 Found 861 hits with Last Name = 'nelson' and Initial = 'a'
Found 861 hits with Last Name = 'nelson' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM81768

(BENZYLIC ACID | CAS_6581-06-2 | NSC_5311391 | Quin...)Show InChI InChI=1S/C14H12O3/c15-13(16)14(17,11-7-3-1-4-8-11)12-9-5-2-6-10-12/h1-10,17H,(H,15,16) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic and Foundation
Curated by PDSP Ki Database
| |
Eur J Pharmacol 103: 197-204 (1984)
Article DOI: 10.1016/0014-2999(84)90478-3
BindingDB Entry DOI: 10.7270/Q2WW7G5B |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM29568

(CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N1CCN(CC1)C(=O)c1ccco1 Show InChI InChI=1S/C19H21N5O4/c1-26-15-10-12-13(11-16(15)27-2)21-19(22-17(12)20)24-7-5-23(6-8-24)18(25)14-4-3-9-28-14/h3-4,9-11H,5-8H2,1-2H3,(H2,20,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic and Foundation
Curated by PDSP Ki Database
| |
Eur J Pharmacol 103: 197-204 (1984)
Article DOI: 10.1016/0014-2999(84)90478-3
BindingDB Entry DOI: 10.7270/Q2WW7G5B |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM29568

(CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N1CCN(CC1)C(=O)c1ccco1 Show InChI InChI=1S/C19H21N5O4/c1-26-15-10-12-13(11-16(15)27-2)21-19(22-17(12)20)24-7-5-23(6-8-24)18(25)14-4-3-9-28-14/h3-4,9-11H,5-8H2,1-2H3,(H2,20,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic Jacksonville
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 114: 559-65 (1994)
Article DOI: 10.1007/BF02244985
BindingDB Entry DOI: 10.7270/Q25X27FZ |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM21397

(8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...)Show SMILES Fc1ccc(cc1)C(=O)CCCN1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C23H26FN3O2/c24-19-10-8-18(9-11-19)21(28)7-4-14-26-15-12-23(13-16-26)22(29)25-17-27(23)20-5-2-1-3-6-20/h1-3,5-6,8-11H,4,7,12-17H2,(H,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic and Foundation
Curated by PDSP Ki Database
| |
Eur J Pharmacol 103: 197-204 (1984)
Article DOI: 10.1016/0014-2999(84)90478-3
BindingDB Entry DOI: 10.7270/Q2WW7G5B |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50079527
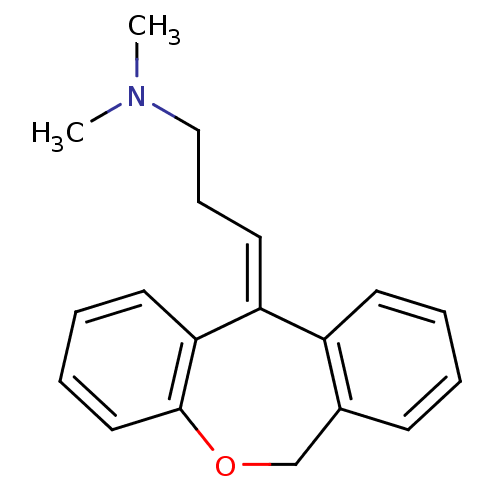
((3E)-3-dibenzo[b,e]oxepin-11(6H)-ylidene-N,N-dimet...)Show InChI InChI=1S/C19H21NO/c1-20(2)13-7-11-17-16-9-4-3-8-15(16)14-21-19-12-6-5-10-18(17)19/h3-6,8-12H,7,13-14H2,1-2H3/b17-11- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic Jacksonville
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 114: 559-65 (1994)
Article DOI: 10.1007/BF02244985
BindingDB Entry DOI: 10.7270/Q25X27FZ |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50081112
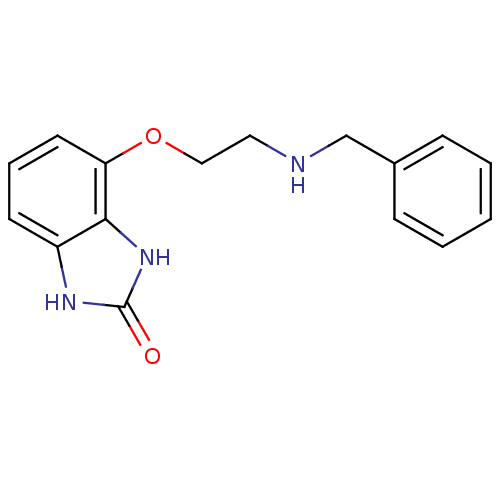
(4-(2-Benzylamino-ethoxy)-1,3-dihydro-benzoimidazol...)Show InChI InChI=1S/C16H17N3O2/c20-16-18-13-7-4-8-14(15(13)19-16)21-10-9-17-11-12-5-2-1-3-6-12/h1-8,17H,9-11H2,(H2,18,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibtion of [3H]-spiperone binding to rat striatal membrane Dopamine receptor D2 low affinity without GTP and sodium |
Bioorg Med Chem Lett 9: 2593-8 (1999)
BindingDB Entry DOI: 10.7270/Q22V2FBG |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50081110
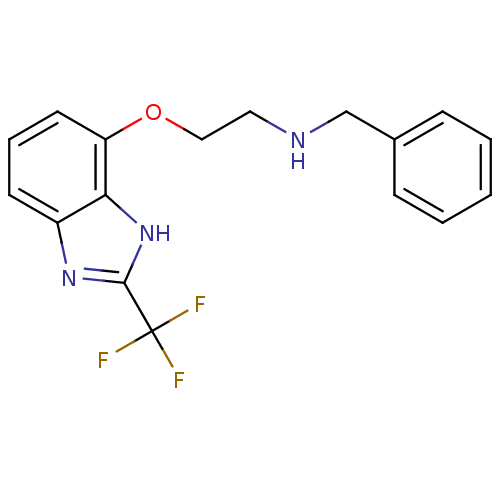
(Benzyl-[2-(2-trifluoromethyl-1H-benzoimidazol-4-yl...)Show InChI InChI=1S/C17H16F3N3O/c18-17(19,20)16-22-13-7-4-8-14(15(13)23-16)24-10-9-21-11-12-5-2-1-3-6-12/h1-8,21H,9-11H2,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibtion of [3H]-spiperone binding to rat striatal membrane Dopamine receptor D2 low affinity without GTP and sodium |
Bioorg Med Chem Lett 9: 2593-8 (1999)
BindingDB Entry DOI: 10.7270/Q22V2FBG |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50081108
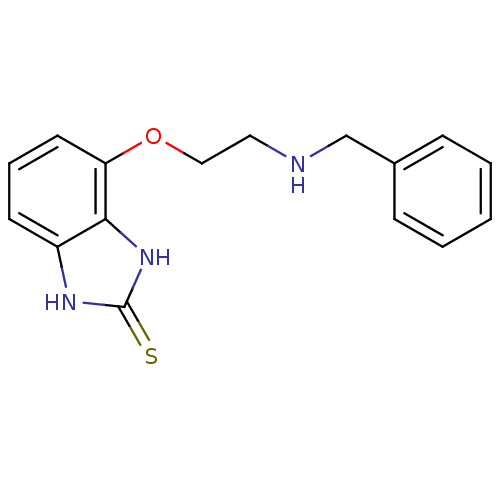
(4-(2-Benzylamino-ethoxy)-1,3-dihydro-benzoimidazol...)Show InChI InChI=1S/C16H17N3OS/c21-16-18-13-7-4-8-14(15(13)19-16)20-10-9-17-11-12-5-2-1-3-6-12/h1-8,17H,9-11H2,(H2,18,19,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-quinpirole binding to rat striatal membrane Dopamine receptor D2 without GTP and sodium |
Bioorg Med Chem Lett 9: 2593-8 (1999)
BindingDB Entry DOI: 10.7270/Q22V2FBG |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50081107

(4-(2-Benzylamino-ethoxy)-6-chloro-1,3-dihydro-benz...)Show InChI InChI=1S/C16H16ClN3OS/c17-12-8-13-15(20-16(22)19-13)14(9-12)21-7-6-18-10-11-4-2-1-3-5-11/h1-5,8-9,18H,6-7,10H2,(H2,19,20,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-quinpirole binding to rat striatal membrane Dopamine receptor D2 without GTP and sodium |
Bioorg Med Chem Lett 9: 2593-8 (1999)
BindingDB Entry DOI: 10.7270/Q22V2FBG |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50077578
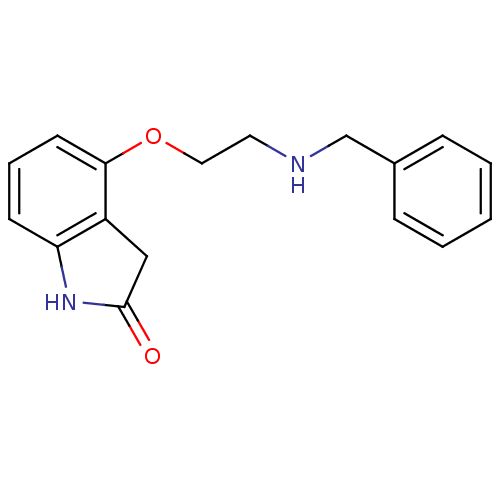
(4-(2-Benzylamino-ethoxy)-1,3-dihydro-indol-2-one |...)Show InChI InChI=1S/C17H18N2O2/c20-17-11-14-15(19-17)7-4-8-16(14)21-10-9-18-12-13-5-2-1-3-6-13/h1-8,18H,9-12H2,(H,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-quinpirole binding to rat striatal membrane Dopamine receptor D2 without GTP and sodium |
Bioorg Med Chem Lett 9: 2593-8 (1999)
BindingDB Entry DOI: 10.7270/Q22V2FBG |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50081106

(4-(2-Benzylamino-ethoxy)-6-chloro-1,3-dihydro-benz...)Show InChI InChI=1S/C16H16ClN3O2/c17-12-8-13-15(20-16(21)19-13)14(9-12)22-7-6-18-10-11-4-2-1-3-5-11/h1-5,8-9,18H,6-7,10H2,(H2,19,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-quinpirole binding to rat striatal membrane Dopamine receptor D2 without GTP and sodium |
Bioorg Med Chem Lett 9: 2593-8 (1999)
BindingDB Entry DOI: 10.7270/Q22V2FBG |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM78576
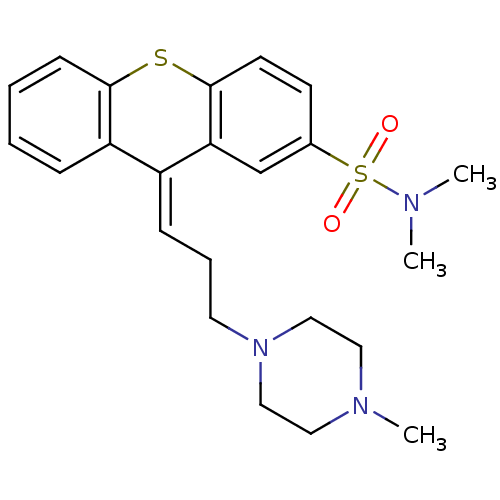
(CIS-THIOTHIXENE | MLS000028463 | SMR000058396 | TH...)Show SMILES CN(C)S(=O)(=O)c1ccc2Sc3ccccc3\C(=C\CCN3CCN(C)CC3)c2c1 Show InChI InChI=1S/C23H29N3O2S2/c1-24(2)30(27,28)18-10-11-23-21(17-18)19(20-7-4-5-9-22(20)29-23)8-6-12-26-15-13-25(3)14-16-26/h4-5,7-11,17H,6,12-16H2,1-3H3/b19-8- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| DrugBank
Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic and Foundation
Curated by PDSP Ki Database
| |
Eur J Pharmacol 103: 197-204 (1984)
Article DOI: 10.1016/0014-2999(84)90478-3
BindingDB Entry DOI: 10.7270/Q2WW7G5B |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50071855

(4-(4-Benzyl-piperazin-1-yl)-2-trifluoromethyl-1H-b...)Show SMILES FC(F)(F)c1nc2c(cccc2[nH]1)N1CCN(Cc2ccccc2)CC1 Show InChI InChI=1S/C19H19F3N4/c20-19(21,22)18-23-15-7-4-8-16(17(15)24-18)26-11-9-25(10-12-26)13-14-5-2-1-3-6-14/h1-8H,9-13H2,(H,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratory
Curated by ChEMBL
| Assay Description
Affinity for Dopamine receptor D2 in rat striatal membranes for high agonist state using [3H]-quinpirole |
Bioorg Med Chem Lett 8: 2675-80 (1999)
BindingDB Entry DOI: 10.7270/Q2C53K0X |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50071856
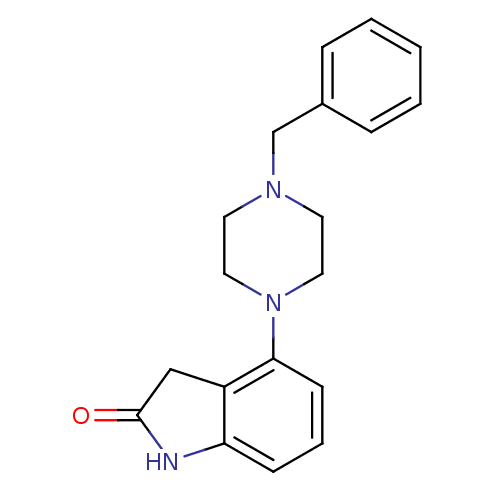
(4-(4-Benzyl-piperazin-1-yl)-1,3-dihydro-indol-2-on...)Show InChI InChI=1S/C19H21N3O/c23-19-13-16-17(20-19)7-4-8-18(16)22-11-9-21(10-12-22)14-15-5-2-1-3-6-15/h1-8H,9-14H2,(H,20,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratory
Curated by ChEMBL
| Assay Description
Affinity for Dopamine receptor D2 in rat striatal membranes for high agonist state using [3H]-quinpirole |
Bioorg Med Chem Lett 8: 2675-80 (1999)
BindingDB Entry DOI: 10.7270/Q2C53K0X |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50081105

(Benzyl-[2-(6-chloro-2-trifluoromethyl-1H-benzoimid...)Show SMILES FC(F)(F)c1nc2c(OCCNCc3ccccc3)cc(Cl)cc2[nH]1 Show InChI InChI=1S/C17H15ClF3N3O/c18-12-8-13-15(24-16(23-13)17(19,20)21)14(9-12)25-7-6-22-10-11-4-2-1-3-5-11/h1-5,8-9,22H,6-7,10H2,(H,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-quinpirole binding to rat striatal membrane Dopamine receptor D2 without GTP and sodium |
Bioorg Med Chem Lett 9: 2593-8 (1999)
BindingDB Entry DOI: 10.7270/Q22V2FBG |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM78433
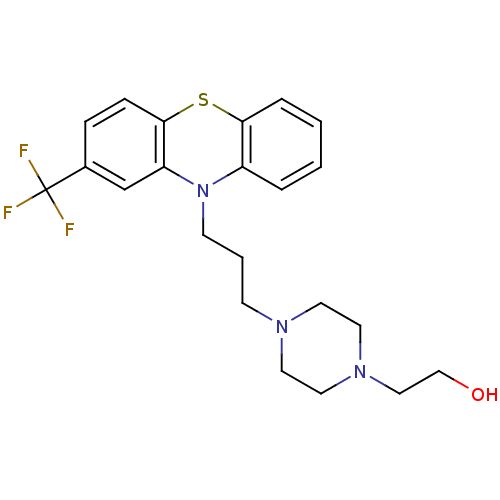
(2-[4-[3-[2-(trifluoromethyl)-10-phenothiazinyl]pro...)Show SMILES OCCN1CCN(CCCN2c3ccccc3Sc3ccc(cc23)C(F)(F)F)CC1 Show InChI InChI=1S/C22H26F3N3OS/c23-22(24,25)17-6-7-21-19(16-17)28(18-4-1-2-5-20(18)30-21)9-3-8-26-10-12-27(13-11-26)14-15-29/h1-2,4-7,16,29H,3,8-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| DrugBank
Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic and Foundation
Curated by PDSP Ki Database
| |
Eur J Pharmacol 103: 197-204 (1984)
Article DOI: 10.1016/0014-2999(84)90478-3
BindingDB Entry DOI: 10.7270/Q2WW7G5B |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50008735

((+)-3-(tert-butyl)-(3S,4aS,13bS)-2,3,4,4a,8,9,13b,...)Show SMILES CC(C)(C)[C@]1(O)CCN2C[C@H]3c4ccccc4CCc4cccc([C@@H]2C1)c34 Show InChI InChI=1S/C25H31NO/c1-24(2,3)25(27)13-14-26-16-21-19-9-5-4-7-17(19)11-12-18-8-6-10-20(23(18)21)22(26)15-25/h4-10,21-22,27H,11-16H2,1-3H3/t21-,22-,25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic and Foundation
Curated by PDSP Ki Database
| |
Eur J Pharmacol 103: 197-204 (1984)
Article DOI: 10.1016/0014-2999(84)90478-3
BindingDB Entry DOI: 10.7270/Q2WW7G5B |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50020712
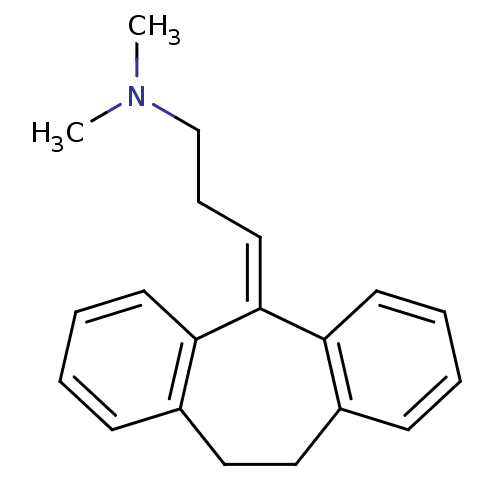
(10,11-dihydro-5-(gamma-dimethylaminopropylidene)-5...)Show SMILES [#6]-[#7](-[#6])-[#6]-[#6]\[#6]=[#6]-1/c2ccccc2-[#6]-[#6]-c2ccccc-12 Show InChI InChI=1S/C20H23N/c1-21(2)15-7-12-20-18-10-5-3-8-16(18)13-14-17-9-4-6-11-19(17)20/h3-6,8-12H,7,13-15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic Jacksonville
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 114: 559-65 (1994)
Article DOI: 10.1007/BF02244985
BindingDB Entry DOI: 10.7270/Q25X27FZ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50091825
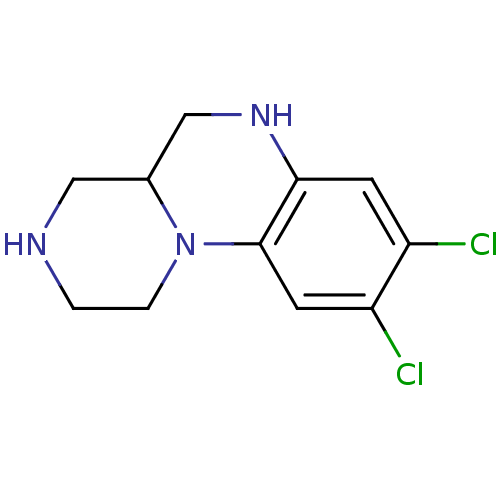
(8,9-Dichloro-2,3,4,4a,5,6-hexahydro-1H-pyrazino[1,...)Show InChI InChI=1S/C11H13Cl2N3/c12-8-3-10-11(4-9(8)13)16-2-1-14-5-7(16)6-15-10/h3-4,7,14-15H,1-2,5-6H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Binding affinity using [125I]-DOI as radioligand with membranes isolated from a CHO-k cell line expressing the human 5-hydroxytryptamine 2C receptor |
Bioorg Med Chem Lett 10: 1991-4 (2001)
BindingDB Entry DOI: 10.7270/Q2Z89BMP |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50081109
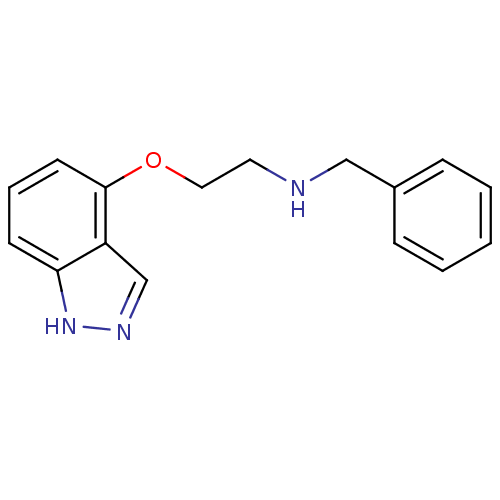
(Benzyl-[2-(1H-indazol-4-yloxy)-ethyl]-amine | CHEM...)Show InChI InChI=1S/C16H17N3O/c1-2-5-13(6-3-1)11-17-9-10-20-16-8-4-7-15-14(16)12-18-19-15/h1-8,12,17H,9-11H2,(H,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-quinpirole binding to rat striatal membrane Dopamine receptor D2 without GTP and sodium |
Bioorg Med Chem Lett 9: 2593-8 (1999)
BindingDB Entry DOI: 10.7270/Q22V2FBG |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM21397

(8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...)Show SMILES Fc1ccc(cc1)C(=O)CCCN1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C23H26FN3O2/c24-19-10-8-18(9-11-19)21(28)7-4-14-26-15-12-23(13-16-26)22(29)25-17-27(23)20-5-2-1-3-6-20/h1-3,5-6,8-11H,4,7,12-17H2,(H,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic and Foundation
Curated by PDSP Ki Database
| |
Eur J Pharmacol 103: 197-204 (1984)
Article DOI: 10.1016/0014-2999(84)90478-3
BindingDB Entry DOI: 10.7270/Q2WW7G5B |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50081110

(Benzyl-[2-(2-trifluoromethyl-1H-benzoimidazol-4-yl...)Show InChI InChI=1S/C17H16F3N3O/c18-17(19,20)16-22-13-7-4-8-14(15(13)23-16)24-10-9-21-11-12-5-2-1-3-6-12/h1-8,21H,9-11H2,(H,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-spiperone binding to human Dopamine receptor D3 in CHO cell membranes |
Bioorg Med Chem Lett 9: 2593-8 (1999)
BindingDB Entry DOI: 10.7270/Q22V2FBG |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50130273
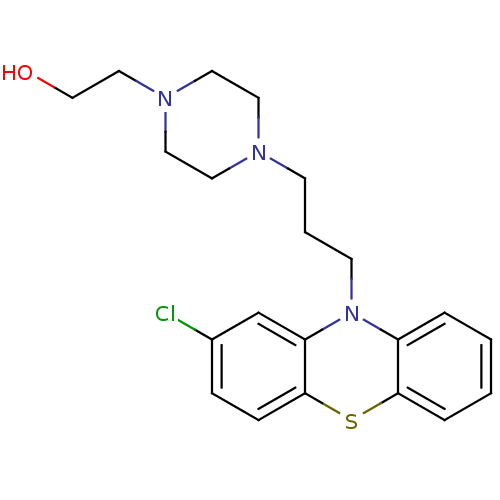
(2-(4-[3-(2-chloro-10H-phenothiazin-10-yl)propyl]-1...)Show InChI InChI=1S/C21H26ClN3OS/c22-17-6-7-21-19(16-17)25(18-4-1-2-5-20(18)27-21)9-3-8-23-10-12-24(13-11-23)14-15-26/h1-2,4-7,16,26H,3,8-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic and Foundation
Curated by PDSP Ki Database
| |
Eur J Pharmacol 103: 197-204 (1984)
Article DOI: 10.1016/0014-2999(84)90478-3
BindingDB Entry DOI: 10.7270/Q2WW7G5B |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM50013515

((+)-yohimbine | (16alpha,17alpha)-17-hydroxyyohimb...)Show SMILES COC(=O)[C@H]1[C@@H](O)CC[C@H]2CN3CCc4c([nH]c5ccccc45)[C@@H]3C[C@H]12 |r| Show InChI InChI=1S/C21H26N2O3/c1-26-21(25)19-15-10-17-20-14(13-4-2-3-5-16(13)22-20)8-9-23(17)11-12(15)6-7-18(19)24/h2-5,12,15,17-19,22,24H,6-11H2,1H3/t12-,15-,17-,18-,19+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic and Foundation
Curated by PDSP Ki Database
| |
Eur J Pharmacol 103: 197-204 (1984)
Article DOI: 10.1016/0014-2999(84)90478-3
BindingDB Entry DOI: 10.7270/Q2WW7G5B |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50071858

(4-(4-Benzyl-piperazin-1-yl)-5-chloro-1H-indole | C...)Show InChI InChI=1S/C19H20ClN3/c20-17-6-7-18-16(8-9-21-18)19(17)23-12-10-22(11-13-23)14-15-4-2-1-3-5-15/h1-9,21H,10-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratory
Curated by ChEMBL
| Assay Description
Affinity towards human Dopamine receptor D4.4 expressed in CHO cells using [3H]-spiperone |
Bioorg Med Chem Lett 8: 2675-80 (1999)
BindingDB Entry DOI: 10.7270/Q2C53K0X |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50131440
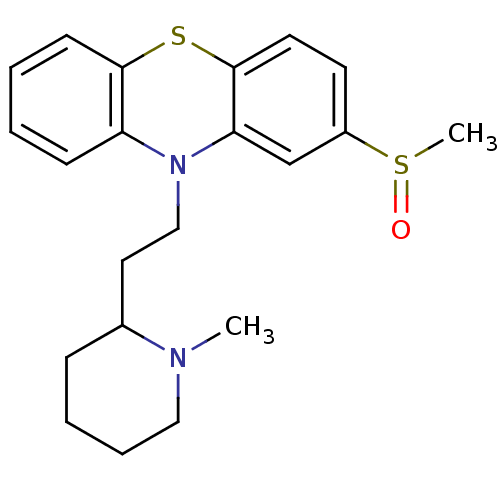
(10-(2-(1-methylpiperidin-2-yl)ethyl)-2-(methylsulf...)Show InChI InChI=1S/C21H26N2OS2/c1-22-13-6-5-7-16(22)12-14-23-18-8-3-4-9-20(18)25-21-11-10-17(26(2)24)15-19(21)23/h3-4,8-11,15-16H,5-7,12-14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic and Foundation
Curated by PDSP Ki Database
| |
Eur J Pharmacol 103: 197-204 (1984)
Article DOI: 10.1016/0014-2999(84)90478-3
BindingDB Entry DOI: 10.7270/Q2WW7G5B |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50081110

(Benzyl-[2-(2-trifluoromethyl-1H-benzoimidazol-4-yl...)Show InChI InChI=1S/C17H16F3N3O/c18-17(19,20)16-22-13-7-4-8-14(15(13)23-16)24-10-9-21-11-12-5-2-1-3-6-12/h1-8,21H,9-11H2,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories
Curated by ChEMBL
| Assay Description
Inihibition of [3H]-spiperone binding to human Dopamine receptor D2 in CHO cell membranes |
Bioorg Med Chem Lett 9: 2593-8 (1999)
BindingDB Entry DOI: 10.7270/Q22V2FBG |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50081110

(Benzyl-[2-(2-trifluoromethyl-1H-benzoimidazol-4-yl...)Show InChI InChI=1S/C17H16F3N3O/c18-17(19,20)16-22-13-7-4-8-14(15(13)23-16)24-10-9-21-11-12-5-2-1-3-6-12/h1-8,21H,9-11H2,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibtion of [3H]-spiperone binding to rat striatal membrane Dopamine receptor D2 low affinity without GTP and sodium |
Bioorg Med Chem Lett 9: 2593-8 (1999)
BindingDB Entry DOI: 10.7270/Q22V2FBG |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM86231

(ATR | ATROPINE | Atropine,(-) | CAS_51-55-8 | CHEM...)Show SMILES CN1C2CCC1CC(C2)OC(=O)C(CO)c1ccccc1 |THB:9:7:1:3.4| Show InChI InChI=1S/C17H23NO3/c1-18-13-7-8-14(18)10-15(9-13)21-17(20)16(11-19)12-5-3-2-4-6-12/h2-6,13-16,19H,7-11H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.96 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic Jacksonville
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 114: 559-65 (1994)
Article DOI: 10.1007/BF02244985
BindingDB Entry DOI: 10.7270/Q25X27FZ |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM67545
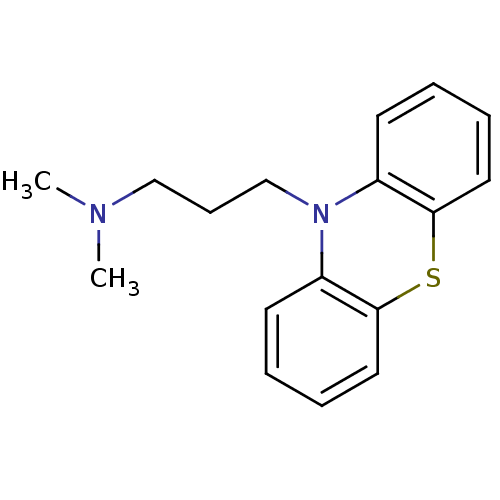
(N,N-dimethyl-3-(10-phenothiazinyl)-1-propanamine;h...)Show InChI InChI=1S/C17H20N2S/c1-18(2)12-7-13-19-14-8-3-5-10-16(14)20-17-11-6-4-9-15(17)19/h3-6,8-11H,7,12-13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic and Foundation
Curated by PDSP Ki Database
| |
Eur J Pharmacol 103: 197-204 (1984)
Article DOI: 10.1016/0014-2999(84)90478-3
BindingDB Entry DOI: 10.7270/Q2WW7G5B |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50131440

(10-(2-(1-methylpiperidin-2-yl)ethyl)-2-(methylsulf...)Show InChI InChI=1S/C21H26N2OS2/c1-22-13-6-5-7-16(22)12-14-23-18-8-3-4-9-20(18)25-21-11-10-17(26(2)24)15-19(21)23/h3-4,8-11,15-16H,5-7,12-14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic and Foundation
Curated by PDSP Ki Database
| |
Eur J Pharmacol 103: 197-204 (1984)
Article DOI: 10.1016/0014-2999(84)90478-3
BindingDB Entry DOI: 10.7270/Q2WW7G5B |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50081108

(4-(2-Benzylamino-ethoxy)-1,3-dihydro-benzoimidazol...)Show InChI InChI=1S/C16H17N3OS/c21-16-18-13-7-4-8-14(15(13)19-16)20-10-9-17-11-12-5-2-1-3-6-12/h1-8,17H,9-11H2,(H2,18,19,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-spiperone binding to human Dopamine receptor D3 in CHO cell membranes |
Bioorg Med Chem Lett 9: 2593-8 (1999)
BindingDB Entry DOI: 10.7270/Q22V2FBG |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM86231

(ATR | ATROPINE | Atropine,(-) | CAS_51-55-8 | CHEM...)Show SMILES CN1C2CCC1CC(C2)OC(=O)C(CO)c1ccccc1 |THB:9:7:1:3.4| Show InChI InChI=1S/C17H23NO3/c1-18-13-7-8-14(18)10-15(9-13)21-17(20)16(11-19)12-5-3-2-4-6-12/h2-6,13-16,19H,7-11H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic and Foundation
Curated by PDSP Ki Database
| |
Eur J Pharmacol 103: 197-204 (1984)
Article DOI: 10.1016/0014-2999(84)90478-3
BindingDB Entry DOI: 10.7270/Q2WW7G5B |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM21395

(3-(2-(4-(4-Fluorobenzoyl)piperidinol)ethyl)-2,4(1H...)Show SMILES Fc1ccc(cc1)C(=O)C1CCN(CCn2c(=O)[nH]c3ccccc3c2=O)CC1 Show InChI InChI=1S/C22H22FN3O3/c23-17-7-5-15(6-8-17)20(27)16-9-11-25(12-10-16)13-14-26-21(28)18-3-1-2-4-19(18)24-22(26)29/h1-8,16H,9-14H2,(H,24,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic Jacksonville
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 114: 559-65 (1994)
Article DOI: 10.1007/BF02244985
BindingDB Entry DOI: 10.7270/Q25X27FZ |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50071853

(4-(4-Benzyl-piperazin-1-yl)-7-bromo-1H-indole | CH...)Show InChI InChI=1S/C19H20BrN3/c20-17-6-7-18(16-8-9-21-19(16)17)23-12-10-22(11-13-23)14-15-4-2-1-3-5-15/h1-9,21H,10-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratory
Curated by ChEMBL
| Assay Description
Affinity towards human Dopamine receptor D4.4 expressed in CHO cells using [3H]-spiperone |
Bioorg Med Chem Lett 8: 2675-80 (1999)
BindingDB Entry DOI: 10.7270/Q2C53K0X |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50081106

(4-(2-Benzylamino-ethoxy)-6-chloro-1,3-dihydro-benz...)Show InChI InChI=1S/C16H16ClN3O2/c17-12-8-13-15(20-16(21)19-13)14(9-12)22-7-6-18-10-11-4-2-1-3-5-11/h1-5,8-9,18H,6-7,10H2,(H2,19,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories
Curated by ChEMBL
| Assay Description
Inihibition of [3H]-spiperone binding to human Dopamine receptor D2 in CHO cell membranes |
Bioorg Med Chem Lett 9: 2593-8 (1999)
BindingDB Entry DOI: 10.7270/Q22V2FBG |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM79181

(10-[3-(4-methyl-1-piperazinyl)propyl]-2-(trifluoro...)Show SMILES CN1CCN(CCCN2c3ccccc3Sc3ccc(cc23)C(F)(F)F)CC1 Show InChI InChI=1S/C21H24F3N3S/c1-25-11-13-26(14-12-25)9-4-10-27-17-5-2-3-6-19(17)28-20-8-7-16(15-18(20)27)21(22,23)24/h2-3,5-8,15H,4,9-14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic and Foundation
Curated by PDSP Ki Database
| |
Eur J Pharmacol 103: 197-204 (1984)
Article DOI: 10.1016/0014-2999(84)90478-3
BindingDB Entry DOI: 10.7270/Q2WW7G5B |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50081108

(4-(2-Benzylamino-ethoxy)-1,3-dihydro-benzoimidazol...)Show InChI InChI=1S/C16H17N3OS/c21-16-18-13-7-4-8-14(15(13)19-16)20-10-9-17-11-12-5-2-1-3-6-12/h1-8,17H,9-11H2,(H2,18,19,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-quinpirole binding to rat striatal membrane Dopamine receptor D2 without GTP and sodium |
Bioorg Med Chem Lett 9: 2593-8 (1999)
BindingDB Entry DOI: 10.7270/Q22V2FBG |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50081106

(4-(2-Benzylamino-ethoxy)-6-chloro-1,3-dihydro-benz...)Show InChI InChI=1S/C16H16ClN3O2/c17-12-8-13-15(20-16(21)19-13)14(9-12)22-7-6-18-10-11-4-2-1-3-5-11/h1-5,8-9,18H,6-7,10H2,(H2,19,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibtion of [3H]-spiperone binding to rat striatal membrane Dopamine receptor D2 low affinity without GTP and sodium |
Bioorg Med Chem Lett 9: 2593-8 (1999)
BindingDB Entry DOI: 10.7270/Q22V2FBG |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM50013515

((+)-yohimbine | (16alpha,17alpha)-17-hydroxyyohimb...)Show SMILES COC(=O)[C@H]1[C@@H](O)CC[C@H]2CN3CCc4c([nH]c5ccccc45)[C@@H]3C[C@H]12 |r| Show InChI InChI=1S/C21H26N2O3/c1-26-21(25)19-15-10-17-20-14(13-4-2-3-5-16(13)22-20)8-9-23(17)11-12(15)6-7-18(19)24/h2-5,12,15,17-19,22,24H,6-11H2,1H3/t12-,15-,17-,18-,19+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic and Foundation
Curated by PDSP Ki Database
| |
Eur J Pharmacol 103: 197-204 (1984)
Article DOI: 10.1016/0014-2999(84)90478-3
BindingDB Entry DOI: 10.7270/Q2WW7G5B |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM67544

(N,N-dimethyl-3-[2-(trifluoromethyl)-10-phenothiazi...)Show InChI InChI=1S/C18H19F3N2S/c1-22(2)10-5-11-23-14-6-3-4-7-16(14)24-17-9-8-13(12-15(17)23)18(19,20)21/h3-4,6-9,12H,5,10-11H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| DrugBank
Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic and Foundation
Curated by PDSP Ki Database
| |
Eur J Pharmacol 103: 197-204 (1984)
Article DOI: 10.1016/0014-2999(84)90478-3
BindingDB Entry DOI: 10.7270/Q2WW7G5B |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM22869

(6-chloro-10-(4-methylpiperazin-1-yl)-2,9-diazatric...)Show SMILES CN1CCN(CC1)C1=c2ccccc2=Nc2ccc(Cl)cc2N1 |c:8,15| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,21H,8-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic and Foundation
Curated by PDSP Ki Database
| |
Eur J Pharmacol 103: 197-204 (1984)
Article DOI: 10.1016/0014-2999(84)90478-3
BindingDB Entry DOI: 10.7270/Q2WW7G5B |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50077578

(4-(2-Benzylamino-ethoxy)-1,3-dihydro-indol-2-one |...)Show InChI InChI=1S/C17H18N2O2/c20-17-11-14-15(19-17)7-4-8-16(14)21-10-9-18-12-13-5-2-1-3-6-13/h1-8,18H,9-12H2,(H,19,20) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-spiperone binding to human Dopamine receptor D3 in CHO cell membranes |
Bioorg Med Chem Lett 9: 2593-8 (1999)
BindingDB Entry DOI: 10.7270/Q22V2FBG |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50071855

(4-(4-Benzyl-piperazin-1-yl)-2-trifluoromethyl-1H-b...)Show SMILES FC(F)(F)c1nc2c(cccc2[nH]1)N1CCN(Cc2ccccc2)CC1 Show InChI InChI=1S/C19H19F3N4/c20-19(21,22)18-23-15-7-4-8-16(17(15)24-18)26-11-9-25(10-12-26)13-14-5-2-1-3-6-14/h1-8H,9-13H2,(H,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratory
Curated by ChEMBL
| Assay Description
Affinity towards human Dopamine receptor D4.4 expressed in CHO cells using [3H]-spiperone |
Bioorg Med Chem Lett 8: 2675-80 (1999)
BindingDB Entry DOI: 10.7270/Q2C53K0X |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50071856

(4-(4-Benzyl-piperazin-1-yl)-1,3-dihydro-indol-2-on...)Show InChI InChI=1S/C19H21N3O/c23-19-13-16-17(20-19)7-4-8-18(16)22-11-9-21(10-12-22)14-15-5-2-1-3-6-15/h1-8H,9-14H2,(H,20,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratory
Curated by ChEMBL
| Assay Description
Affinity towards human Dopamine receptor D4.4 expressed in CHO cells using [3H]-spiperone |
Bioorg Med Chem Lett 8: 2675-80 (1999)
BindingDB Entry DOI: 10.7270/Q2C53K0X |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50081108

(4-(2-Benzylamino-ethoxy)-1,3-dihydro-benzoimidazol...)Show InChI InChI=1S/C16H17N3OS/c21-16-18-13-7-4-8-14(15(13)19-16)20-10-9-17-11-12-5-2-1-3-6-12/h1-8,17H,9-11H2,(H2,18,19,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories
Curated by ChEMBL
| Assay Description
Inihibition of [3H]-spiperone binding to human Dopamine receptor D2 in CHO cell membranes |
Bioorg Med Chem Lett 9: 2593-8 (1999)
BindingDB Entry DOI: 10.7270/Q22V2FBG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50091831

((R)-8,9-Dichloro-2,3,4,4a-tetrahydro-1H,6H-pyrazin...)Show InChI InChI=1S/C11H11Cl2N3O/c12-6-3-8-9(4-7(6)13)16-2-1-14-5-10(16)11(17)15-8/h3-4,10,14H,1-2,5H2,(H,15,17)/t10-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Binding affinity using [3H]-mesulergine as radioligand with receptor membranes isolated from a CHO-k cell line expressing the human 5-hydroxytryptami... |
Bioorg Med Chem Lett 10: 1991-4 (2001)
BindingDB Entry DOI: 10.7270/Q2Z89BMP |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50081110

(Benzyl-[2-(2-trifluoromethyl-1H-benzoimidazol-4-yl...)Show InChI InChI=1S/C17H16F3N3O/c18-17(19,20)16-22-13-7-4-8-14(15(13)23-16)24-10-9-21-11-12-5-2-1-3-6-12/h1-8,21H,9-11H2,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-spiperone binding to human Dopamine receptor D4.4 expressed in CHO cell membranes |
Bioorg Med Chem Lett 9: 2593-8 (1999)
BindingDB Entry DOI: 10.7270/Q22V2FBG |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50071857

(4-(4-Benzyl-piperazin-1-yl)-1H-indole | CHEMBL3280...)Show InChI InChI=1S/C19H21N3/c1-2-5-16(6-3-1)15-21-11-13-22(14-12-21)19-8-4-7-18-17(19)9-10-20-18/h1-10,20H,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratory
Curated by ChEMBL
| Assay Description
Affinity for Dopamine receptor D2 in rat striatal membranes for high agonist state using [3H]-quinpirole |
Bioorg Med Chem Lett 8: 2675-80 (1999)
BindingDB Entry DOI: 10.7270/Q2C53K0X |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50071855

(4-(4-Benzyl-piperazin-1-yl)-2-trifluoromethyl-1H-b...)Show SMILES FC(F)(F)c1nc2c(cccc2[nH]1)N1CCN(Cc2ccccc2)CC1 Show InChI InChI=1S/C19H19F3N4/c20-19(21,22)18-23-15-7-4-8-16(17(15)24-18)26-11-9-25(10-12-26)13-14-5-2-1-3-6-14/h1-8H,9-13H2,(H,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratory
Curated by ChEMBL
| Assay Description
Affinity of compound for the Dopamine receptor D2 in rat striatal membranes was determined for low agonist state in rat striatal membranes using [3H]... |
Bioorg Med Chem Lett 8: 2675-80 (1999)
BindingDB Entry DOI: 10.7270/Q2C53K0X |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data