

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Proprotein convertase subtilisin/kexin type 9 (Homo sapiens (Human)) | BDBM50581548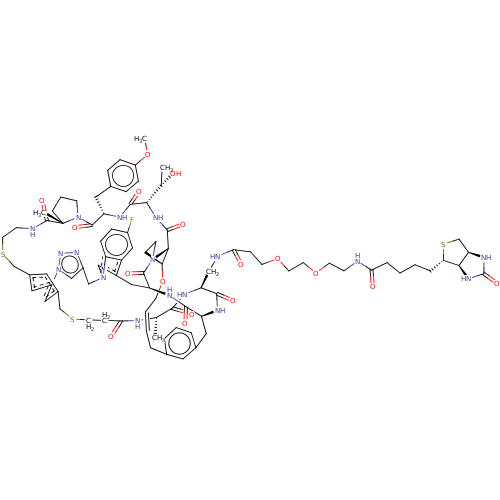 (CHEMBL5085124) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.000600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET ultra assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01599 BindingDB Entry DOI: 10.7270/Q2TM7G0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 9 (Homo sapiens (Human)) | BDBM50581547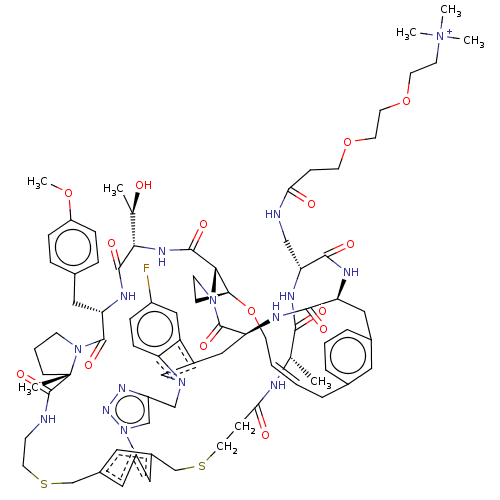 (CHEMBL5081349) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.000930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET ultra assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01599 BindingDB Entry DOI: 10.7270/Q2TM7G0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50147818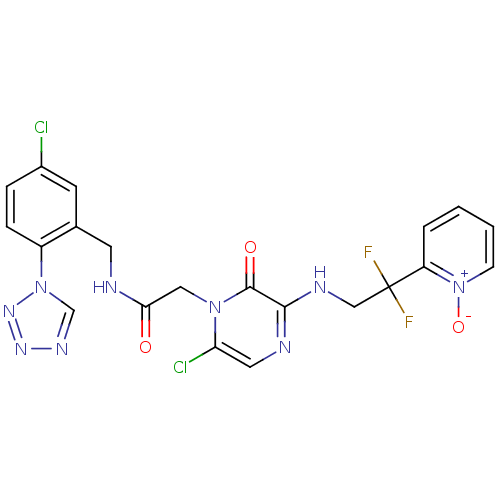 ((2-[6-CHLORO-3-{[2,2-DIFLUORO-2-(1-OXIDOPYRIDIN-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.00140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hefei University of Technology Curated by ChEMBL | Assay Description Binding affinity to thrombin (unknown origin) | Eur J Med Chem 146: 299-317 (2018) Article DOI: 10.1016/j.ejmech.2018.01.067 BindingDB Entry DOI: 10.7270/Q2251MTJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50147824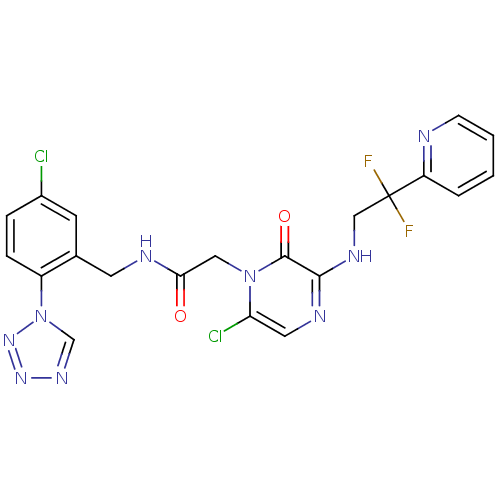 (2-[6-Chloro-3-(2,2-difluoro-2-pyridin-2-yl-ethylam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hefei University of Technology Curated by ChEMBL | Assay Description Binding affinity to thrombin (unknown origin) | Eur J Med Chem 146: 299-317 (2018) Article DOI: 10.1016/j.ejmech.2018.01.067 BindingDB Entry DOI: 10.7270/Q2251MTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50147818 ((2-[6-CHLORO-3-{[2,2-DIFLUORO-2-(1-OXIDOPYRIDIN-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.00140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hefei University of Technology Curated by ChEMBL | Assay Description Inhibition of thrombin (unknown origin) | Eur J Med Chem 146: 299-317 (2018) Article DOI: 10.1016/j.ejmech.2018.01.067 BindingDB Entry DOI: 10.7270/Q2251MTJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50147824 (2-[6-Chloro-3-(2,2-difluoro-2-pyridin-2-yl-ethylam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hefei University of Technology Curated by ChEMBL | Assay Description Inhibition of thrombin (unknown origin) | Eur J Med Chem 146: 299-317 (2018) Article DOI: 10.1016/j.ejmech.2018.01.067 BindingDB Entry DOI: 10.7270/Q2251MTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor 3 (RAT) | BDBM50166288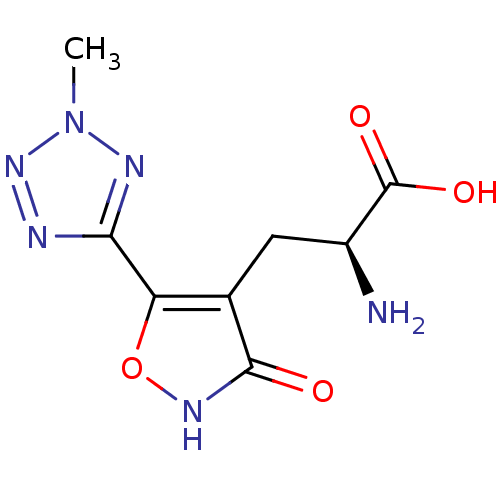 ((S)-2-Amino-3-[3-hydroxy-5-(2-methyl-2H-tetrazol-5...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | Article PubMed | 0.00220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Displacement of [3H]AMPA from rat recombinant GluR3 expressed in Sf9 cells | J Med Chem 50: 2408-14 (2007) Article DOI: 10.1021/jm061439q BindingDB Entry DOI: 10.7270/Q2DZ0809 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 9 (Homo sapiens (Human)) | BDBM50581546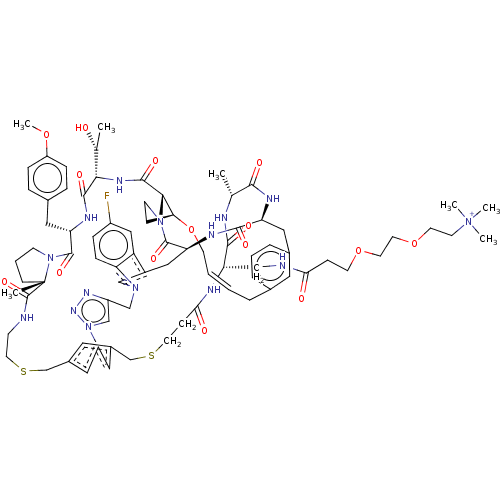 (CHEMBL5084416) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.00239 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET ultra assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01599 BindingDB Entry DOI: 10.7270/Q2TM7G0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor 4 (Rattus norvegicus) | BDBM50166288 ((S)-2-Amino-3-[3-hydroxy-5-(2-methyl-2H-tetrazol-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | MMDB Article PubMed | 0.00270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Displacement of [3H]AMPA from rat recombinant GluR4 expressed in Sf9 cells | J Med Chem 50: 2408-14 (2007) Article DOI: 10.1021/jm061439q BindingDB Entry DOI: 10.7270/Q2DZ0809 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50111101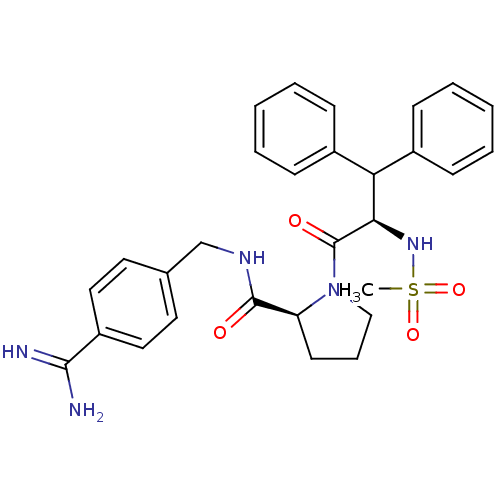 ((S)-1-((R)-2-Methanesulfonylamino-3,3-diphenyl-pro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50111110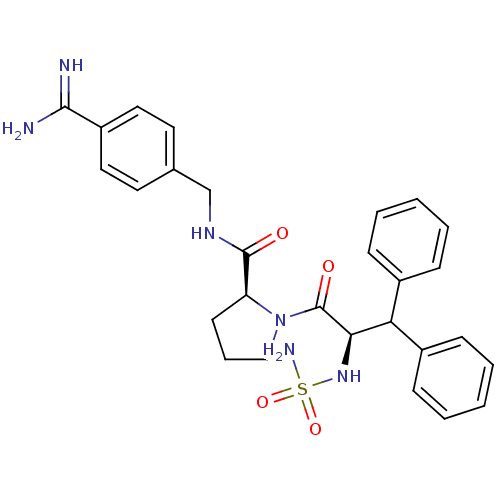 (2N-(4-Benzamidinemethyl)-1-[2-aminosulfonamido-3,3...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor 2 (Rattus norvegicus) | BDBM50166288 ((S)-2-Amino-3-[3-hydroxy-5-(2-methyl-2H-tetrazol-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | MMDB Article PubMed | 0.00390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Displacement of [3H]AMPA from rat recombinant GluR2 expressed in Sf9 cells | J Med Chem 50: 2408-14 (2007) Article DOI: 10.1021/jm061439q BindingDB Entry DOI: 10.7270/Q2DZ0809 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50131789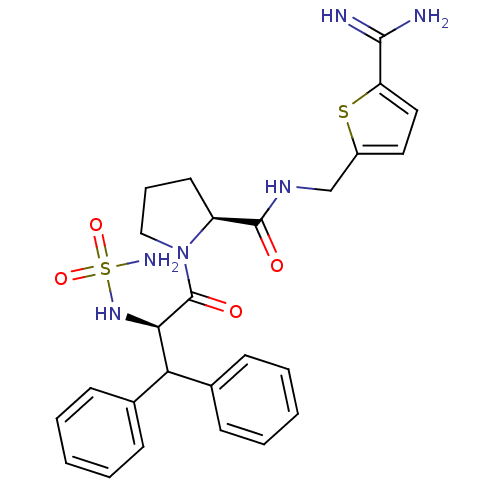 (1-(2-sulfonamide-amino-3,3-diphenyl-propionyl)-pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50111105 ((S)-1-((R)-2-Methanesulfonylamino-3,3-diphenyl-pro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor 1 (Rattus norvegicus (Rat)) | BDBM50166288 ((S)-2-Amino-3-[3-hydroxy-5-(2-methyl-2H-tetrazol-5...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | Article PubMed | 0.00520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Displacement of [3H]AMPA from rat recombinant GluR1 expressed in Sf9 cells | J Med Chem 50: 2408-14 (2007) Article DOI: 10.1021/jm061439q BindingDB Entry DOI: 10.7270/Q2DZ0809 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM554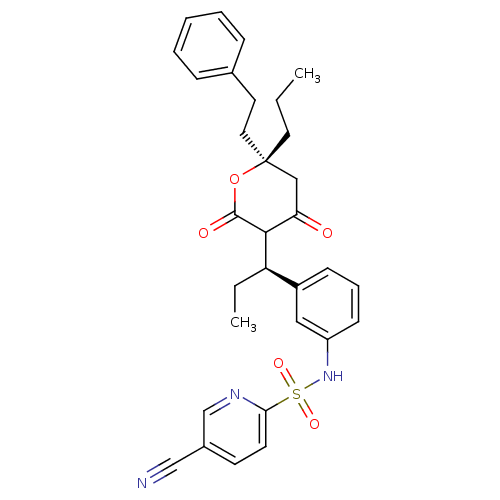 (5-cyano-N-{3-[(1R)-1-[(6R)-4-hydroxy-2-oxo-6-(2-ph...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.00700 | -63.0 | n/a | n/a | n/a | n/a | n/a | 5.0 | 22 |
Upjohn | Assay Description HIV-1 protease was purified and refolded from E. coli inclusion bodies. The substrate used spans the p17-p24 processing site (R-V-S-Q-N-Y-P-I-V-Q-N-K... | J Med Chem 39: 4349-53 (1996) Article DOI: 10.1021/jm960541s BindingDB Entry DOI: 10.7270/Q2HQ3X35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1501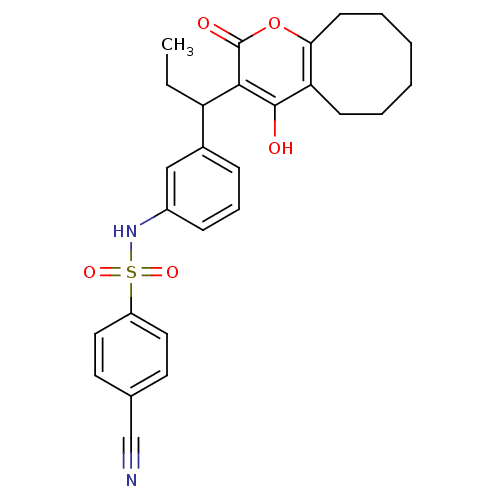 (4-Cyano-N-[3-[1-(5,6,7,8,9,10-hexahydro-4-hydroxy-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia and Upjohn Curated by ChEMBL | Assay Description Binding affinity of the compound towards HIV protease was determined | J Med Chem 39: 4125-30 (1996) Article DOI: 10.1021/jm960296c BindingDB Entry DOI: 10.7270/Q2KH0MFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 9 (Homo sapiens (Human)) | BDBM50581547 (CHEMBL5081349) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00736 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET plus assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01599 BindingDB Entry DOI: 10.7270/Q2TM7G0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50156455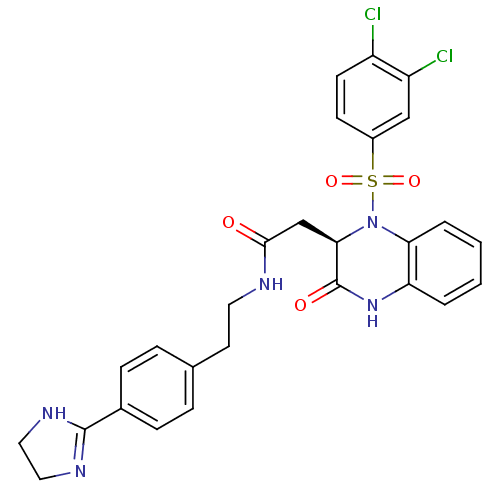 ((R)-N-(4-(4,5-dihydro-1H-imidazol-2-yl)phenethyl)-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human BK1 receptor E273 mutant | Bioorg Med Chem Lett 16: 2791-5 (2006) Article DOI: 10.1016/j.bmcl.2006.01.112 BindingDB Entry DOI: 10.7270/Q28915GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM558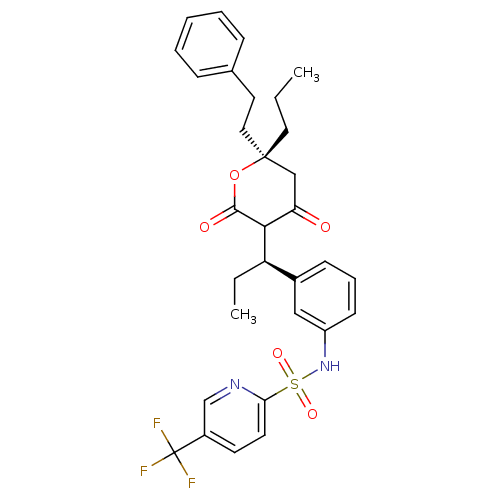 (N-{3-[(1R)-1-[(6R)-4-hydroxy-2-oxo-6-(2-phenylethy...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 0.00800 | -62.7 | n/a | n/a | n/a | n/a | n/a | 5.0 | 22 |
Upjohn | Assay Description HIV-1 protease was purified and refolded from E. coli inclusion bodies. The substrate used spans the p17-p24 processing site (R-V-S-Q-N-Y-P-I-V-Q-N-K... | J Med Chem 39: 4349-53 (1996) Article DOI: 10.1021/jm960541s BindingDB Entry DOI: 10.7270/Q2HQ3X35 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50131790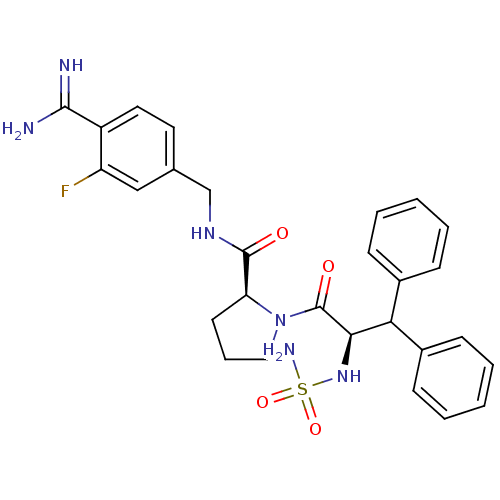 (1-(2-sulfonamideamino-3,3-diphenyl-propionyl)-pyrr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 9 (Homo sapiens (Human)) | BDBM50581545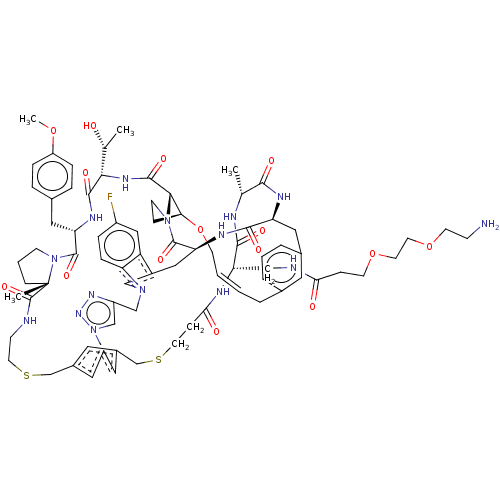 (CHEMBL5084902) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00813 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET ultra assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01599 BindingDB Entry DOI: 10.7270/Q2TM7G0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 9 (Homo sapiens (Human)) | BDBM50581544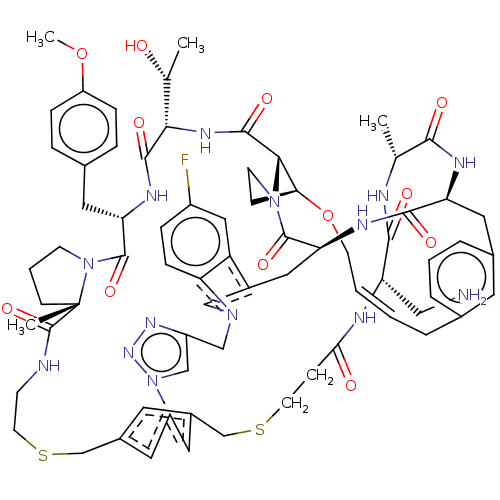 (CHEMBL5086475) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00826 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET plus assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01599 BindingDB Entry DOI: 10.7270/Q2TM7G0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 9 (Homo sapiens (Human)) | BDBM50581544 (CHEMBL5086475) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET ultra assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01599 BindingDB Entry DOI: 10.7270/Q2TM7G0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM85357 (2-[[(2R,3S)-2-[[4-[(2-Oxo-2,3-dihydro-1H-benzimida...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by PDSP Ki Database | Proc Natl Acad Sci U S A 95: 10836-41 (1998) Article DOI: 10.1073/pnas.95.18.10836 BindingDB Entry DOI: 10.7270/Q2XW4HCM | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 9 (Homo sapiens (Human)) | BDBM50581548 (CHEMBL5085124) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET plus assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01599 BindingDB Entry DOI: 10.7270/Q2TM7G0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50457933 (CHEMBL327265) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hefei University of Technology Curated by ChEMBL | Assay Description Inhibition of thrombin (unknown origin) | Eur J Med Chem 146: 299-317 (2018) Article DOI: 10.1016/j.ejmech.2018.01.067 BindingDB Entry DOI: 10.7270/Q2251MTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50457929 (CHEMBL104951) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hefei University of Technology Curated by ChEMBL | Assay Description Inhibition of thrombin (unknown origin) | Eur J Med Chem 146: 299-317 (2018) Article DOI: 10.1016/j.ejmech.2018.01.067 BindingDB Entry DOI: 10.7270/Q2251MTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50457933 (CHEMBL327265) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hefei University of Technology Curated by ChEMBL | Assay Description Binding affinity to thrombin (unknown origin) | Eur J Med Chem 146: 299-317 (2018) Article DOI: 10.1016/j.ejmech.2018.01.067 BindingDB Entry DOI: 10.7270/Q2251MTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50457929 (CHEMBL104951) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hefei University of Technology Curated by ChEMBL | Assay Description Binding affinity to thrombin (unknown origin) | Eur J Med Chem 146: 299-317 (2018) Article DOI: 10.1016/j.ejmech.2018.01.067 BindingDB Entry DOI: 10.7270/Q2251MTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50131795 (1-(2-sulfonamideamino-3,3-diphenyl-propionyl)-pyrr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50131778 (3-(1-Benzhydryl-2-{2-[(5-carbamimidoyl-thiophen-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic receptor M1 (Bos taurus) | BDBM50055976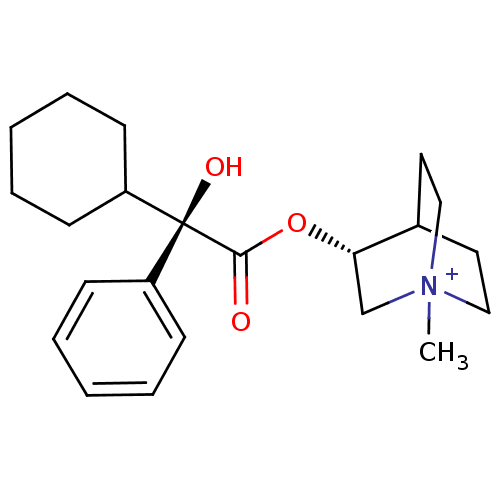 ((R)-3-((R)-2-Cyclohexyl-2-hydroxy-2-phenyl-acetoxy...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Groningen University Hospital Curated by ChEMBL | Assay Description Binding affinity towards Muscarinic acetylcholine receptor M1 was determined in calf brain membrane | J Med Chem 40: 117-24 (1997) Article DOI: 10.1021/jm960374w BindingDB Entry DOI: 10.7270/Q2VH5PG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50055978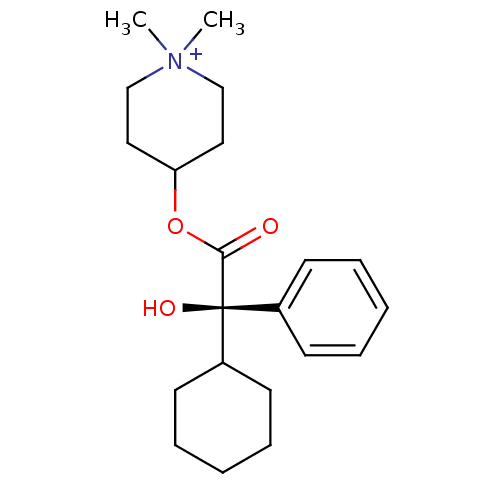 (4-((R)-2-Cyclohexyl-2-hydroxy-2-phenyl-acetoxy)-1,...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Groningen University Hospital Curated by ChEMBL | Assay Description Binding affinity towards rat Muscarinic acetylcholine receptor M1 was determined | J Med Chem 40: 117-24 (1997) Article DOI: 10.1021/jm960374w BindingDB Entry DOI: 10.7270/Q2VH5PG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50337878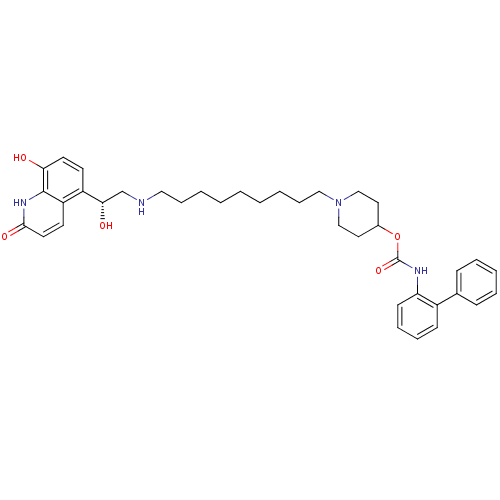 ((R)-1-(9-(2-hydroxy-2-(8-hydroxy-2-oxo-1,2-dihydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-N-methyl-scopolamine from human muscarinic M3 receptor after 6 hrs by cell based assay | Bioorg Med Chem Lett 21: 1354-8 (2011) Checked by Author Article DOI: 10.1016/j.bmcl.2011.01.043 BindingDB Entry DOI: 10.7270/Q2NC61HQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082842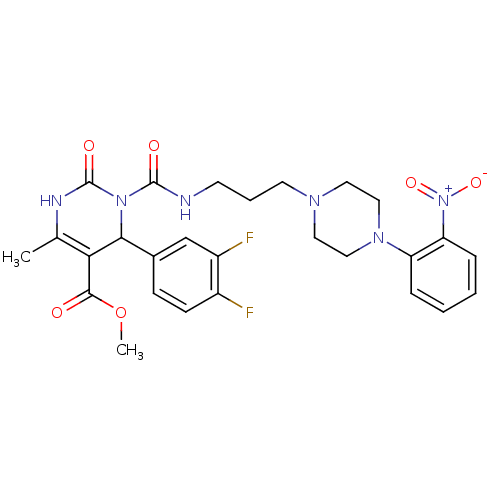 (4-(3,4-Difluoro-phenyl)-6-methyl-3-{3-[4-(2-nitro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against Alpha-1A adrenergic receptor of human liver microsomes. | J Med Chem 42: 4794-803 (1999) BindingDB Entry DOI: 10.7270/Q25B01P0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 9 (Homo sapiens (Human)) | BDBM50581546 (CHEMBL5084416) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.0114 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET plus assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01599 BindingDB Entry DOI: 10.7270/Q2TM7G0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50111120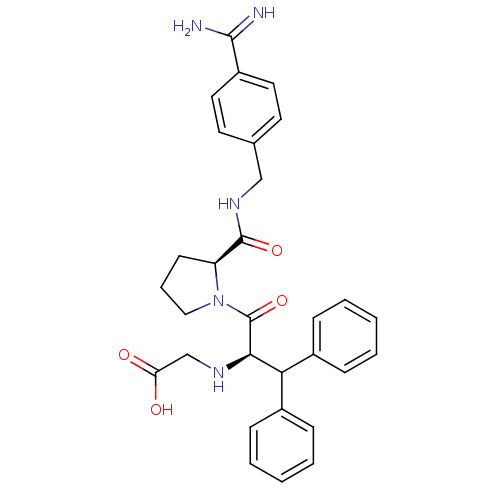 (2N-(4-Benzamidinemethyl)-1-[2-Aminoaceticacid-3,3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM591137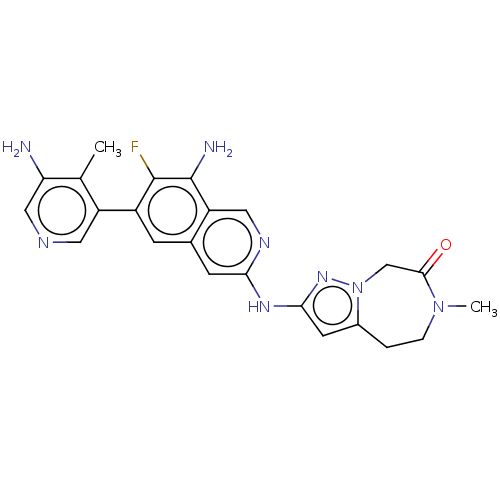 (2-((8-amino-6-(5-amino-4- methylpyridin-3-yl)-7- f...) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To a 384 well Proxiplate with 80 nL compound or DMSO spotted on was added 4 μl/well kinase mix. The mixture was preincubated for 30 minutes and ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GF0ZFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM591137 (2-((8-amino-6-(5-amino-4- methylpyridin-3-yl)-7- f...) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For the binding assay, 4 ul 2×HPK1 and Eu-anti-GST antibody were added to each well of the assay plate using a Multidrop reagent dispenser. The solut... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GF0ZFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM591244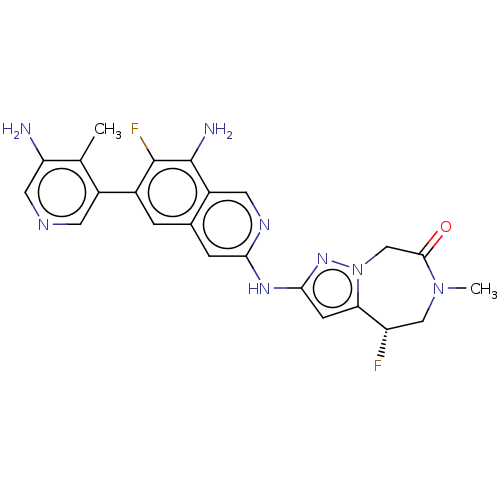 ((S)-2-((8-amino-6-(5-amino-4- methylpyridin-3-yl)-...) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To a 384 well Proxiplate with 80 nL compound or DMSO spotted on was added 4 μl/well kinase mix. The mixture was preincubated for 30 minutes and ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GF0ZFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM591242 (2-((8-amino-6-(5-amino-4- methylpyridin-3-yl)-7-fl...) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To a 384 well Proxiplate with 80 nL compound or DMSO spotted on was added 4 μl/well kinase mix. The mixture was preincubated for 30 minutes and ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GF0ZFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM591240 (2-((8-amino-6-(5-amino-4- chloropyridin-3-yl)-7-fl...) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To a 384 well Proxiplate with 80 nL compound or DMSO spotted on was added 4 μl/well kinase mix. The mixture was preincubated for 30 minutes and ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GF0ZFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM591225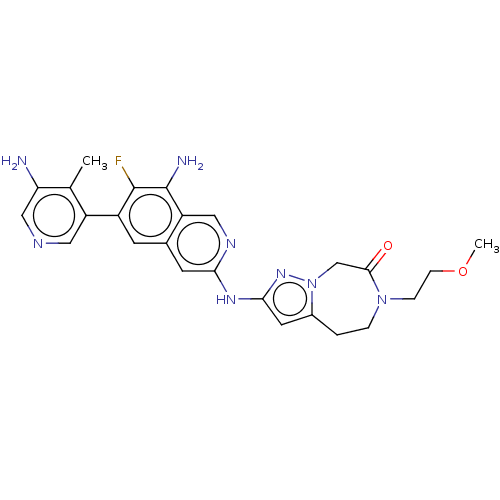 (2-((8-amino-6-(5-amino-4- methylpyridin-3-yl)-7-fl...) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To a 384 well Proxiplate with 80 nL compound or DMSO spotted on was added 4 μl/well kinase mix. The mixture was preincubated for 30 minutes and ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GF0ZFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM591222 (2-((8-amino-6-(5-amino-4- methylpyridin-3-yl)-7-fl...) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To a 384 well Proxiplate with 80 nL compound or DMSO spotted on was added 4 μl/well kinase mix. The mixture was preincubated for 30 minutes and ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GF0ZFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM591220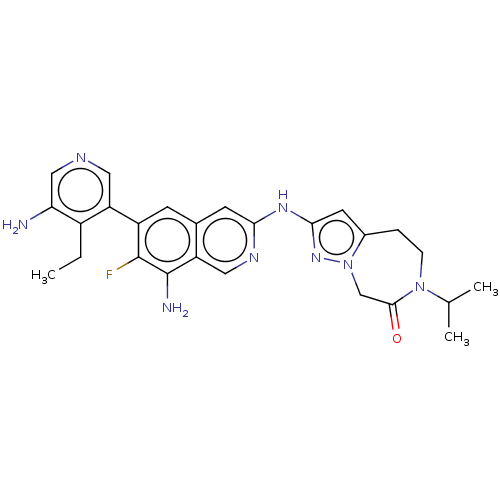 (2-((8-amino-6-(5-amino-4-ethylpyridin- 3-yl)-7-flu...) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To a 384 well Proxiplate with 80 nL compound or DMSO spotted on was added 4 μl/well kinase mix. The mixture was preincubated for 30 minutes and ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GF0ZFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM591217 (2-((8-amino-6-(5-amino-4- methylpyridin-3-yl)-7-fl...) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To a 384 well Proxiplate with 80 nL compound or DMSO spotted on was added 4 μl/well kinase mix. The mixture was preincubated for 30 minutes and ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GF0ZFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM591211 (2-((8-amino-6-(5-amino-4- methylpyridin-3-yl)-7-fl...) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To a 384 well Proxiplate with 80 nL compound or DMSO spotted on was added 4 μl/well kinase mix. The mixture was preincubated for 30 minutes and ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GF0ZFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM591252 (2-((8-amino-7-fluoro-6-(4-methyl-6- (oxazol-2-yl)p...) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To a 384 well Proxiplate with 80 nL compound or DMSO spotted on was added 4 μl/well kinase mix. The mixture was preincubated for 30 minutes and ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GF0ZFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM591250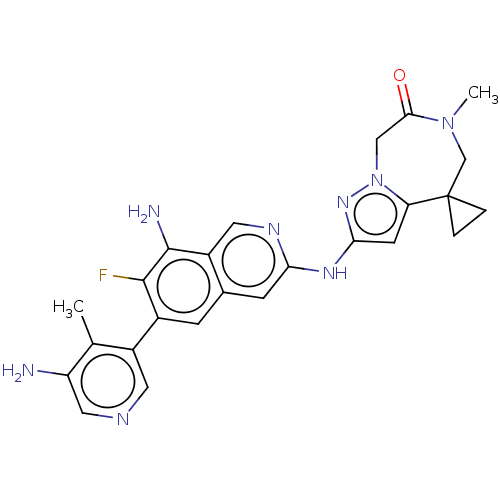 (2'-((8-amino-6-(5-amino-4- methylpyridin-3-yl)-7-f...) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To a 384 well Proxiplate with 80 nL compound or DMSO spotted on was added 4 μl/well kinase mix. The mixture was preincubated for 30 minutes and ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GF0ZFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 102156 total ) | Next | Last >> |