

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50279769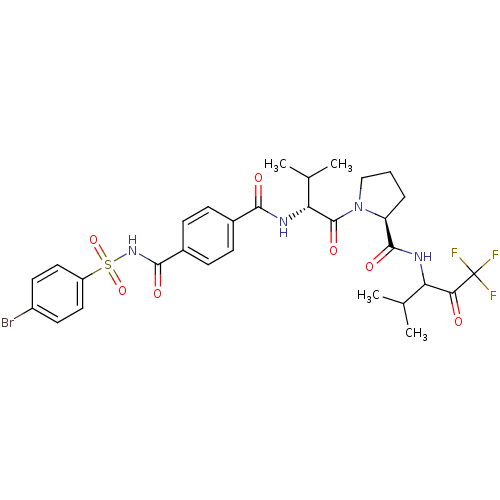 ((S)-1-{(R)-2-[4-(4-Bromo-benzenesulfonylaminocarbo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Inhibitory activity against human leukocyte elastase | J Med Chem 40: 3408-22 (1997) Article DOI: 10.1021/jm970251r BindingDB Entry DOI: 10.7270/Q26D5S4T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50192958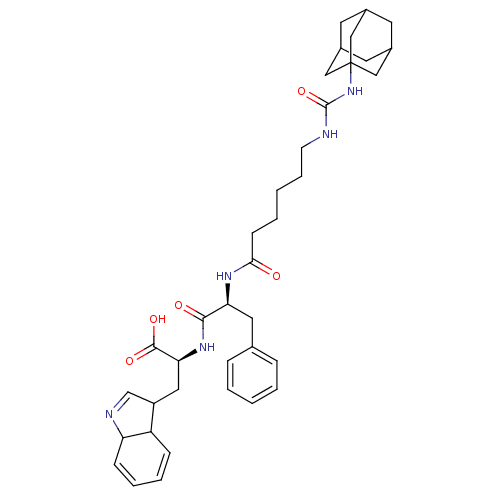 (CHEMBL385894 | N-[6-(3-adamantyl-ureido)-hexanoyl]...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble Epoxide hydrolase | Bioorg Med Chem Lett 16: 5439-44 (2006) Article DOI: 10.1016/j.bmcl.2006.07.073 BindingDB Entry DOI: 10.7270/Q2183643 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50279767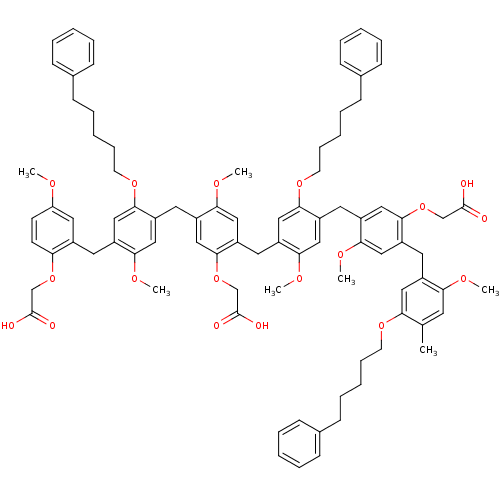 (CHEMBL268343 | {5-[4-(2-Carboxymethoxy-5-methoxy-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Inhibitory activity against human leukocyte elastase | J Med Chem 40: 3408-22 (1997) Article DOI: 10.1021/jm970251r BindingDB Entry DOI: 10.7270/Q26D5S4T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50279765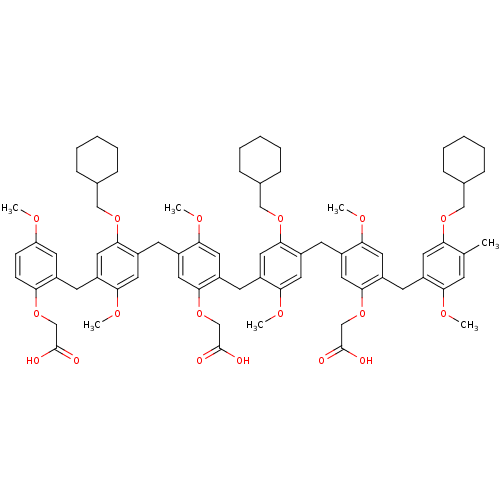 (CHEMBL215405 | {2-{4-[5-Carboxymethoxy-4-(5-cycloh...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Inhibitory activity against human leukocyte elastase | J Med Chem 40: 3408-22 (1997) Article DOI: 10.1021/jm970251r BindingDB Entry DOI: 10.7270/Q26D5S4T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50060330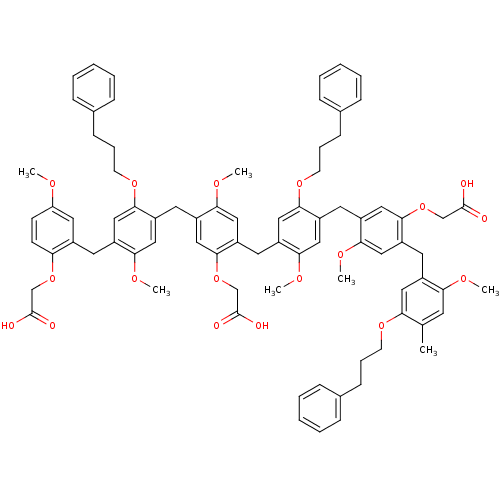 (CHEMBL265335 | {5-[4-(2-Carboxymethoxy-5-methoxy-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Inhibitory activity against human leukocyte elastase | J Med Chem 40: 3408-22 (1997) Article DOI: 10.1021/jm970251r BindingDB Entry DOI: 10.7270/Q26D5S4T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50279770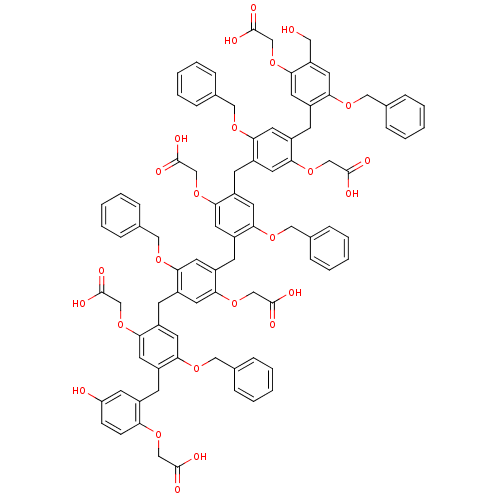 (2-{4-benzyloxy-5-[5-benzyloxy-4-(5-benzyloxy-4-{5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Inhibitory activity against human leukocyte elastase | J Med Chem 40: 3408-22 (1997) Article DOI: 10.1021/jm970251r BindingDB Entry DOI: 10.7270/Q26D5S4T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50279771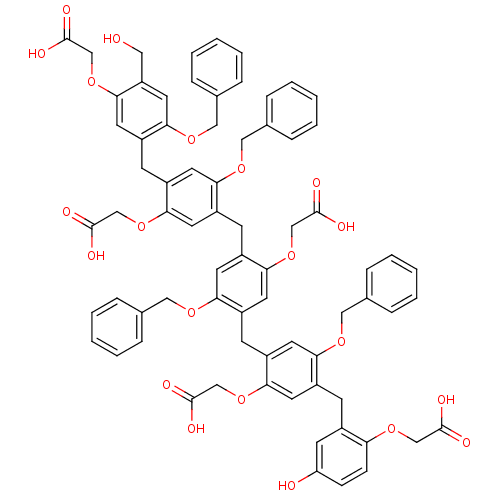 (CHEMBL217744 | [4-Benzyloxy-5-{5-benzyloxy-4-[5-be...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Inhibitory activity against human leukocyte elastase | J Med Chem 40: 3408-22 (1997) Article DOI: 10.1021/jm970251r BindingDB Entry DOI: 10.7270/Q26D5S4T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50279770 (2-{4-benzyloxy-5-[5-benzyloxy-4-(5-benzyloxy-4-{5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Binding Affinity of the compound to inhibit HLE | J Med Chem 40: 3408-22 (1997) Article DOI: 10.1021/jm970251r BindingDB Entry DOI: 10.7270/Q26D5S4T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50279766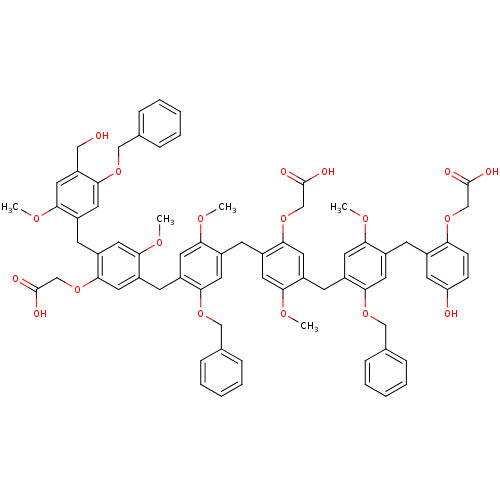 (CHEMBL213783 | {2-{5-Benzyloxy-4-[4-(5-benzyloxy-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Inhibitory activity against human leukocyte elastase | J Med Chem 40: 3408-22 (1997) Article DOI: 10.1021/jm970251r BindingDB Entry DOI: 10.7270/Q26D5S4T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50060332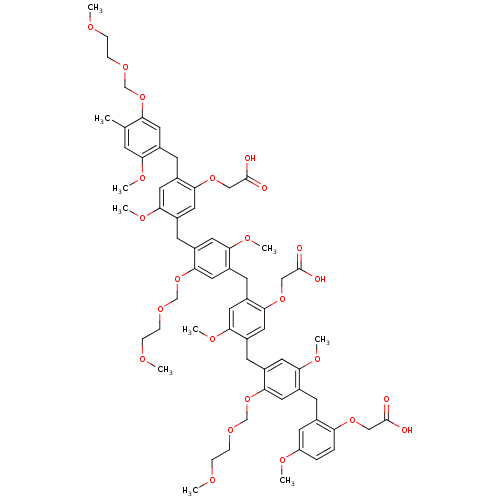 (CHEMBL215927) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 240 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Inhibition of thrombin at pH 7.5 in HEPES buffer containing 200 mM NaCl with substrate Bz-Phe-Val-Arg-pNA. | J Med Chem 40: 3408-22 (1997) Article DOI: 10.1021/jm970251r BindingDB Entry DOI: 10.7270/Q26D5S4T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50118750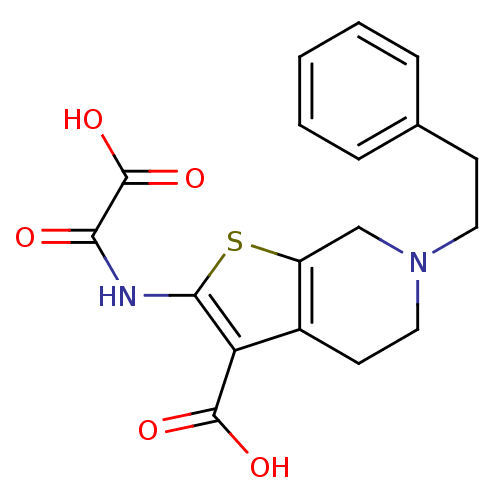 (2-(Oxalyl-amino)-6-phenethyl-4,5,6,7-tetrahydro-th...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 270 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. | J Med Chem 45: 4443-59 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50118792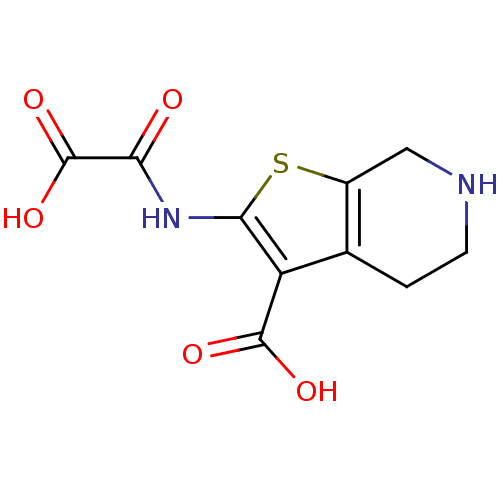 (2-(OXALYL-AMINO)-4,5,6,7-TETRAHYDRO-THIENO[2,3-C]P...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 290 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. | J Med Chem 45: 4443-59 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GM5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50118786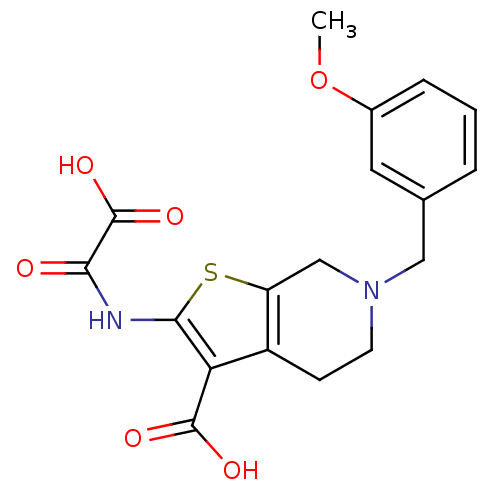 (6-(3-Methoxy-benzyl)-2-(oxalyl-amino)-4,5,6,7-tetr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. | J Med Chem 45: 4443-59 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50279771 (CHEMBL217744 | [4-Benzyloxy-5-{5-benzyloxy-4-[5-be...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 750 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Inhibition of thrombin at pH 7.5 in HEPES buffer containing 200 mM NaCl with substrate Bz-Phe-Val-Arg-pNA. | J Med Chem 40: 3408-22 (1997) Article DOI: 10.1021/jm970251r BindingDB Entry DOI: 10.7270/Q26D5S4T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50118794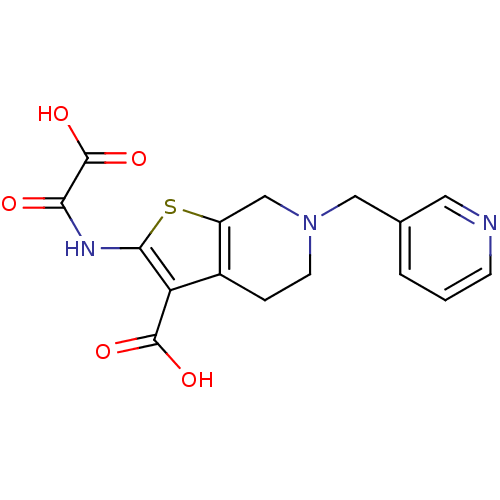 (2-(Oxalyl-amino)-6-pyridin-3-ylmethyl-4,5,6,7-tetr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 900 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. | J Med Chem 45: 4443-59 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50060332 (CHEMBL215927) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Inhibitory activity against human leukocyte elastase | J Med Chem 40: 3408-22 (1997) Article DOI: 10.1021/jm970251r BindingDB Entry DOI: 10.7270/Q26D5S4T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50118793 (6-Methyl-2-(oxalyl-amino)-4,5,6,7-tetrahydro-thien...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. | J Med Chem 45: 4443-59 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50118774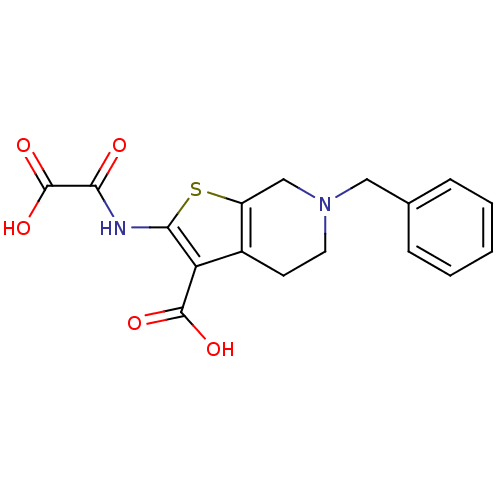 (6-Benzyl-2-(oxalyl-amino)-4,5,6,7-tetrahydro-thien...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. | J Med Chem 45: 4443-59 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50118742 (2-(Oxalyl-amino)-6-pyridin-2-ylmethyl-4,5,6,7-tetr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. | J Med Chem 45: 4443-59 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50118755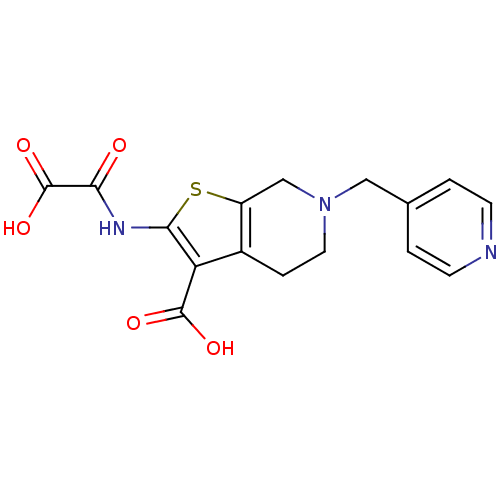 (2-(Oxalyl-amino)-6-pyridin-4-ylmethyl-4,5,6,7-tetr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. | J Med Chem 45: 4443-59 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM912 ((3S,4S)-3-hydroxy-4-[(2S)-2-[(3S,4S)-3-hydroxy-6-m...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli | J Nat Prod 64: 265-77 (2001) Article DOI: 10.1021/np0003995 BindingDB Entry DOI: 10.7270/Q2HT2S52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50060329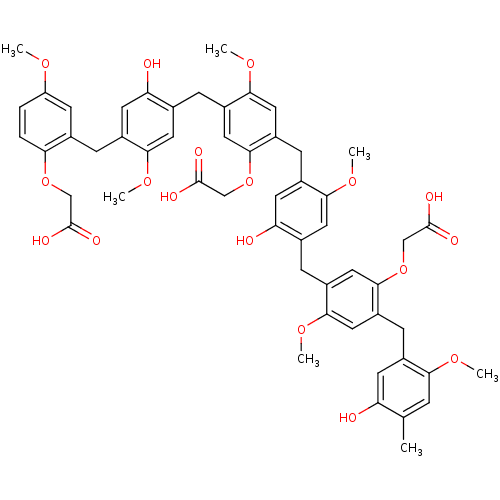 (CHEMBL385442 | {2-{4-[5-Carboxymethoxy-4-(5-hydrox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Inhibitory activity against human leukocyte elastase | J Med Chem 40: 3408-22 (1997) Article DOI: 10.1021/jm970251r BindingDB Entry DOI: 10.7270/Q26D5S4T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50060331 (CHEMBL412744 | bis-[4-tert-Butyl-2-[5-tert-butyl-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Inhibitory activity against human leukocyte elastase | J Med Chem 40: 3408-22 (1997) Article DOI: 10.1021/jm970251r BindingDB Entry DOI: 10.7270/Q26D5S4T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50118772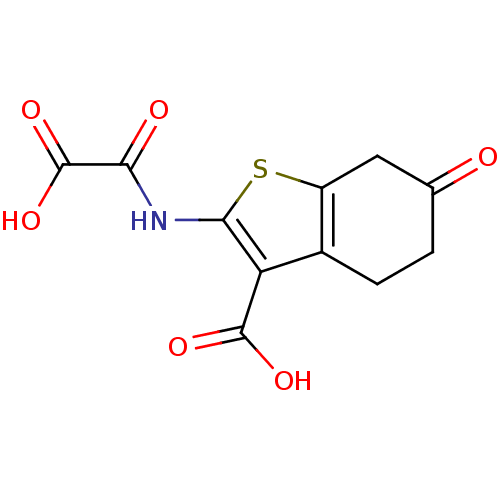 (2-(Oxalyl-amino)-6-oxo-4,5,6,7-tetrahydro-benzo[b]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. | J Med Chem 45: 4443-59 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50279767 (CHEMBL268343 | {5-[4-(2-Carboxymethoxy-5-methoxy-b...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Inhibition of thrombin at pH 7.5 in HEPES buffer containing 200 mM NaCl with substrate Bz-Phe-Val-Arg-pNA. | J Med Chem 40: 3408-22 (1997) Article DOI: 10.1021/jm970251r BindingDB Entry DOI: 10.7270/Q26D5S4T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin G (Homo sapiens (Human)) | BDBM50279766 (CHEMBL213783 | {2-{5-Benzyloxy-4-[4-(5-benzyloxy-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Inhibition of cathepsin G at pH 7.5 in HEPES buffer containing 200 mM NaCl with substrate Suc-Ala-Ala-Pro-Phe-pNA. | J Med Chem 40: 3408-22 (1997) Article DOI: 10.1021/jm970251r BindingDB Entry DOI: 10.7270/Q26D5S4T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50060330 (CHEMBL265335 | {5-[4-(2-Carboxymethoxy-5-methoxy-b...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Inhibition of thrombin at pH 7.5 in HEPES buffer containing 200 mM NaCl with substrate Bz-Phe-Val-Arg-pNA. | J Med Chem 40: 3408-22 (1997) Article DOI: 10.1021/jm970251r BindingDB Entry DOI: 10.7270/Q26D5S4T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50118767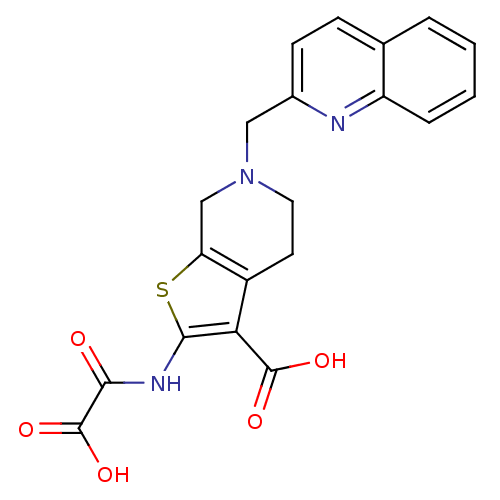 (2-(Oxalyl-amino)-6-quinolin-2-ylmethyl-4,5,6,7-tet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. | J Med Chem 45: 4443-59 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50118771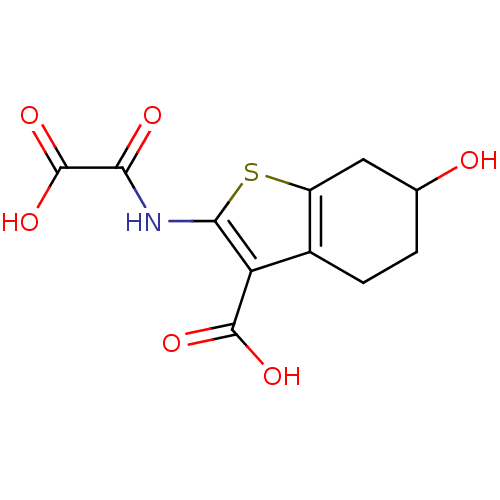 (2-(carboxyformamido)-6-hydroxy-4,5,6,7-tetrahydrob...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. | J Med Chem 45: 4443-59 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50118741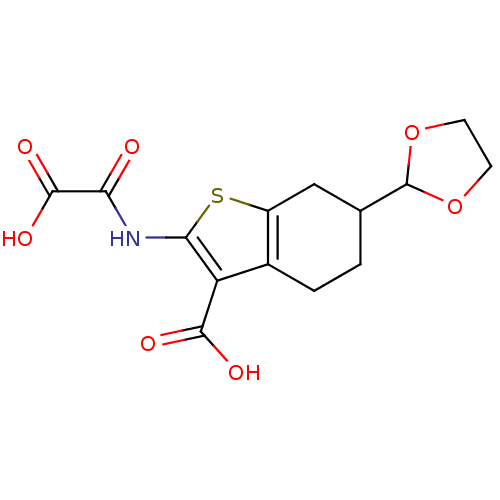 (6-[1,3]Dioxolan-2-yl-2-(oxalyl-amino)-4,5,6,7-tetr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. | J Med Chem 45: 4443-59 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50118765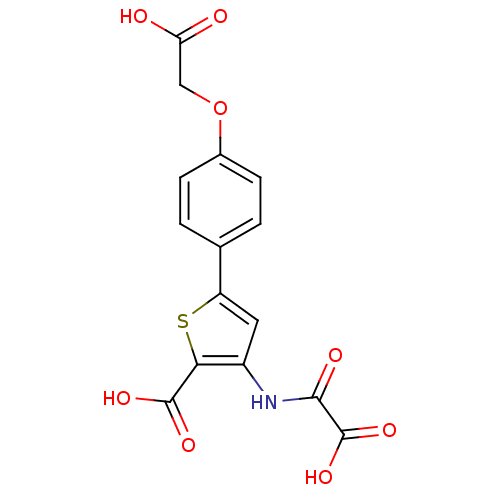 (3-(carboxyformamido)-5-(4-(carboxymethoxy)phenyl)t...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. | J Med Chem 45: 4443-59 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50118750 (2-(Oxalyl-amino)-6-phenethyl-4,5,6,7-tetrahydro-th...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibition of human recombinant Protein-tyrosine phosphatase 1B. | J Med Chem 45: 4443-59 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50118750 (2-(Oxalyl-amino)-6-phenethyl-4,5,6,7-tetrahydro-th...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibition of human recombinant Protein-tyrosine phosphatase 1B. | J Med Chem 45: 4443-59 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50118782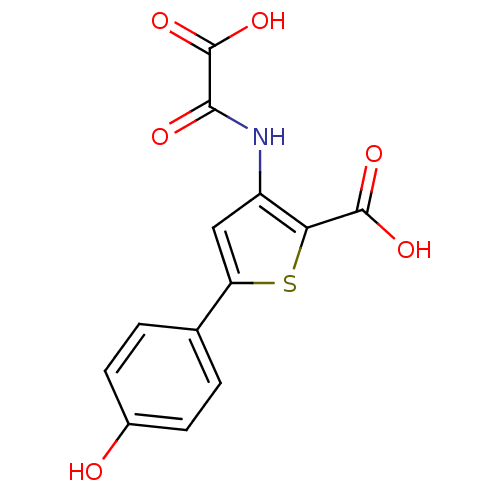 (3-(carboxyformamido)-5-(4-hydroxyphenyl)thiophene-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. | J Med Chem 45: 4443-59 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50118780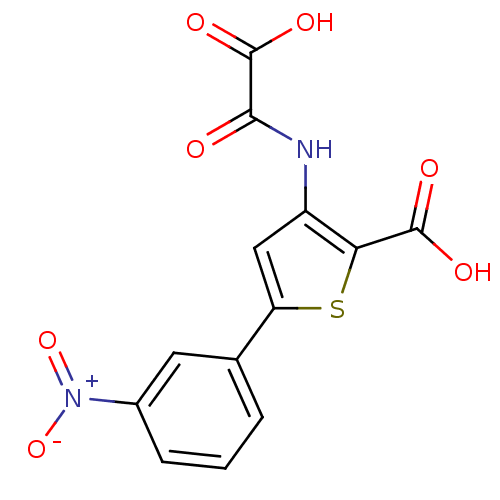 (3-(carboxyformamido)-5-(3-nitrophenyl)thiophene-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. | J Med Chem 45: 4443-59 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50118792 (2-(OXALYL-AMINO)-4,5,6,7-TETRAHYDRO-THIENO[2,3-C]P...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibition of human recombinant Protein-tyrosine phosphatase 1B. | J Med Chem 45: 4443-59 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GM5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50118783 (2-(Oxalyl-amino)-6-oxo-4,5,6,7-tetrahydro-6lambda*...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. | J Med Chem 45: 4443-59 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase alpha (Homo sapiens (Human)) | BDBM50118752 (6-(3,3-Diphenyl-propyl)-2-(oxalyl-amino)-4,5,6,7-t...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory effect against T cell protein tyrosine phosphatase (TC-PTP) using p-nitrophenyl phosphate as substrate at pH 7.0 | J Med Chem 45: 4443-59 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50118752 (6-(3,3-Diphenyl-propyl)-2-(oxalyl-amino)-4,5,6,7-t...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibition of human recombinant Protein-tyrosine phosphatase 1B. | J Med Chem 45: 4443-59 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50118763 (2-(Oxalyl-amino)-6,6-dioxo-4,5,6,7-tetrahydro-6lam...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. | J Med Chem 45: 4443-59 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50118758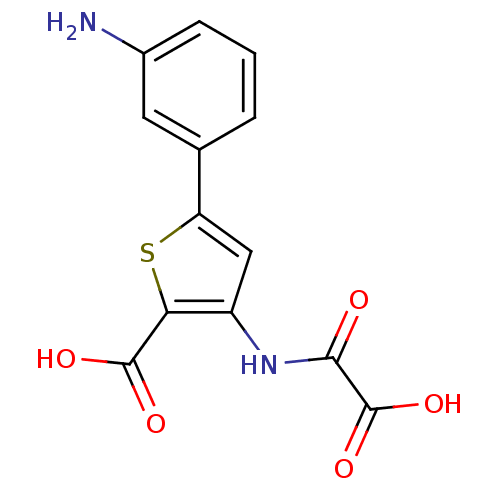 (5-(3-Amino-phenyl)-3-(oxalyl-amino)-thiophene-2-ca...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. | J Med Chem 45: 4443-59 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50118790 (2-(Oxalyl-amino)-6-(3-phenyl-propyl)-4,5,6,7-tetra...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibition of human recombinant Protein-tyrosine phosphatase 1B. | J Med Chem 45: 4443-59 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50118756 (3-(carboxyformamido)-5-(3,5-dimethoxyphenyl)thioph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. | J Med Chem 45: 4443-59 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50118796 (6-(OXALYL-AMINO)-1H-INDOLE-5-CARBOXYLIC ACID | 6-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB PubMed | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. | J Med Chem 45: 4443-59 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GM5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50118740 (3-(carboxyformamido)-5-(4-fluorophenyl)thiophene-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. | J Med Chem 45: 4443-59 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50118754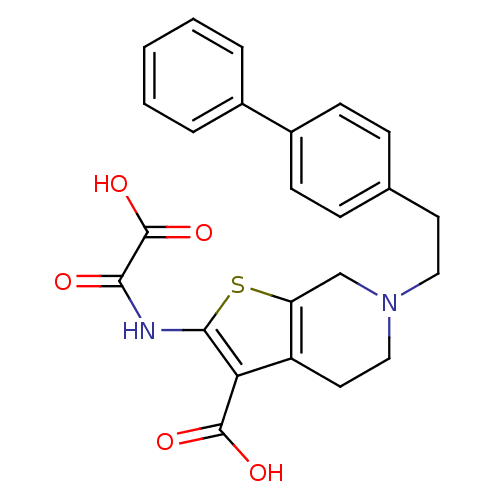 (6-(2-Biphenyl-4-yl-ethyl)-2-(oxalyl-amino)-4,5,6,7...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibition of human recombinant Protein-tyrosine phosphatase 1B. | J Med Chem 45: 4443-59 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50118762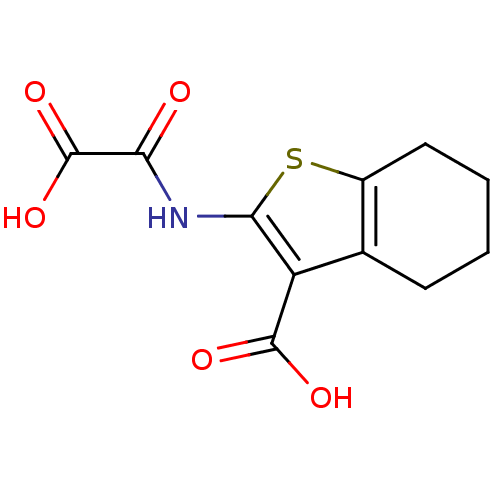 (2-(Oxalyl-amino)-4,5,6,7-tetrahydro-benzo[b]thioph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. | J Med Chem 45: 4443-59 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50279768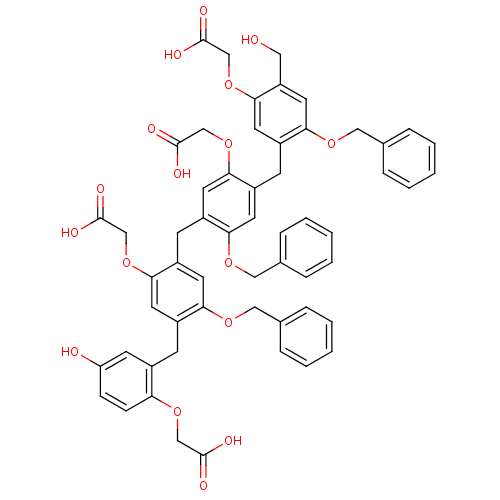 ((2-{2-Benzyloxy-4-[2-benzyloxy-4-(2-benzyloxy-5-ca...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Inhibitory activity against human leukocyte elastase | J Med Chem 40: 3408-22 (1997) Article DOI: 10.1021/jm970251r BindingDB Entry DOI: 10.7270/Q26D5S4T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50118787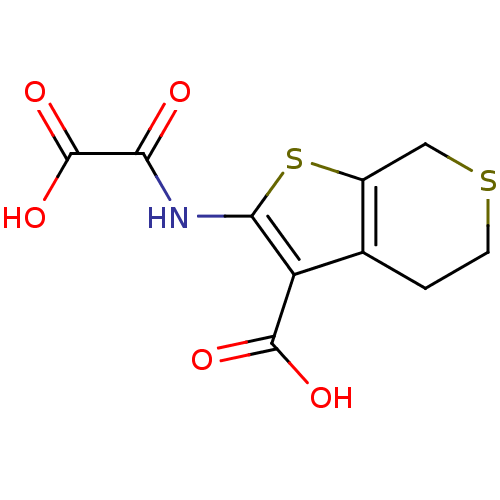 (2-(Oxalyl-amino)-4,7-dihydro-5H-thieno[2,3-c]thiop...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. | J Med Chem 45: 4443-59 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GM5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50118786 (6-(3-Methoxy-benzyl)-2-(oxalyl-amino)-4,5,6,7-tetr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibition of human recombinant Protein-tyrosine phosphatase 1B. | J Med Chem 45: 4443-59 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 566 total ) | Next | Last >> |