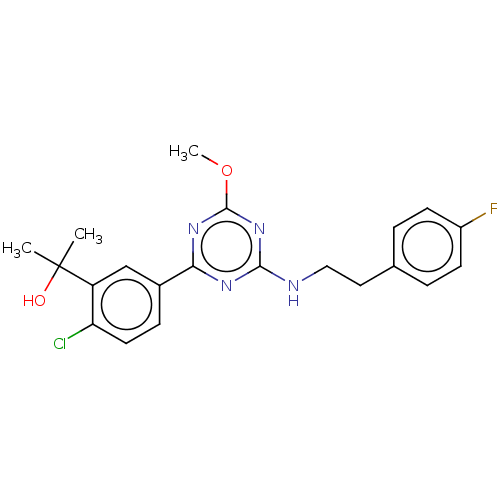 (US9115121, 13) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Patent | Assay Description Affinity binding tests were performed according to the experimental conditions described by M. Rinaldi-Carmona in J. Pharmacol. Exp. Therap. 1998, 28... | US Patent US9115121 (2015) BindingDB Entry DOI: 10.7270/Q23N225X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM176622 (US9115121, 13) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D Curated by ChEMBL | Assay Description Binding affinity to human CB2 receptor | Bioorg Med Chem Lett 24: 283-7 (2014) Article DOI: 10.1016/j.bmcl.2013.11.023 BindingDB Entry DOI: 10.7270/Q2ZC85VD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM10652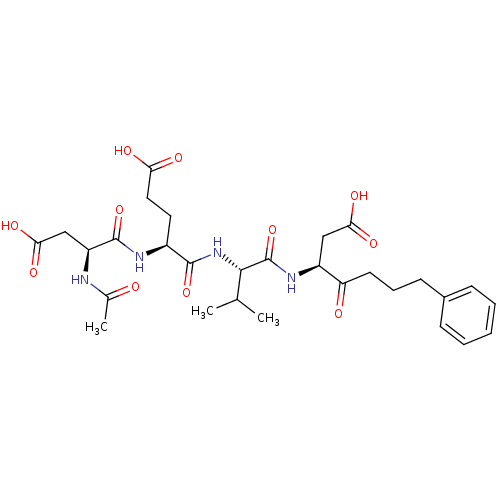 ((3S)-3-[(2S)-2-[(4S)-4-[(3S)-3-acetamido-3-formami...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.771 | n/a | n/a | n/a | n/a | 7.0 | 37 |
Merck Research Laboratories | Assay Description The substrate peptides terminating in AMC are processed by caspases with or without inhibitors, and the accumulation of AMC was assessed with a Cytof... | J Med Chem 47: 2466-74 (2004) Article DOI: 10.1021/jm0305523 BindingDB Entry DOI: 10.7270/Q24F1NZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM176645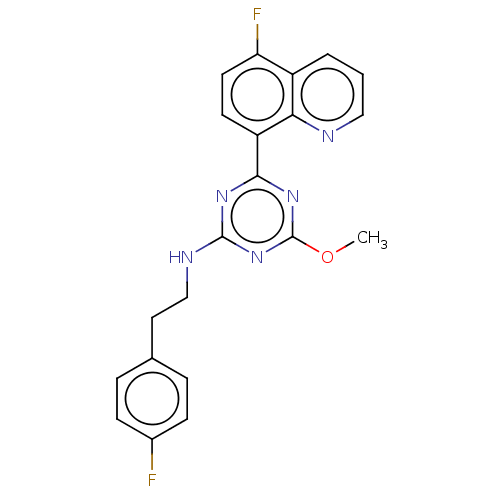 (US9115121, 37) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Patent | Assay Description Affinity binding tests were performed according to the experimental conditions described by M. Rinaldi-Carmona in J. Pharmacol. Exp. Therap. 1998, 28... | US Patent US9115121 (2015) BindingDB Entry DOI: 10.7270/Q23N225X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM176668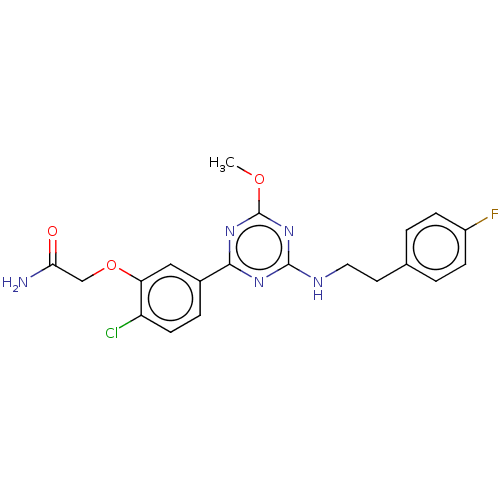 (US9115121, 65) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Patent | Assay Description Affinity binding tests were performed according to the experimental conditions described by M. Rinaldi-Carmona in J. Pharmacol. Exp. Therap. 1998, 28... | US Patent US9115121 (2015) BindingDB Entry DOI: 10.7270/Q23N225X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM176606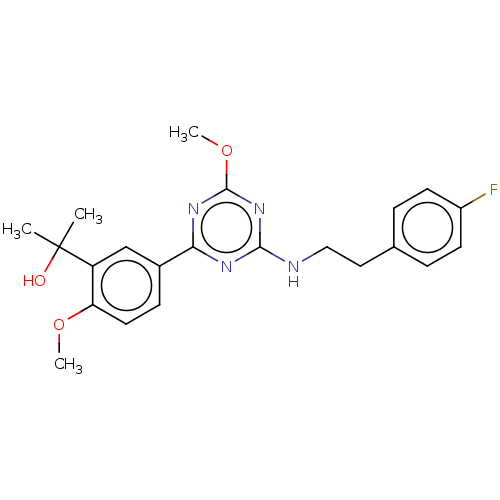 (US9115121, 58) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Patent | Assay Description Affinity binding tests were performed according to the experimental conditions described by M. Rinaldi-Carmona in J. Pharmacol. Exp. Therap. 1998, 28... | US Patent US9115121 (2015) BindingDB Entry DOI: 10.7270/Q23N225X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM176604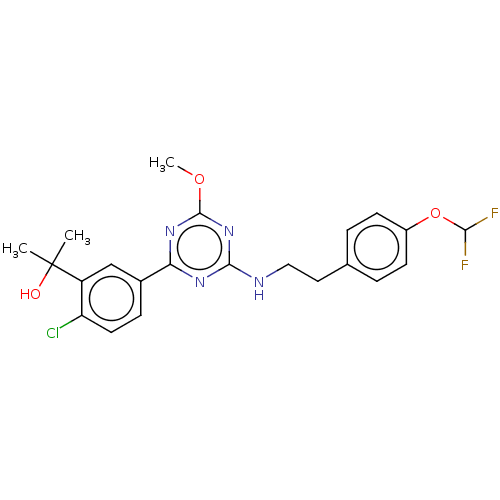 (US9115121, 46) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Patent | Assay Description Affinity binding tests were performed according to the experimental conditions described by M. Rinaldi-Carmona in J. Pharmacol. Exp. Therap. 1998, 28... | US Patent US9115121 (2015) BindingDB Entry DOI: 10.7270/Q23N225X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Homo sapiens (Human)) | BDBM50308090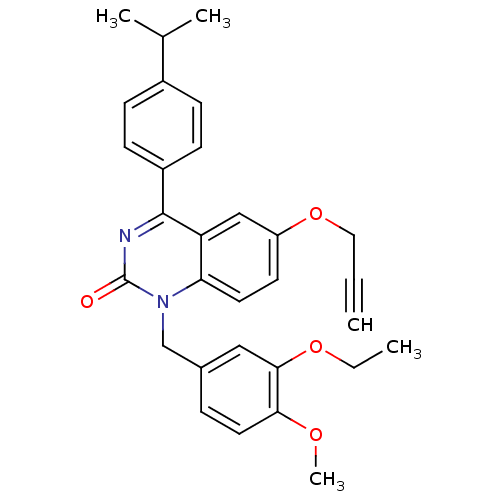 (1-(3-Ethoxy-4-methoxy-benzyl)-4-(4-isopropyl-pheny...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at human CaSR expressed in CCL39 cells assessed as inhibition of extracellular calcium-induced intracellular calcium transient by... | J Med Chem 53: 2250-63 (2010) Article DOI: 10.1021/jm901811v BindingDB Entry DOI: 10.7270/Q2Q240BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50494951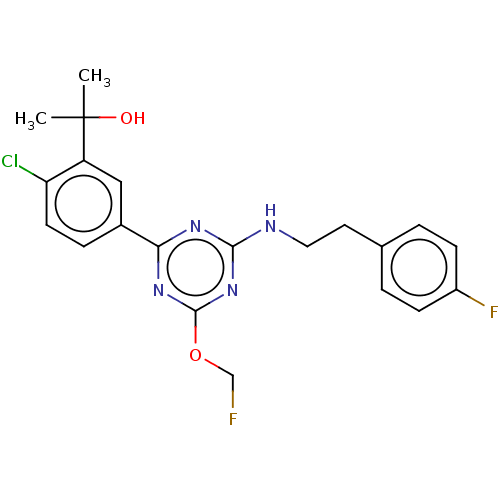 (CHEMBL3099054) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D Curated by ChEMBL | Assay Description Binding affinity to human CB2 receptor expressed in CHO cells | Bioorg Med Chem Lett 24: 283-7 (2014) Article DOI: 10.1016/j.bmcl.2013.11.023 BindingDB Entry DOI: 10.7270/Q2ZC85VD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Homo sapiens (Human)) | BDBM50308081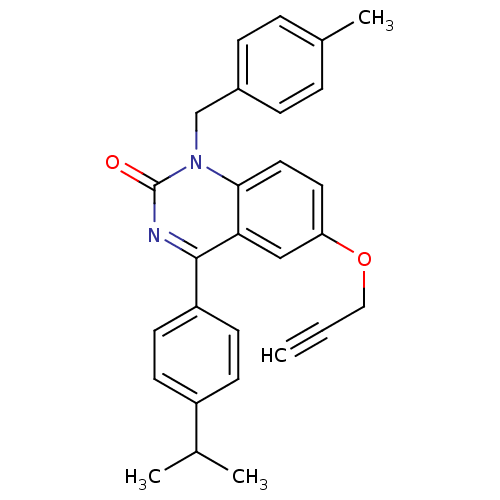 (4-(4-Isopropyl-phenyl)-1-(4-methyl-benzyl)-6-propa...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at human CaSR expressed in CCL39 cells assessed as inhibition of extracellular calcium-induced intracellular calcium transient by... | J Med Chem 53: 2250-63 (2010) Article DOI: 10.1021/jm901811v BindingDB Entry DOI: 10.7270/Q2Q240BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Homo sapiens (Human)) | BDBM50308087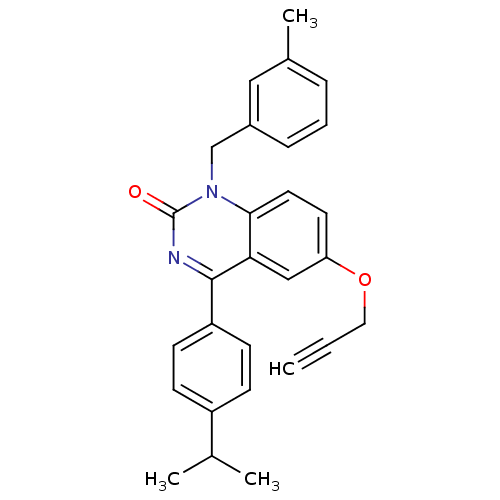 (4-(4-Isopropyl-phenyl)-1-(3-methyl-benzyl)-6-propa...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at human CaSR expressed in CCL39 cells assessed as inhibition of extracellular calcium-induced intracellular calcium transient by... | J Med Chem 53: 2250-63 (2010) Article DOI: 10.1021/jm901811v BindingDB Entry DOI: 10.7270/Q2Q240BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM10227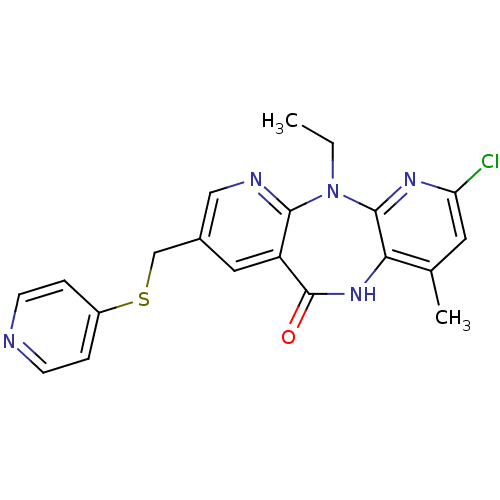 (5-chloro-2-ethyl-7-methyl-13-[(pyridin-4-ylsulfany...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Boehringer Ingelheim (Canada) Ltd. | Assay Description IC50 values for wild-type and mutant RTs were obtained from a scintillation proximity assay using poly rC/biotin-dG15 and 3H-dGTP. Each value represe... | Bioorg Med Chem Lett 14: 739-42 (2004) Article DOI: 10.1016/j.bmcl.2003.11.049 BindingDB Entry DOI: 10.7270/Q2C53J2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Homo sapiens (Human)) | BDBM50308103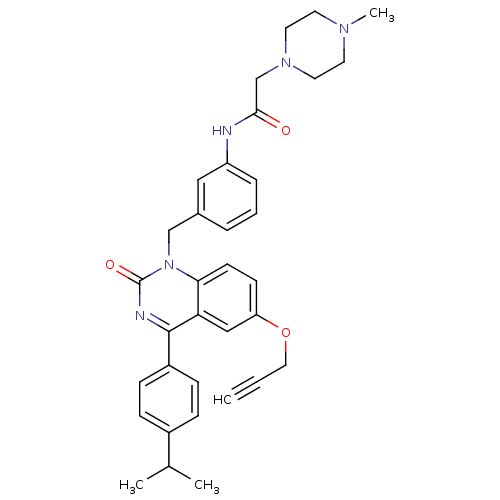 (CHEMBL590539 | N-{3-[4-(4-Isopropyl-pheny)-2-oxo-6...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at human CaSR expressed in CCL39 cells assessed as inhibition of extracellular calcium-induced intracellular calcium transient by... | J Med Chem 53: 2250-63 (2010) Article DOI: 10.1021/jm901811v BindingDB Entry DOI: 10.7270/Q2Q240BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Homo sapiens (Human)) | BDBM50308108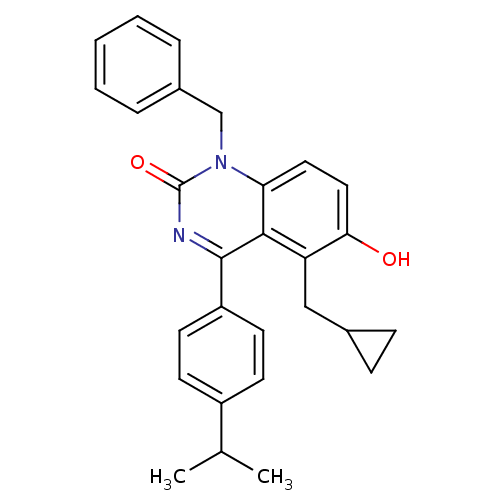 (1-Benzyl-5-cyclopropylmethyl-6-hydroxy-4-(4-isopro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at human CaSR expressed in CCL39 cells assessed as inhibition of extracellular calcium-induced intracellular calcium transient by... | J Med Chem 53: 2250-63 (2010) Article DOI: 10.1021/jm901811v BindingDB Entry DOI: 10.7270/Q2Q240BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM176620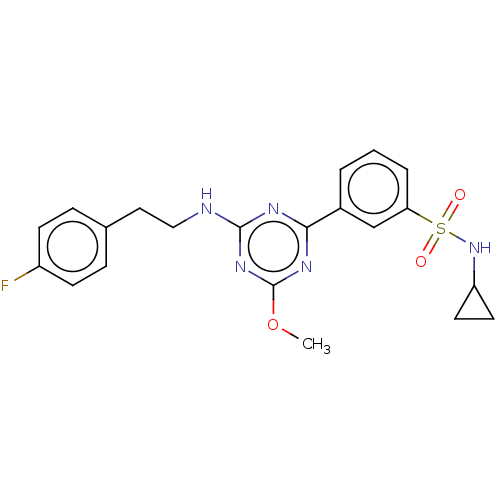 (US9115121, 11) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Patent | Assay Description Affinity binding tests were performed according to the experimental conditions described by M. Rinaldi-Carmona in J. Pharmacol. Exp. Therap. 1998, 28... | US Patent US9115121 (2015) BindingDB Entry DOI: 10.7270/Q23N225X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Homo sapiens (Human)) | BDBM50308089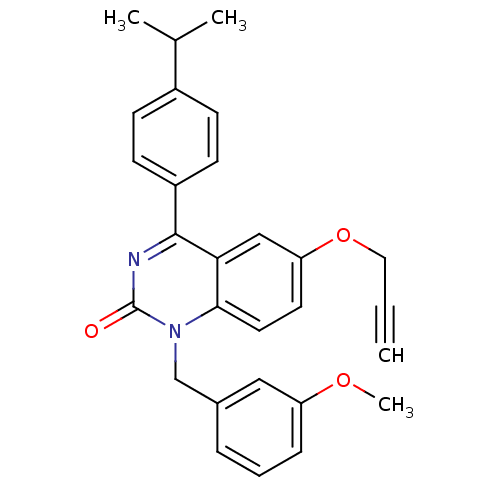 (1-(3-Methoxy-benzyl)-4-(4-isopropyl-phenyl)-6-prop...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at human CaSR expressed in CCL39 cells assessed as inhibition of extracellular calcium-induced intracellular calcium transient by... | J Med Chem 53: 2250-63 (2010) Article DOI: 10.1021/jm901811v BindingDB Entry DOI: 10.7270/Q2Q240BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM176660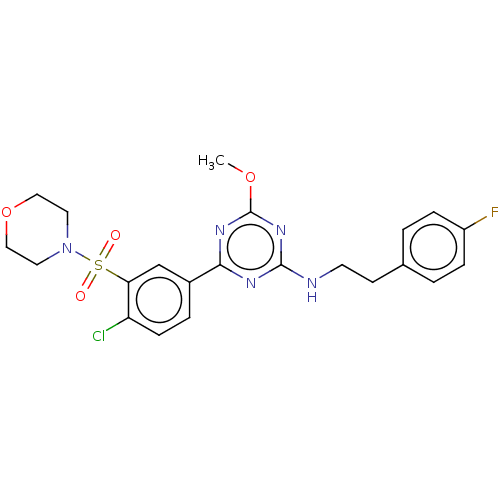 (US9115121, 53) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Patent | Assay Description Affinity binding tests were performed according to the experimental conditions described by M. Rinaldi-Carmona in J. Pharmacol. Exp. Therap. 1998, 28... | US Patent US9115121 (2015) BindingDB Entry DOI: 10.7270/Q23N225X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM10654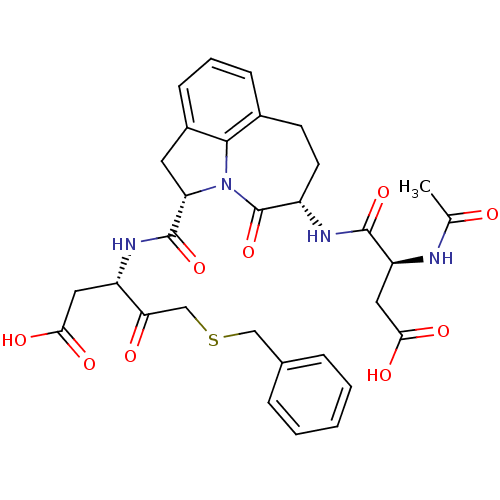 ((3S)-3-[({(2S)-5-[(N-ACETYL-L-ALPHA-ASPARTYL)AMINO...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.0 | 37 |
Merck Research Laboratories | Assay Description The substrate peptides terminating in AMC are processed by caspases with or without inhibitors, and the accumulation of AMC was assessed with a Cytof... | J Med Chem 47: 2466-74 (2004) Article DOI: 10.1021/jm0305523 BindingDB Entry DOI: 10.7270/Q24F1NZ4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM2058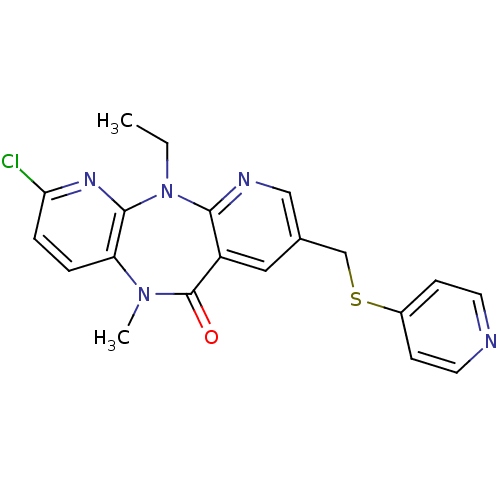 (2,8-disubstituted dipyridodiazepinone 41 | 2-Chlor...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Boehringer Ingelheim (Canada) Ltd. | Assay Description IC50 values for wild-type and mutant RTs were obtained from a scintillation proximity assay using poly rC/biotin-dG15 and 3H-dGTP. Each value represe... | Bioorg Med Chem Lett 14: 739-42 (2004) Article DOI: 10.1016/j.bmcl.2003.11.049 BindingDB Entry DOI: 10.7270/Q2C53J2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-1 (Homo sapiens (Human)) | BDBM50203364 (CHEMBL248310 | N-((S)-3-(2-(3-(1H-tetrazol-5-ylami...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of human recombinant caspase 1 | Bioorg Med Chem Lett 17: 1671-4 (2007) Article DOI: 10.1016/j.bmcl.2006.12.110 BindingDB Entry DOI: 10.7270/Q22R3RBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Homo sapiens (Human)) | BDBM50308091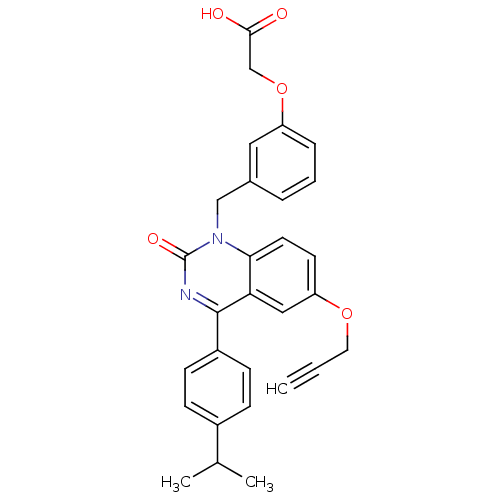 (CHEMBL597194 | {3-[4-(4-Isopropyl-phenyl)-2-oxo-6-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at human CaSR expressed in CCL39 cells assessed as inhibition of extracellular calcium-induced intracellular calcium transient by... | J Med Chem 53: 2250-63 (2010) Article DOI: 10.1021/jm901811v BindingDB Entry DOI: 10.7270/Q2Q240BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM176610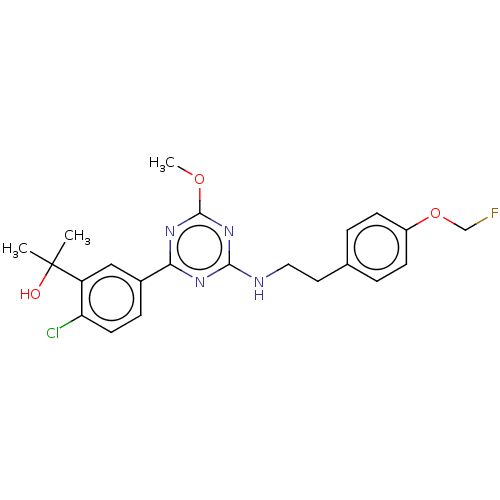 (US9115121, 69) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Patent | Assay Description Affinity binding tests were performed according to the experimental conditions described by M. Rinaldi-Carmona in J. Pharmacol. Exp. Therap. 1998, 28... | US Patent US9115121 (2015) BindingDB Entry DOI: 10.7270/Q23N225X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Homo sapiens (Human)) | BDBM50308106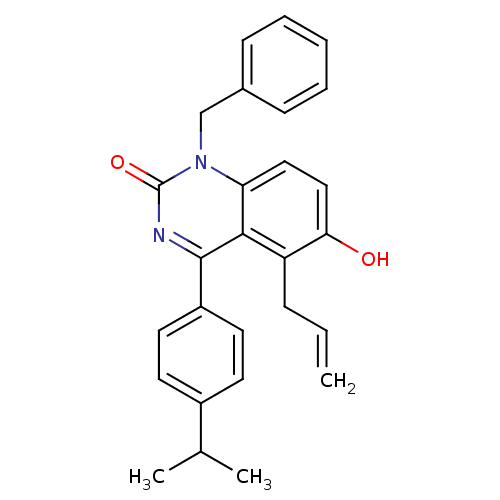 (5-Allyl-1-benzyl-6-hydroxy-4-(4-isopropyl-phenyl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at human CaSR expressed in CCL39 cells assessed as inhibition of extracellular calcium-induced intracellular calcium transient by... | J Med Chem 53: 2250-63 (2010) Article DOI: 10.1021/jm901811v BindingDB Entry DOI: 10.7270/Q2Q240BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM176654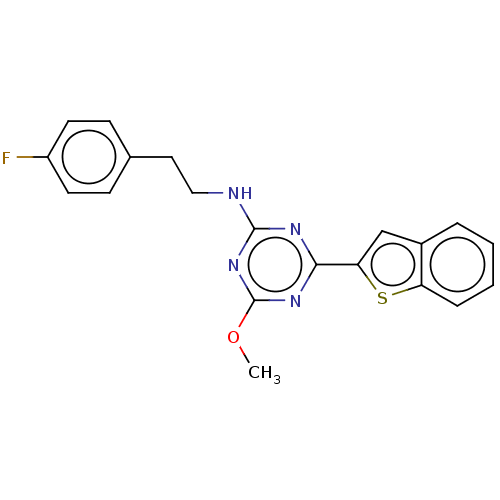 (US9115121, 47) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Patent | Assay Description Affinity binding tests were performed according to the experimental conditions described by M. Rinaldi-Carmona in J. Pharmacol. Exp. Therap. 1998, 28... | US Patent US9115121 (2015) BindingDB Entry DOI: 10.7270/Q23N225X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM176656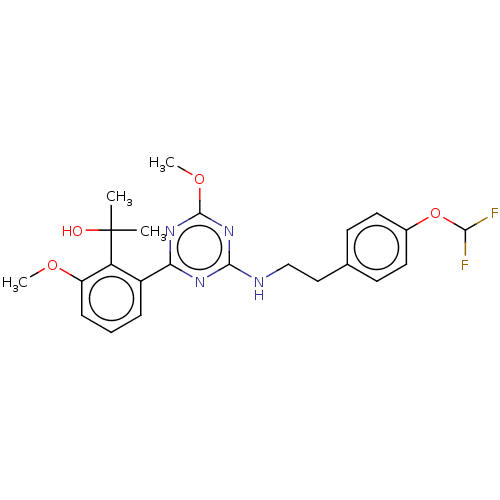 (US9115121, 49) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Patent | Assay Description Affinity binding tests were performed according to the experimental conditions described by M. Rinaldi-Carmona in J. Pharmacol. Exp. Therap. 1998, 28... | US Patent US9115121 (2015) BindingDB Entry DOI: 10.7270/Q23N225X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM20606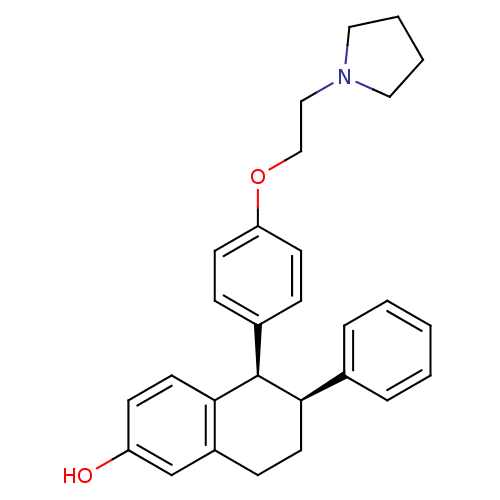 ((5R,6S)-6-phenyl-5-[4-(2-pyrrolidin-1-ylethoxy)phe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 4 | n/a | 3.10 | n/a | n/a | 7.4 | 22 |
Novartis Pharmaceuticals | Assay Description Radioligand binding assay was performed by using 96-well microtiterplates containing ER, 17beta-estradiol, and the test compound to be tested and SPA... | J Med Chem 46: 2945-57 (2003) Article DOI: 10.1021/jm030086h BindingDB Entry DOI: 10.7270/Q2H41PQQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM20606 ((5R,6S)-6-phenyl-5-[4-(2-pyrrolidin-1-ylethoxy)phe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 4 | n/a | 3.10 | n/a | n/a | 7.4 | 22 |
Novartis Pharmaceuticals | Assay Description Radioligand binding assay was performed by using 96-well microtiterplates containing ER, 17beta-estradiol, and the test compound to be tested and SPA... | J Med Chem 48: 364-79 (2005) Article DOI: 10.1021/jm040858p BindingDB Entry DOI: 10.7270/Q2CC0XZJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM176666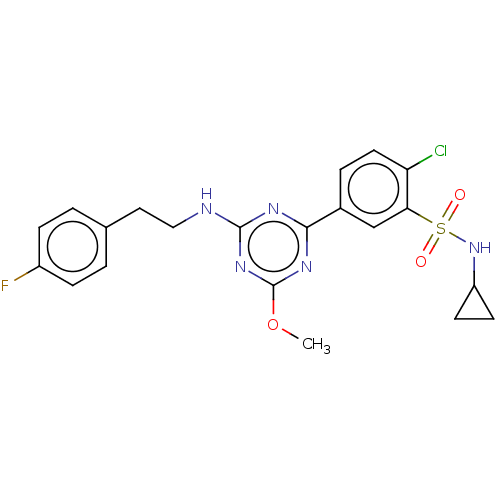 (US9115121, 62) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Patent | Assay Description Affinity binding tests were performed according to the experimental conditions described by M. Rinaldi-Carmona in J. Pharmacol. Exp. Therap. 1998, 28... | US Patent US9115121 (2015) BindingDB Entry DOI: 10.7270/Q23N225X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM10235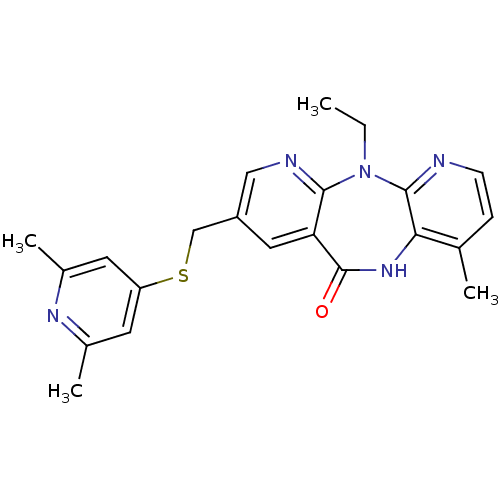 (13-{[(2,6-dimethylpyridin-4-yl)sulfanyl]methyl}-2-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Boehringer Ingelheim (Canada) Ltd. | Assay Description IC50 values for wild-type and mutant RTs were obtained from a scintillation proximity assay using poly rC/biotin-dG15 and 3H-dGTP. Each value represe... | Bioorg Med Chem Lett 14: 739-42 (2004) Article DOI: 10.1016/j.bmcl.2003.11.049 BindingDB Entry DOI: 10.7270/Q2C53J2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Homo sapiens (Human)) | BDBM50308082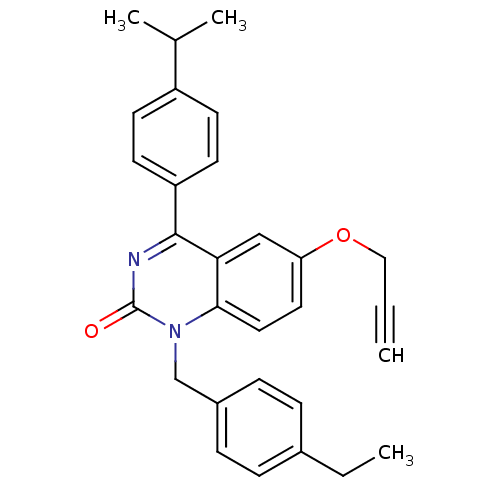 (1-(4-Ethyl-benzyl)-4-(4-isopropyl-phenyl)-6-propar...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at human CaSR expressed in CCL39 cells assessed as inhibition of extracellular calcium-induced intracellular calcium transient by... | J Med Chem 53: 2250-63 (2010) Article DOI: 10.1021/jm901811v BindingDB Entry DOI: 10.7270/Q2Q240BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Homo sapiens (Human)) | BDBM50308084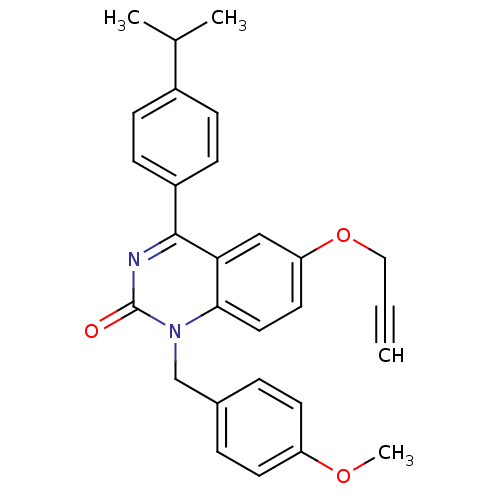 (4-(4-Isopropyl-phenyl)-1-(4-methoxy-benzyl)-6-prop...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at human CaSR expressed in CCL39 cells assessed as inhibition of extracellular calcium-induced intracellular calcium transient by... | J Med Chem 53: 2250-63 (2010) Article DOI: 10.1021/jm901811v BindingDB Entry DOI: 10.7270/Q2Q240BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Homo sapiens (Human)) | BDBM50308080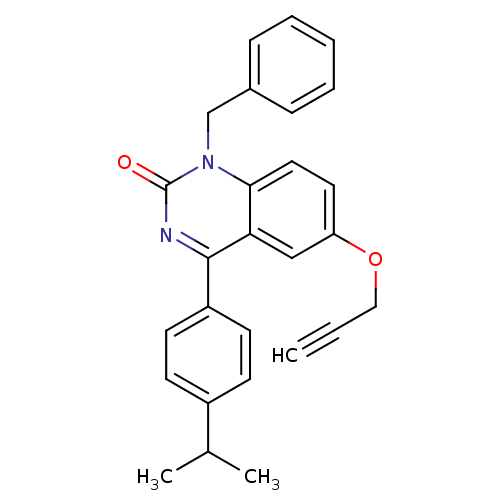 (1-Benzyl-4-(4-isopropyl-phenyl)-6-propargyloxy-1H-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at human CaSR expressed in CCL39 cells assessed as inhibition of extracellular calcium-induced intracellular calcium transient by... | J Med Chem 53: 2250-63 (2010) Article DOI: 10.1021/jm901811v BindingDB Entry DOI: 10.7270/Q2Q240BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM50203361 ((3S)-3-(2-(3-(1H-tetrazol-5-ylamino)-5-tert-butyl-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of human recombinant caspase 3 | Bioorg Med Chem Lett 17: 1671-4 (2007) Article DOI: 10.1016/j.bmcl.2006.12.110 BindingDB Entry DOI: 10.7270/Q22R3RBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM176638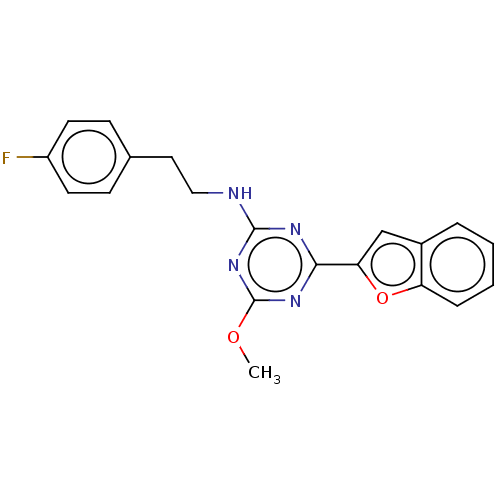 (US9115121, 30) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Patent | Assay Description Affinity binding tests were performed according to the experimental conditions described by M. Rinaldi-Carmona in J. Pharmacol. Exp. Therap. 1998, 28... | US Patent US9115121 (2015) BindingDB Entry DOI: 10.7270/Q23N225X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM10232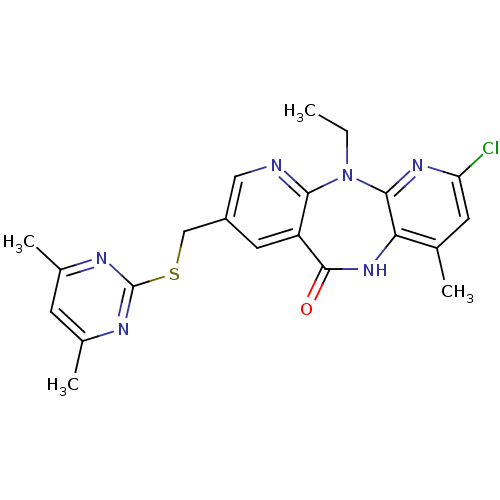 (5-chloro-13-{[(4,6-dimethylpyrimidin-2-yl)sulfanyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Boehringer Ingelheim (Canada) Ltd. | Assay Description IC50 values for wild-type and mutant RTs were obtained from a scintillation proximity assay using poly rC/biotin-dG15 and 3H-dGTP. Each value represe... | Bioorg Med Chem Lett 14: 739-42 (2004) Article DOI: 10.1016/j.bmcl.2003.11.049 BindingDB Entry DOI: 10.7270/Q2C53J2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50113667 (4-Benzyloxy-N-{1-[2-(4-methoxy-phenylamino)-ethylc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant human cathepsin K in fluorescence assay | J Med Chem 45: 2352-4 (2002) BindingDB Entry DOI: 10.7270/Q2DV1J73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50113669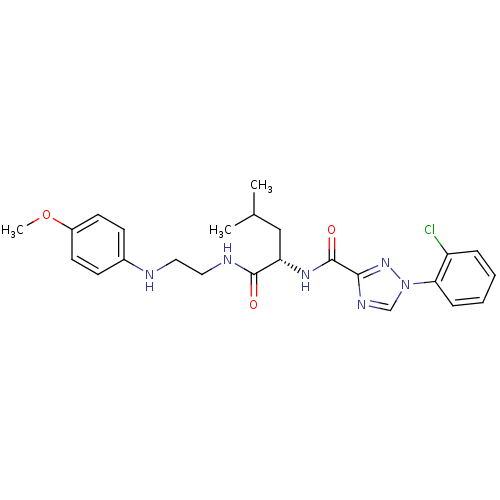 (1-(2-Chloro-phenyl)-1H-[1,2,4]triazole-3-carboxyli...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant human cathepsin K in fluorescence assay | J Med Chem 45: 2352-4 (2002) BindingDB Entry DOI: 10.7270/Q2DV1J73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Homo sapiens (Human)) | BDBM50308107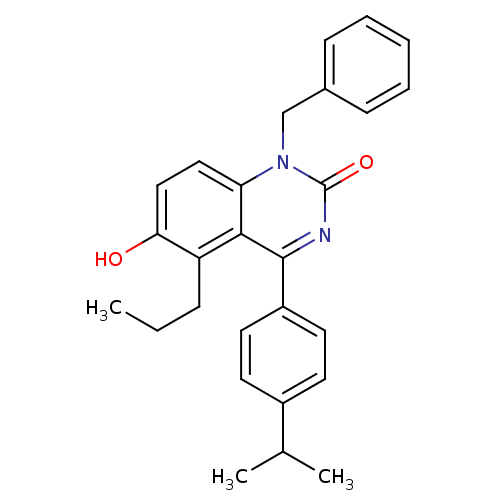 (1-Benzyl-6-hydroxy-4-(4-isopropyl-phenyl)-5-propyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at human CaSR expressed in CCL39 cells assessed as inhibition of extracellular calcium-induced intracellular calcium transient by... | J Med Chem 53: 2250-63 (2010) Article DOI: 10.1021/jm901811v BindingDB Entry DOI: 10.7270/Q2Q240BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM50203364 (CHEMBL248310 | N-((S)-3-(2-(3-(1H-tetrazol-5-ylami...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of human recombinant caspase 3 | Bioorg Med Chem Lett 17: 1671-4 (2007) Article DOI: 10.1016/j.bmcl.2006.12.110 BindingDB Entry DOI: 10.7270/Q22R3RBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM10234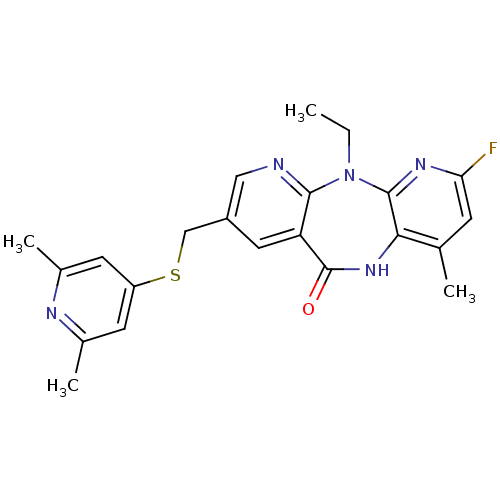 (13-{[(2,6-dimethylpyridin-4-yl)sulfanyl]methyl}-2-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Boehringer Ingelheim (Canada) Ltd. | Assay Description IC50 values for wild-type and mutant RTs were obtained from a scintillation proximity assay using poly rC/biotin-dG15 and 3H-dGTP. Each value represe... | Bioorg Med Chem Lett 14: 739-42 (2004) Article DOI: 10.1016/j.bmcl.2003.11.049 BindingDB Entry DOI: 10.7270/Q2C53J2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM176602 (US9115121, 18) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Patent | Assay Description Affinity binding tests were performed according to the experimental conditions described by M. Rinaldi-Carmona in J. Pharmacol. Exp. Therap. 1998, 28... | US Patent US9115121 (2015) BindingDB Entry DOI: 10.7270/Q23N225X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM10230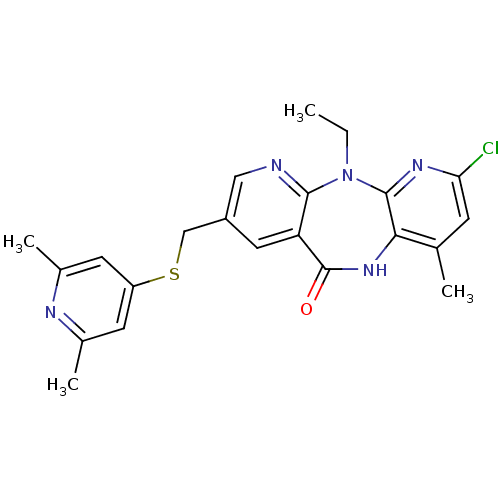 (5-chloro-13-{[(2,6-dimethylpyridin-4-yl)sulfanyl]m...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Boehringer Ingelheim (Canada) Ltd. | Assay Description IC50 values for wild-type and mutant RTs were obtained from a scintillation proximity assay using poly rC/biotin-dG15 and 3H-dGTP. Each value represe... | Bioorg Med Chem Lett 14: 739-42 (2004) Article DOI: 10.1016/j.bmcl.2003.11.049 BindingDB Entry DOI: 10.7270/Q2C53J2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Homo sapiens (Human)) | BDBM50308092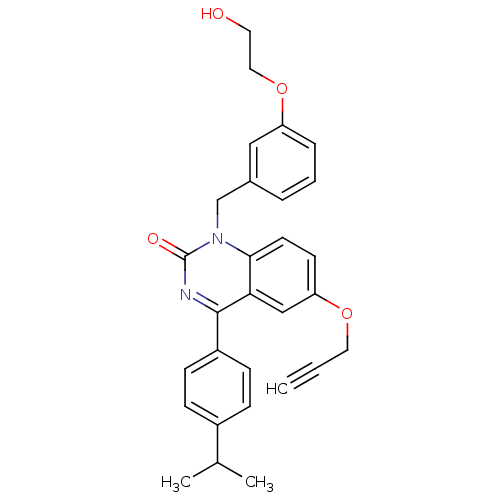 (1-[3-(2-Hydroxy-ethoxy)-benzyl]-4-(4-isopropyl-phe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at human CaSR expressed in CCL39 cells assessed as inhibition of extracellular calcium-induced intracellular calcium transient by... | J Med Chem 53: 2250-63 (2010) Article DOI: 10.1021/jm901811v BindingDB Entry DOI: 10.7270/Q2Q240BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM10653 ((3S)-5-[(2-CHLORO-6-FLUOROBENZYL)SULFANYL]-3-{[N-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 7.95 | n/a | n/a | n/a | n/a | 7.0 | 37 |
Merck Research Laboratories | Assay Description The substrate peptides terminating in AMC are processed by caspases with or without inhibitors, and the accumulation of AMC was assessed with a Cytof... | J Med Chem 47: 2466-74 (2004) Article DOI: 10.1021/jm0305523 BindingDB Entry DOI: 10.7270/Q24F1NZ4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM176623 (US9115121, 14) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Patent | Assay Description Affinity binding tests were performed according to the experimental conditions described by M. Rinaldi-Carmona in J. Pharmacol. Exp. Therap. 1998, 28... | US Patent US9115121 (2015) BindingDB Entry DOI: 10.7270/Q23N225X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM10233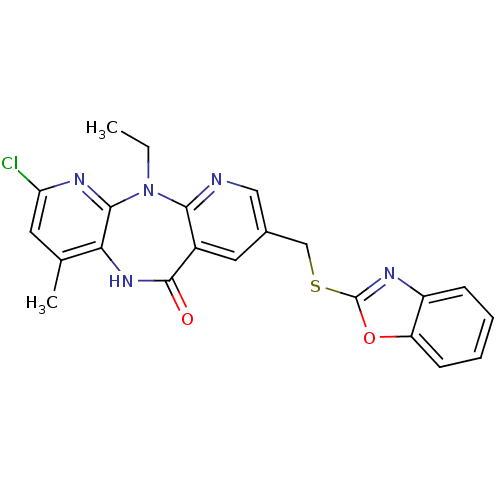 (13-[(1,3-benzoxazol-2-ylsulfanyl)methyl]-5-chloro-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Boehringer Ingelheim (Canada) Ltd. | Assay Description IC50 values for wild-type and mutant RTs were obtained from a scintillation proximity assay using poly rC/biotin-dG15 and 3H-dGTP. Each value represe... | Bioorg Med Chem Lett 14: 739-42 (2004) Article DOI: 10.1016/j.bmcl.2003.11.049 BindingDB Entry DOI: 10.7270/Q2C53J2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-7 (Homo sapiens (Human)) | BDBM50203361 ((3S)-3-(2-(3-(1H-tetrazol-5-ylamino)-5-tert-butyl-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of human recombinant caspase 7 | Bioorg Med Chem Lett 17: 1671-4 (2007) Article DOI: 10.1016/j.bmcl.2006.12.110 BindingDB Entry DOI: 10.7270/Q22R3RBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 493 total ) | Next | Last >> |