

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prothrombin (Homo sapiens (Human)) | BDBM14073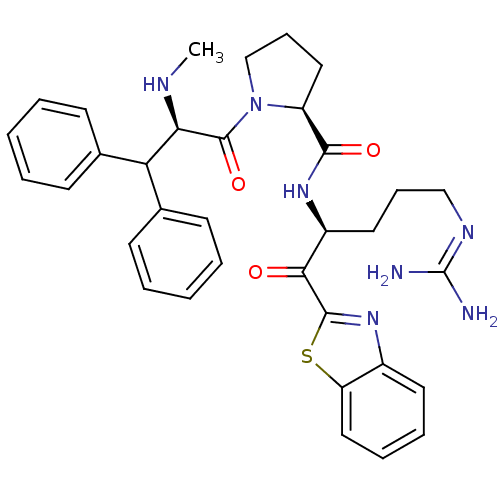 ((2S)-N-[(2S)-1-(1,3-benzothiazol-2-yl)-5-carbamimi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.000650 | -72.4 | 4.5 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Johnson & Johnson Pharmaceutical | Assay Description Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50096105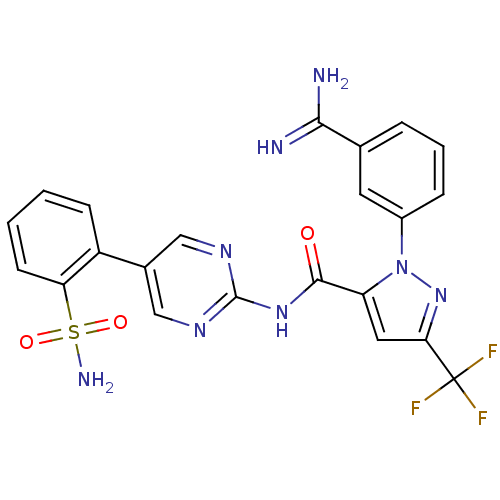 (2-(3-Carbamimidoyl-phenyl)-5-trifluoromethyl-2H-py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity against human coagulation factor X | J Med Chem 44: 566-78 (2001) BindingDB Entry DOI: 10.7270/Q2RF5VQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM110961 (US8614206, 518) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of recombinant human PIM1 using FAM-pimtide as substrate after 90 mins by Z-LYTE assay | J Med Chem 62: 2140-2153 (2019) Article DOI: 10.1021/acs.jmedchem.8b01857 BindingDB Entry DOI: 10.7270/Q2Q52SVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50098576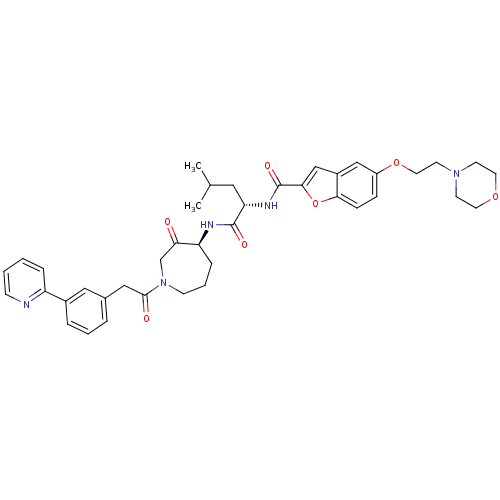 (5-(2-MORPHOLIN-4-YLETHOXY)BENZOFURAN-2-CARBOXYLIC ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB PubMed | 0.00480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Human cathepsin K | J Med Chem 44: 1380-95 (2001) BindingDB Entry DOI: 10.7270/Q2QR4WDC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50377655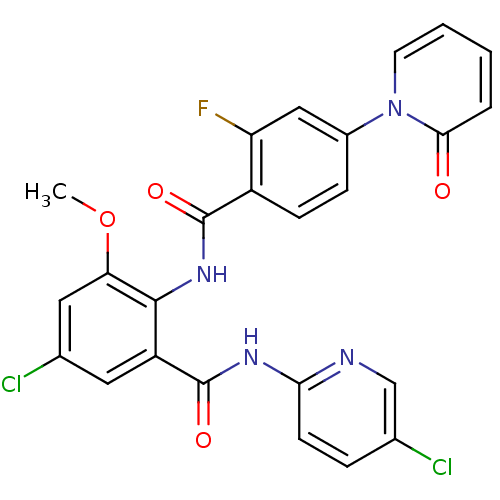 (CHEMBL260160) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human factor 10a | Bioorg Med Chem Lett 18: 2845-9 (2008) Article DOI: 10.1016/j.bmcl.2008.03.092 BindingDB Entry DOI: 10.7270/Q2611169 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50096099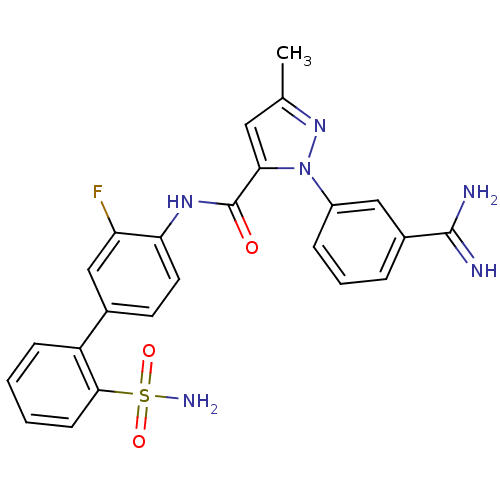 (2-(3-Carbamimidoyl-phenyl)-5-methyl-2H-pyrazole-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity against human coagulation factor X | J Med Chem 44: 566-78 (2001) BindingDB Entry DOI: 10.7270/Q2RF5VQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50377655 (CHEMBL260160) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human factor 10a | Bioorg Med Chem Lett 18: 2845-9 (2008) Article DOI: 10.1016/j.bmcl.2008.03.092 BindingDB Entry DOI: 10.7270/Q2611169 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM50505054 (CHEMBL4455188) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of recombinant human PIM3 using FAM-pimtide as substrate after 90 mins by Z-LYTE assay | J Med Chem 62: 2140-2153 (2019) Article DOI: 10.1021/acs.jmedchem.8b01857 BindingDB Entry DOI: 10.7270/Q2Q52SVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50505052 (CHEMBL3623150) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of recombinant human PIM1 using FAM-pimtide as substrate after 90 mins by Z-LYTE assay | J Med Chem 62: 2140-2153 (2019) Article DOI: 10.1021/acs.jmedchem.8b01857 BindingDB Entry DOI: 10.7270/Q2Q52SVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM227170 (US9328106, 118) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of recombinant human PIM3 using FAM-pimtide as substrate after 90 mins by Z-LYTE assay | J Med Chem 62: 2140-2153 (2019) Article DOI: 10.1021/acs.jmedchem.8b01857 BindingDB Entry DOI: 10.7270/Q2Q52SVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14065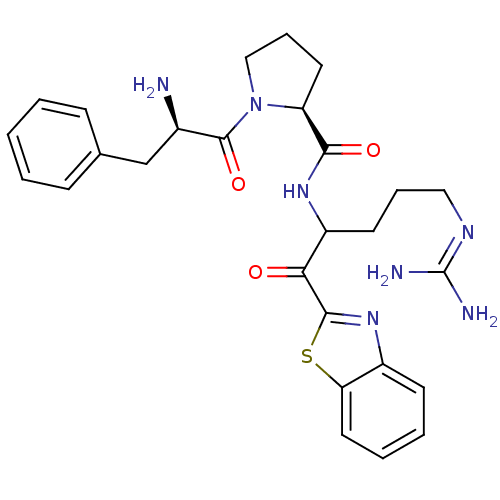 ((2S)-1-[(2R)-2-amino-3-phenylpropanoyl]-N-[1-(1,3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.00550 | -66.9 | 21 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Johnson & Johnson Pharmaceutical | Assay Description Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma intracellular receptor 2 (Mus musculus (Mouse)) | BDBM312199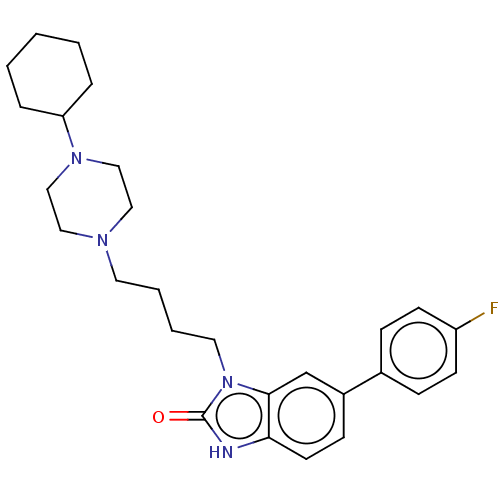 (US9604926, Compound CM-366) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
THE UNIVERSITY OF MISSISSIPPI US Patent | Assay Description Compounds were evaluated for σ-1 and σ-2 binding in rat brain homogenates. Twelve concentrations of each test ligand (0.001-1,000 nM) were ... | US Patent US9604926 (2017) BindingDB Entry DOI: 10.7270/Q27D2X6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM227170 (US9328106, 118) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of recombinant human PIM1 using FAM-pimtide as substrate after 90 mins by Z-LYTE assay | J Med Chem 62: 2140-2153 (2019) Article DOI: 10.1021/acs.jmedchem.8b01857 BindingDB Entry DOI: 10.7270/Q2Q52SVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma intracellular receptor 2 (Rattus norvegicus (Rat)) | BDBM330349 (US9724435, Compound CM-366) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
THE UNIVERSITY OF MISSISSIPPI; THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIVERSITY US Patent | Assay Description Compounds were evaluated for σ-1 and σ-2 binding in rat brain homogenates. Twelve concentrations of each test ligand (0.001-1,000 nM) were ... | US Patent US9724435 (2017) BindingDB Entry DOI: 10.7270/Q2BZ6856 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14127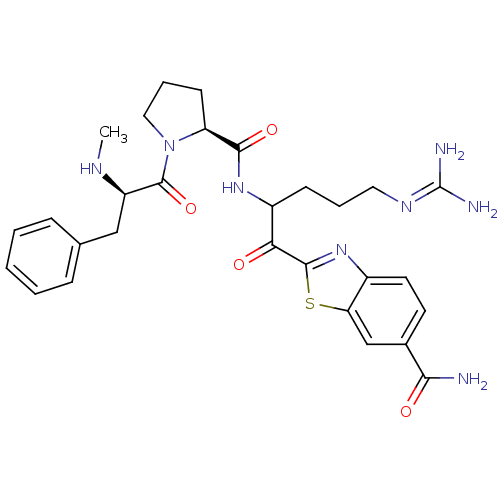 (2-(5-carbamimidamido-2-{[(2S)-1-[(2R)-2-(methylami...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.00700 | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM50505051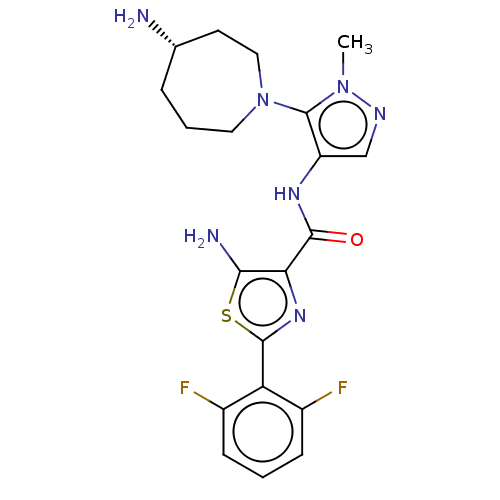 (CHEMBL4437940) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of recombinant human PIM3 using FAM-pimtide as substrate after 90 mins by Z-LYTE assay | J Med Chem 62: 2140-2153 (2019) Article DOI: 10.1021/acs.jmedchem.8b01857 BindingDB Entry DOI: 10.7270/Q2Q52SVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50505059 (CHEMBL4459538) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of recombinant human PIM1 using FAM-pimtide as substrate after 90 mins by Z-LYTE assay | J Med Chem 62: 2140-2153 (2019) Article DOI: 10.1021/acs.jmedchem.8b01857 BindingDB Entry DOI: 10.7270/Q2Q52SVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50096101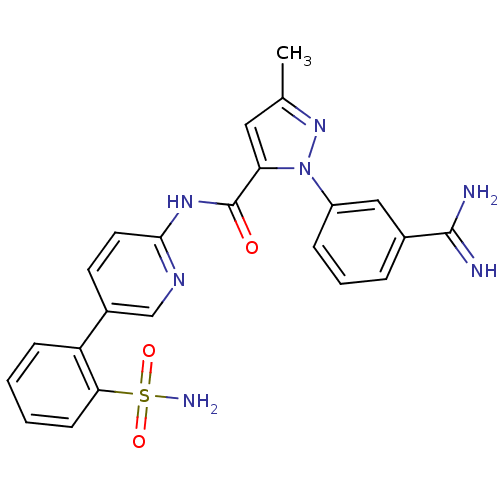 (2-(3-Carbamimidoyl-phenyl)-5-methyl-2H-pyrazole-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity against human coagulation factor X | J Med Chem 44: 566-78 (2001) BindingDB Entry DOI: 10.7270/Q2RF5VQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50156455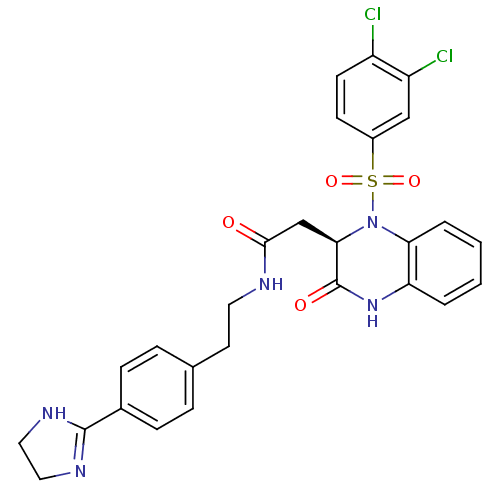 ((R)-N-(4-(4,5-dihydro-1H-imidazol-2-yl)phenethyl)-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human BK1 receptor E273 mutant | Bioorg Med Chem Lett 16: 2791-5 (2006) Article DOI: 10.1016/j.bmcl.2006.01.112 BindingDB Entry DOI: 10.7270/Q28915GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50096091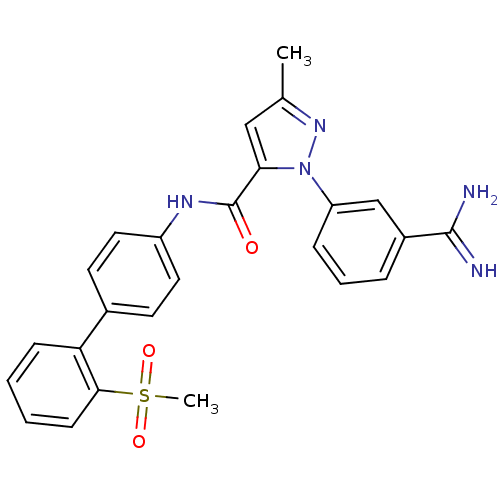 (2-(3-Carbamimidoyl-phenyl)-5-methyl-2H-pyrazole-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity against human coagulation factor X | J Med Chem 44: 566-78 (2001) BindingDB Entry DOI: 10.7270/Q2RF5VQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50096110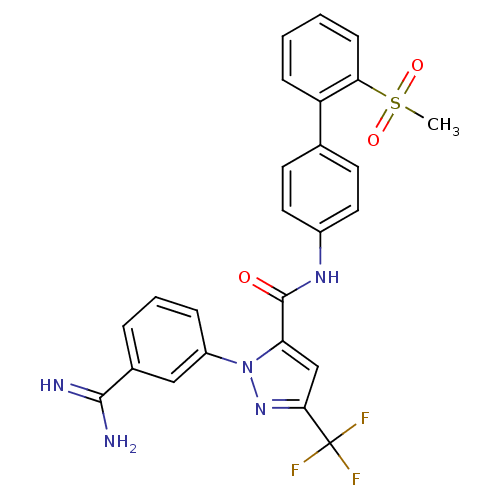 (2-(3-Carbamimidoyl-phenyl)-5-trifluoromethyl-2H-py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity against human coagulation factor X | J Med Chem 44: 566-78 (2001) BindingDB Entry DOI: 10.7270/Q2RF5VQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50096085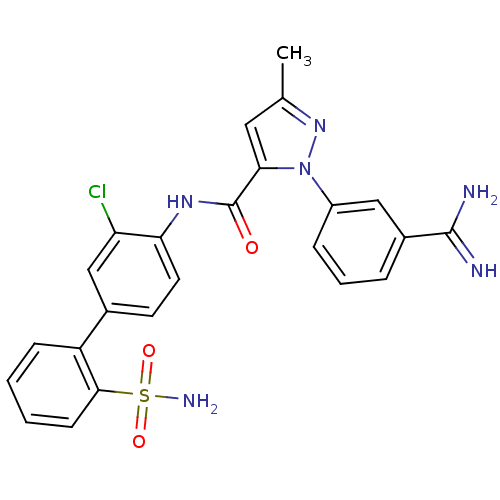 (2-(3-Carbamimidoyl-phenyl)-5-methyl-2H-pyrazole-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity against human coagulation factor X | J Med Chem 44: 566-78 (2001) BindingDB Entry DOI: 10.7270/Q2RF5VQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50096108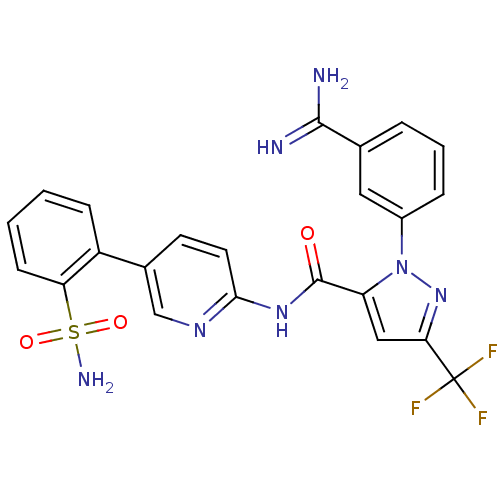 (2-(3-Carbamimidoyl-phenyl)-5-trifluoromethyl-2H-py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity against human coagulation factor X | J Med Chem 44: 566-78 (2001) BindingDB Entry DOI: 10.7270/Q2RF5VQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM50505052 (CHEMBL3623150) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of recombinant human PIM3 using FAM-pimtide as substrate after 90 mins by Z-LYTE assay | J Med Chem 62: 2140-2153 (2019) Article DOI: 10.1021/acs.jmedchem.8b01857 BindingDB Entry DOI: 10.7270/Q2Q52SVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM110700 (US8614206, 120 | US8614206, 125 | US8614206, 400) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of recombinant human PIM3 using FAM-pimtide as substrate after 90 mins by Z-LYTE assay | J Med Chem 62: 2140-2153 (2019) Article DOI: 10.1021/acs.jmedchem.8b01857 BindingDB Entry DOI: 10.7270/Q2Q52SVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19770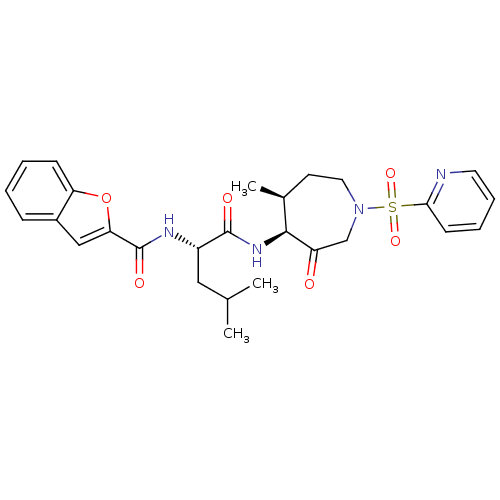 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00990 | -62.2 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082842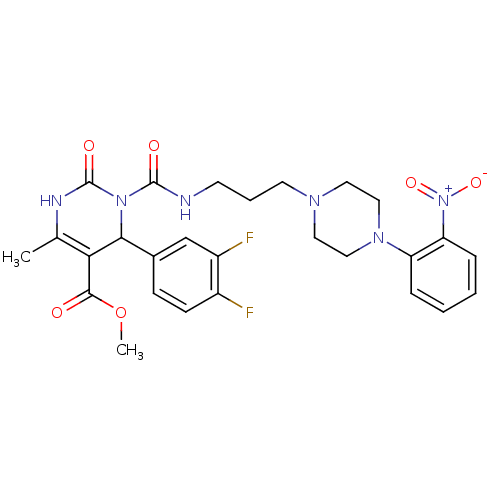 (4-(3,4-Difluoro-phenyl)-6-methyl-3-{3-[4-(2-nitro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against Alpha-1A adrenergic receptor of human liver microsomes. | J Med Chem 42: 4794-803 (1999) BindingDB Entry DOI: 10.7270/Q25B01P0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM36647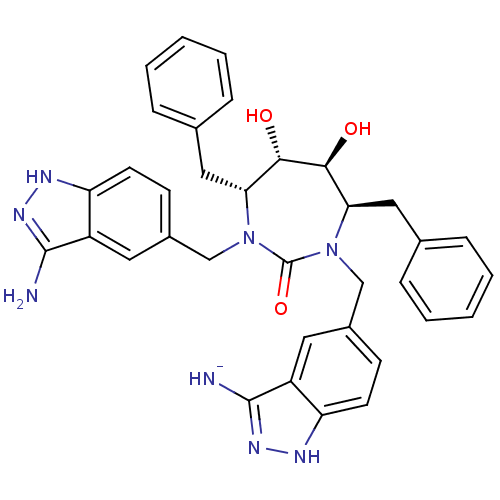 (3-Aminoindazole, 2) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
DuPont Merck Pharmaceutical Company | Assay Description Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease. | Chem Biol 5: 597-608 (1998) Article DOI: 10.1016/s1074-5521(98)90117-x BindingDB Entry DOI: 10.7270/Q2R78CK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50096098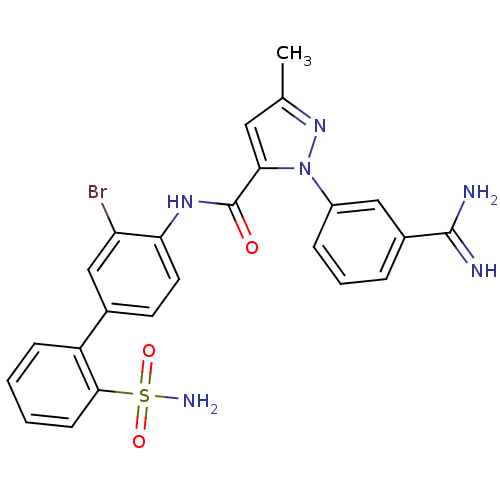 (2-(3-Carbamimidoyl-phenyl)-5-methyl-2H-pyrazole-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity against human coagulation factor X | J Med Chem 44: 566-78 (2001) BindingDB Entry DOI: 10.7270/Q2RF5VQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50214385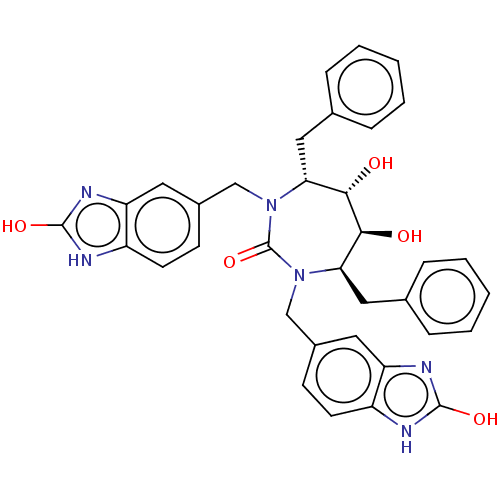 (CHEMBL316681) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of S. cerevisiae glyoxalase-I by using enzymatic assay at each of 6 substrate concentrations between 0.1 mM and... | Bioorg Med Chem Lett 8: 715-20 (1999) BindingDB Entry DOI: 10.7270/Q2FQ9VRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50214385 (CHEMBL316681) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity was evaluated against HIV protease | Bioorg Med Chem Lett 6: 2919-2924 (1996) Article DOI: 10.1016/S0960-894X(96)00531-8 BindingDB Entry DOI: 10.7270/Q2QF8SVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073223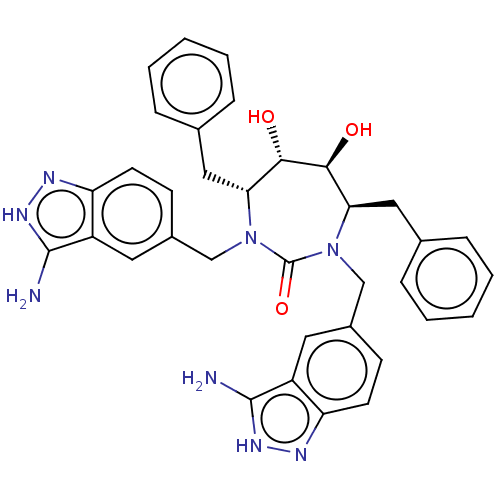 (CHEMBL73240) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description The compound was evaluated for inhibition of HIV protease | Bioorg Med Chem Lett 8: 715-20 (1999) BindingDB Entry DOI: 10.7270/Q2FQ9VRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM36648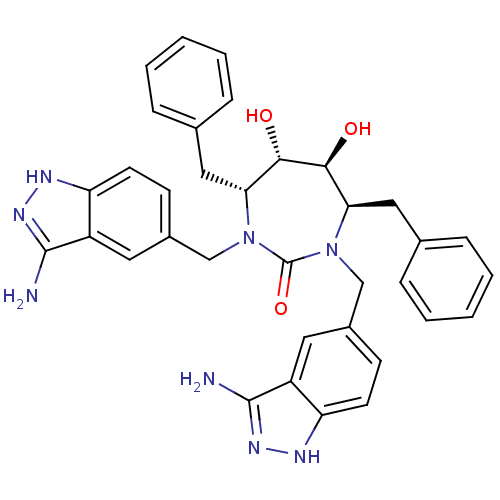 (3-alkylaminoindazole cyclic urea, (H)) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
DuPont Merck Pharmaceutical Company | Assay Description Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease. | Chem Biol 5: 597-608 (1998) Article DOI: 10.1016/s1074-5521(98)90117-x BindingDB Entry DOI: 10.7270/Q2R78CK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM50162774 (ABT-199 | US11420968, Example ABT-199 | Venetoclax) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | Article PubMed | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of BCL2 (unknown origin) by TR-FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00242 BindingDB Entry DOI: 10.7270/Q2KD22HB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599] (Human immunodeficiency virus type 1) | BDBM177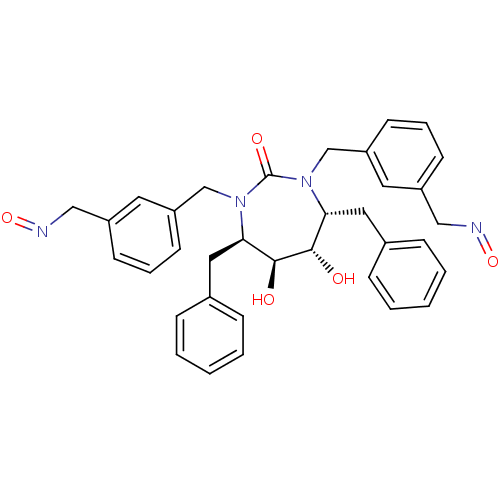 ((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis({...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | -65.3 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
DuPont Merck Pharmaceutical Company | Assay Description Ki values were determined with recombinant single-chain dimeric HIV protease and a fluorescent substrate. The use of single-chain dimeric protease a... | J Med Chem 39: 3514-25 (1996) Article DOI: 10.1021/jm9602571 BindingDB Entry DOI: 10.7270/Q2ZW1J3M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50505050 (CHEMBL4439756) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of recombinant human PIM1 using FAM-pimtide as substrate after 90 mins by Z-LYTE assay | J Med Chem 62: 2140-2153 (2019) Article DOI: 10.1021/acs.jmedchem.8b01857 BindingDB Entry DOI: 10.7270/Q2Q52SVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM50505053 (CHEMBL4469964) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of recombinant human PIM3 using FAM-pimtide as substrate after 90 mins by Z-LYTE assay | J Med Chem 62: 2140-2153 (2019) Article DOI: 10.1021/acs.jmedchem.8b01857 BindingDB Entry DOI: 10.7270/Q2Q52SVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50096906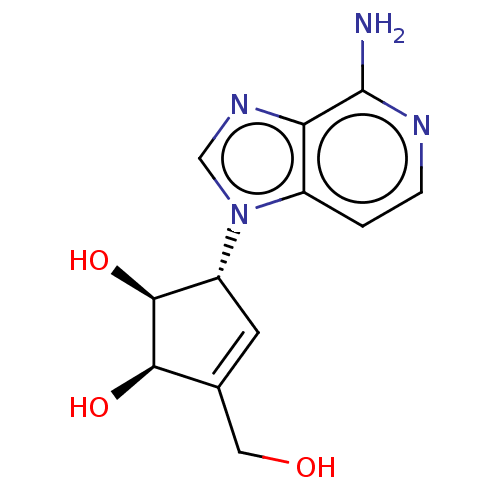 (CHEMBL154745 | US10227373, Compound D-3-Deazaisone...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00047 BindingDB Entry DOI: 10.7270/Q27D3059 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM50505050 (CHEMBL4439756) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of recombinant human PIM3 using FAM-pimtide as substrate after 90 mins by Z-LYTE assay | J Med Chem 62: 2140-2153 (2019) Article DOI: 10.1021/acs.jmedchem.8b01857 BindingDB Entry DOI: 10.7270/Q2Q52SVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM110961 (US8614206, 518) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of recombinant human PIM3 using FAM-pimtide as substrate after 90 mins by Z-LYTE assay | J Med Chem 62: 2140-2153 (2019) Article DOI: 10.1021/acs.jmedchem.8b01857 BindingDB Entry DOI: 10.7270/Q2Q52SVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM50505061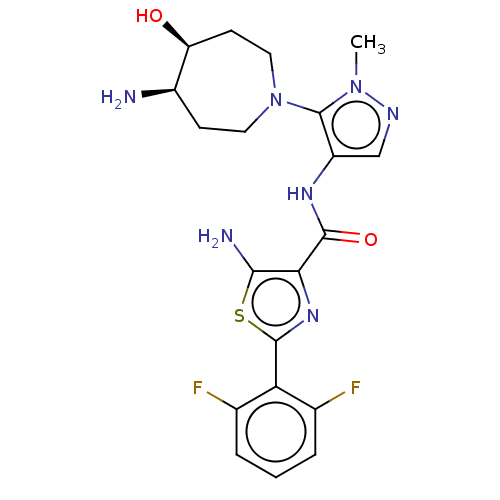 (CHEMBL4453890) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of recombinant human PIM3 using FAM-pimtide as substrate after 90 mins by Z-LYTE assay | J Med Chem 62: 2140-2153 (2019) Article DOI: 10.1021/acs.jmedchem.8b01857 BindingDB Entry DOI: 10.7270/Q2Q52SVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-6 (Homo sapiens (Human)) | BDBM50464108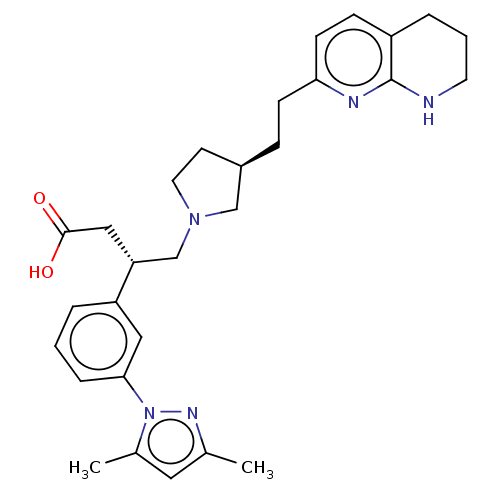 (CHEMBL4241824) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde Curated by ChEMBL | Assay Description Binding affinity to human integrin alphaVbeta6 assessed as dissociation constant up to 48 hrs by liquid scintillation counting | J Med Chem 61: 8417-8443 (2018) Article DOI: 10.1021/acs.jmedchem.8b00959 BindingDB Entry DOI: 10.7270/Q24T6N25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599] (Human immunodeficiency virus type 1) | BDBM162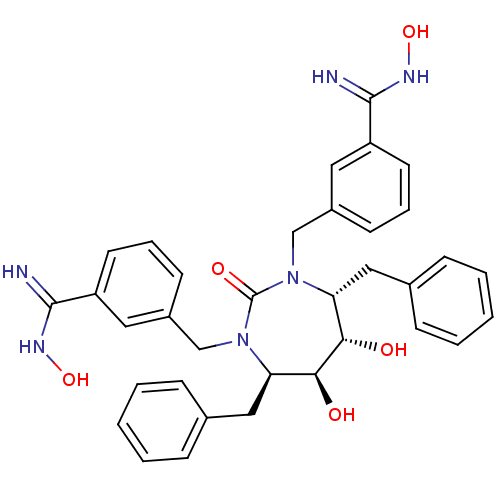 (3-{[(4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-3-{[3...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | -65.3 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
DuPont Merck Pharmaceutical Company | J Med Chem 39: 3514-25 (1996) Article DOI: 10.1021/jm9602571 BindingDB Entry DOI: 10.7270/Q2ZW1J3M | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50505057 (CHEMBL3676285) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of recombinant human PIM1 using FAM-pimtide as substrate after 90 mins by Z-LYTE assay | J Med Chem 62: 2140-2153 (2019) Article DOI: 10.1021/acs.jmedchem.8b01857 BindingDB Entry DOI: 10.7270/Q2Q52SVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM29611 (2-Iodomelatonin | CHEMBL289233 | Melatonin,2-Iodo) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development, Ltd. Curated by PDSP Ki Database | J Pharmacol Exp Ther 285: 1239-45 (1998) BindingDB Entry DOI: 10.7270/Q2PK0DPM | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM177 ((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis({...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of HIV protease, measured by assaying the cleavage of a fluorescent peptide substrate using HPLC | J Med Chem 41: 2019-28 (1998) Article DOI: 10.1021/jm9704199 BindingDB Entry DOI: 10.7270/Q29K49B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12751 (1-(3-carbamimidoylphenyl)-3-methyl-N-[4-(2-sulfamo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0130 | -61.5 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were determined ... | J Med Chem 46: 4405-18 (2003) Article DOI: 10.1021/jm020578e BindingDB Entry DOI: 10.7270/Q2TT4P78 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM591137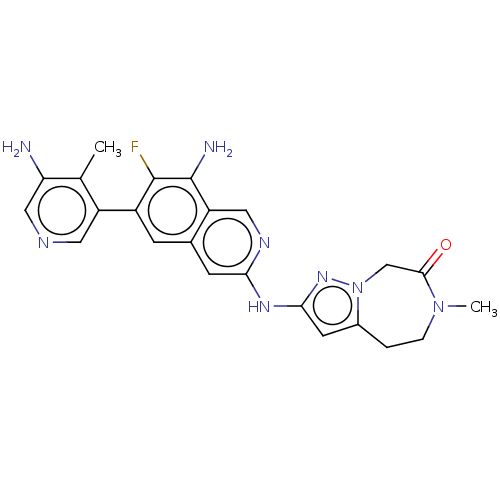 (2-((8-amino-6-(5-amino-4- methylpyridin-3-yl)-7- f...) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To a 384 well Proxiplate with 80 nL compound or DMSO spotted on was added 4 μl/well kinase mix. The mixture was preincubated for 30 minutes and ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GF0ZFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM591155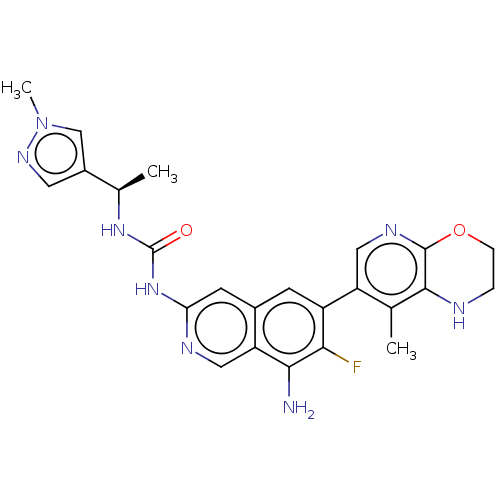 (US11566003, Compound 111) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To a 384 well Proxiplate with 80 nL compound or DMSO spotted on was added 4 μl/well kinase mix. The mixture was preincubated for 30 minutes and ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GF0ZFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM591146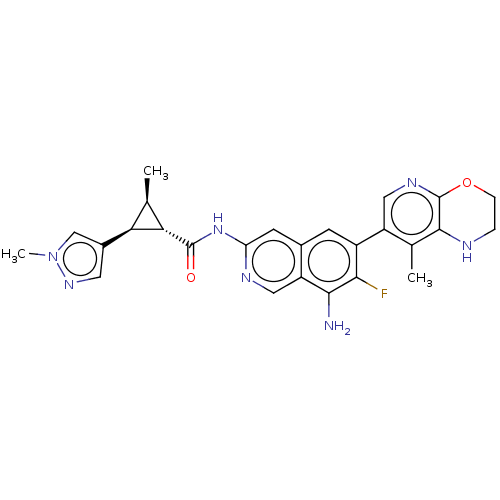 (US11566003, Compound 35) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To a 384 well Proxiplate with 80 nL compound or DMSO spotted on was added 4 μl/well kinase mix. The mixture was preincubated for 30 minutes and ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GF0ZFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 185822 total ) | Next | Last >> |