 Found 67 hits with Last Name = 'nellans' and Initial = 'hn'
Found 67 hits with Last Name = 'nellans' and Initial = 'hn' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Lutropin-choriogonadotropic hormone receptor
(Rattus norvegicus) | BDBM50230127
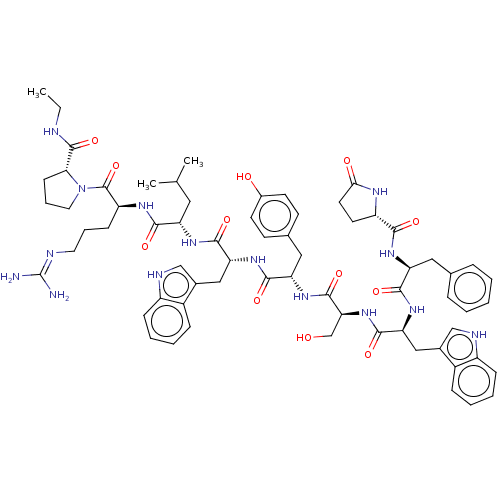
(CHEMBL407606)Show SMILES CCNC(=O)[C@H]1CCCN1C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCC(=O)N1 |wU:45.57,31.44,63.79,23.28,5.4,88.94,wD:57.63,77.91,12.20,(27.09,-8.95,;27.47,-10.44,;28.96,-10.86,;30.04,-11.96,;31.53,-11.6,;29.61,-13.45,;30.56,-14.67,;29.69,-15.95,;28.21,-15.53,;28.16,-13.97,;26.88,-13.11,;26.98,-11.58,;25.49,-13.78,;25.38,-15.33,;26.66,-16.2,;26.55,-17.73,;27.83,-18.59,;27.72,-20.13,;29,-21,;26.33,-20.8,;24.22,-12.92,;22.82,-13.6,;22.72,-15.13,;21.54,-12.73,;21.65,-11.2,;23.05,-10.52,;24.32,-11.38,;23.15,-8.98,;20.15,-13.41,;18.89,-12.55,;18.99,-11.01,;17.49,-13.23,;17.39,-14.76,;18.14,-16.08,;19.64,-16.43,;19.78,-17.94,;18.38,-18.56,;17.91,-20.01,;16.43,-20.33,;15.39,-19.17,;15.88,-17.73,;17.36,-17.42,;16.22,-12.36,;14.83,-13.03,;14.71,-14.57,;13.55,-12.17,;13.66,-10.62,;15.04,-9.95,;15.16,-8.42,;16.54,-7.74,;17.82,-8.6,;19.2,-7.93,;17.71,-10.14,;16.32,-10.81,;12.16,-12.85,;10.88,-11.97,;10.99,-10.44,;9.49,-12.65,;9.39,-14.19,;10.67,-15.06,;8.21,-11.79,;6.83,-12.46,;6.71,-14.01,;5.54,-11.6,;5.65,-10.07,;7.05,-9.39,;8.58,-9.18,;8.85,-7.67,;7.49,-6.93,;7.12,-5.44,;5.63,-5,;4.53,-6.07,;4.89,-7.56,;6.37,-8,;4.17,-12.27,;2.88,-11.41,;3,-9.87,;1.49,-12.09,;1.38,-13.62,;2.15,-14.95,;3.66,-14.95,;4.41,-16.27,;3.65,-17.59,;2.12,-17.59,;1.37,-16.26,;.22,-11.23,;-1.18,-11.9,;-1.28,-13.44,;-2.34,-11.11,;-2.34,-9.69,;-3.87,-9.46,;-4.55,-10.83,;-6.06,-11.09,;-3.45,-11.92,)| Show InChI InChI=1S/C67H85N15O12/c1-4-70-65(93)56-21-13-29-82(56)66(94)49(20-12-28-71-67(68)69)75-59(87)50(30-38(2)3)76-62(90)53(33-41-35-72-46-18-10-8-16-44(41)46)79-61(89)52(32-40-22-24-43(84)25-23-40)78-64(92)55(37-83)81-63(91)54(34-42-36-73-47-19-11-9-17-45(42)47)80-60(88)51(31-39-14-6-5-7-15-39)77-58(86)48-26-27-57(85)74-48/h5-11,14-19,22-25,35-36,38,48-56,72-73,83-84H,4,12-13,20-21,26-34,37H2,1-3H3,(H,70,93)(H,74,85)(H,75,87)(H,76,90)(H,77,86)(H,78,92)(H,79,89)(H,80,88)(H,81,91)(H4,68,69,71)/t48-,49-,50-,51-,52-,53+,54-,55-,56+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0245 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Negative logarithm of equilibrium dissociation constant in the rat pituitary luteinizing releasing hormone receptor binding assay |
J Med Chem 35: 3890-4 (1992)
BindingDB Entry DOI: 10.7270/Q24Q7X6M |
More data for this
Ligand-Target Pair | |
Lutropin-choriogonadotropic hormone receptor
(Rattus norvegicus) | BDBM50230129

(CHEMBL407123)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](Cc1ccc2ccccc2c1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)N(C)C(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@@H]1CCC(=O)N1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N1CCC[C@@H]1C(=O)NCC(O)=O |wU:42.57,23.34,8.21,4.4,89.97,66.71,wD:56.68,75.81,35.38,(22.87,-8.85,;21.6,-7.99,;21.71,-6.46,;20.2,-8.67,;20.11,-10.2,;18.72,-10.89,;17.44,-10.03,;17.55,-8.48,;16.05,-10.7,;15.95,-12.23,;16.7,-13.56,;18.23,-13.58,;19,-14.91,;18.21,-16.25,;18.95,-17.58,;18.18,-18.9,;16.64,-18.88,;15.89,-17.55,;16.68,-16.23,;15.91,-14.89,;14.77,-9.83,;13.39,-10.5,;13.28,-12.05,;12.12,-9.64,;12.22,-8.11,;13.61,-7.44,;14.89,-8.3,;16.27,-7.62,;16.37,-6.08,;17.77,-5.41,;15.1,-5.21,;13.72,-5.9,;10.73,-10.31,;9.45,-9.46,;9.55,-7.9,;8.06,-10.13,;7.95,-11.66,;9.24,-12.54,;6.79,-9.27,;6.88,-7.74,;5.39,-9.94,;5.28,-11.48,;4.12,-9.08,;4.23,-7.54,;5.62,-6.87,;7.15,-6.65,;7.41,-5.14,;6.06,-4.41,;5.7,-2.91,;4.21,-2.48,;3.1,-3.55,;3.47,-5.05,;4.94,-5.48,;2.74,-9.75,;1.45,-8.89,;1.56,-7.34,;.08,-9.56,;-.04,-11.1,;1.24,-11.96,;1.28,-13.51,;2.77,-13.94,;3.63,-12.66,;2.68,-11.43,;-1.21,-8.71,;-2.59,-9.38,;-2.71,-10.92,;-3.76,-8.59,;-3.76,-7.16,;-5.29,-6.93,;-5.97,-8.31,;-7.49,-8.57,;-4.87,-9.39,;21.39,-11.07,;21.27,-12.61,;22.77,-10.4,;24.05,-11.27,;23.94,-12.8,;25.22,-13.67,;25.1,-15.21,;26.38,-16.06,;26.26,-17.6,;27.56,-18.46,;24.89,-18.28,;25.43,-10.59,;25.54,-9.05,;26.7,-11.45,;26.77,-13,;28.24,-13.43,;29.11,-12.14,;28.17,-10.92,;28.58,-9.43,;30.09,-9.08,;27.52,-8.34,;26.03,-7.92,;25.64,-6.44,;24.17,-6.02,;26.73,-5.36,)| Show InChI InChI=1S/C67H84N16O14/c1-37(2)26-49(59(90)76-48(14-8-24-71-67(68)69)66(97)83-25-9-15-54(83)63(94)73-34-57(87)88)77-61(92)51(29-39-16-19-40-10-4-5-11-41(40)27-39)78-60(91)50(28-38-17-20-44(85)21-18-38)80-64(95)55(35-84)82(3)65(96)53(30-42-32-72-46-13-7-6-12-45(42)46)81-62(93)52(31-43-33-70-36-74-43)79-58(89)47-22-23-56(86)75-47/h4-7,10-13,16-21,27,32-33,36-37,47-55,72,84-85H,8-9,14-15,22-26,28-31,34-35H2,1-3H3,(H,70,74)(H,73,94)(H,75,86)(H,76,90)(H,77,92)(H,78,91)(H,79,89)(H,80,95)(H,81,93)(H,87,88)(H4,68,69,71)/t47-,48-,49-,50-,51+,52-,53-,54+,55-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0427 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Negative logarithm of equilibrium dissociation constant in the rat pituitary luteinizing releasing hormone receptor binding assay |
J Med Chem 35: 3890-4 (1992)
BindingDB Entry DOI: 10.7270/Q24Q7X6M |
More data for this
Ligand-Target Pair | |
Lutropin-choriogonadotropic hormone receptor
(Rattus norvegicus) | BDBM50230120
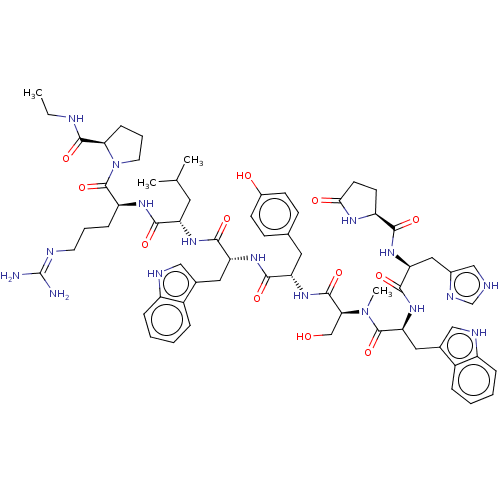
(CHEMBL405737)Show SMILES CCNC(=O)[C@H]1CCCN1C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)N(C)C(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@@H]1CCC(=O)N1 |wU:64.80,45.57,31.44,23.28,5.4,88.94,wD:78.91,12.20,57.61,(25.64,-6.44,;26.03,-7.92,;27.52,-8.34,;28.58,-9.43,;30.09,-9.08,;28.17,-10.92,;29.11,-12.14,;28.24,-13.43,;26.77,-13,;26.7,-11.45,;25.43,-10.59,;25.54,-9.05,;24.05,-11.27,;23.94,-12.8,;25.22,-13.67,;25.1,-15.21,;26.38,-16.06,;26.26,-17.6,;27.56,-18.46,;24.89,-18.28,;22.77,-10.4,;21.39,-11.07,;21.27,-12.61,;20.11,-10.2,;20.2,-8.67,;21.6,-7.99,;22.87,-8.85,;21.71,-6.46,;18.72,-10.89,;17.44,-10.03,;17.55,-8.48,;16.05,-10.7,;15.95,-12.23,;16.7,-13.56,;18.2,-13.91,;18.35,-15.42,;16.95,-16.04,;16.47,-17.49,;14.98,-17.81,;13.96,-16.65,;14.44,-15.21,;15.93,-14.9,;14.77,-9.83,;13.39,-10.5,;13.28,-12.05,;12.12,-9.64,;12.22,-8.11,;13.61,-7.44,;13.72,-5.9,;15.1,-5.21,;16.37,-6.08,;17.77,-5.41,;16.27,-7.62,;14.89,-8.3,;10.73,-10.31,;9.45,-9.46,;9.55,-7.9,;8.06,-10.13,;7.95,-11.66,;9.24,-12.54,;6.79,-9.27,;6.88,-7.74,;5.39,-9.94,;5.28,-11.48,;4.12,-9.08,;4.23,-7.54,;5.62,-6.87,;7.15,-6.65,;7.41,-5.14,;6.06,-4.41,;5.7,-2.91,;4.21,-2.48,;3.1,-3.55,;3.47,-5.05,;4.94,-5.48,;2.74,-9.75,;1.45,-8.89,;1.56,-7.34,;.08,-9.56,;-.04,-11.1,;1.24,-11.96,;1.28,-13.51,;2.77,-13.94,;3.63,-12.66,;2.68,-11.43,;-1.21,-8.71,;-2.59,-9.38,;-2.71,-10.92,;-3.76,-8.59,;-3.76,-7.16,;-5.29,-6.93,;-5.97,-8.31,;-7.49,-8.57,;-4.87,-9.39,)| Show InChI InChI=1S/C65H85N17O12/c1-5-69-61(91)53-17-11-25-82(53)64(94)47(16-10-24-70-65(66)67)75-57(87)48(26-36(2)3)76-59(89)50(28-38-31-71-44-14-8-6-12-42(38)44)77-58(88)49(27-37-18-20-41(84)21-19-37)79-62(92)54(34-83)81(4)63(93)52(29-39-32-72-45-15-9-7-13-43(39)45)80-60(90)51(30-40-33-68-35-73-40)78-56(86)46-22-23-55(85)74-46/h6-9,12-15,18-21,31-33,35-36,46-54,71-72,83-84H,5,10-11,16-17,22-30,34H2,1-4H3,(H,68,73)(H,69,91)(H,74,85)(H,75,87)(H,76,89)(H,77,88)(H,78,86)(H,79,92)(H,80,90)(H4,66,67,70)/t46-,47-,48-,49-,50+,51-,52-,53+,54-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0776 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Negative logarithm of equilibrium dissociation constant in the rat pituitary luteinizing releasing hormone receptor binding assay |
J Med Chem 35: 3890-4 (1992)
BindingDB Entry DOI: 10.7270/Q24Q7X6M |
More data for this
Ligand-Target Pair | |
Lutropin-choriogonadotropic hormone receptor
(Rattus norvegicus) | BDBM50230123

(CHEMBL385042)Show SMILES CCNC(=O)[C@H]1CCCN1C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](CC(C)C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1cccc2ccccc12)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@@H]1CCC(=O)N1 |wU:39.49,57.72,31.36,23.28,5.4,82.86,wD:51.55,72.83,12.20,(29.65,-8.55,;30.04,-10.04,;31.53,-10.45,;32.6,-11.55,;34.09,-11.18,;32.18,-13.04,;33.11,-14.25,;32.25,-15.54,;30.77,-15.11,;30.72,-13.57,;29.44,-12.71,;29.55,-11.16,;28.05,-13.38,;27.95,-14.92,;29.23,-15.78,;29.12,-17.32,;30.4,-18.18,;30.28,-19.72,;31.56,-20.59,;28.9,-20.4,;26.77,-12.51,;25.39,-13.18,;25.28,-14.73,;24.12,-12.32,;24.22,-10.79,;25.61,-10.11,;26.89,-10.97,;25.72,-8.57,;22.72,-12.99,;21.45,-12.14,;21.54,-10.6,;20.06,-12.81,;19.94,-14.34,;20.71,-15.68,;22.24,-15.69,;19.93,-17.01,;18.78,-11.95,;17.39,-12.62,;17.27,-14.16,;16.11,-11.76,;16.22,-10.22,;17.61,-9.55,;17.71,-8,;19.1,-7.33,;20.38,-8.19,;21.77,-7.51,;20.27,-9.73,;18.89,-10.41,;14.73,-12.44,;13.44,-11.57,;13.55,-10.02,;12.06,-12.24,;11.95,-13.78,;13.23,-14.64,;10.78,-11.38,;9.39,-12.06,;9.28,-13.6,;8.11,-11.18,;8.22,-9.65,;9.3,-8.56,;10.79,-8.95,;11.87,-7.86,;11.46,-6.37,;9.97,-5.99,;9.56,-4.51,;8.07,-4.14,;7,-5.24,;7.42,-6.71,;8.9,-7.09,;6.72,-11.86,;5.44,-11,;5.56,-9.46,;4.07,-11.67,;3.95,-13.22,;5.23,-14.08,;5.28,-15.62,;6.76,-16.05,;7.63,-14.78,;6.68,-13.55,;2.77,-10.81,;1.39,-11.48,;1.29,-13.03,;.22,-10.69,;.22,-9.28,;-1.3,-9.05,;-1.99,-10.42,;-3.5,-10.67,;-.9,-11.51,)| Show InChI InChI=1S/C61H85N15O12/c1-6-65-59(87)50-17-11-25-76(50)60(88)43(16-10-24-66-61(62)63)69-53(81)44(26-34(2)3)70-54(82)45(27-35(4)5)71-55(83)46(28-36-18-20-40(78)21-19-36)72-58(86)49(32-77)75-56(84)47(29-38-14-9-13-37-12-7-8-15-41(37)38)73-57(85)48(30-39-31-64-33-67-39)74-52(80)42-22-23-51(79)68-42/h7-9,12-15,18-21,31,33-35,42-50,77-78H,6,10-11,16-17,22-30,32H2,1-5H3,(H,64,67)(H,65,87)(H,68,79)(H,69,81)(H,70,82)(H,71,83)(H,72,86)(H,73,85)(H,74,80)(H,75,84)(H4,62,63,66)/t42-,43-,44-,45+,46-,47-,48-,49-,50+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0933 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Negative logarithm of equilibrium dissociation constant in the rat pituitary luteinizing releasing hormone receptor binding assay |
J Med Chem 35: 3890-4 (1992)
BindingDB Entry DOI: 10.7270/Q24Q7X6M |
More data for this
Ligand-Target Pair | |
Lutropin-choriogonadotropic hormone receptor
(Rattus norvegicus) | BDBM50230122

(CHEMBL415571)Show SMILES CCNC(=O)[C@H]1CCCN1C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](CC(C)C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(OC)cc1)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@@H]1CCC(=O)N1 |wU:39.49,57.69,23.28,31.36,5.4,80.83,wD:51.55,70.80,12.20,(24.19,-10.58,;24.58,-12.06,;26.07,-12.48,;27.14,-13.57,;28.64,-13.22,;26.72,-15.06,;27.66,-16.28,;26.79,-17.57,;25.32,-17.14,;25.25,-15.59,;23.98,-14.73,;24.09,-13.19,;22.6,-15.41,;22.49,-16.94,;23.77,-17.81,;23.65,-19.35,;24.93,-20.21,;24.81,-21.75,;26.11,-22.61,;23.44,-22.42,;21.32,-14.54,;19.94,-15.21,;19.82,-16.75,;18.66,-14.34,;18.75,-12.81,;20.14,-12.13,;21.42,-12.99,;20.26,-10.6,;17.26,-15.03,;15.99,-14.17,;16.1,-12.62,;14.59,-14.84,;14.49,-16.37,;15.26,-17.71,;16.79,-17.72,;14.48,-19.03,;13.32,-13.97,;11.94,-14.64,;11.82,-16.19,;10.66,-13.78,;10.76,-12.25,;12.15,-11.57,;13.43,-12.43,;14.82,-11.76,;14.92,-10.22,;16.31,-9.55,;13.64,-9.34,;12.26,-10.03,;9.27,-14.45,;7.99,-13.59,;8.09,-12.05,;6.6,-14.27,;6.49,-15.8,;7.78,-16.68,;5.33,-13.41,;3.93,-14.08,;3.82,-15.62,;2.66,-13.22,;2.77,-11.68,;3.84,-10.59,;5.34,-10.97,;6.42,-9.88,;6.02,-8.39,;7.08,-7.32,;6.67,-5.84,;4.51,-8.01,;3.45,-9.11,;1.28,-13.89,;-.02,-13.03,;.1,-11.48,;-1.39,-13.7,;-1.5,-15.24,;-.22,-16.1,;-.18,-17.65,;1.31,-18.08,;2.17,-16.8,;1.22,-15.57,;-2.67,-12.84,;-4.06,-13.52,;-4.17,-15.06,;-5.23,-12.73,;-5.23,-11.3,;-6.75,-11.06,;-7.43,-12.45,;-8.96,-12.7,;-6.34,-13.53,)| Show InChI InChI=1S/C58H85N15O13/c1-7-62-56(84)47-11-9-23-73(47)57(85)40(10-8-22-63-58(59)60)66-50(78)41(24-32(2)3)67-51(79)42(25-33(4)5)68-52(80)43(26-34-12-16-37(75)17-13-34)70-55(83)46(30-74)72-53(81)44(27-35-14-18-38(86-6)19-15-35)69-54(82)45(28-36-29-61-31-64-36)71-49(77)39-20-21-48(76)65-39/h12-19,29,31-33,39-47,74-75H,7-11,20-28,30H2,1-6H3,(H,61,64)(H,62,84)(H,65,76)(H,66,78)(H,67,79)(H,68,80)(H,69,82)(H,70,83)(H,71,77)(H,72,81)(H4,59,60,63)/t39-,40-,41-,42+,43-,44-,45-,46-,47+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.155 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Negative logarithm of equilibrium dissociation constant in the rat pituitary luteinizing releasing hormone receptor binding assay |
J Med Chem 35: 3890-4 (1992)
BindingDB Entry DOI: 10.7270/Q24Q7X6M |
More data for this
Ligand-Target Pair | |
Lutropin-choriogonadotropic hormone receptor
(Rattus norvegicus) | BDBM50230126

(CHEMBL385468)Show SMILES CCNC(=O)[C@H]1CCCN1C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](CC(C)C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@@H]1CCC(=O)N1 |wU:39.49,57.71,31.36,23.28,5.4,81.85,wD:51.55,71.82,12.20,(25.64,-6.44,;26.03,-7.92,;27.52,-8.34,;28.58,-9.43,;30.09,-9.08,;28.17,-10.92,;29.11,-12.14,;28.24,-13.43,;26.77,-13,;26.7,-11.45,;25.43,-10.59,;25.54,-9.05,;24.05,-11.27,;23.94,-12.8,;25.22,-13.67,;25.1,-15.21,;26.38,-16.06,;26.26,-17.6,;27.56,-18.46,;24.89,-18.28,;22.77,-10.4,;21.39,-11.07,;21.27,-12.61,;20.11,-10.2,;20.2,-8.67,;21.6,-7.99,;22.87,-8.85,;21.71,-6.46,;18.72,-10.89,;17.44,-10.03,;17.55,-8.48,;16.05,-10.7,;15.95,-12.23,;16.71,-13.56,;18.25,-13.58,;15.93,-14.89,;14.77,-9.83,;13.39,-10.5,;13.28,-12.05,;12.12,-9.64,;12.22,-8.11,;13.61,-7.44,;14.89,-8.3,;16.27,-7.62,;16.37,-6.08,;17.77,-5.41,;15.1,-5.21,;13.72,-5.9,;10.73,-10.31,;9.45,-9.46,;9.55,-7.9,;8.06,-10.13,;7.95,-11.66,;9.24,-12.54,;6.79,-9.27,;5.39,-9.94,;5.28,-11.48,;4.12,-9.08,;4.23,-7.54,;5.62,-6.87,;7.15,-6.65,;7.41,-5.14,;6.06,-4.41,;5.7,-2.91,;4.21,-2.48,;3.1,-3.55,;3.47,-5.05,;4.94,-5.48,;2.74,-9.75,;1.45,-8.89,;1.56,-7.34,;.08,-9.56,;-.04,-11.1,;1.24,-11.96,;1.28,-13.51,;2.77,-13.94,;3.63,-12.66,;2.68,-11.43,;-1.21,-8.71,;-2.59,-9.38,;-2.71,-10.92,;-3.76,-8.59,;-3.76,-7.16,;-5.29,-6.93,;-5.97,-8.31,;-7.49,-8.57,;-4.87,-9.39,)| Show InChI InChI=1S/C59H84N16O12/c1-6-63-57(86)48-14-10-22-75(48)58(87)41(13-9-21-64-59(60)61)68-51(80)42(23-32(2)3)69-52(81)43(24-33(4)5)70-53(82)44(25-34-15-17-37(77)18-16-34)71-56(85)47(30-76)74-54(83)45(26-35-28-65-39-12-8-7-11-38(35)39)72-55(84)46(27-36-29-62-31-66-36)73-50(79)40-19-20-49(78)67-40/h7-8,11-12,15-18,28-29,31-33,40-48,65,76-77H,6,9-10,13-14,19-27,30H2,1-5H3,(H,62,66)(H,63,86)(H,67,78)(H,68,80)(H,69,81)(H,70,82)(H,71,85)(H,72,84)(H,73,79)(H,74,83)(H4,60,61,64)/t40-,41-,42-,43+,44-,45-,46-,47-,48+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.186 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Negative logarithm of equilibrium dissociation constant in the rat pituitary luteinizing releasing hormone receptor binding assay |
J Med Chem 35: 3890-4 (1992)
BindingDB Entry DOI: 10.7270/Q24Q7X6M |
More data for this
Ligand-Target Pair | |
Lutropin-choriogonadotropic hormone receptor
(Rattus norvegicus) | BDBM50230124

(CHEMBL429240)Show SMILES CCNC(=O)[C@H]1CCCN1C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccccc1)N(C)C(=O)[C@@H]1CCC(=O)N1 |wU:89.95,63.79,45.57,31.44,23.28,5.4,wD:57.63,12.20,77.83,(27.09,-8.95,;27.47,-10.44,;28.96,-10.86,;30.04,-11.96,;31.53,-11.6,;29.61,-13.45,;30.56,-14.67,;29.69,-15.95,;28.21,-15.53,;28.16,-13.97,;26.88,-13.11,;26.98,-11.58,;25.49,-13.78,;25.38,-15.33,;26.66,-16.2,;26.55,-17.73,;27.83,-18.59,;27.72,-20.13,;29,-21,;26.33,-20.8,;24.22,-12.92,;22.82,-13.6,;22.72,-15.13,;21.54,-12.73,;21.65,-11.2,;23.05,-10.52,;23.15,-8.98,;24.32,-11.38,;20.15,-13.41,;18.89,-12.55,;18.99,-11.01,;17.49,-13.23,;17.39,-14.76,;18.14,-16.08,;19.64,-16.43,;19.78,-17.94,;18.38,-18.56,;17.91,-20.01,;16.43,-20.33,;15.39,-19.17,;15.88,-17.73,;17.36,-17.42,;16.22,-12.36,;14.83,-13.03,;14.71,-14.57,;13.55,-12.17,;13.66,-10.62,;15.04,-9.95,;16.32,-10.81,;17.71,-10.14,;17.82,-8.6,;19.2,-7.93,;16.54,-7.74,;15.16,-8.42,;12.16,-12.85,;10.88,-11.97,;10.99,-10.44,;9.49,-12.65,;9.39,-14.19,;10.67,-15.06,;8.21,-11.79,;6.83,-12.46,;6.71,-14.01,;5.54,-11.6,;5.65,-10.07,;7.05,-9.39,;8.58,-9.18,;8.85,-7.67,;7.49,-6.93,;7.12,-5.44,;5.63,-5,;4.53,-6.07,;4.89,-7.56,;6.37,-8,;4.17,-12.27,;2.88,-11.41,;3,-9.87,;1.49,-12.09,;1.38,-13.62,;2.15,-14.95,;1.37,-16.26,;2.12,-17.59,;3.65,-17.59,;4.41,-16.27,;3.66,-14.95,;.22,-11.23,;.31,-9.7,;-1.18,-11.9,;-1.28,-13.44,;-2.34,-11.11,;-2.34,-9.69,;-3.87,-9.46,;-4.55,-10.83,;-6.06,-11.09,;-3.45,-11.92,)| Show InChI InChI=1S/C68H87N15O12/c1-5-71-64(92)56-22-14-30-83(56)67(95)49(21-13-29-72-68(69)70)76-59(87)51(31-39(2)3)77-61(89)53(34-42-36-73-47-19-11-9-17-45(42)47)79-60(88)52(32-41-23-25-44(85)26-24-41)78-63(91)55(38-84)81-62(90)54(35-43-37-74-48-20-12-10-18-46(43)48)80-65(93)57(33-40-15-7-6-8-16-40)82(4)66(94)50-27-28-58(86)75-50/h6-12,15-20,23-26,36-37,39,49-57,73-74,84-85H,5,13-14,21-22,27-35,38H2,1-4H3,(H,71,92)(H,75,86)(H,76,87)(H,77,89)(H,78,91)(H,79,88)(H,80,93)(H,81,90)(H4,69,70,72)/t49-,50-,51-,52-,53+,54-,55-,56+,57-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.209 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Negative logarithm of equilibrium dissociation constant in the rat pituitary luteinizing releasing hormone receptor binding assay |
J Med Chem 35: 3890-4 (1992)
BindingDB Entry DOI: 10.7270/Q24Q7X6M |
More data for this
Ligand-Target Pair | |
Lutropin-choriogonadotropic hormone receptor
(Rattus norvegicus) | BDBM50230128
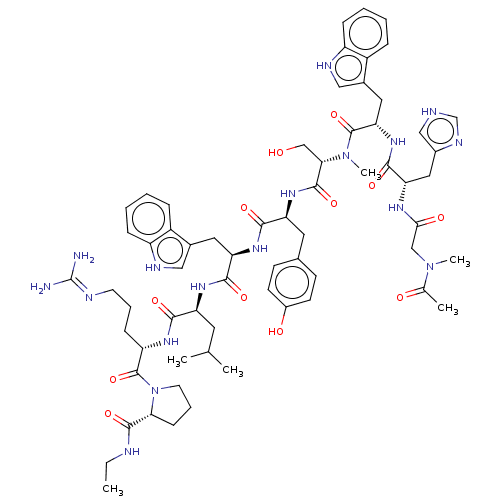
(CHEMBL437798)Show SMILES CCNC(=O)[C@H]1CCCN1C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)N(C)C(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)CN(C)C(C)=O |wU:64.80,45.57,31.44,23.28,5.4,wD:12.20,78.91,57.61,(25.45,-7.16,;25.84,-8.64,;27.33,-9.06,;28.4,-10.16,;29.9,-9.79,;27.98,-11.65,;28.92,-12.86,;28.05,-14.15,;26.58,-13.71,;26.52,-12.17,;25.24,-11.31,;25.35,-9.77,;23.86,-11.99,;23.75,-13.53,;25.03,-14.39,;24.91,-15.92,;26.19,-16.78,;26.07,-18.33,;27.37,-19.2,;24.7,-19,;22.58,-11.11,;21.2,-11.79,;21.08,-13.34,;19.92,-10.93,;20.01,-9.39,;21.4,-8.72,;21.52,-7.18,;22.68,-9.58,;18.52,-11.6,;17.25,-10.74,;17.35,-9.21,;15.85,-11.41,;15.75,-12.95,;16.5,-14.29,;18,-14.62,;18.15,-16.15,;16.76,-16.76,;16.27,-18.2,;14.78,-18.52,;13.76,-17.38,;14.24,-15.92,;15.74,-15.62,;14.57,-10.56,;13.2,-11.23,;13.08,-12.76,;11.92,-10.37,;12.02,-8.83,;13.41,-8.14,;13.52,-6.61,;14.9,-5.93,;16.18,-6.79,;17.57,-6.12,;16.08,-8.34,;14.69,-9.01,;10.53,-11.04,;9.25,-10.18,;9.35,-8.63,;7.86,-10.85,;7.74,-12.39,;9.04,-13.25,;6.58,-9.99,;6.68,-8.46,;5.19,-10.66,;5.07,-12.2,;3.91,-9.79,;4.03,-8.26,;5.41,-7.58,;6.95,-7.37,;7.21,-5.85,;5.85,-5.12,;5.49,-3.63,;4,-3.19,;2.89,-4.26,;3.26,-5.76,;4.74,-6.19,;2.53,-10.46,;1.24,-9.6,;1.36,-8.07,;-.13,-10.28,;-.25,-11.83,;1.03,-12.68,;1.08,-14.23,;2.56,-14.66,;3.42,-13.38,;2.47,-12.16,;-1.42,-9.42,;-2.8,-10.09,;-2.92,-11.64,;-3.97,-9.3,;-5.09,-10.11,;-5.2,-11.65,;-6.18,-9.02,;-5.5,-7.65,;-7.71,-9.28,)| Show InChI InChI=1S/C65H87N17O12/c1-7-69-61(91)54-19-13-25-82(54)64(94)48(18-12-24-70-65(66)67)75-57(87)49(26-37(2)3)76-59(89)51(28-40-31-71-46-16-10-8-14-44(40)46)77-58(88)50(27-39-20-22-43(85)23-21-39)78-62(92)55(35-83)81(6)63(93)53(29-41-32-72-47-17-11-9-15-45(41)47)79-60(90)52(30-42-33-68-36-73-42)74-56(86)34-80(5)38(4)84/h8-11,14-17,20-23,31-33,36-37,48-55,71-72,83,85H,7,12-13,18-19,24-30,34-35H2,1-6H3,(H,68,73)(H,69,91)(H,74,86)(H,75,87)(H,76,89)(H,77,88)(H,78,92)(H,79,90)(H4,66,67,70)/t48-,49-,50-,51+,52-,53-,54+,55-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Negative logarithm of equilibrium dissociation constant in the rat pituitary luteinizing releasing hormone receptor binding assay |
J Med Chem 35: 3890-4 (1992)
BindingDB Entry DOI: 10.7270/Q24Q7X6M |
More data for this
Ligand-Target Pair | |
Lutropin-choriogonadotropic hormone receptor
(Rattus norvegicus) | BDBM50230130

(CHEMBL266205)Show SMILES CCNC(=O)[C@H]1CCCN1C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](CC(C)C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)N(C)C(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@@H]1CCC(=O)N1 |wU:58.72,39.49,31.36,23.28,5.4,82.86,wD:72.83,12.20,51.53,(25.64,-6.44,;26.03,-7.92,;27.52,-8.34,;28.58,-9.43,;30.09,-9.08,;28.17,-10.92,;29.11,-12.14,;28.24,-13.43,;26.77,-13,;26.7,-11.45,;25.43,-10.59,;25.54,-9.05,;24.05,-11.27,;23.94,-12.8,;25.22,-13.67,;25.1,-15.21,;26.38,-16.06,;26.26,-17.6,;27.56,-18.46,;24.89,-18.28,;22.77,-10.4,;21.39,-11.07,;21.27,-12.61,;20.11,-10.2,;20.2,-8.67,;21.6,-7.99,;21.71,-6.46,;22.87,-8.85,;18.72,-10.89,;17.44,-10.03,;17.55,-8.48,;16.05,-10.7,;15.95,-12.23,;16.71,-13.56,;18.25,-13.58,;15.93,-14.89,;14.77,-9.83,;13.39,-10.5,;13.28,-12.05,;12.12,-9.64,;12.22,-8.11,;13.61,-7.44,;13.72,-5.9,;15.1,-5.21,;16.37,-6.08,;17.77,-5.41,;16.27,-7.62,;14.89,-8.3,;10.73,-10.31,;9.45,-9.46,;9.55,-7.9,;8.06,-10.13,;7.95,-11.66,;9.24,-12.54,;6.79,-9.27,;6.88,-7.74,;5.39,-9.94,;5.28,-11.48,;4.12,-9.08,;4.23,-7.54,;5.62,-6.87,;7.15,-6.65,;7.41,-5.14,;6.06,-4.41,;5.7,-2.91,;4.21,-2.48,;3.1,-3.55,;3.47,-5.05,;4.94,-5.48,;2.74,-9.75,;1.45,-8.89,;1.56,-7.34,;.08,-9.56,;-.04,-11.1,;1.24,-11.96,;1.28,-13.51,;2.77,-13.94,;3.63,-12.66,;2.68,-11.43,;-1.21,-8.71,;-2.59,-9.38,;-2.71,-10.92,;-3.76,-8.59,;-3.76,-7.16,;-5.29,-6.93,;-5.97,-8.31,;-7.49,-8.57,;-4.87,-9.39,)| Show InChI InChI=1S/C60H86N16O12/c1-7-64-56(85)48-15-11-23-76(48)59(88)42(14-10-22-65-60(61)62)69-52(81)43(24-33(2)3)70-53(82)44(25-34(4)5)71-54(83)45(26-35-16-18-38(78)19-17-35)73-57(86)49(31-77)75(6)58(87)47(27-36-29-66-40-13-9-8-12-39(36)40)74-55(84)46(28-37-30-63-32-67-37)72-51(80)41-20-21-50(79)68-41/h8-9,12-13,16-19,29-30,32-34,41-49,66,77-78H,7,10-11,14-15,20-28,31H2,1-6H3,(H,63,67)(H,64,85)(H,68,79)(H,69,81)(H,70,82)(H,71,83)(H,72,80)(H,73,86)(H,74,84)(H4,61,62,65)/t41-,42-,43-,44+,45-,46-,47-,48+,49-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Negative logarithm of equilibrium dissociation constant in the rat pituitary luteinizing releasing hormone receptor binding assay |
J Med Chem 35: 3890-4 (1992)
BindingDB Entry DOI: 10.7270/Q24Q7X6M |
More data for this
Ligand-Target Pair | |
Lutropin-choriogonadotropic hormone receptor
(Rattus norvegicus) | BDBM50230121

(CHEMBL217405)Show SMILES CCNC(=O)[C@H]1CCCN1C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](CC(C)C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCC(=O)N1 |wU:39.49,57.71,31.36,23.28,5.4,82.86,wD:51.55,71.83,12.20,(27.09,-8.95,;27.46,-10.44,;28.95,-10.86,;30.04,-11.96,;31.53,-11.6,;29.6,-13.45,;30.55,-14.67,;29.69,-15.94,;28.21,-15.52,;28.15,-13.97,;26.88,-13.11,;26.98,-11.58,;25.49,-13.78,;25.37,-15.33,;26.65,-16.2,;26.55,-17.73,;27.83,-18.59,;27.71,-20.12,;29,-21,;26.33,-20.8,;24.21,-12.92,;22.82,-13.6,;22.72,-15.13,;21.54,-12.73,;21.65,-11.2,;23.04,-10.52,;24.32,-11.38,;23.14,-8.97,;20.15,-13.41,;18.89,-12.55,;18.99,-11.01,;17.49,-13.23,;17.38,-14.76,;18.15,-16.1,;17.36,-17.42,;19.68,-16.11,;16.21,-12.36,;14.83,-13.03,;14.71,-14.57,;13.55,-12.17,;13.66,-10.62,;15.04,-9.95,;15.15,-8.42,;16.54,-7.74,;17.82,-8.6,;19.2,-7.93,;17.71,-10.14,;16.31,-10.81,;12.16,-12.85,;10.88,-11.97,;10.99,-10.44,;9.49,-12.64,;9.38,-14.19,;10.67,-15.06,;8.21,-11.79,;6.83,-12.46,;6.71,-14.01,;5.54,-11.6,;5.65,-10.07,;7.05,-9.39,;8.58,-9.18,;8.85,-7.67,;7.49,-6.93,;7.12,-5.44,;5.63,-5,;4.53,-6.07,;4.89,-7.56,;6.37,-8,;4.17,-12.27,;2.88,-11.41,;3,-9.87,;1.49,-12.09,;1.38,-13.62,;2.14,-14.94,;3.65,-14.94,;4.41,-16.27,;3.65,-17.59,;2.12,-17.58,;1.37,-16.26,;.22,-11.23,;-1.18,-11.9,;-1.28,-13.43,;-2.34,-11.11,;-2.34,-9.69,;-3.87,-9.45,;-4.55,-10.83,;-6.06,-11.09,;-3.45,-11.92,)| Show InChI InChI=1S/C62H86N14O12/c1-6-65-60(87)51-19-13-27-76(51)61(88)44(18-12-26-66-62(63)64)69-54(81)45(28-35(2)3)70-55(82)46(29-36(4)5)71-56(83)48(31-38-20-22-40(78)23-21-38)73-59(86)50(34-77)75-58(85)49(32-39-33-67-42-17-11-10-16-41(39)42)74-57(84)47(30-37-14-8-7-9-15-37)72-53(80)43-24-25-52(79)68-43/h7-11,14-17,20-23,33,35-36,43-51,67,77-78H,6,12-13,18-19,24-32,34H2,1-5H3,(H,65,87)(H,68,79)(H,69,81)(H,70,82)(H,71,83)(H,72,80)(H,73,86)(H,74,84)(H,75,85)(H4,63,64,66)/t43-,44-,45-,46+,47-,48-,49-,50-,51+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Negative logarithm of equilibrium dissociation constant in the rat pituitary luteinizing releasing hormone receptor binding assay |
J Med Chem 35: 3890-4 (1992)
BindingDB Entry DOI: 10.7270/Q24Q7X6M |
More data for this
Ligand-Target Pair | |
Lutropin-choriogonadotropic hormone receptor
(Rattus norvegicus) | BDBM50230125

(CHEMBL274682)Show SMILES CCNC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](CC(C)C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)CN(C)C(C)=O |wU:39.49,57.71,31.36,23.28,wD:51.55,12.20,5.4,71.82,(26.08,-5.67,;26.47,-7.16,;27.96,-7.58,;29.03,-8.67,;30.53,-8.31,;28.61,-10.15,;29.55,-11.37,;28.68,-12.66,;27.21,-12.23,;27.15,-10.69,;25.87,-9.83,;25.98,-8.29,;24.49,-10.5,;24.38,-12.03,;25.66,-12.91,;25.54,-14.44,;26.82,-15.3,;26.7,-16.84,;28,-17.7,;25.33,-17.51,;23.21,-9.63,;21.83,-10.31,;21.71,-11.85,;20.55,-9.43,;20.65,-7.9,;22.04,-7.23,;23.31,-8.09,;22.15,-5.7,;19.16,-10.12,;17.88,-9.26,;17.99,-7.71,;16.49,-9.94,;16.39,-11.47,;17.14,-12.8,;18.67,-12.82,;16.37,-14.14,;15.21,-9.06,;13.84,-9.73,;13.72,-11.28,;12.56,-8.88,;12.66,-7.34,;14.05,-6.67,;15.33,-7.53,;16.71,-6.86,;16.81,-5.32,;18.21,-4.64,;15.54,-4.44,;14.16,-5.13,;11.17,-9.55,;9.89,-8.69,;9.99,-7.15,;8.5,-9.36,;8.39,-10.9,;9.68,-11.77,;7.23,-8.5,;5.83,-9.18,;5.72,-10.71,;4.56,-8.31,;4.67,-6.78,;6.06,-6.11,;7.59,-5.88,;7.85,-4.37,;6.5,-3.64,;6.14,-2.14,;4.65,-1.72,;3.54,-2.79,;3.91,-4.28,;5.39,-4.71,;3.18,-8.98,;1.89,-8.12,;2,-6.58,;.52,-8.8,;.4,-10.34,;1.68,-11.2,;1.73,-12.74,;3.21,-13.17,;4.07,-11.89,;3.12,-10.66,;-.77,-7.94,;-2.15,-8.61,;-2.27,-10.15,;-3.32,-7.82,;-4.43,-8.62,;-4.55,-10.15,;-5.52,-7.54,;-7,-7.95,;-5.13,-6.07,)| Show InChI InChI=1S/C59H86N16O12/c1-8-63-57(86)49-16-12-22-75(49)58(87)42(15-11-21-64-59(60)61)68-51(80)43(23-33(2)3)69-52(81)44(24-34(4)5)70-53(82)45(25-36-17-19-39(78)20-18-36)71-56(85)48(31-76)73-54(83)46(26-37-28-65-41-14-10-9-13-40(37)41)72-55(84)47(27-38-29-62-32-66-38)67-50(79)30-74(7)35(6)77/h9-10,13-14,17-20,28-29,32-34,42-49,65,76,78H,8,11-12,15-16,21-27,30-31H2,1-7H3,(H,62,66)(H,63,86)(H,67,79)(H,68,80)(H,69,81)(H,70,82)(H,71,85)(H,72,84)(H,73,83)(H4,60,61,64)/t42-,43-,44+,45-,46-,47-,48-,49-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Negative logarithm of equilibrium dissociation constant in the rat pituitary luteinizing releasing hormone receptor binding assay |
J Med Chem 35: 3890-4 (1992)
BindingDB Entry DOI: 10.7270/Q24Q7X6M |
More data for this
Ligand-Target Pair | |
Lutropin-choriogonadotropic hormone receptor
(Rattus norvegicus) | BDBM50230119
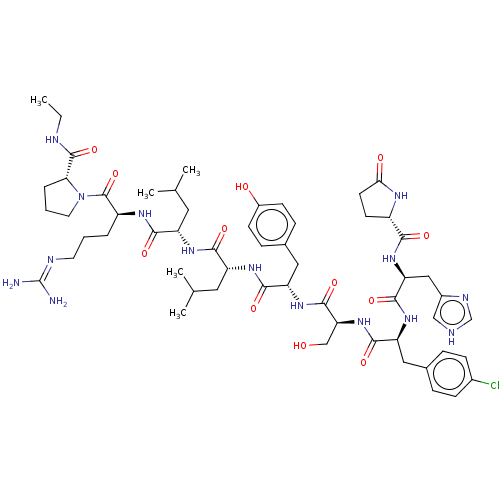
(CHEMBL414071)Show SMILES CCNC(=O)[C@H]1CCCN1C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](CC(C)C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(Cl)cc1)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@@H]1CCC(=O)N1 |wU:39.49,57.68,23.28,31.36,5.4,79.82,wD:51.55,69.79,12.20,(29.65,-8.55,;30.04,-10.04,;31.53,-10.45,;32.6,-11.55,;34.09,-11.18,;32.18,-13.04,;33.11,-14.25,;32.25,-15.54,;30.77,-15.11,;30.72,-13.57,;29.44,-12.71,;29.55,-11.16,;28.05,-13.38,;27.95,-14.92,;29.23,-15.78,;29.12,-17.32,;30.4,-18.18,;30.28,-19.72,;31.56,-20.59,;28.9,-20.4,;26.77,-12.51,;25.39,-13.18,;25.28,-14.73,;24.12,-12.32,;24.22,-10.79,;25.61,-10.11,;26.89,-10.97,;25.72,-8.57,;22.72,-12.99,;21.45,-12.14,;21.54,-10.6,;20.06,-12.81,;19.94,-14.34,;20.71,-15.68,;19.93,-17.01,;22.24,-15.69,;18.78,-11.95,;17.39,-12.62,;17.27,-14.16,;16.11,-11.76,;16.22,-10.22,;17.61,-9.55,;17.71,-8,;19.1,-7.33,;20.38,-8.19,;21.77,-7.51,;20.27,-9.73,;18.89,-10.41,;14.73,-12.44,;13.44,-11.57,;13.55,-10.02,;12.06,-12.24,;11.95,-13.78,;13.23,-14.64,;10.78,-11.38,;9.39,-12.06,;9.28,-13.6,;8.11,-11.18,;8.22,-9.65,;9.3,-8.56,;10.79,-8.95,;11.87,-7.86,;11.46,-6.37,;12.55,-5.28,;9.97,-5.98,;8.9,-7.09,;6.72,-11.86,;5.44,-11,;5.56,-9.46,;4.07,-11.67,;3.95,-13.22,;5.23,-14.08,;5.28,-15.62,;6.76,-16.05,;7.63,-14.78,;6.68,-13.55,;2.77,-10.81,;1.39,-11.48,;1.29,-13.03,;.22,-10.69,;.22,-9.28,;-1.3,-9.05,;-1.99,-10.42,;-3.5,-10.67,;-.9,-11.51,)| Show InChI InChI=1S/C57H82ClN15O12/c1-6-62-55(84)46-10-8-22-73(46)56(85)39(9-7-21-63-57(59)60)66-49(78)40(23-31(2)3)67-50(79)41(24-32(4)5)68-51(80)42(26-34-13-17-37(75)18-14-34)70-54(83)45(29-74)72-52(81)43(25-33-11-15-35(58)16-12-33)69-53(82)44(27-36-28-61-30-64-36)71-48(77)38-19-20-47(76)65-38/h11-18,28,30-32,38-46,74-75H,6-10,19-27,29H2,1-5H3,(H,61,64)(H,62,84)(H,65,76)(H,66,78)(H,67,79)(H,68,80)(H,69,82)(H,70,83)(H,71,77)(H,72,81)(H4,59,60,63)/t38-,39-,40-,41+,42-,43-,44-,45-,46+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 9.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Negative logarithm of equilibrium dissociation constant in the rat pituitary luteinizing releasing hormone receptor binding assay |
J Med Chem 35: 3890-4 (1992)
BindingDB Entry DOI: 10.7270/Q24Q7X6M |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50017780

(CHEMBL284451 | {1-[1-(4-Azido-1-cyclohexylmethyl-2...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](CC1CCCCC1)[C@H](O)[C@H](O)CN=[N+]=[N-] Show InChI InChI=1S/C31H46N8O6/c1-31(2,3)45-30(44)38-24(15-21-12-8-5-9-13-21)28(42)37-25(16-22-17-33-19-34-22)29(43)36-23(14-20-10-6-4-7-11-20)27(41)26(40)18-35-39-32/h5,8-9,12-13,17,19-20,23-27,40-41H,4,6-7,10-11,14-16,18H2,1-3H3,(H,33,34)(H,36,43)(H,37,42)(H,38,44)/t23-,24+,25+,26-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against purified human renal renin at pH 6.0 |
J Med Chem 32: 1371-8 (1989)
BindingDB Entry DOI: 10.7270/Q2474BFR |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50017780

(CHEMBL284451 | {1-[1-(4-Azido-1-cyclohexylmethyl-2...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](CC1CCCCC1)[C@H](O)[C@H](O)CN=[N+]=[N-] Show InChI InChI=1S/C31H46N8O6/c1-31(2,3)45-30(44)38-24(15-21-12-8-5-9-13-21)28(42)37-25(16-22-17-33-19-34-22)29(43)36-23(14-20-10-6-4-7-11-20)27(41)26(40)18-35-39-32/h5,8-9,12-13,17,19-20,23-27,40-41H,4,6-7,10-11,14-16,18H2,1-3H3,(H,33,34)(H,36,43)(H,37,42)(H,38,44)/t23-,24+,25+,26-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against purified human plasma renin at pH 6.0 |
J Med Chem 32: 1371-8 (1989)
BindingDB Entry DOI: 10.7270/Q2474BFR |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50017783

(CHEMBL440779 | N-{1-[1-(4-Azido-1-cyclohexylmethyl...)Show SMILES CC(C)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](CC1CCCCC1)[C@H](O)[C@H](O)CN=[N+]=[N-] Show InChI InChI=1S/C30H44N8O5/c1-19(2)28(41)36-24(14-21-11-7-4-8-12-21)29(42)37-25(15-22-16-32-18-33-22)30(43)35-23(13-20-9-5-3-6-10-20)27(40)26(39)17-34-38-31/h4,7-8,11-12,16,18-20,23-27,39-40H,3,5-6,9-10,13-15,17H2,1-2H3,(H,32,33)(H,35,43)(H,36,41)(H,37,42)/t23-,24+,25+,26-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against purified human plasma renin at pH 6.0 |
J Med Chem 32: 1371-8 (1989)
BindingDB Entry DOI: 10.7270/Q2474BFR |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50017783

(CHEMBL440779 | N-{1-[1-(4-Azido-1-cyclohexylmethyl...)Show SMILES CC(C)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](CC1CCCCC1)[C@H](O)[C@H](O)CN=[N+]=[N-] Show InChI InChI=1S/C30H44N8O5/c1-19(2)28(41)36-24(14-21-11-7-4-8-12-21)29(42)37-25(15-22-16-32-18-33-22)30(43)35-23(13-20-9-5-3-6-10-20)27(40)26(39)17-34-38-31/h4,7-8,11-12,16,18-20,23-27,39-40H,3,5-6,9-10,13-15,17H2,1-2H3,(H,32,33)(H,35,43)(H,36,41)(H,37,42)/t23-,24+,25+,26-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against purified human plasma renin at pH 6.0 |
J Med Chem 32: 1371-8 (1989)
BindingDB Entry DOI: 10.7270/Q2474BFR |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50017782

(CHEMBL280744 | Tetrahydro-pyran-4-carboxylic acid ...)Show SMILES O[C@H](CN=[N+]=[N-])[C@@H](O)[C@@H](CC1CCCCC1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)C1CCOCC1 Show InChI InChI=1S/C32H46N8O6/c33-40-36-19-28(41)29(42)25(15-21-7-3-1-4-8-21)37-32(45)27(17-24-18-34-20-35-24)39-31(44)26(16-22-9-5-2-6-10-22)38-30(43)23-11-13-46-14-12-23/h2,5-6,9-10,18,20-21,23,25-29,41-42H,1,3-4,7-8,11-17,19H2,(H,34,35)(H,37,45)(H,38,43)(H,39,44)/t25-,26+,27+,28-,29+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against purified human plasma renin at pH 6.0 |
J Med Chem 32: 1371-8 (1989)
BindingDB Entry DOI: 10.7270/Q2474BFR |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50050056

(2-[(R)-1-[(S)-2-(3-Cyclohexyl-3-methyl-ureido)-4-m...)Show SMILES CC(C)C[C@H](NC(=O)N(C)C1CCCCC1)C(=O)N[C@H](Cc1cn(C)c2ccccc12)c1nc(C(O)=O)c(C)[nH]1 Show InChI InChI=1S/C30H42N6O4/c1-18(2)15-24(33-30(40)36(5)21-11-7-6-8-12-21)28(37)32-23(27-31-19(3)26(34-27)29(38)39)16-20-17-35(4)25-14-10-9-13-22(20)25/h9-10,13-14,17-18,21,23-24H,6-8,11-12,15-16H2,1-5H3,(H,31,34)(H,32,37)(H,33,40)(H,38,39)/t23-,24+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity was evaluated against human Endothelin A receptor |
J Med Chem 39: 982-91 (1996)
Article DOI: 10.1021/jm9505932
BindingDB Entry DOI: 10.7270/Q2DB80WG |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50017777

(CHEMBL281436 | N-[1-[1-(4-Azido-1-cyclohexylmethyl...)Show SMILES COc1ccc(C[C@H](NC(=O)C(C)C)C(=O)N[C@@H](Cc2cnc[nH]2)C(=O)N[C@H](CC2CCCCC2)[C@H](O)[C@H](O)CN=[N+]=[N-])cc1 Show InChI InChI=1S/C31H46N8O6/c1-19(2)29(42)37-25(14-21-9-11-23(45-3)12-10-21)30(43)38-26(15-22-16-33-18-34-22)31(44)36-24(13-20-7-5-4-6-8-20)28(41)27(40)17-35-39-32/h9-12,16,18-20,24-28,40-41H,4-8,13-15,17H2,1-3H3,(H,33,34)(H,36,44)(H,37,42)(H,38,43)/t24-,25+,26+,27-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human plasma renin at pH 7.4 |
J Med Chem 32: 1371-8 (1989)
BindingDB Entry DOI: 10.7270/Q2474BFR |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50017778
(CHEMBL282757 | Morpholine-4-carboxylic acid {1-[1-...)Show SMILES O[C@H](CN=[N+]=[N-])[C@@H](O)[C@@H](CC1CCCCC1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)N1CCOCC1 Show InChI InChI=1S/C31H45N9O6/c32-39-35-19-27(41)28(42)24(15-21-7-3-1-4-8-21)36-30(44)26(17-23-18-33-20-34-23)37-29(43)25(16-22-9-5-2-6-10-22)38-31(45)40-11-13-46-14-12-40/h2,5-6,9-10,18,20-21,24-28,41-42H,1,3-4,7-8,11-17,19H2,(H,33,34)(H,36,44)(H,37,43)(H,38,45)/t24-,25+,26+,27-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human plasma renin at pH 7.4 |
J Med Chem 32: 1371-8 (1989)
BindingDB Entry DOI: 10.7270/Q2474BFR |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50017778

(CHEMBL282757 | Morpholine-4-carboxylic acid {1-[1-...)Show SMILES O[C@H](CN=[N+]=[N-])[C@@H](O)[C@@H](CC1CCCCC1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)N1CCOCC1 Show InChI InChI=1S/C31H45N9O6/c32-39-35-19-27(41)28(42)24(15-21-7-3-1-4-8-21)36-30(44)26(17-23-18-33-20-34-23)37-29(43)25(16-22-9-5-2-6-10-22)38-31(45)40-11-13-46-14-12-40/h2,5-6,9-10,18,20-21,24-28,41-42H,1,3-4,7-8,11-17,19H2,(H,33,34)(H,36,44)(H,37,43)(H,38,45)/t24-,25+,26+,27-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against purified human plasma renin at pH 6.0 |
J Med Chem 32: 1371-8 (1989)
BindingDB Entry DOI: 10.7270/Q2474BFR |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50017773

(CHEMBL280503 | Morpholine-4-carboxylic acid [1-[1-...)Show SMILES COc1ccc(C[C@H](NC(=O)N2CCOCC2)C(=O)N[C@@H](Cc2cnc[nH]2)C(=O)N[C@H](CC2CCCCC2)[C@H](O)[C@H](O)CN=[N+]=[N-])cc1 Show InChI InChI=1S/C32H47N9O7/c1-47-24-9-7-22(8-10-24)16-26(39-32(46)41-11-13-48-14-12-41)30(44)38-27(17-23-18-34-20-35-23)31(45)37-25(15-21-5-3-2-4-6-21)29(43)28(42)19-36-40-33/h7-10,18,20-21,25-29,42-43H,2-6,11-17,19H2,1H3,(H,34,35)(H,37,45)(H,38,44)(H,39,46)/t25-,26+,27+,28-,29+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against purified human plasma renin at pH 6.0 |
J Med Chem 32: 1371-8 (1989)
BindingDB Entry DOI: 10.7270/Q2474BFR |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50050063
(2-[(R)-1-[(S)-2-(Cyclohexyl-methyl-carbamoyloxy)-4...)Show SMILES CC(C)C[C@H](OC(=O)N(C)C1CCCCC1)C(=O)N[C@H](Cc1cn(C)c2ccccc12)c1nc(C(O)=O)c(C)o1 Show InChI InChI=1S/C30H40N4O6/c1-18(2)15-25(40-30(38)34(5)21-11-7-6-8-12-21)27(35)31-23(28-32-26(29(36)37)19(3)39-28)16-20-17-33(4)24-14-10-9-13-22(20)24/h9-10,13-14,17-18,21,23,25H,6-8,11-12,15-16H2,1-5H3,(H,31,35)(H,36,37)/t23-,25+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity was evaluated against human Endothelin A receptor |
J Med Chem 39: 982-91 (1996)
Article DOI: 10.1021/jm9505932
BindingDB Entry DOI: 10.7270/Q2DB80WG |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50017774
(CHEMBL29004 | {1-[1-(4-Azido-1-cyclohexylmethyl-2,...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](CC1CCCCC1)[C@H](O)C(O)(CO)CN=[N+]=[N-] Show InChI InChI=1S/C32H48N8O7/c1-31(2,3)47-30(45)39-25(15-22-12-8-5-9-13-22)28(43)38-26(16-23-17-34-20-35-23)29(44)37-24(14-21-10-6-4-7-11-21)27(42)32(46,19-41)18-36-40-33/h5,8-9,12-13,17,20-21,24-27,41-42,46H,4,6-7,10-11,14-16,18-19H2,1-3H3,(H,34,35)(H,37,44)(H,38,43)(H,39,45)/t24-,25+,26+,27+,32?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against purified human renal renin at pH 6.0 |
J Med Chem 32: 1371-8 (1989)
BindingDB Entry DOI: 10.7270/Q2474BFR |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50017779

(CHEMBL28679 | {1-[1-(4-Azido-1-cyclohexylmethyl-2-...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](CC1CCCCC1)[C@H](O)C(=O)CN=[N+]=[N-] Show InChI InChI=1S/C31H44N8O6/c1-31(2,3)45-30(44)38-24(15-21-12-8-5-9-13-21)28(42)37-25(16-22-17-33-19-34-22)29(43)36-23(14-20-10-6-4-7-11-20)27(41)26(40)18-35-39-32/h5,8-9,12-13,17,19-20,23-25,27,41H,4,6-7,10-11,14-16,18H2,1-3H3,(H,33,34)(H,36,43)(H,37,42)(H,38,44)/t23-,24+,25+,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against purified human renal renin at pH 6.0 |
J Med Chem 32: 1371-8 (1989)
BindingDB Entry DOI: 10.7270/Q2474BFR |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50017775

(CHEMBL29315 | {1-[1-(3-Azido-1-cyclohexylmethyl-2-...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](CC1CCCCC1)[C@H](O)CN=[N+]=[N-] Show InChI InChI=1S/C30H44N8O5/c1-30(2,3)43-29(42)37-24(15-21-12-8-5-9-13-21)27(40)36-25(16-22-17-32-19-33-22)28(41)35-23(26(39)18-34-38-31)14-20-10-6-4-7-11-20/h5,8-9,12-13,17,19-20,23-26,39H,4,6-7,10-11,14-16,18H2,1-3H3,(H,32,33)(H,35,41)(H,36,40)(H,37,42)/t23-,24+,25+,26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against purified human renal renin at pH 6.0 |
J Med Chem 32: 1371-8 (1989)
BindingDB Entry DOI: 10.7270/Q2474BFR |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50017782

(CHEMBL280744 | Tetrahydro-pyran-4-carboxylic acid ...)Show SMILES O[C@H](CN=[N+]=[N-])[C@@H](O)[C@@H](CC1CCCCC1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)C1CCOCC1 Show InChI InChI=1S/C32H46N8O6/c33-40-36-19-28(41)29(42)25(15-21-7-3-1-4-8-21)37-32(45)27(17-24-18-34-20-35-24)39-31(44)26(16-22-9-5-2-6-10-22)38-30(43)23-11-13-46-14-12-23/h2,5-6,9-10,18,20-21,23,25-29,41-42H,1,3-4,7-8,11-17,19H2,(H,34,35)(H,37,45)(H,38,43)(H,39,44)/t25-,26+,27+,28-,29+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against purified human plasma renin at pH 6.0 |
J Med Chem 32: 1371-8 (1989)
BindingDB Entry DOI: 10.7270/Q2474BFR |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50017780

(CHEMBL284451 | {1-[1-(4-Azido-1-cyclohexylmethyl-2...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](CC1CCCCC1)[C@H](O)[C@H](O)CN=[N+]=[N-] Show InChI InChI=1S/C31H46N8O6/c1-31(2,3)45-30(44)38-24(15-21-12-8-5-9-13-21)28(42)37-25(16-22-17-33-19-34-22)29(43)36-23(14-20-10-6-4-7-11-20)27(41)26(40)18-35-39-32/h5,8-9,12-13,17,19-20,23-27,40-41H,4,6-7,10-11,14-16,18H2,1-3H3,(H,33,34)(H,36,43)(H,37,42)(H,38,44)/t23-,24+,25+,26-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human plasma renin at pH 7.4 |
J Med Chem 32: 1371-8 (1989)
BindingDB Entry DOI: 10.7270/Q2474BFR |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50050058

(2-[(R)-1-((S)-2-Cyclohexylcarbamoyloxy-4-methyl-pe...)Show SMILES CC(C)C[C@H](OC(=O)NC1CCCCC1)C(=O)N[C@H](Cc1cn(C)c2ccccc12)c1nc(C(O)=O)c(C)[nH]1 Show InChI InChI=1S/C29H39N5O5/c1-17(2)14-24(39-29(38)31-20-10-6-5-7-11-20)27(35)32-22(26-30-18(3)25(33-26)28(36)37)15-19-16-34(4)23-13-9-8-12-21(19)23/h8-9,12-13,16-17,20,22,24H,5-7,10-11,14-15H2,1-4H3,(H,30,33)(H,31,38)(H,32,35)(H,36,37)/t22-,24+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity was evaluated against human Endothelin A receptor |
J Med Chem 39: 982-91 (1996)
Article DOI: 10.1021/jm9505932
BindingDB Entry DOI: 10.7270/Q2DB80WG |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50017786
(CHEMBL408084 | {1-[1-(4-Azido-1-cyclohexylmethyl-2...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](CC1CCCCC1)[C@H](O)[C@H](CO)CN=[N+]=[N-] Show InChI InChI=1S/C32H48N8O6/c1-32(2,3)46-31(45)39-26(15-22-12-8-5-9-13-22)29(43)38-27(16-24-18-34-20-35-24)30(44)37-25(14-21-10-6-4-7-11-21)28(42)23(19-41)17-36-40-33/h5,8-9,12-13,18,20-21,23,25-28,41-42H,4,6-7,10-11,14-17,19H2,1-3H3,(H,34,35)(H,37,44)(H,38,43)(H,39,45)/t23-,25+,26-,27-,28+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against purified human renal renin at pH 6.0 |
J Med Chem 32: 1371-8 (1989)
BindingDB Entry DOI: 10.7270/Q2474BFR |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50017777

(CHEMBL281436 | N-[1-[1-(4-Azido-1-cyclohexylmethyl...)Show SMILES COc1ccc(C[C@H](NC(=O)C(C)C)C(=O)N[C@@H](Cc2cnc[nH]2)C(=O)N[C@H](CC2CCCCC2)[C@H](O)[C@H](O)CN=[N+]=[N-])cc1 Show InChI InChI=1S/C31H46N8O6/c1-19(2)29(42)37-25(14-21-9-11-23(45-3)12-10-21)30(43)38-26(15-22-16-33-18-34-22)31(44)36-24(13-20-7-5-4-6-8-20)28(41)27(40)17-35-39-32/h9-12,16,18-20,24-28,40-41H,4-8,13-15,17H2,1-3H3,(H,33,34)(H,36,44)(H,37,42)(H,38,43)/t24-,25+,26+,27-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human plasma renin at pH 7.4 |
J Med Chem 32: 1371-8 (1989)
BindingDB Entry DOI: 10.7270/Q2474BFR |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50050047
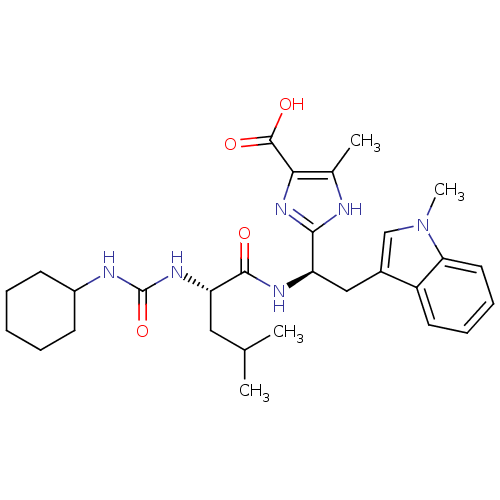
(2-[(R)-1-[(S)-2-(3-Cyclohexyl-ureido)-4-methyl-pen...)Show SMILES CC(C)C[C@H](NC(=O)NC1CCCCC1)C(=O)N[C@H](Cc1cn(C)c2ccccc12)c1nc(C(O)=O)c(C)[nH]1 Show InChI InChI=1S/C29H40N6O4/c1-17(2)14-23(33-29(39)31-20-10-6-5-7-11-20)27(36)32-22(26-30-18(3)25(34-26)28(37)38)15-19-16-35(4)24-13-9-8-12-21(19)24/h8-9,12-13,16-17,20,22-23H,5-7,10-11,14-15H2,1-4H3,(H,30,34)(H,32,36)(H,37,38)(H2,31,33,39)/t22-,23+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity was evaluated against human Endothelin A receptor |
J Med Chem 39: 982-91 (1996)
Article DOI: 10.1021/jm9505932
BindingDB Entry DOI: 10.7270/Q2DB80WG |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50050064

(2-[(R)-1-((S)-2-Cyclohexyloxycarbonyloxy-4-methyl-...)Show SMILES CC(C)C[C@H](OC(=O)OC1CCCCC1)C(=O)N[C@H](Cc1cn(C)c2ccccc12)c1nc(C(O)=O)c(C)o1 Show InChI InChI=1S/C29H37N3O7/c1-17(2)14-24(39-29(36)38-20-10-6-5-7-11-20)26(33)30-22(27-31-25(28(34)35)18(3)37-27)15-19-16-32(4)23-13-9-8-12-21(19)23/h8-9,12-13,16-17,20,22,24H,5-7,10-11,14-15H2,1-4H3,(H,30,33)(H,34,35)/t22-,24+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity was evaluated against human Endothelin A receptor |
J Med Chem 39: 982-91 (1996)
Article DOI: 10.1021/jm9505932
BindingDB Entry DOI: 10.7270/Q2DB80WG |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50017773

(CHEMBL280503 | Morpholine-4-carboxylic acid [1-[1-...)Show SMILES COc1ccc(C[C@H](NC(=O)N2CCOCC2)C(=O)N[C@@H](Cc2cnc[nH]2)C(=O)N[C@H](CC2CCCCC2)[C@H](O)[C@H](O)CN=[N+]=[N-])cc1 Show InChI InChI=1S/C32H47N9O7/c1-47-24-9-7-22(8-10-24)16-26(39-32(46)41-11-13-48-14-12-41)30(44)38-27(17-23-18-34-20-35-23)31(45)37-25(15-21-5-3-2-4-6-21)29(43)28(42)19-36-40-33/h7-10,18,20-21,25-29,42-43H,2-6,11-17,19H2,1H3,(H,34,35)(H,37,45)(H,38,44)(H,39,46)/t25-,26+,27+,28-,29+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human plasma renin at pH 7.4 |
J Med Chem 32: 1371-8 (1989)
BindingDB Entry DOI: 10.7270/Q2474BFR |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50017776
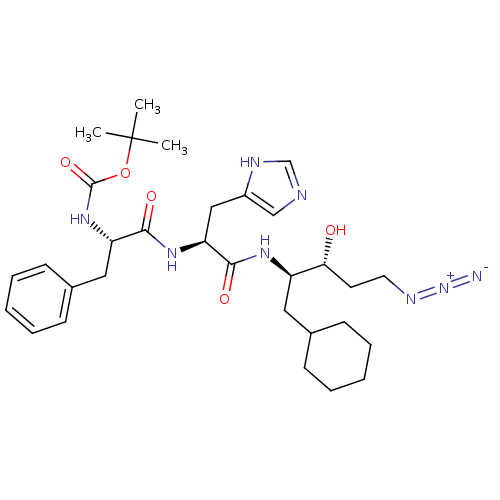
(CHEMBL28701 | {1-[1-(4-Azido-1-cyclohexylmethyl-2-...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](CC1CCCCC1)[C@H](O)CCN=[N+]=[N-] Show InChI InChI=1S/C31H46N8O5/c1-31(2,3)44-30(43)38-25(17-22-12-8-5-9-13-22)28(41)37-26(18-23-19-33-20-34-23)29(42)36-24(27(40)14-15-35-39-32)16-21-10-6-4-7-11-21/h5,8-9,12-13,19-21,24-27,40H,4,6-7,10-11,14-18H2,1-3H3,(H,33,34)(H,36,42)(H,37,41)(H,38,43)/t24-,25+,26+,27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against purified human renal renin at pH 6.0 |
J Med Chem 32: 1371-8 (1989)
BindingDB Entry DOI: 10.7270/Q2474BFR |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50050051

(2-[(R)-1-((S)-2-Cyclohexylcarbamoyloxy-4-methyl-pe...)Show SMILES CC(C)C[C@H](OC(=O)NC1CCCCC1)C(=O)N[C@H](Cc1cn(C)c2ccccc12)c1nc(C(O)=O)c(C)o1 Show InChI InChI=1S/C29H38N4O6/c1-17(2)14-24(39-29(37)30-20-10-6-5-7-11-20)26(34)31-22(27-32-25(28(35)36)18(3)38-27)15-19-16-33(4)23-13-9-8-12-21(19)23/h8-9,12-13,16-17,20,22,24H,5-7,10-11,14-15H2,1-4H3,(H,30,37)(H,31,34)(H,35,36)/t22-,24+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity was evaluated against human Endothelin A receptor |
J Med Chem 39: 982-91 (1996)
Article DOI: 10.1021/jm9505932
BindingDB Entry DOI: 10.7270/Q2DB80WG |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50050055
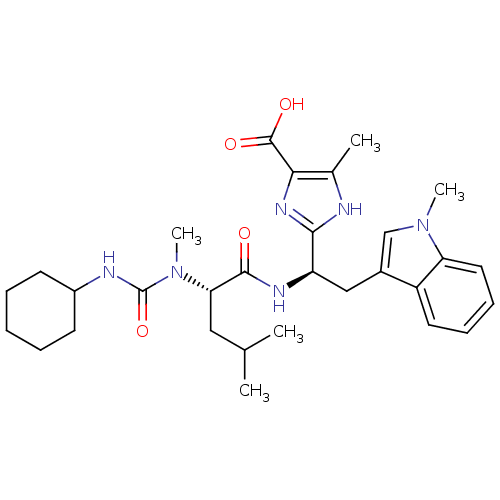
(2-[(R)-1-[(S)-2-(3-Cyclohexyl-1-methyl-ureido)-4-m...)Show SMILES CC(C)C[C@H](N(C)C(=O)NC1CCCCC1)C(=O)N[C@H](Cc1cn(C)c2ccccc12)c1nc(C(O)=O)c(C)[nH]1 Show InChI InChI=1S/C30H42N6O4/c1-18(2)15-25(36(5)30(40)32-21-11-7-6-8-12-21)28(37)33-23(27-31-19(3)26(34-27)29(38)39)16-20-17-35(4)24-14-10-9-13-22(20)24/h9-10,13-14,17-18,21,23,25H,6-8,11-12,15-16H2,1-5H3,(H,31,34)(H,32,40)(H,33,37)(H,38,39)/t23-,25+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity was evaluated against human Endothelin A receptor |
J Med Chem 39: 982-91 (1996)
Article DOI: 10.1021/jm9505932
BindingDB Entry DOI: 10.7270/Q2DB80WG |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50050059

(2-[(R)-1-[(S)-2-(3-Cyclohexyl-3-methyl-ureido)-4-m...)Show SMILES CC(C)C[C@H](NC(=O)N(C)C1CCCCC1)C(=O)N[C@H](Cc1cn(C)c2ccccc12)c1nc(C(O)=O)c(C)o1 Show InChI InChI=1S/C30H41N5O5/c1-18(2)15-23(32-30(39)35(5)21-11-7-6-8-12-21)27(36)31-24(28-33-26(29(37)38)19(3)40-28)16-20-17-34(4)25-14-10-9-13-22(20)25/h9-10,13-14,17-18,21,23-24H,6-8,11-12,15-16H2,1-5H3,(H,31,36)(H,32,39)(H,37,38)/t23-,24+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity was evaluated against human Endothelin A receptor |
J Med Chem 39: 982-91 (1996)
Article DOI: 10.1021/jm9505932
BindingDB Entry DOI: 10.7270/Q2DB80WG |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50017781

(CHEMBL432280 | {1-[1-(4-Azido-1-cyclohexylmethyl-2...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](CC1CCCCC1)[C@H](O)[C@@H](O)CN=[N+]=[N-] Show InChI InChI=1S/C31H46N8O6/c1-31(2,3)45-30(44)38-24(15-21-12-8-5-9-13-21)28(42)37-25(16-22-17-33-19-34-22)29(43)36-23(14-20-10-6-4-7-11-20)27(41)26(40)18-35-39-32/h5,8-9,12-13,17,19-20,23-27,40-41H,4,6-7,10-11,14-16,18H2,1-3H3,(H,33,34)(H,36,43)(H,37,42)(H,38,44)/t23-,24+,25+,26+,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against purified human renal renin at pH 6.0 |
J Med Chem 32: 1371-8 (1989)
BindingDB Entry DOI: 10.7270/Q2474BFR |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50050061

(2-[(R)-1-((R)-2-Cyclohexylcarbamoylmethyl-4-methyl...)Show SMILES CC(C)C[C@H](CC(=O)NC1CCCCC1)C(=O)N[C@H](Cc1cn(C)c2ccccc12)c1nc(C(O)=O)c(C)[nH]1 Show InChI InChI=1S/C30H41N5O4/c1-18(2)14-20(16-26(36)32-22-10-6-5-7-11-22)29(37)33-24(28-31-19(3)27(34-28)30(38)39)15-21-17-35(4)25-13-9-8-12-23(21)25/h8-9,12-13,17-18,20,22,24H,5-7,10-11,14-16H2,1-4H3,(H,31,34)(H,32,36)(H,33,37)(H,38,39)/t20-,24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity was evaluated against human Endothelin A receptor |
J Med Chem 39: 982-91 (1996)
Article DOI: 10.1021/jm9505932
BindingDB Entry DOI: 10.7270/Q2DB80WG |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50050060
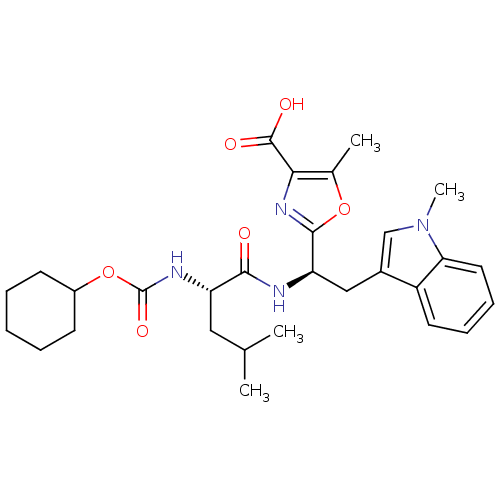
(2-[(R)-1-((S)-2-Cyclohexyloxycarbonylamino-4-methy...)Show SMILES CC(C)C[C@H](NC(=O)OC1CCCCC1)C(=O)N[C@H](Cc1cn(C)c2ccccc12)c1nc(C(O)=O)c(C)o1 Show InChI InChI=1S/C29H38N4O6/c1-17(2)14-22(31-29(37)39-20-10-6-5-7-11-20)26(34)30-23(27-32-25(28(35)36)18(3)38-27)15-19-16-33(4)24-13-9-8-12-21(19)24/h8-9,12-13,16-17,20,22-23H,5-7,10-11,14-15H2,1-4H3,(H,30,34)(H,31,37)(H,35,36)/t22-,23+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity was evaluated against human Endothelin A receptor |
J Med Chem 39: 982-91 (1996)
Article DOI: 10.1021/jm9505932
BindingDB Entry DOI: 10.7270/Q2DB80WG |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50017785
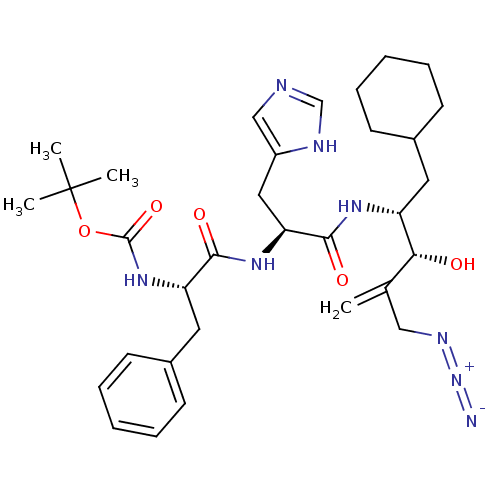
(CHEMBL284891 | {1-[1-(3-Azidomethyl-1-cyclohexylme...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](CC1CCCCC1)[C@H](O)C(=C)CN=[N+]=[N-] Show InChI InChI=1S/C32H46N8O5/c1-21(18-36-40-33)28(41)25(15-22-11-7-5-8-12-22)37-30(43)27(17-24-19-34-20-35-24)38-29(42)26(16-23-13-9-6-10-14-23)39-31(44)45-32(2,3)4/h6,9-10,13-14,19-20,22,25-28,41H,1,5,7-8,11-12,15-18H2,2-4H3,(H,34,35)(H,37,43)(H,38,42)(H,39,44)/t25-,26+,27+,28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against purified human renal renin at pH 6.0 |
J Med Chem 32: 1371-8 (1989)
BindingDB Entry DOI: 10.7270/Q2474BFR |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50050062
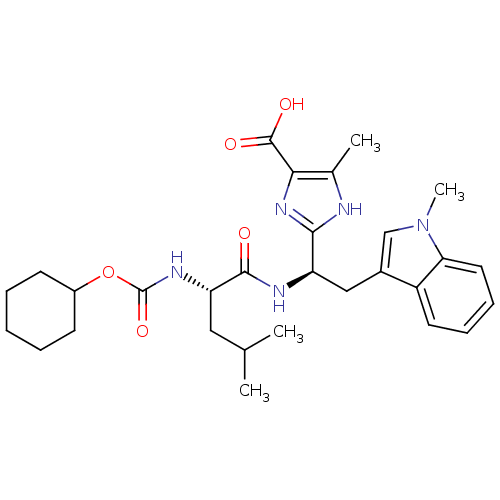
(2-[(R)-1-((S)-2-Cyclohexyloxycarbonylamino-4-methy...)Show SMILES CC(C)C[C@H](NC(=O)OC1CCCCC1)C(=O)N[C@H](Cc1cn(C)c2ccccc12)c1nc(C(O)=O)c(C)[nH]1 Show InChI InChI=1S/C29H39N5O5/c1-17(2)14-23(32-29(38)39-20-10-6-5-7-11-20)27(35)31-22(26-30-18(3)25(33-26)28(36)37)15-19-16-34(4)24-13-9-8-12-21(19)24/h8-9,12-13,16-17,20,22-23H,5-7,10-11,14-15H2,1-4H3,(H,30,33)(H,31,35)(H,32,38)(H,36,37)/t22-,23+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity was evaluated against human Endothelin A receptor |
J Med Chem 39: 982-91 (1996)
Article DOI: 10.1021/jm9505932
BindingDB Entry DOI: 10.7270/Q2DB80WG |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50050042

(2-[(R)-1-[(S)-2-(3-Cyclohexyl-ureido)-4-methyl-pen...)Show SMILES CC(C)C[C@H](NC(=O)NC1CCCCC1)C(=O)N[C@H](Cc1cn(C)c2ccccc12)c1nc(C(O)=O)c(C)o1 Show InChI InChI=1S/C29H39N5O5/c1-17(2)14-22(32-29(38)30-20-10-6-5-7-11-20)26(35)31-23(27-33-25(28(36)37)18(3)39-27)15-19-16-34(4)24-13-9-8-12-21(19)24/h8-9,12-13,16-17,20,22-23H,5-7,10-11,14-15H2,1-4H3,(H,31,35)(H,36,37)(H2,30,32,38)/t22-,23+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity was evaluated against human Endothelin A receptor |
J Med Chem 39: 982-91 (1996)
Article DOI: 10.1021/jm9505932
BindingDB Entry DOI: 10.7270/Q2DB80WG |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50050054
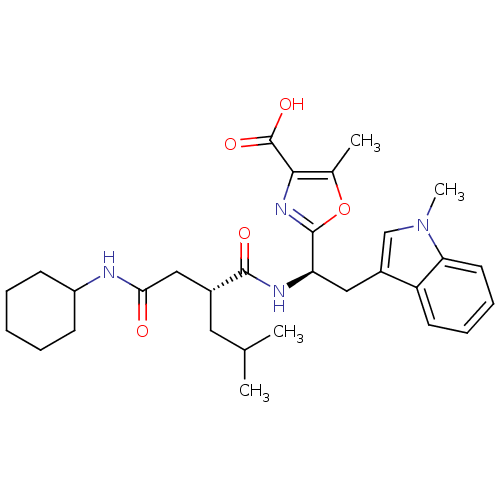
(2-[(R)-1-((R)-2-Cyclohexylcarbamoylmethyl-4-methyl...)Show SMILES CC(C)C[C@H](CC(=O)NC1CCCCC1)C(=O)N[C@H](Cc1cn(C)c2ccccc12)c1nc(C(O)=O)c(C)o1 Show InChI InChI=1S/C30H40N4O5/c1-18(2)14-20(16-26(35)31-22-10-6-5-7-11-22)28(36)32-24(29-33-27(30(37)38)19(3)39-29)15-21-17-34(4)25-13-9-8-12-23(21)25/h8-9,12-13,17-18,20,22,24H,5-7,10-11,14-16H2,1-4H3,(H,31,35)(H,32,36)(H,37,38)/t20-,24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity was evaluated against human Endothelin A receptor |
J Med Chem 39: 982-91 (1996)
Article DOI: 10.1021/jm9505932
BindingDB Entry DOI: 10.7270/Q2DB80WG |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50017784

(CHEMBL265319 | {1-[1-(4-Azido-1-cyclohexylmethyl-2...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](CC1CCCCC1)[C@H](O)[C@@H](CO)CN=[N+]=[N-] Show InChI InChI=1S/C32H48N8O6/c1-32(2,3)46-31(45)39-26(15-22-12-8-5-9-13-22)29(43)38-27(16-24-18-34-20-35-24)30(44)37-25(14-21-10-6-4-7-11-21)28(42)23(19-41)17-36-40-33/h5,8-9,12-13,18,20-21,23,25-28,41-42H,4,6-7,10-11,14-17,19H2,1-3H3,(H,34,35)(H,37,44)(H,38,43)(H,39,45)/t23-,25-,26+,27+,28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 370 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against purified human renal renin at pH 6.0 |
J Med Chem 32: 1371-8 (1989)
BindingDB Entry DOI: 10.7270/Q2474BFR |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50050052

(2-[(R)-1-[(S)-2-(3-Cyclohexyl-1-methyl-ureido)-4-m...)Show SMILES CC(C)C[C@H](N(C)C(=O)NC1CCCCC1)C(=O)N[C@H](Cc1cn(C)c2ccccc12)c1nc(C(O)=O)c(C)o1 Show InChI InChI=1S/C30H41N5O5/c1-18(2)15-25(35(5)30(39)31-21-11-7-6-8-12-21)27(36)32-23(28-33-26(29(37)38)19(3)40-28)16-20-17-34(4)24-14-10-9-13-22(20)24/h9-10,13-14,17-18,21,23,25H,6-8,11-12,15-16H2,1-5H3,(H,31,39)(H,32,36)(H,37,38)/t23-,25+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity was evaluated against human Endothelin A receptor |
J Med Chem 39: 982-91 (1996)
Article DOI: 10.1021/jm9505932
BindingDB Entry DOI: 10.7270/Q2DB80WG |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50050057
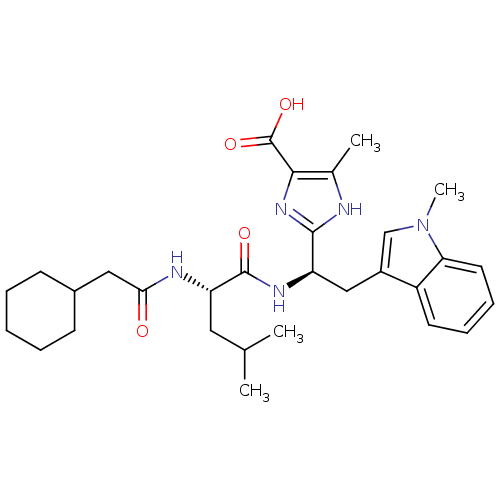
(2-[(R)-1-[(S)-2-(2-Cyclohexyl-acetylamino)-4-methy...)Show SMILES CC(C)C[C@H](NC(=O)CC1CCCCC1)C(=O)N[C@H](Cc1cn(C)c2ccccc12)c1nc(C(O)=O)c(C)[nH]1 Show InChI InChI=1S/C30H41N5O4/c1-18(2)14-24(32-26(36)15-20-10-6-5-7-11-20)29(37)33-23(28-31-19(3)27(34-28)30(38)39)16-21-17-35(4)25-13-9-8-12-22(21)25/h8-9,12-13,17-18,20,23-24H,5-7,10-11,14-16H2,1-4H3,(H,31,34)(H,32,36)(H,33,37)(H,38,39)/t23-,24+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity was evaluated against human Endothelin A receptor |
J Med Chem 39: 982-91 (1996)
Article DOI: 10.1021/jm9505932
BindingDB Entry DOI: 10.7270/Q2DB80WG |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50050053

(2-[(R)-1-[(S)-2-(2-Cyclohexyl-acetylamino)-4-methy...)Show SMILES CC(C)C[C@H](NC(=O)CC1CCCCC1)C(=O)N[C@H](Cc1cn(C)c2ccccc12)c1nc(C(O)=O)c(C)o1 Show InChI InChI=1S/C30H40N4O5/c1-18(2)14-23(31-26(35)15-20-10-6-5-7-11-20)28(36)32-24(29-33-27(30(37)38)19(3)39-29)16-21-17-34(4)25-13-9-8-12-22(21)25/h8-9,12-13,17-18,20,23-24H,5-7,10-11,14-16H2,1-4H3,(H,31,35)(H,32,36)(H,37,38)/t23-,24+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity was evaluated against human Endothelin A receptor |
J Med Chem 39: 982-91 (1996)
Article DOI: 10.1021/jm9505932
BindingDB Entry DOI: 10.7270/Q2DB80WG |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM912

((3S,4S)-3-hydroxy-4-[(2S)-2-[(3S,4S)-3-hydroxy-6-m...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CC(C)C)C(C)C)C(C)C)[C@@H](O)CC(O)=O |r| Show InChI InChI=1S/C34H63N5O9/c1-17(2)12-23(37-33(47)31(21(9)10)39-34(48)30(20(7)8)38-27(42)14-19(5)6)25(40)15-28(43)35-22(11)32(46)36-24(13-18(3)4)26(41)16-29(44)45/h17-26,30-31,40-41H,12-16H2,1-11H3,(H,35,43)(H,36,46)(H,37,47)(H,38,42)(H,39,48)(H,44,45)/t22-,23-,24-,25-,26-,30-,31-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human plasma renin at pH 7.4 |
J Med Chem 32: 1371-8 (1989)
BindingDB Entry DOI: 10.7270/Q2474BFR |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

















































