

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM85790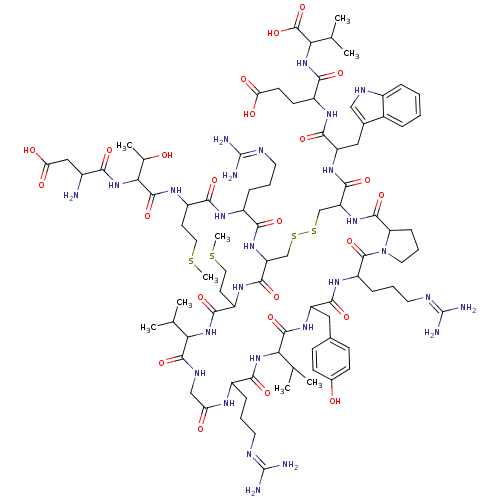 (Salmon MCH) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by PDSP Ki Database | Proc Natl Acad Sci U S A 98: 7564-9 (2001) Article DOI: 10.1073/pnas.121170598 BindingDB Entry DOI: 10.7270/Q2RJ4H14 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50049478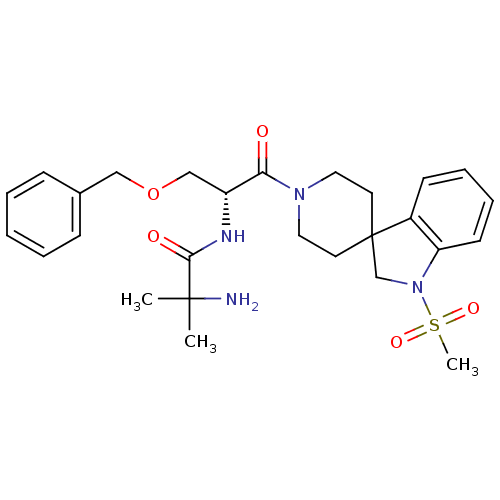 (1-{[(2R)-3-(benzyloxy)-1-{1-methanesulfonyl-1,2-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding Affinity of the compound against Growth hormone secretagogue receptor of swine using [35S]-MK-0677 as radioligand | J Med Chem 39: 1767-70 (1996) Article DOI: 10.1021/jm960054c BindingDB Entry DOI: 10.7270/Q2BK1BDG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM85789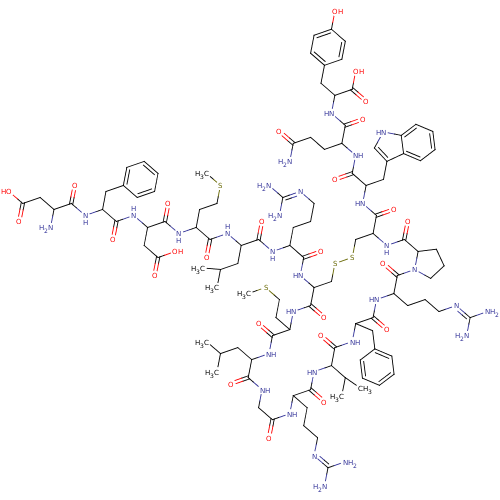 ([Phe13,Tyr19]MCH) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by PDSP Ki Database | Proc Natl Acad Sci U S A 98: 7564-9 (2001) Article DOI: 10.1073/pnas.121170598 BindingDB Entry DOI: 10.7270/Q2RJ4H14 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 2 (Homo sapiens (Human)) | BDBM85789 ([Phe13,Tyr19]MCH) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by PDSP Ki Database | Proc Natl Acad Sci U S A 98: 7564-9 (2001) Article DOI: 10.1073/pnas.121170598 BindingDB Entry DOI: 10.7270/Q2RJ4H14 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50049091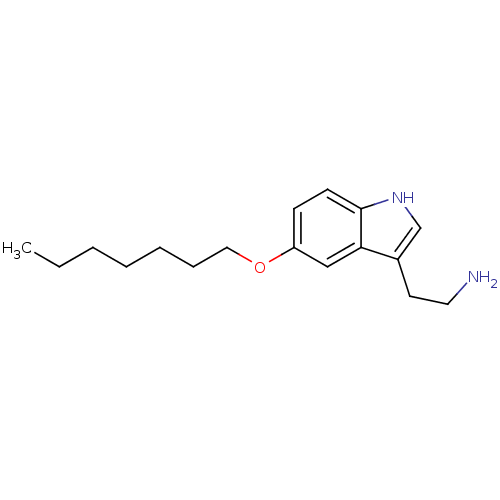 (2-(5-Heptyloxy-1H-indol-3-yl)-ethylamine | CHEMBL3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity for recombinant human 5-hydroxytryptamine 1D receptor beta was determined using [3H]-5-HT as radioligand | J Med Chem 39: 314-22 (1996) Article DOI: 10.1021/jm950498t BindingDB Entry DOI: 10.7270/Q2DJ5DRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50049093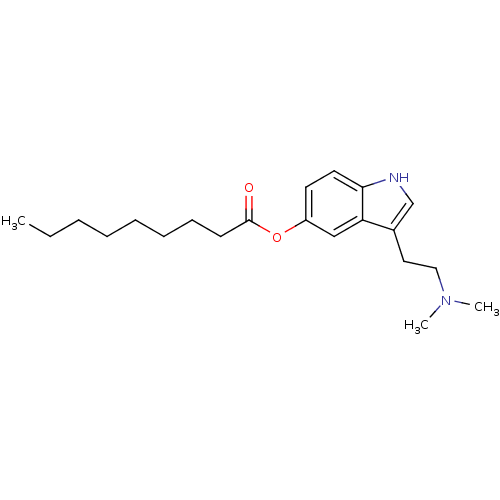 (CHEMBL321190 | Nonanoic acid 3-(2-dimethylamino-et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity for recombinant human 5-hydroxytryptamine 1D receptor beta was determined using [3H]-5-HT as radioligand | J Med Chem 39: 314-22 (1996) Article DOI: 10.1021/jm950498t BindingDB Entry DOI: 10.7270/Q2DJ5DRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM86516 (ETPDCFWKYCV | Human U-II | L-Ala-Gly-L-Thr-L-Ala-L...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd. Curated by PDSP Ki Database | J Pharmacol Exp Ther 311: 204-12 (2004) Article DOI: 10.1124/jpet.104.068320 BindingDB Entry DOI: 10.7270/Q2H993RW | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50039947 (2-(5-Nonyloxy-1H-indol-3-yl)-ethylamine | CHEMBL97...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical College of Virginia/Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity was measured on 5-hydroxytryptamine 1D receptor beta in CHO cells transfected with human 5-HT1D beta gene labeled with [3H]-5-HT | J Med Chem 37: 2828-30 (1994) BindingDB Entry DOI: 10.7270/Q2GH9H0R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50049085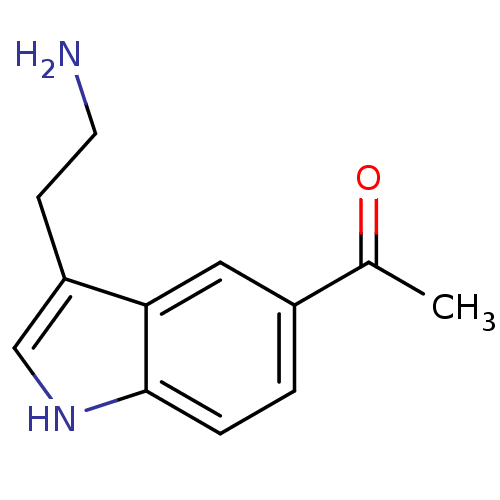 (1-[3-(2-Amino-ethyl)-1H-indol-5-yl]-ethanone | Ace...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description In vitro affinity at human cloned 5-hydroxytryptamine 1A receptor by [3H]-8-OH-DPAT displacement. | J Med Chem 39: 314-22 (1996) Article DOI: 10.1021/jm950498t BindingDB Entry DOI: 10.7270/Q2DJ5DRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50049077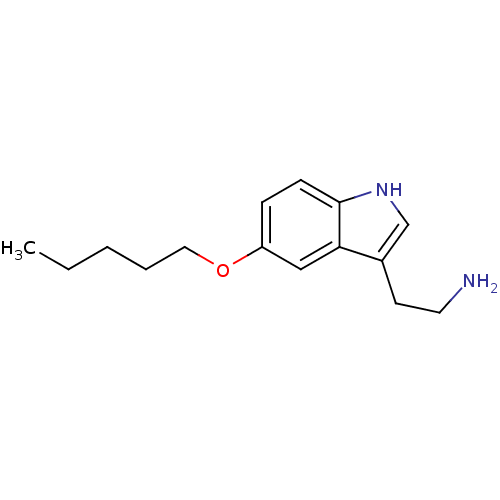 (2-(5-Pentyloxy-1H-indol-3-yl)-ethylamine | CHEMBL1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description In vitro affitnity human cloned 5-hydroxytryptamine 1D receptor alpha by [3H]-5-HT displacement. | J Med Chem 39: 314-22 (1996) Article DOI: 10.1021/jm950498t BindingDB Entry DOI: 10.7270/Q2DJ5DRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 2 (Homo sapiens (Human)) | BDBM85788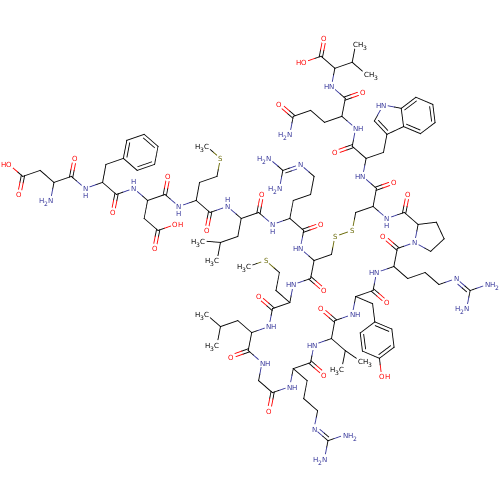 (MCH | hMCH) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by PDSP Ki Database | Proc Natl Acad Sci U S A 98: 7564-9 (2001) Article DOI: 10.1073/pnas.121170598 BindingDB Entry DOI: 10.7270/Q2RJ4H14 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50049077 (2-(5-Pentyloxy-1H-indol-3-yl)-ethylamine | CHEMBL1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity for recombinant human 5-hydroxytryptamine 1D receptor beta was determined using [3H]-5-HT as radioligand | J Med Chem 39: 314-22 (1996) Article DOI: 10.1021/jm950498t BindingDB Entry DOI: 10.7270/Q2DJ5DRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50049082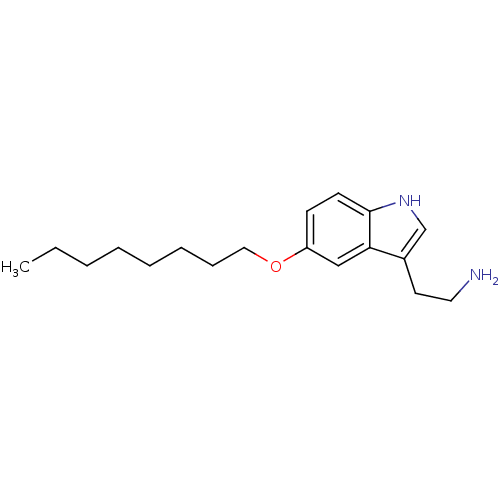 (2-(5-Octyloxy-1H-indol-3-yl)-ethylamine | CHEMBL32...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description In vitro affitnity human cloned 5-hydroxytryptamine 1D receptor alpha by [3H]-5-HT displacement. | J Med Chem 39: 314-22 (1996) Article DOI: 10.1021/jm950498t BindingDB Entry DOI: 10.7270/Q2DJ5DRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM10755 (14C-5-hydroxy tryptamine creatinine disulfate | 2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description In vitro affitnity human cloned 5-hydroxytryptamine 1D receptor alpha by [3H]-5-HT displacement. | J Med Chem 39: 314-22 (1996) Article DOI: 10.1021/jm950498t BindingDB Entry DOI: 10.7270/Q2DJ5DRK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM10755 (14C-5-hydroxy tryptamine creatinine disulfate | 2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | PDB PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical College of Virginia/Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity was measured on 5-hydroxytryptamine 1A receptor in AK cells transfected with human 5-HT1A gene labeled with [3H]-8-OH-DPAT | J Med Chem 37: 2828-30 (1994) BindingDB Entry DOI: 10.7270/Q2GH9H0R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50049081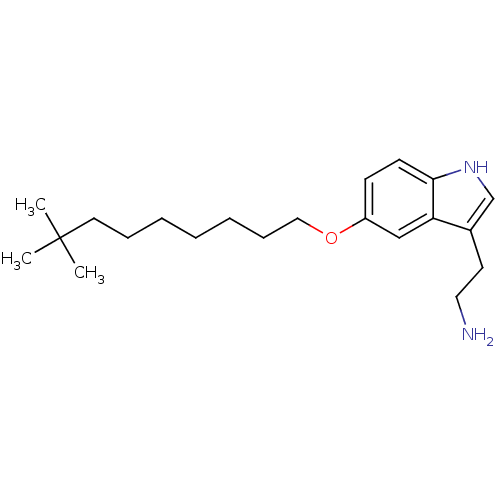 (2-[5-(8,8-Dimethyl-nonyloxy)-1H-indol-3-yl]-ethyla...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity for recombinant human 5-hydroxytryptamine 1D receptor beta was determined using [3H]-5-HT as radioligand | J Med Chem 39: 314-22 (1996) Article DOI: 10.1021/jm950498t BindingDB Entry DOI: 10.7270/Q2DJ5DRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM50355500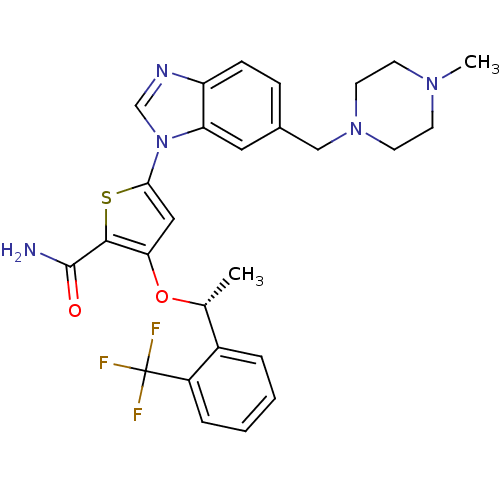 (CHEMBL1908394 | US9695172, GSK461364) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00533 BindingDB Entry DOI: 10.7270/Q23X8BM3 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50049084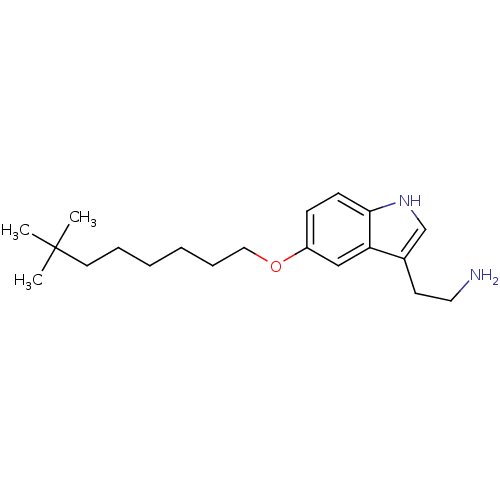 (2-[5-(7,7-Dimethyl-octyloxy)-1H-indol-3-yl]-ethyla...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity for recombinant human 5-hydroxytryptamine 1D receptor beta was determined using [3H]-5-HT as radioligand | J Med Chem 39: 314-22 (1996) Article DOI: 10.1021/jm950498t BindingDB Entry DOI: 10.7270/Q2DJ5DRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50049082 (2-(5-Octyloxy-1H-indol-3-yl)-ethylamine | CHEMBL32...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity for recombinant human 5-hydroxytryptamine 1D receptor beta was determined using [3H]-5-HT as radioligand | J Med Chem 39: 314-22 (1996) Article DOI: 10.1021/jm950498t BindingDB Entry DOI: 10.7270/Q2DJ5DRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50049082 (2-(5-Octyloxy-1H-indol-3-yl)-ethylamine | CHEMBL32...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity for recombinant human 5-hydroxytryptamine 1D receptor beta was determined using [3H]-5-HT as radioligand | J Med Chem 39: 314-22 (1996) Article DOI: 10.1021/jm950498t BindingDB Entry DOI: 10.7270/Q2DJ5DRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM82087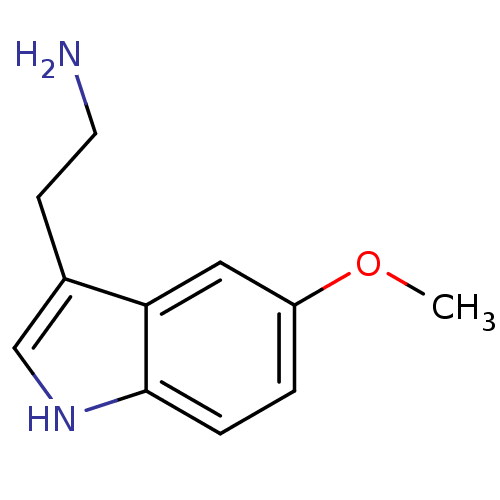 (2-(5-methoxy-1H-indol-3-yl)ethanamine | 5-MT | 5-M...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description In vitro affinity at human cloned 5-hydroxytryptamine 1A receptor by [3H]-8-OH-DPAT displacement. | J Med Chem 39: 314-22 (1996) Article DOI: 10.1021/jm950498t BindingDB Entry DOI: 10.7270/Q2DJ5DRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM82087 (2-(5-methoxy-1H-indol-3-yl)ethanamine | 5-MT | 5-M...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity for recombinant human 5-hydroxytryptamine 1D receptor beta was determined using [3H]-5-HT as radioligand | J Med Chem 39: 314-22 (1996) Article DOI: 10.1021/jm950498t BindingDB Entry DOI: 10.7270/Q2DJ5DRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2C adrenergic receptor (Homo sapiens (Human)) | BDBM50173162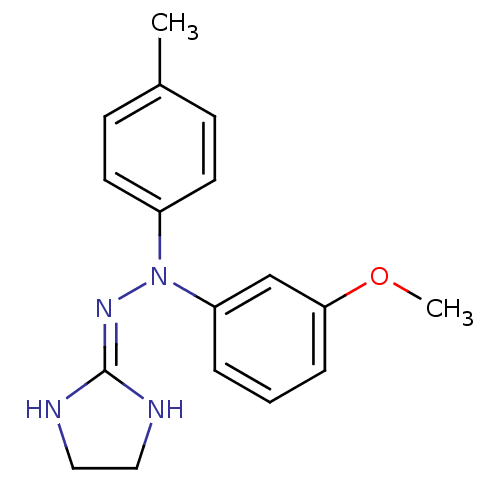 (CHEMBL362954 | N'-(4,5-Dihydro-1H-imidazol-2-yl)-N...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Binding affinity towards human alpha-2C adrenergic receptor expressed in Chinese Hamster ovary (CHO) cells | Bioorg Med Chem Lett 15: 4691-5 (2005) Article DOI: 10.1016/j.bmcl.2005.07.083 BindingDB Entry DOI: 10.7270/Q2D799ZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM10755 (14C-5-hydroxy tryptamine creatinine disulfate | 2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical College of Virginia/Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity was measured on 5-hydroxytryptamine 1D receptor beta in CHO cells transfected with human 5-HT1D beta gene labeled with [3H]-5-HT | J Med Chem 37: 2828-30 (1994) BindingDB Entry DOI: 10.7270/Q2GH9H0R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM10755 (14C-5-hydroxy tryptamine creatinine disulfate | 2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity of the compound towards cloned human 5-hydroxytryptamine 1D receptor beta was determined using [3H]-8-OH-DPAT as radioligand | J Med Chem 39: 314-22 (1996) Article DOI: 10.1021/jm950498t BindingDB Entry DOI: 10.7270/Q2DJ5DRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM82087 (2-(5-methoxy-1H-indol-3-yl)ethanamine | 5-MT | 5-M...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity of the compound towards 5-hydroxytryptamine 1D receptor was determined in calf striatum homogenate | J Med Chem 39: 314-22 (1996) Article DOI: 10.1021/jm950498t BindingDB Entry DOI: 10.7270/Q2DJ5DRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM31046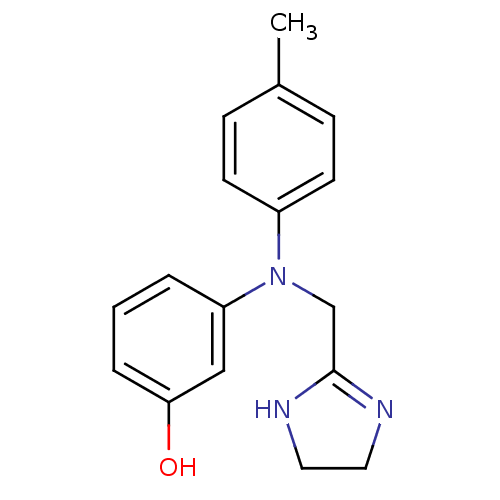 (3-[4,5-dihydro-1H-imidazol-2-ylmethyl-(4-methylphe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Similars | DrugBank Article PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Binding affinity towards alpha-1A adrenergic receptor expressed in human embryonic kidney (HEK293) cells | Bioorg Med Chem Lett 15: 4691-5 (2005) Article DOI: 10.1016/j.bmcl.2005.07.083 BindingDB Entry DOI: 10.7270/Q2D799ZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50173165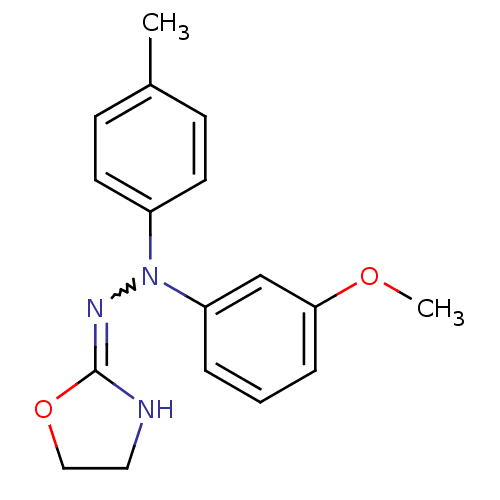 (CHEMBL194323 | N'-(4,5-Dihydro-oxazol-2-yl)-N-(3-m...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Binding affinity towards human alpha-2A adrenergic receptor expressed in Chinese Hamster ovary (CHO) cells | Bioorg Med Chem Lett 15: 4691-5 (2005) Article DOI: 10.1016/j.bmcl.2005.07.083 BindingDB Entry DOI: 10.7270/Q2D799ZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50049089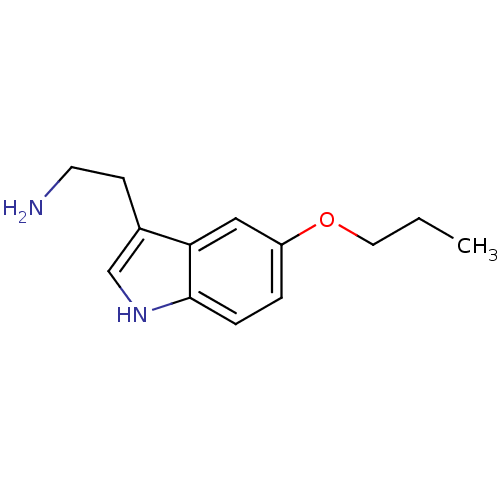 (2-(5-Propoxy-1H-indol-3-yl)-ethylamine | CHEMBL109...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity for recombinant human 5-hydroxytryptamine 1D receptor beta was determined using [3H]-5-HT as radioligand | J Med Chem 39: 314-22 (1996) Article DOI: 10.1021/jm950498t BindingDB Entry DOI: 10.7270/Q2DJ5DRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50173163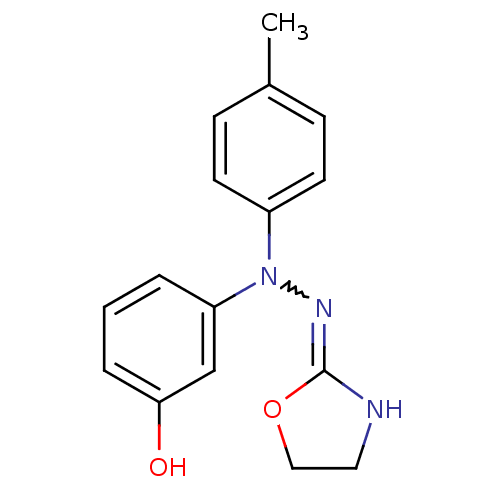 (3-[N'-(4,5-Dihydro-oxazol-2-yl)-N-p-tolyl-hydrazin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Binding affinity towards human alpha-2A adrenergic receptor expressed in Chinese Hamster ovary (CHO) cells | Bioorg Med Chem Lett 15: 4691-5 (2005) Article DOI: 10.1016/j.bmcl.2005.07.083 BindingDB Entry DOI: 10.7270/Q2D799ZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50049091 (2-(5-Heptyloxy-1H-indol-3-yl)-ethylamine | CHEMBL3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description In vitro affitnity human cloned 5-hydroxytryptamine 1D receptor alpha by [3H]-5-HT displacement. | J Med Chem 39: 314-22 (1996) Article DOI: 10.1021/jm950498t BindingDB Entry DOI: 10.7270/Q2DJ5DRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50250387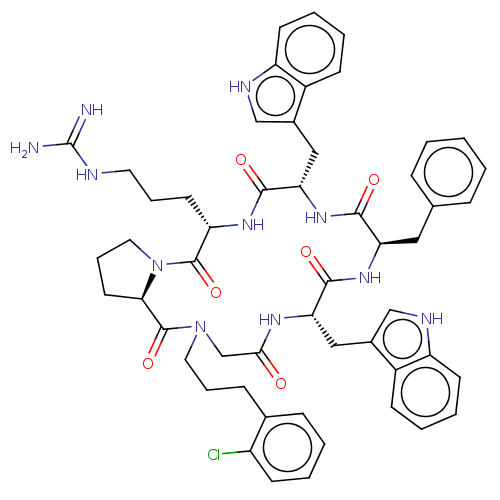 (CHEMBL4102791) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-CXCL12 from human CXCR7 expressed in CHOK1 cell membranes after 2 hrs by scintillation counting method | J Med Chem 60: 9653-9663 (2017) Article DOI: 10.1021/acs.jmedchem.7b01028 BindingDB Entry DOI: 10.7270/Q2ZG6VPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50250387 (CHEMBL4102791) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Modulation of ProLink-fused human CXCR7 expressed in CHOK1 cell membranes assessed as induction of EA-tagged beta-arrestin recruitment after 30 mins ... | J Med Chem 60: 9653-9663 (2017) Article DOI: 10.1021/acs.jmedchem.7b01028 BindingDB Entry DOI: 10.7270/Q2ZG6VPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50173163 (3-[N'-(4,5-Dihydro-oxazol-2-yl)-N-p-tolyl-hydrazin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Binding affinity towards alpha-1A adrenergic receptor expressed in human embryonic kidney (HEK293) cells | Bioorg Med Chem Lett 15: 4691-5 (2005) Article DOI: 10.1016/j.bmcl.2005.07.083 BindingDB Entry DOI: 10.7270/Q2D799ZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50173165 (CHEMBL194323 | N'-(4,5-Dihydro-oxazol-2-yl)-N-(3-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Binding affinity towards alpha-1A adrenergic receptor expressed in human embryonic kidney (HEK293) cells | Bioorg Med Chem Lett 15: 4691-5 (2005) Article DOI: 10.1016/j.bmcl.2005.07.083 BindingDB Entry DOI: 10.7270/Q2D799ZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Rattus norvegicus (Rat)) | BDBM10755 (14C-5-hydroxy tryptamine creatinine disulfate | 2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | PubMed | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical College of Virginia/Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity was measured on 5-hydroxytryptamine 1B receptor in rat striatum labeled with [3H]-5-HT | J Med Chem 37: 2828-30 (1994) BindingDB Entry DOI: 10.7270/Q2GH9H0R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM82087 (2-(5-methoxy-1H-indol-3-yl)ethanamine | 5-MT | 5-M...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Selectivity of the compound towards 5-hydroxytryptamine 1D receptor beta to that of 5-hydroxytryptamine 1D receptor alpha | J Med Chem 39: 314-22 (1996) Article DOI: 10.1021/jm950498t BindingDB Entry DOI: 10.7270/Q2DJ5DRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM10755 (14C-5-hydroxy tryptamine creatinine disulfate | 2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | PDB PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical College of Virginia/Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity was measured on 5-hydroxytryptamine 1D receptor alpha in COS cells transfected with human 5-HT1D alpha gene labeled with [3H]-5-HT | J Med Chem 37: 2828-30 (1994) BindingDB Entry DOI: 10.7270/Q2GH9H0R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50005835 ((3-[2-(dimethylamino)ethyl]-1H-indol-5-yl)-N-methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents | DrugBank PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical College of Virginia/Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity was measured on 5-hydroxytryptamine 1D receptor beta in CHO cells transfected with human 5-HT1D beta gene labeled with [3H]-5-HT | J Med Chem 37: 2828-30 (1994) BindingDB Entry DOI: 10.7270/Q2GH9H0R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50049099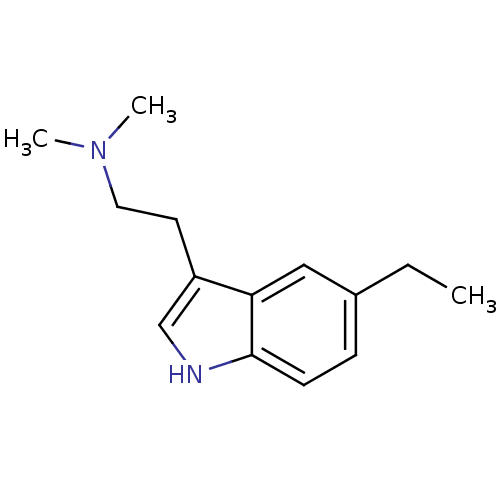 (CHEMBL50492 | [2-(5-Ethyl-1H-indol-3-yl)-ethyl]-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity for recombinant human 5-hydroxytryptamine 1D receptor beta was determined using [3H]-5-HT as radioligand | J Med Chem 39: 314-22 (1996) Article DOI: 10.1021/jm950498t BindingDB Entry DOI: 10.7270/Q2DJ5DRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50049479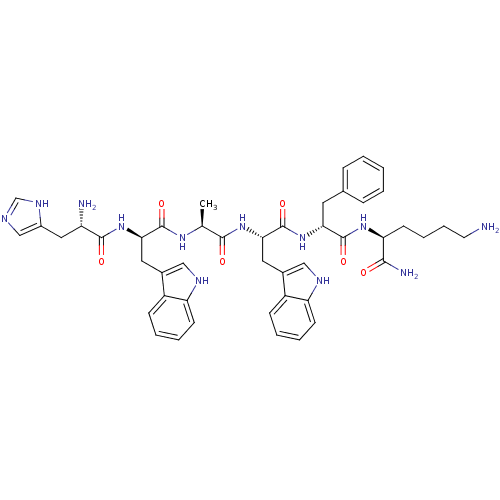 ((S)-6-Amino-2-{(R)-2-[(S)-2-{(S)-2-[(R)-2-[(S)-2-a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding Affinity of the compound against Growth hormone secretagogue receptor of swine using [35S]-MK-0677 as radioligand | J Med Chem 39: 1767-70 (1996) Article DOI: 10.1021/jm960054c BindingDB Entry DOI: 10.7270/Q2BK1BDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50173162 (CHEMBL362954 | N'-(4,5-Dihydro-1H-imidazol-2-yl)-N...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Binding affinity towards human alpha-2A adrenergic receptor expressed in Chinese Hamster ovary (CHO) cells | Bioorg Med Chem Lett 15: 4691-5 (2005) Article DOI: 10.1016/j.bmcl.2005.07.083 BindingDB Entry DOI: 10.7270/Q2D799ZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM86516 (ETPDCFWKYCV | Human U-II | L-Ala-Gly-L-Thr-L-Ala-L...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd. Curated by PDSP Ki Database | J Pharmacol Exp Ther 311: 204-12 (2004) Article DOI: 10.1124/jpet.104.068320 BindingDB Entry DOI: 10.7270/Q2H993RW | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2B adrenergic receptor (Homo sapiens (Human)) | BDBM50173162 (CHEMBL362954 | N'-(4,5-Dihydro-1H-imidazol-2-yl)-N...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Binding affinity towards human alpha-2B adrenergic receptor expressed in Chinese Hamster ovary (CHO) cells | Bioorg Med Chem Lett 15: 4691-5 (2005) Article DOI: 10.1016/j.bmcl.2005.07.083 BindingDB Entry DOI: 10.7270/Q2D799ZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50049085 (1-[3-(2-Amino-ethyl)-1H-indol-5-yl]-ethanone | Ace...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity for recombinant human 5-hydroxytryptamine 1D receptor beta was determined using [3H]-5-HT as radioligand | J Med Chem 39: 314-22 (1996) Article DOI: 10.1021/jm950498t BindingDB Entry DOI: 10.7270/Q2DJ5DRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50049086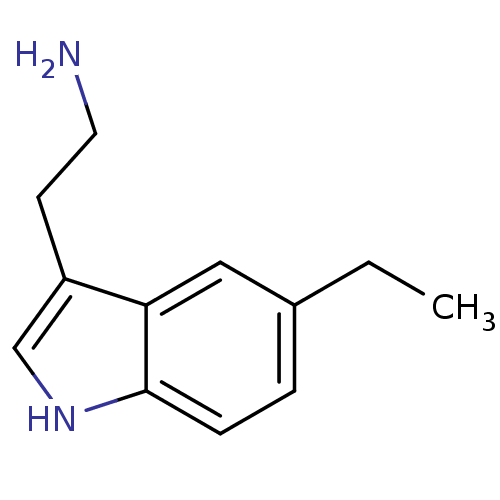 (2-(5-Ethyl-1H-indol-3-yl)-ethylamine | CHEMBL10751...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity for recombinant human 5-hydroxytryptamine 1D receptor beta was determined using [3H]-5-HT as radioligand | J Med Chem 39: 314-22 (1996) Article DOI: 10.1021/jm950498t BindingDB Entry DOI: 10.7270/Q2DJ5DRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50250402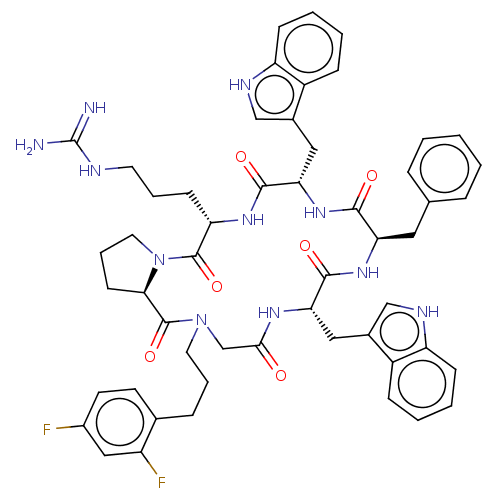 (CHEMBL4084835) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Modulation of ProLink-fused human CXCR7 expressed in CHOK1 cell membranes assessed as induction of EA-tagged beta-arrestin recruitment after 30 mins ... | J Med Chem 60: 9653-9663 (2017) Article DOI: 10.1021/acs.jmedchem.7b01028 BindingDB Entry DOI: 10.7270/Q2ZG6VPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50250402 (CHEMBL4084835) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-CXCL12 from human CXCR7 expressed in CHOK1 cell membranes after 2 hrs by scintillation counting method | J Med Chem 60: 9653-9663 (2017) Article DOI: 10.1021/acs.jmedchem.7b01028 BindingDB Entry DOI: 10.7270/Q2ZG6VPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50250395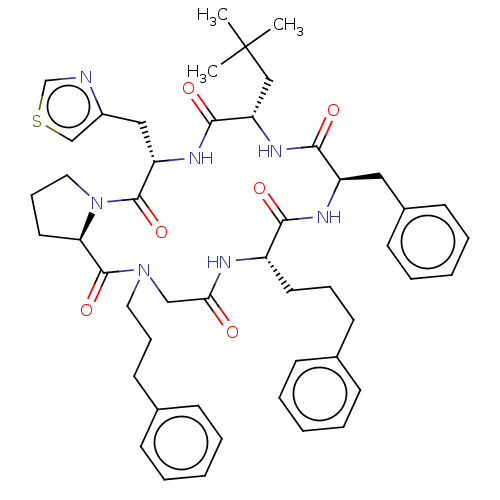 (CHEMBL4103373) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Modulation of ProLink-fused human CXCR7 expressed in CHOK1 cell membranes assessed as induction of EA-tagged beta-arrestin recruitment after 30 mins ... | J Med Chem 60: 9653-9663 (2017) Article DOI: 10.1021/acs.jmedchem.7b01028 BindingDB Entry DOI: 10.7270/Q2ZG6VPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50250375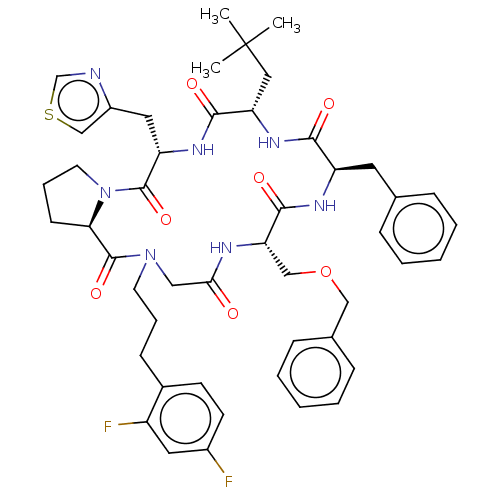 (CHEMBL4077017) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-CXCL12 from human CXCR7 expressed in CHOK1 cell membranes after 2 hrs by scintillation counting method | J Med Chem 60: 9653-9663 (2017) Article DOI: 10.1021/acs.jmedchem.7b01028 BindingDB Entry DOI: 10.7270/Q2ZG6VPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1760 total ) | Next | Last >> |