

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448761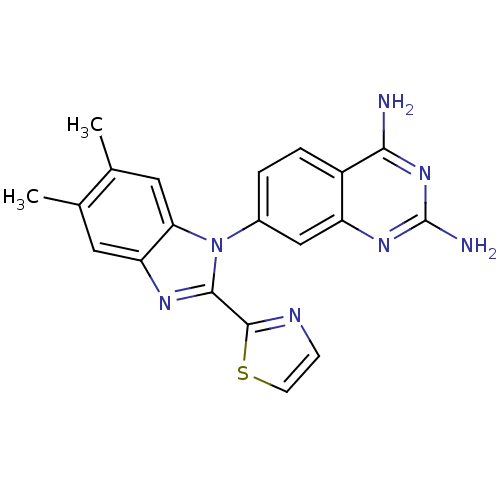 (CHEMBL3128021 | US8835445, 22) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics Inc. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR using dihydrofolate as substrate preincubated for 10 mins followed by substrate addition by spectrophotometr... | J Med Chem 57: 651-68 (2014) Article DOI: 10.1021/jm401204g BindingDB Entry DOI: 10.7270/Q2PN974G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448757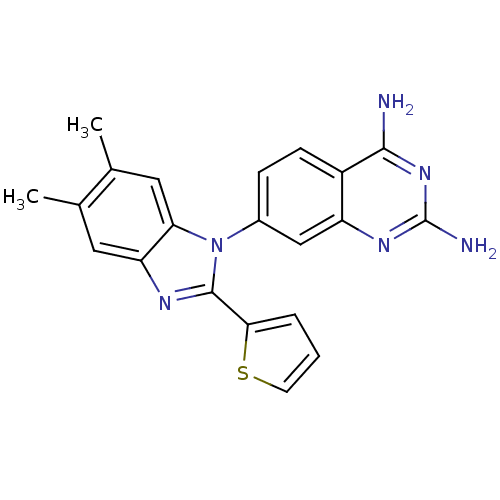 (CHEMBL3128025 | US8835445, 18) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics Inc. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR using dihydrofolate as substrate preincubated for 10 mins followed by substrate addition by spectrophotometr... | J Med Chem 57: 651-68 (2014) Article DOI: 10.1021/jm401204g BindingDB Entry DOI: 10.7270/Q2PN974G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448759 (CHEMBL3128023 | US8835445, 17) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics Inc. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR using dihydrofolate as substrate preincubated for 10 mins followed by substrate addition by spectrophotometr... | J Med Chem 57: 651-68 (2014) Article DOI: 10.1021/jm401204g BindingDB Entry DOI: 10.7270/Q2PN974G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448758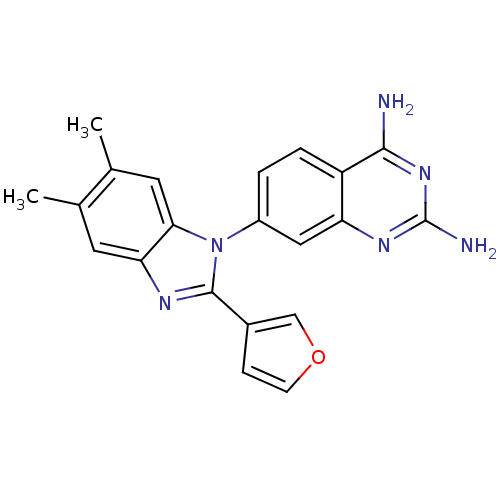 (CHEMBL3128024) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics Inc. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR using dihydrofolate as substrate preincubated for 10 mins followed by substrate addition by spectrophotometr... | J Med Chem 57: 651-68 (2014) Article DOI: 10.1021/jm401204g BindingDB Entry DOI: 10.7270/Q2PN974G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448743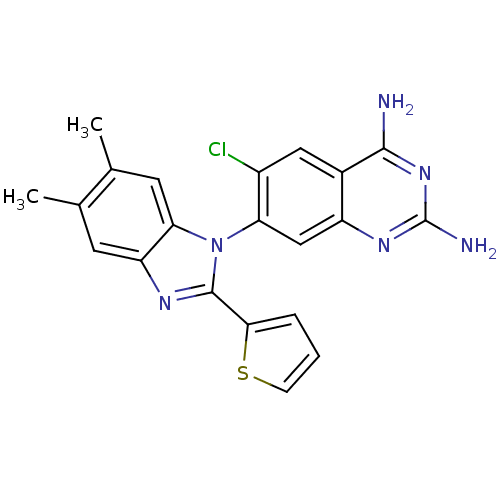 (CHEMBL3128015) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics Inc. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR using dihydrofolate as substrate preincubated for 10 mins followed by substrate addition by spectrophotometr... | J Med Chem 57: 651-68 (2014) Article DOI: 10.1021/jm401204g BindingDB Entry DOI: 10.7270/Q2PN974G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448744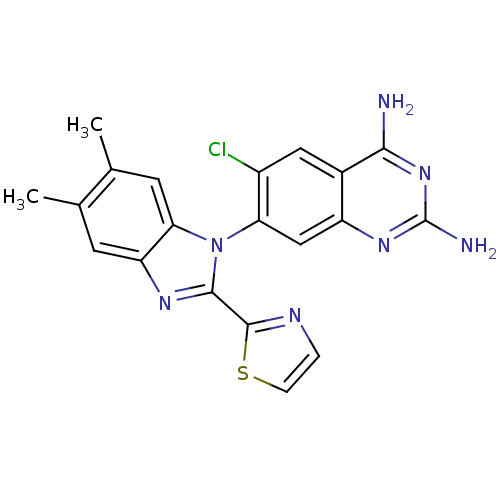 (CHEMBL3128014 | US8835445, 25 | US8835445, 34) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics Inc. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR using dihydrofolate as substrate preincubated for 10 mins followed by substrate addition by spectrophotometr... | J Med Chem 57: 651-68 (2014) Article DOI: 10.1021/jm401204g BindingDB Entry DOI: 10.7270/Q2PN974G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448740 (CHEMBL3128018) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics Inc. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR using dihydrofolate as substrate preincubated for 10 mins followed by substrate addition by spectrophotometr... | J Med Chem 57: 651-68 (2014) Article DOI: 10.1021/jm401204g BindingDB Entry DOI: 10.7270/Q2PN974G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448756 (CHEMBL3128026 | US8835445, 21) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics Inc. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR using dihydrofolate as substrate preincubated for 10 mins followed by substrate addition by spectrophotometr... | J Med Chem 57: 651-68 (2014) Article DOI: 10.1021/jm401204g BindingDB Entry DOI: 10.7270/Q2PN974G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448741 (CHEMBL3128017) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics Inc. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR using dihydrofolate as substrate preincubated for 10 mins followed by substrate addition by spectrophotometr... | J Med Chem 57: 651-68 (2014) Article DOI: 10.1021/jm401204g BindingDB Entry DOI: 10.7270/Q2PN974G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448753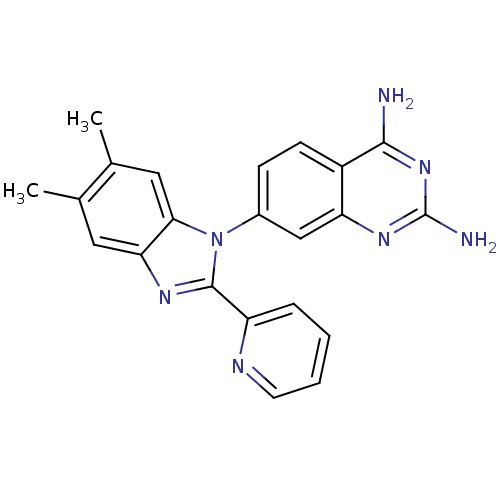 (CHEMBL3127912 | US8835445, 16) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics Inc. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR using dihydrofolate as substrate preincubated for 10 mins followed by substrate addition by spectrophotometr... | J Med Chem 57: 651-68 (2014) Article DOI: 10.1021/jm401204g BindingDB Entry DOI: 10.7270/Q2PN974G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448738 (CHEMBL3127909) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics Inc. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR using dihydrofolate as substrate preincubated for 10 mins followed by substrate addition by spectrophotometr... | J Med Chem 57: 651-68 (2014) Article DOI: 10.1021/jm401204g BindingDB Entry DOI: 10.7270/Q2PN974G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448751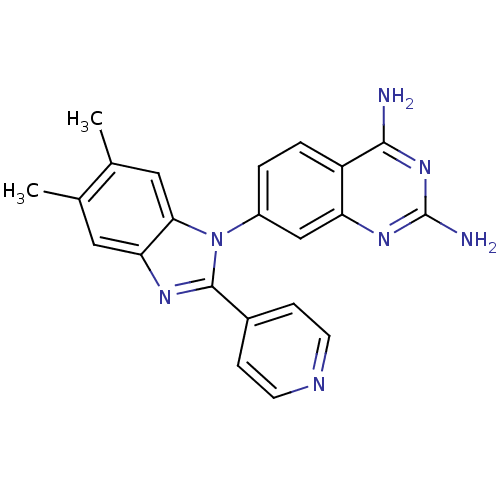 (CHEMBL3127914) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics Inc. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR using dihydrofolate as substrate preincubated for 10 mins followed by substrate addition by spectrophotometr... | J Med Chem 57: 651-68 (2014) Article DOI: 10.1021/jm401204g BindingDB Entry DOI: 10.7270/Q2PN974G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448745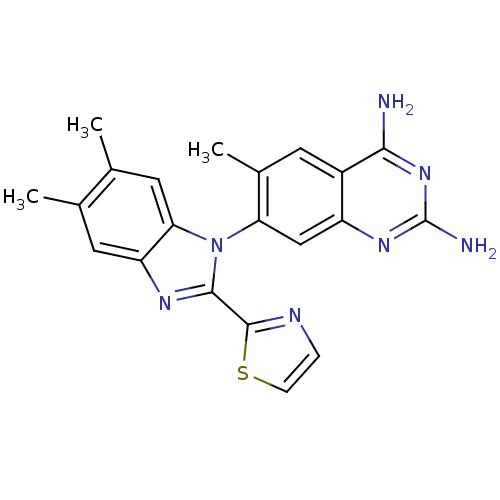 (CHEMBL3128013 | US8835445, 26) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics Inc. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR using dihydrofolate as substrate preincubated for 10 mins followed by substrate addition by spectrophotometr... | J Med Chem 57: 651-68 (2014) Article DOI: 10.1021/jm401204g BindingDB Entry DOI: 10.7270/Q2PN974G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448739 (CHEMBL3128019) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics Inc. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR using dihydrofolate as substrate preincubated for 10 mins followed by substrate addition by spectrophotometr... | J Med Chem 57: 651-68 (2014) Article DOI: 10.1021/jm401204g BindingDB Entry DOI: 10.7270/Q2PN974G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM18070 (5-[(2-cyclopropyl-7,8-dimethoxy-2H-chromen-5-yl)me...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics Inc. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR using dihydrofolate as substrate preincubated for 10 mins followed by substrate addition by spectrophotometr... | J Med Chem 57: 651-68 (2014) Article DOI: 10.1021/jm401204g BindingDB Entry DOI: 10.7270/Q2PN974G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448760 (CHEMBL3128022) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | PDB Article PubMed | 0.0960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics Inc. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR using dihydrofolate as substrate preincubated for 10 mins followed by substrate addition by spectrophotometr... | J Med Chem 57: 651-68 (2014) Article DOI: 10.1021/jm401204g BindingDB Entry DOI: 10.7270/Q2PN974G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50064340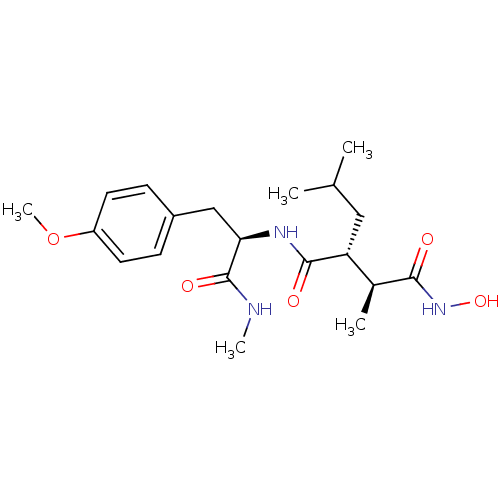 ((2R,3S)-N*4*-Hydroxy-2-isobutyl-N*1*-[(R)-2-(4-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of MMP-9 (Matrix metalloproteinase-9) | J Med Chem 41: 1745-8 (1998) Article DOI: 10.1021/jm970849z BindingDB Entry DOI: 10.7270/Q2GB235N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448749 (CHEMBL3127916 | US8835445, 15) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics Inc. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR using dihydrofolate as substrate preincubated for 10 mins followed by substrate addition by spectrophotometr... | J Med Chem 57: 651-68 (2014) Article DOI: 10.1021/jm401204g BindingDB Entry DOI: 10.7270/Q2PN974G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448742 (CHEMBL3128016) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics Inc. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR using dihydrofolate as substrate preincubated for 10 mins followed by substrate addition by spectrophotometr... | J Med Chem 57: 651-68 (2014) Article DOI: 10.1021/jm401204g BindingDB Entry DOI: 10.7270/Q2PN974G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50081112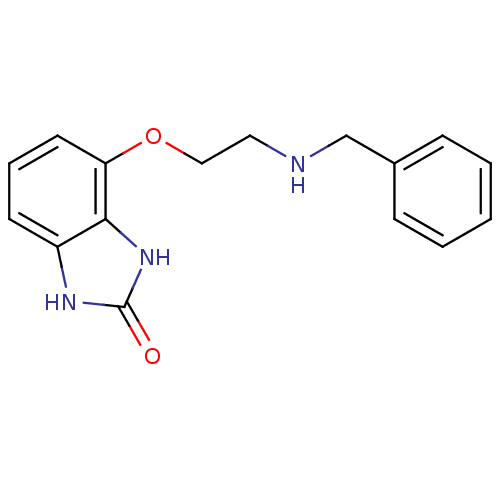 (4-(2-Benzylamino-ethoxy)-1,3-dihydro-benzoimidazol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description Inhibtion of [3H]-spiperone binding to rat striatal membrane Dopamine receptor D2 low affinity without GTP and sodium | Bioorg Med Chem Lett 9: 2593-8 (1999) BindingDB Entry DOI: 10.7270/Q22V2FBG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50081110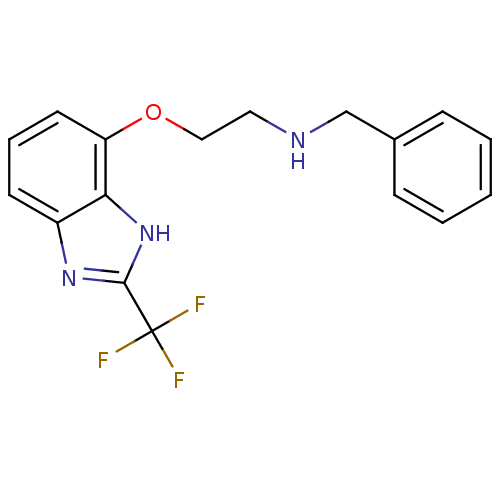 (Benzyl-[2-(2-trifluoromethyl-1H-benzoimidazol-4-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description Inhibtion of [3H]-spiperone binding to rat striatal membrane Dopamine receptor D2 low affinity without GTP and sodium | Bioorg Med Chem Lett 9: 2593-8 (1999) BindingDB Entry DOI: 10.7270/Q22V2FBG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50081108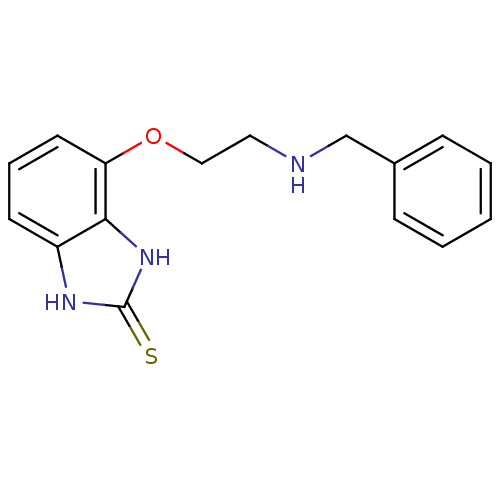 (4-(2-Benzylamino-ethoxy)-1,3-dihydro-benzoimidazol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]-quinpirole binding to rat striatal membrane Dopamine receptor D2 without GTP and sodium | Bioorg Med Chem Lett 9: 2593-8 (1999) BindingDB Entry DOI: 10.7270/Q22V2FBG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448746 (CHEMBL3127919) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics Inc. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR using dihydrofolate as substrate preincubated for 10 mins followed by substrate addition by spectrophotometr... | J Med Chem 57: 651-68 (2014) Article DOI: 10.1021/jm401204g BindingDB Entry DOI: 10.7270/Q2PN974G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50077578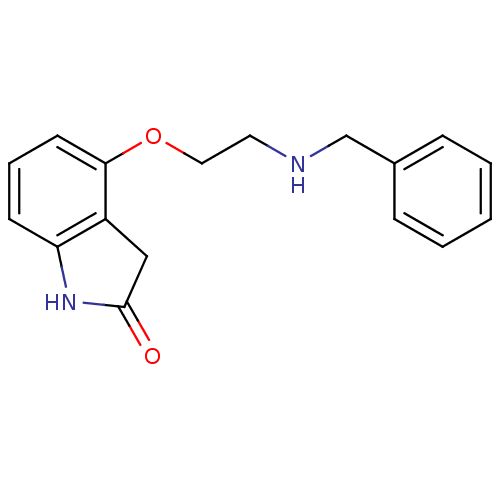 (4-(2-Benzylamino-ethoxy)-1,3-dihydro-indol-2-one |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]-quinpirole binding to rat striatal membrane Dopamine receptor D2 without GTP and sodium | Bioorg Med Chem Lett 9: 2593-8 (1999) BindingDB Entry DOI: 10.7270/Q22V2FBG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50081107 (4-(2-Benzylamino-ethoxy)-6-chloro-1,3-dihydro-benz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]-quinpirole binding to rat striatal membrane Dopamine receptor D2 without GTP and sodium | Bioorg Med Chem Lett 9: 2593-8 (1999) BindingDB Entry DOI: 10.7270/Q22V2FBG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50081106 (4-(2-Benzylamino-ethoxy)-6-chloro-1,3-dihydro-benz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]-quinpirole binding to rat striatal membrane Dopamine receptor D2 without GTP and sodium | Bioorg Med Chem Lett 9: 2593-8 (1999) BindingDB Entry DOI: 10.7270/Q22V2FBG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448752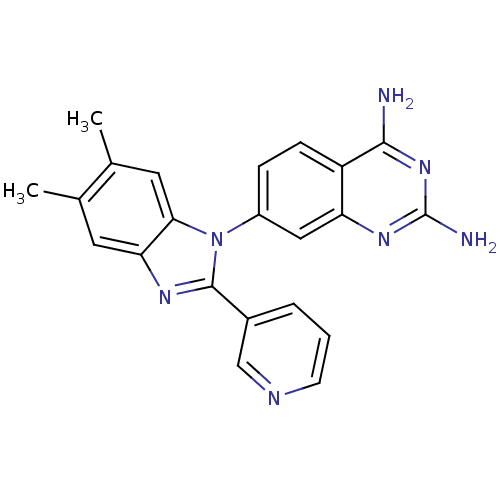 (CHEMBL3127913) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics Inc. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR using dihydrofolate as substrate preincubated for 10 mins followed by substrate addition by spectrophotometr... | J Med Chem 57: 651-68 (2014) Article DOI: 10.1021/jm401204g BindingDB Entry DOI: 10.7270/Q2PN974G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448750 (CHEMBL3127915) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics Inc. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR using dihydrofolate as substrate preincubated for 10 mins followed by substrate addition by spectrophotometr... | J Med Chem 57: 651-68 (2014) Article DOI: 10.1021/jm401204g BindingDB Entry DOI: 10.7270/Q2PN974G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor (RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50228318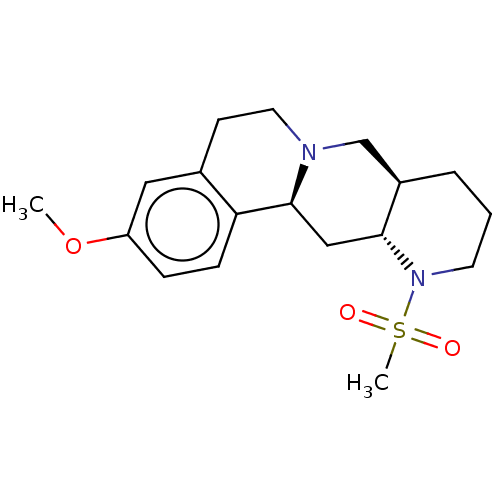 (CHEMBL279807) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.355 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Binding affinity to alpha-2 adrenergic receptor determined by measurement of [3H]yohimbine displacement from rat cortical membrane | J Med Chem 34: 705-17 (1991) BindingDB Entry DOI: 10.7270/Q2MW2KBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448737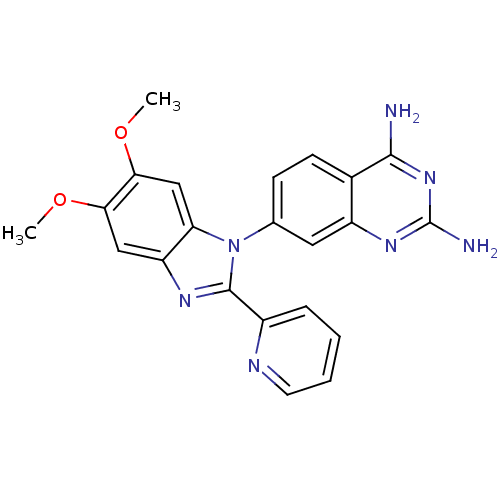 (CHEMBL3127910) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics Inc. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR using dihydrofolate as substrate preincubated for 10 mins followed by substrate addition by spectrophotometr... | J Med Chem 57: 651-68 (2014) Article DOI: 10.1021/jm401204g BindingDB Entry DOI: 10.7270/Q2PN974G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50102594 (7-Isobutyl-8-oxo-2-oxa-9-aza-bicyclo[10.2.2]hexade...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Inhibition of matrix metalloprotease-13 | J Med Chem 44: 2636-60 (2001) BindingDB Entry DOI: 10.7270/Q2FB53NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor (RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50229080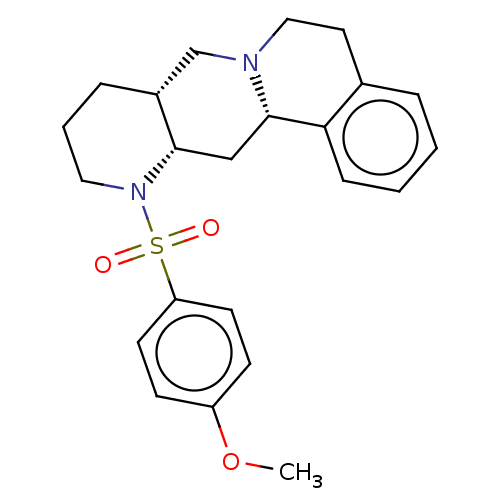 (CHEMBL349665) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.457 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Binding affinity to alpha-2 adrenergic receptor determined by measurement of [3H]yohimbine displacement from rat cortical membrane | J Med Chem 34: 705-17 (1991) BindingDB Entry DOI: 10.7270/Q2MW2KBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil collagenase (Homo sapiens (Human)) | BDBM50102594 (7-Isobutyl-8-oxo-2-oxa-9-aza-bicyclo[10.2.2]hexade...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Inhibition of matrix metalloprotease-8 | J Med Chem 44: 2636-60 (2001) BindingDB Entry DOI: 10.7270/Q2FB53NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50071855 (4-(4-Benzyl-piperazin-1-yl)-2-trifluoromethyl-1H-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratory Curated by ChEMBL | Assay Description Affinity for Dopamine receptor D2 in rat striatal membranes for high agonist state using [3H]-quinpirole | Bioorg Med Chem Lett 8: 2675-80 (1999) BindingDB Entry DOI: 10.7270/Q2C53K0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50071856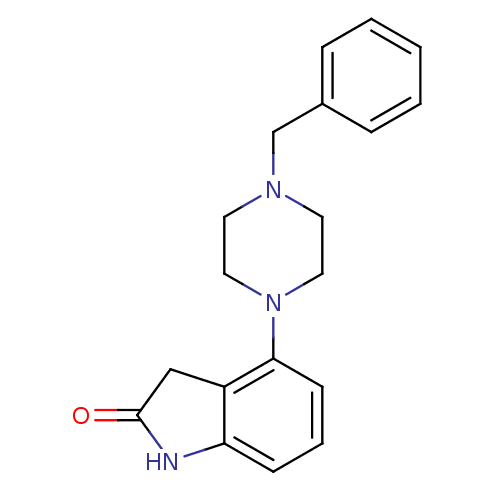 (4-(4-Benzyl-piperazin-1-yl)-1,3-dihydro-indol-2-on...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratory Curated by ChEMBL | Assay Description Affinity for Dopamine receptor D2 in rat striatal membranes for high agonist state using [3H]-quinpirole | Bioorg Med Chem Lett 8: 2675-80 (1999) BindingDB Entry DOI: 10.7270/Q2C53K0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448748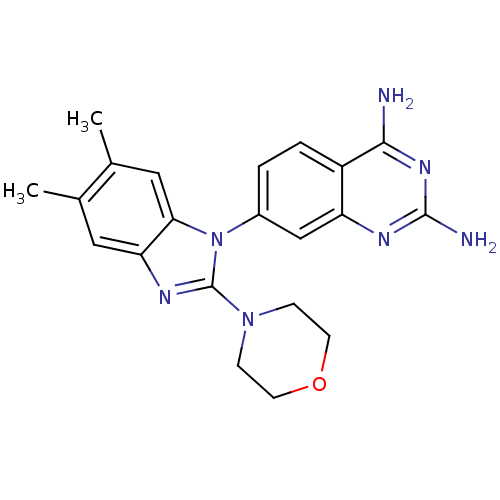 (CHEMBL3127917 | US8835445, 19) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics Inc. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR using dihydrofolate as substrate preincubated for 10 mins followed by substrate addition by spectrophotometr... | J Med Chem 57: 651-68 (2014) Article DOI: 10.1021/jm401204g BindingDB Entry DOI: 10.7270/Q2PN974G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor (RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50229162 (CHEMBL349747) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.603 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Binding affinity to alpha-2 adrenergic receptor determined by measurement of [3H]yohimbine displacement from rat cortical membrane | J Med Chem 34: 705-17 (1991) BindingDB Entry DOI: 10.7270/Q2MW2KBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor (RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50229147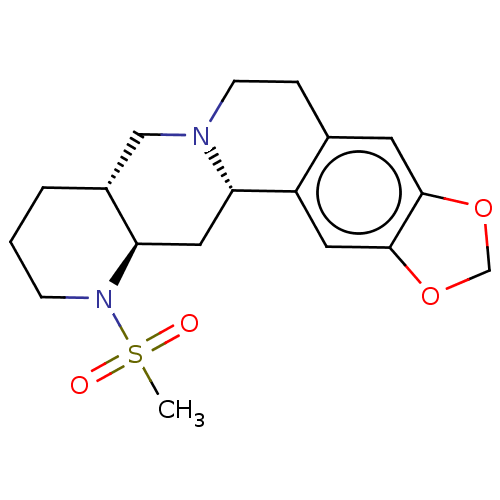 (CHEMBL348793) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.646 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Binding affinity to alpha-2 adrenergic receptor determined by measurement of [3H]yohimbine displacement from rat cortical membrane | J Med Chem 34: 705-17 (1991) BindingDB Entry DOI: 10.7270/Q2MW2KBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor (RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50229143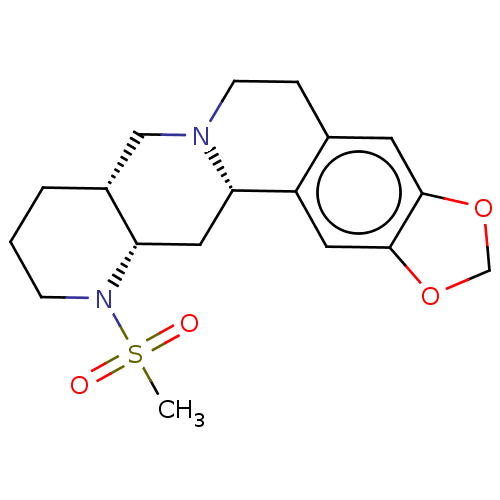 (CHEMBL162971) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.661 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Binding affinity to alpha-2 adrenergic receptor determined by measurement of [3H]yohimbine displacement from rat cortical membrane | J Med Chem 34: 705-17 (1991) BindingDB Entry DOI: 10.7270/Q2MW2KBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor (RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50229179 (Delequamine) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.661 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Binding affinity to alpha-2 adrenergic receptor determined by measurement of [3H]yohimbine displacement from rat cortical membrane | J Med Chem 34: 705-17 (1991) BindingDB Entry DOI: 10.7270/Q2MW2KBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor (RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50229152 (CHEMBL349004) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.708 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Binding affinity to alpha-2 adrenergic receptor determined by measurement of [3H]yohimbine displacement from rat cortical membrane | J Med Chem 34: 705-17 (1991) BindingDB Entry DOI: 10.7270/Q2MW2KBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50081105 (Benzyl-[2-(6-chloro-2-trifluoromethyl-1H-benzoimid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]-quinpirole binding to rat striatal membrane Dopamine receptor D2 without GTP and sodium | Bioorg Med Chem Lett 9: 2593-8 (1999) BindingDB Entry DOI: 10.7270/Q22V2FBG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrilysin (Homo sapiens (Human)) | BDBM50102594 (7-Isobutyl-8-oxo-2-oxa-9-aza-bicyclo[10.2.2]hexade...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Inhibition of matrix metalloprotease-7 | J Med Chem 44: 2636-60 (2001) BindingDB Entry DOI: 10.7270/Q2FB53NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50064340 ((2R,3S)-N*4*-Hydroxy-2-isobutyl-N*1*-[(R)-2-(4-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of MMP-1 (Matrix metalloproteinase-1) | J Med Chem 41: 1745-8 (1998) Article DOI: 10.1021/jm970849z BindingDB Entry DOI: 10.7270/Q2GB235N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50102594 (7-Isobutyl-8-oxo-2-oxa-9-aza-bicyclo[10.2.2]hexade...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description In vitro inhibition of human MMP-9. | J Med Chem 44: 2636-60 (2001) BindingDB Entry DOI: 10.7270/Q2FB53NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50064340 ((2R,3S)-N*4*-Hydroxy-2-isobutyl-N*1*-[(R)-2-(4-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description In vitro inhibition of human MMP-9. | J Med Chem 44: 2636-60 (2001) BindingDB Entry DOI: 10.7270/Q2FB53NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50091825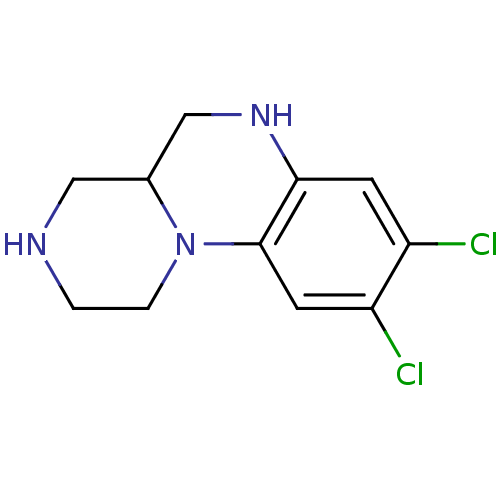 (8,9-Dichloro-2,3,4,4a,5,6-hexahydro-1H-pyrazino[1,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Binding affinity using [125I]-DOI as radioligand with membranes isolated from a CHO-k cell line expressing the human 5-hydroxytryptamine 2C receptor | Bioorg Med Chem Lett 10: 1991-4 (2001) BindingDB Entry DOI: 10.7270/Q2Z89BMP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50102610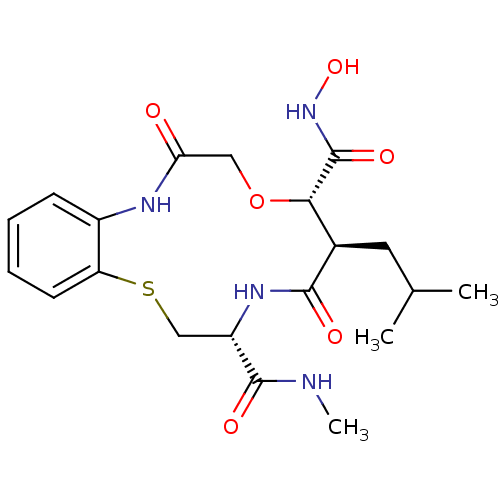 (10-Isobutyl-6,11-dioxo-6,7,9,10,11,12,13,14-octahy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description In vitro inhibition of human MMP-1. | J Med Chem 44: 2636-60 (2001) BindingDB Entry DOI: 10.7270/Q2FB53NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50102630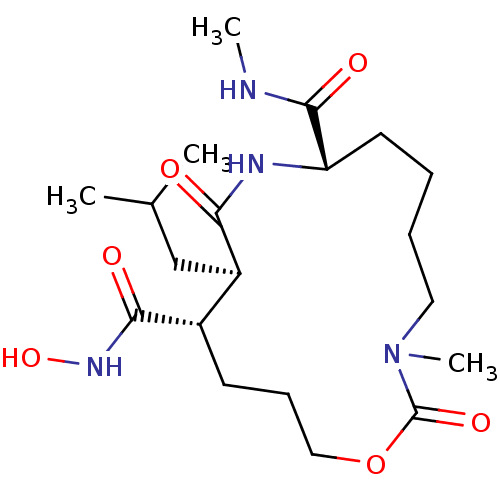 (11-Isobutyl-3-methyl-2,10-dioxo-1-oxa-3,9-diaza-cy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description In vitro inhibition of human MMP-1. | J Med Chem 44: 2636-60 (2001) BindingDB Entry DOI: 10.7270/Q2FB53NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50076993 ((2S,11S,12R)-12-Isobutyl-13-oxo-1,7diaza-cyclotrid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description In vitro inhibition of human MMP-1. | J Med Chem 44: 2636-60 (2001) BindingDB Entry DOI: 10.7270/Q2FB53NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2462 total ) | Next | Last >> |