

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50260806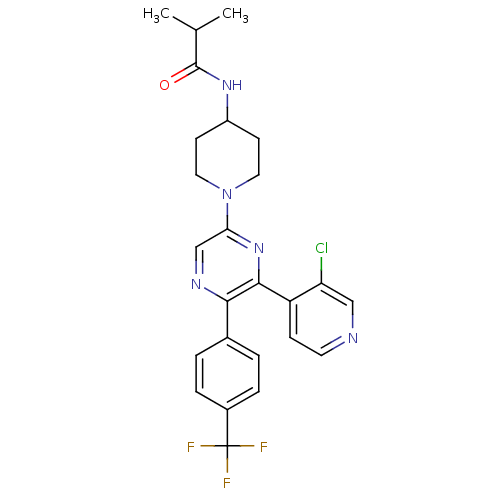 (CHEMBL497557 | N-(1-(6-(3-chloropyridin-4-yl)-5-(4...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at rat CB1 receptor in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50260806 (CHEMBL497557 | N-(1-(6-(3-chloropyridin-4-yl)-5-(4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at rat CB1 receptor in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50260767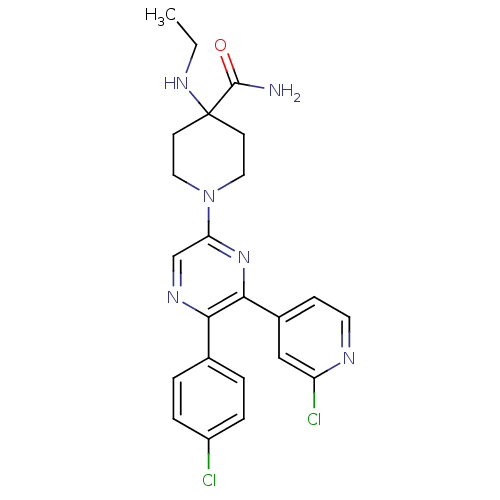 (1-(5-(4-chlorophenyl)-6-(2-chloropyridin-4-yl)pyra...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50260805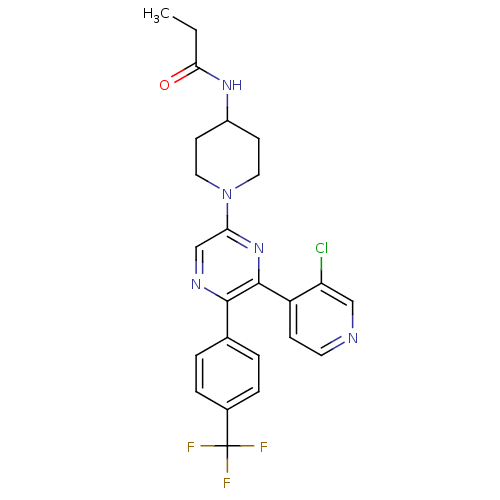 (CHEMBL524804 | N-(1-(6-(3-chloropyridin-4-yl)-5-(4...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50260681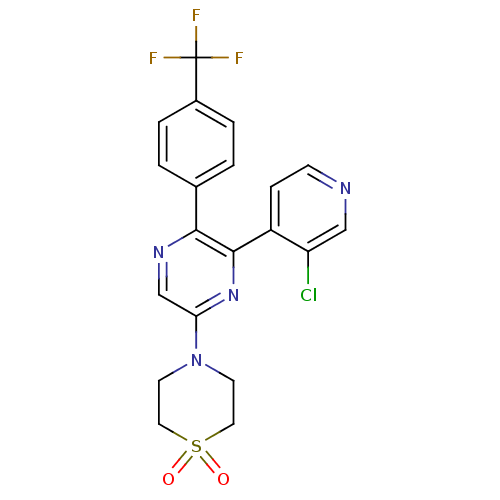 (4-[6-(3-Chloro-pyridin-4-yl)-5-(4-trifluoromethyl-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50260681 (4-[6-(3-Chloro-pyridin-4-yl)-5-(4-trifluoromethyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at rat CB1 receptor in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50260768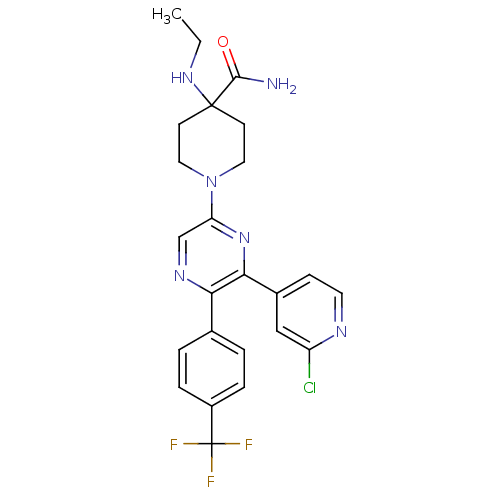 (1-(6-(2-chloropyridin-4-yl)-5-(4-(trifluoromethyl)...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50260682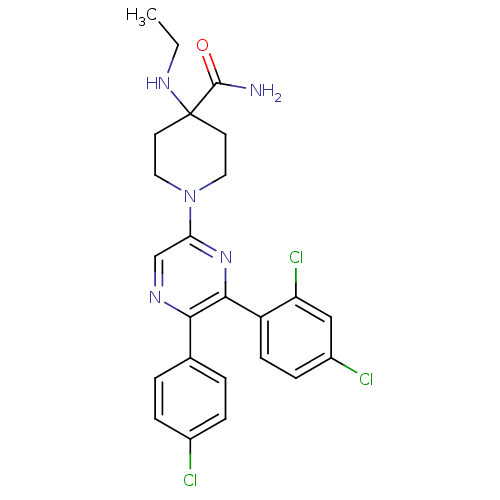 (1-(5-(4-chlorophenyl)-6-(2,4-dichlorophenyl)pyrazi...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C delta type (Homo sapiens (Human)) | BDBM50258529 (CHEMBL449158 | bryostatin 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University Curated by ChEMBL | Assay Description Inhibition of [3H]PDBu binding to recombinant full length human PKCdelta expressed in baculovirus expression system incubated for 5 mins by scintilla... | J Nat Prod 79: 680-4 (2016) Article DOI: 10.1021/acs.jnatprod.5b01017 BindingDB Entry DOI: 10.7270/Q2BK1GXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50260803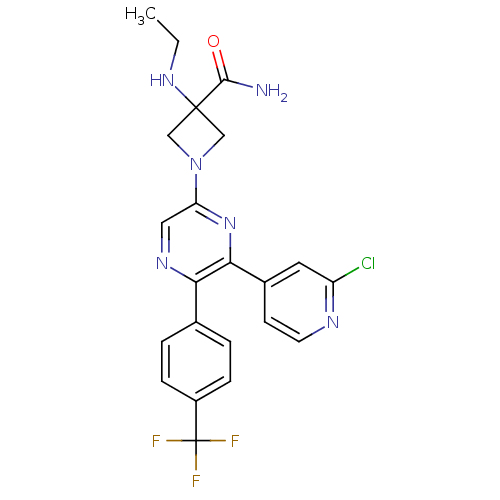 (1-(6-(2-chloropyridin-4-yl)-5-(4-(trifluoromethyl)...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C delta type (Homo sapiens (Human)) | BDBM50535777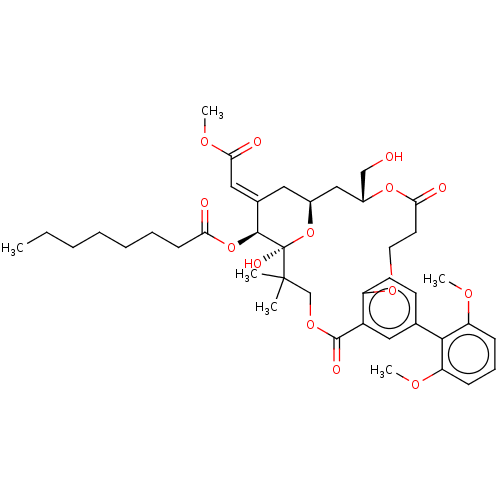 (CHEMBL4565484) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University Curated by ChEMBL | Assay Description Inhibition of [3H]PDBu binding to recombinant full length human PKCdelta expressed in baculovirus expression system incubated for 5 mins by scintilla... | J Nat Prod 79: 680-4 (2016) Article DOI: 10.1021/acs.jnatprod.5b01017 BindingDB Entry DOI: 10.7270/Q2BK1GXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50260718 (1-(6-(2-chlorophenyl)-5-(4-chlorophenyl)pyrazin-2-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50260717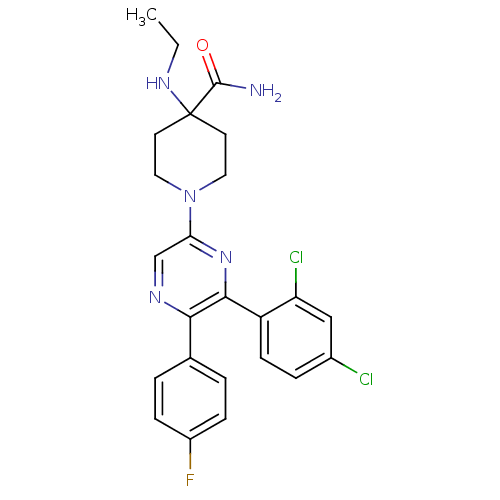 (1-(6-(2,4-dichlorophenyl)-5-(4-fluorophenyl)pyrazi...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C delta type (Homo sapiens (Human)) | BDBM50535778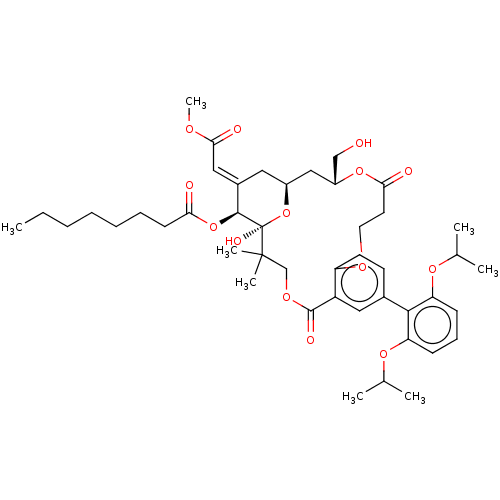 (CHEMBL4533216) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University Curated by ChEMBL | Assay Description Inhibition of [3H]PDBu binding to recombinant full length human PKCdelta expressed in baculovirus expression system incubated for 5 mins by scintilla... | J Nat Prod 79: 680-4 (2016) Article DOI: 10.1021/acs.jnatprod.5b01017 BindingDB Entry DOI: 10.7270/Q2BK1GXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50260804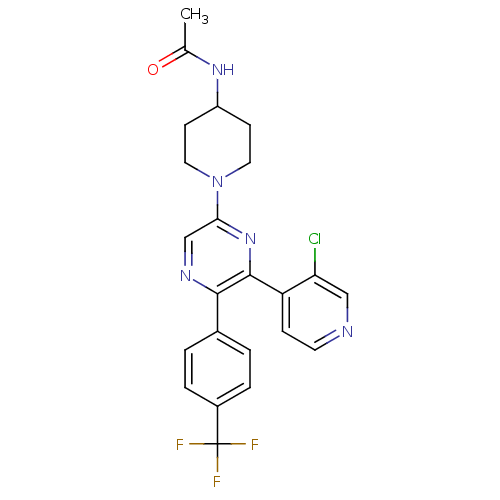 (CHEMBL497556 | N-(1-(6-(3-chloropyridin-4-yl)-5-(4...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.97 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50260803 (1-(6-(2-chloropyridin-4-yl)-5-(4-(trifluoromethyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.06 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at rat CB1 receptor in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C delta type (Homo sapiens (Human)) | BDBM50535789 (CHEMBL4574406) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University Curated by ChEMBL | Assay Description Inhibition of [3H]PDBu binding to recombinant full length human PKCdelta expressed in baculovirus expression system incubated for 5 mins by scintilla... | J Nat Prod 79: 680-4 (2016) Article DOI: 10.1021/acs.jnatprod.5b01017 BindingDB Entry DOI: 10.7270/Q2BK1GXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C delta type (Homo sapiens (Human)) | BDBM50535791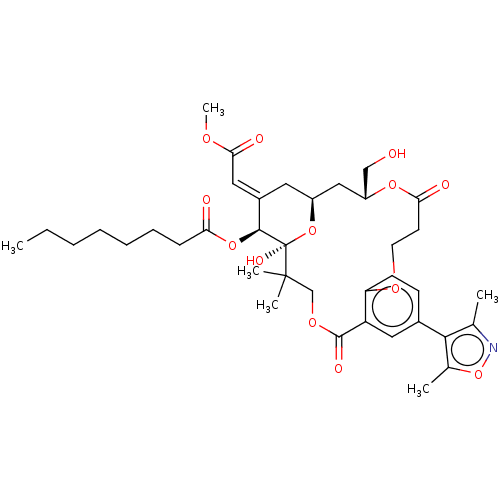 (CHEMBL4593581) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University Curated by ChEMBL | Assay Description Inhibition of [3H]PDBu binding to recombinant full length human PKCdelta expressed in baculovirus expression system incubated for 5 mins by scintilla... | J Nat Prod 79: 680-4 (2016) Article DOI: 10.1021/acs.jnatprod.5b01017 BindingDB Entry DOI: 10.7270/Q2BK1GXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50260720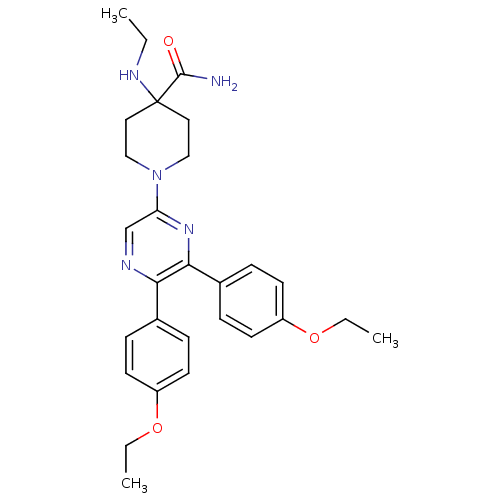 (1-(5,6-bis(4-ethoxyphenyl)pyrazin-2-yl)-4-(ethylam...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C delta type (Homo sapiens (Human)) | BDBM50535790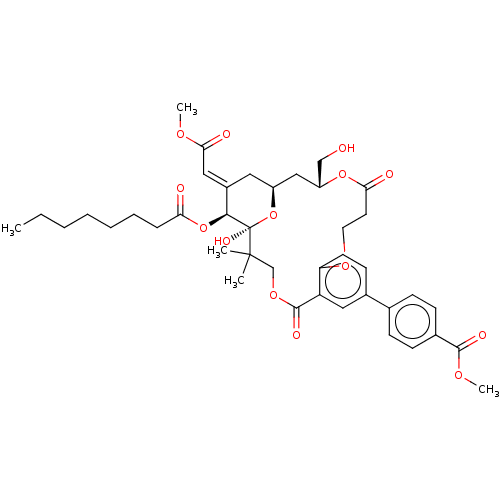 (CHEMBL4536091) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University Curated by ChEMBL | Assay Description Inhibition of [3H]PDBu binding to recombinant full length human PKCdelta expressed in baculovirus expression system incubated for 5 mins by scintilla... | J Nat Prod 79: 680-4 (2016) Article DOI: 10.1021/acs.jnatprod.5b01017 BindingDB Entry DOI: 10.7270/Q2BK1GXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C delta type (Homo sapiens (Human)) | BDBM50535785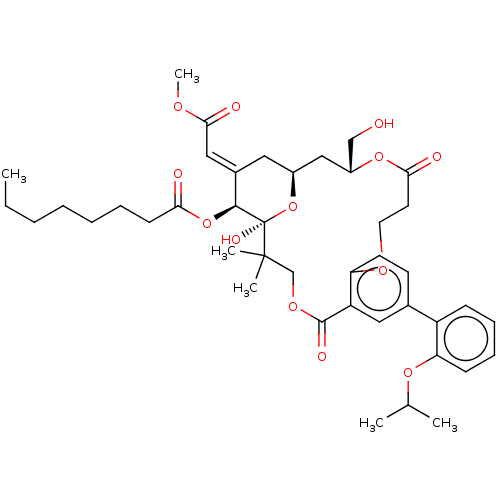 (CHEMBL4526756) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University Curated by ChEMBL | Assay Description Inhibition of [3H]PDBu binding to recombinant full length human PKCdelta expressed in baculovirus expression system incubated for 5 mins by scintilla... | J Nat Prod 79: 680-4 (2016) Article DOI: 10.1021/acs.jnatprod.5b01017 BindingDB Entry DOI: 10.7270/Q2BK1GXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C delta type (Homo sapiens (Human)) | BDBM50535782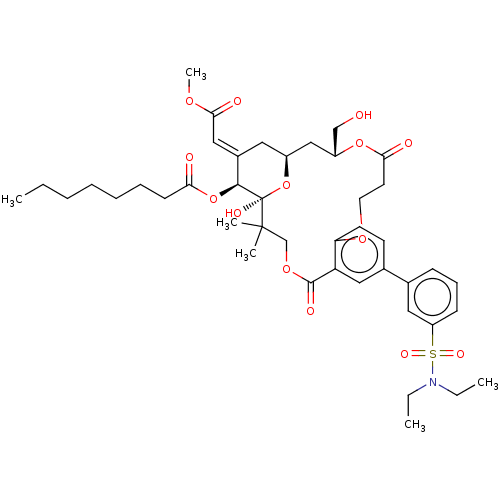 (CHEMBL4576842) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University Curated by ChEMBL | Assay Description Inhibition of [3H]PDBu binding to recombinant full length human PKCdelta expressed in baculovirus expression system incubated for 5 mins by scintilla... | J Nat Prod 79: 680-4 (2016) Article DOI: 10.1021/acs.jnatprod.5b01017 BindingDB Entry DOI: 10.7270/Q2BK1GXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C delta type (Homo sapiens (Human)) | BDBM50535775 (CHEMBL4525186) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University Curated by ChEMBL | Assay Description Inhibition of [3H]PDBu binding to recombinant full length human PKCdelta expressed in baculovirus expression system incubated for 5 mins by scintilla... | J Nat Prod 79: 680-4 (2016) Article DOI: 10.1021/acs.jnatprod.5b01017 BindingDB Entry DOI: 10.7270/Q2BK1GXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C delta type (Homo sapiens (Human)) | BDBM50535772 (CHEMBL4574986) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University Curated by ChEMBL | Assay Description Inhibition of [3H]PDBu binding to recombinant full length human PKCdelta expressed in baculovirus expression system incubated for 5 mins by scintilla... | J Nat Prod 79: 680-4 (2016) Article DOI: 10.1021/acs.jnatprod.5b01017 BindingDB Entry DOI: 10.7270/Q2BK1GXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C delta type (Homo sapiens (Human)) | BDBM50535776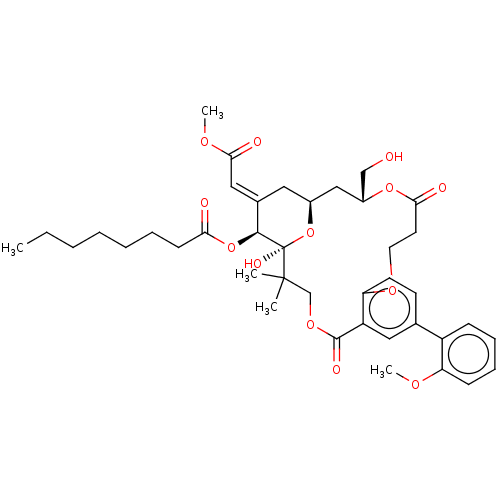 (CHEMBL4550131) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University Curated by ChEMBL | Assay Description Inhibition of [3H]PDBu binding to recombinant full length human PKCdelta expressed in baculovirus expression system incubated for 5 mins by scintilla... | J Nat Prod 79: 680-4 (2016) Article DOI: 10.1021/acs.jnatprod.5b01017 BindingDB Entry DOI: 10.7270/Q2BK1GXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C delta type (Homo sapiens (Human)) | BDBM50535786 (CHEMBL4542079) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University Curated by ChEMBL | Assay Description Inhibition of [3H]PDBu binding to recombinant full length human PKCdelta expressed in baculovirus expression system incubated for 5 mins by scintilla... | J Nat Prod 79: 680-4 (2016) Article DOI: 10.1021/acs.jnatprod.5b01017 BindingDB Entry DOI: 10.7270/Q2BK1GXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C delta type (Homo sapiens (Human)) | BDBM50535780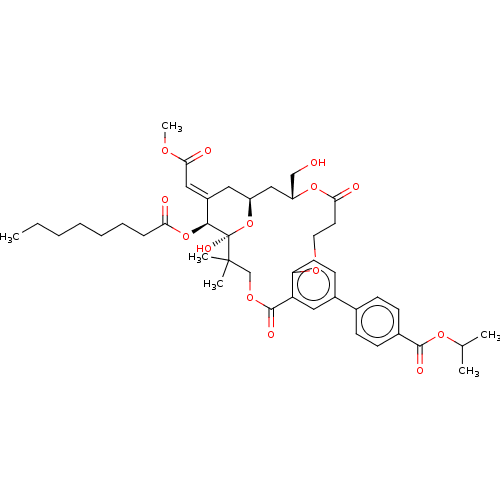 (CHEMBL4560593) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University Curated by ChEMBL | Assay Description Inhibition of [3H]PDBu binding to recombinant full length human PKCdelta expressed in baculovirus expression system incubated for 5 mins by scintilla... | J Nat Prod 79: 680-4 (2016) Article DOI: 10.1021/acs.jnatprod.5b01017 BindingDB Entry DOI: 10.7270/Q2BK1GXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C delta type (Homo sapiens (Human)) | BDBM50535781 (CHEMBL4535813) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University Curated by ChEMBL | Assay Description Inhibition of [3H]PDBu binding to recombinant full length human PKCdelta expressed in baculovirus expression system incubated for 5 mins by scintilla... | J Nat Prod 79: 680-4 (2016) Article DOI: 10.1021/acs.jnatprod.5b01017 BindingDB Entry DOI: 10.7270/Q2BK1GXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C delta type (Homo sapiens (Human)) | BDBM50535788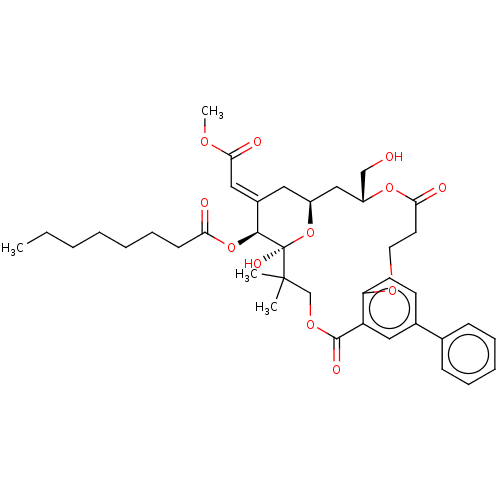 (CHEMBL4564420) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University Curated by ChEMBL | Assay Description Inhibition of [3H]PDBu binding to recombinant full length human PKCdelta expressed in baculovirus expression system incubated for 5 mins by scintilla... | J Nat Prod 79: 680-4 (2016) Article DOI: 10.1021/acs.jnatprod.5b01017 BindingDB Entry DOI: 10.7270/Q2BK1GXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50260766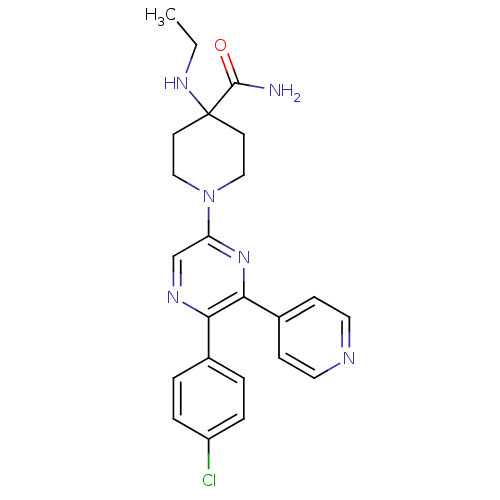 (1-(5-(4-chlorophenyl)-6-(pyridin-4-yl)pyrazin-2-yl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.81 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C delta type (Homo sapiens (Human)) | BDBM50535779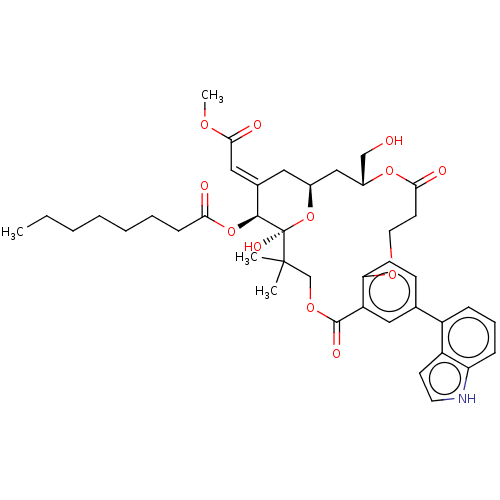 (CHEMBL4519769) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University Curated by ChEMBL | Assay Description Inhibition of [3H]PDBu binding to recombinant full length human PKCdelta expressed in baculovirus expression system incubated for 5 mins by scintilla... | J Nat Prod 79: 680-4 (2016) Article DOI: 10.1021/acs.jnatprod.5b01017 BindingDB Entry DOI: 10.7270/Q2BK1GXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50260719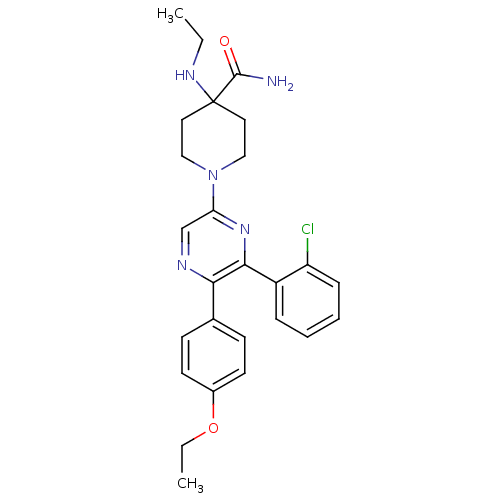 (1-(6-(2-chlorophenyl)-5-(4-ethoxyphenyl)pyrazin-2-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15.0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C delta type (Homo sapiens (Human)) | BDBM50535771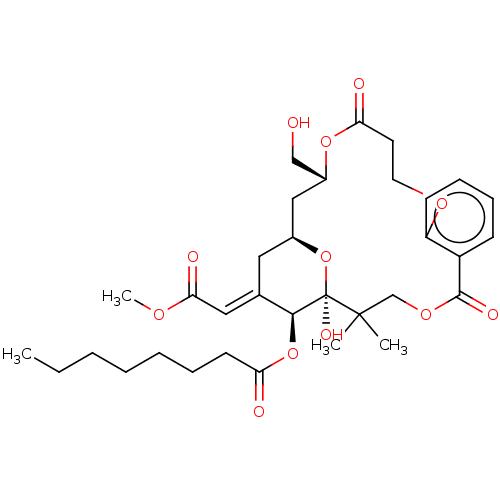 (CHEMBL4541641) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University Curated by ChEMBL | Assay Description Inhibition of [3H]PDBu binding to recombinant full length human PKCdelta expressed in baculovirus expression system incubated for 5 mins by scintilla... | J Nat Prod 79: 680-4 (2016) Article DOI: 10.1021/acs.jnatprod.5b01017 BindingDB Entry DOI: 10.7270/Q2BK1GXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C delta type (Homo sapiens (Human)) | BDBM50535774 (CHEMBL4559327) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University Curated by ChEMBL | Assay Description Inhibition of [3H]PDBu binding to recombinant full length human PKCdelta expressed in baculovirus expression system incubated for 5 mins by scintilla... | J Nat Prod 79: 680-4 (2016) Article DOI: 10.1021/acs.jnatprod.5b01017 BindingDB Entry DOI: 10.7270/Q2BK1GXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50260765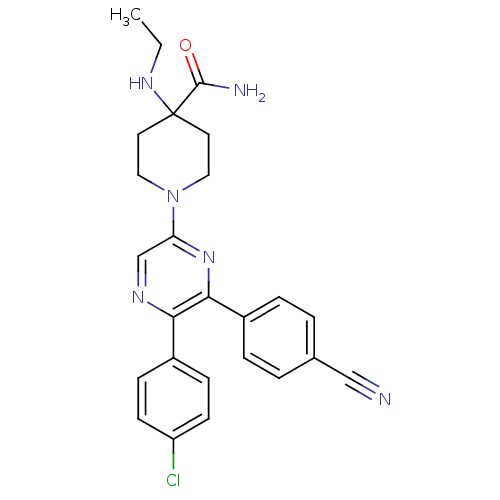 (1-(5-(4-chlorophenyl)-6-(4-cyanophenyl)pyrazin-2-y...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 32.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C delta type (Homo sapiens (Human)) | BDBM50535787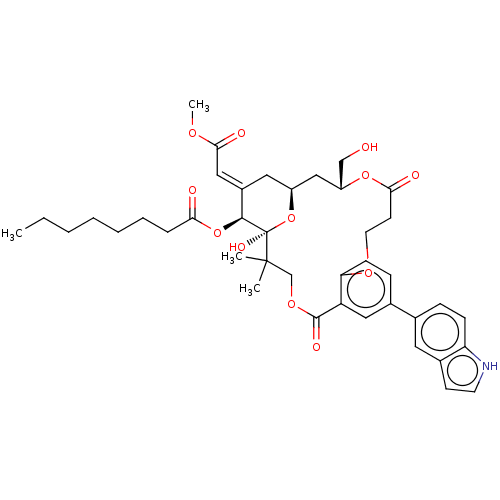 (CHEMBL4550478) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University Curated by ChEMBL | Assay Description Inhibition of [3H]PDBu binding to recombinant full length human PKCdelta expressed in baculovirus expression system incubated for 5 mins by scintilla... | J Nat Prod 79: 680-4 (2016) Article DOI: 10.1021/acs.jnatprod.5b01017 BindingDB Entry DOI: 10.7270/Q2BK1GXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 815 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at human CB2 receptor in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C delta type (Homo sapiens (Human)) | BDBM50535773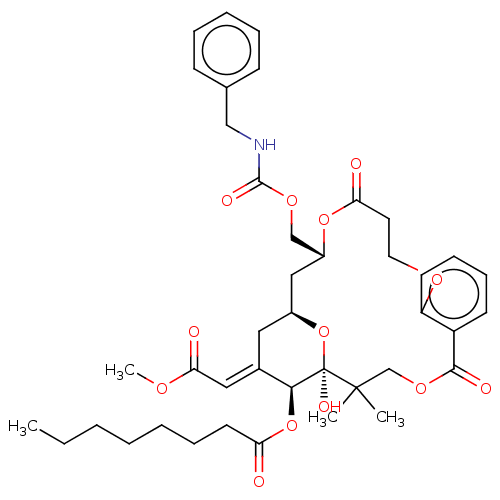 (CHEMBL4575057) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University Curated by ChEMBL | Assay Description Inhibition of [3H]PDBu binding to recombinant full length human PKCdelta expressed in baculovirus expression system incubated for 5 mins by scintilla... | J Nat Prod 79: 680-4 (2016) Article DOI: 10.1021/acs.jnatprod.5b01017 BindingDB Entry DOI: 10.7270/Q2BK1GXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C delta type (Homo sapiens (Human)) | BDBM50535784 (CHEMBL4212128) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University Curated by ChEMBL | Assay Description Inhibition of [3H]PDBu binding to recombinant full length human PKCdelta expressed in baculovirus expression system incubated for 5 mins by scintilla... | J Nat Prod 79: 680-4 (2016) Article DOI: 10.1021/acs.jnatprod.5b01017 BindingDB Entry DOI: 10.7270/Q2BK1GXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C delta type (Homo sapiens (Human)) | BDBM50535783 (CHEMBL4482943) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University Curated by ChEMBL | Assay Description Inhibition of [3H]PDBu binding to recombinant full length human PKCdelta expressed in baculovirus expression system incubated for 5 mins by scintilla... | J Nat Prod 79: 680-4 (2016) Article DOI: 10.1021/acs.jnatprod.5b01017 BindingDB Entry DOI: 10.7270/Q2BK1GXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50260681 (4-[6-(3-Chloro-pyridin-4-yl)-5-(4-trifluoromethyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at human CB2 receptor in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50260806 (CHEMBL497557 | N-(1-(6-(3-chloropyridin-4-yl)-5-(4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at human CB2 receptor in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50260803 (1-(6-(2-chloropyridin-4-yl)-5-(4-(trifluoromethyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at human CB2 receptor in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50260806 (CHEMBL497557 | N-(1-(6-(3-chloropyridin-4-yl)-5-(4...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Inverse agonist at human CB1 receptor expressed in SF9 cells assessed as decrease in GTPgammaS level | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 1.35 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Inverse agonist at human CB1 receptor expressed in SF9 cells assessed as decrease in GTPgammaS level | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50260803 (1-(6-(2-chloropyridin-4-yl)-5-(4-(trifluoromethyl)...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.07 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Inverse agonist at human CB1 receptor expressed in SF9 cells assessed as decrease in GTPgammaS level | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50260681 (4-[6-(3-Chloro-pyridin-4-yl)-5-(4-trifluoromethyl-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Inverse agonist at human CB1 receptor expressed in SF9 cells assessed as decrease in GTPgammaS level | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||