 Found 378 hits with Last Name = 'ngo' and Initial = 'vt'
Found 378 hits with Last Name = 'ngo' and Initial = 'vt' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50049553
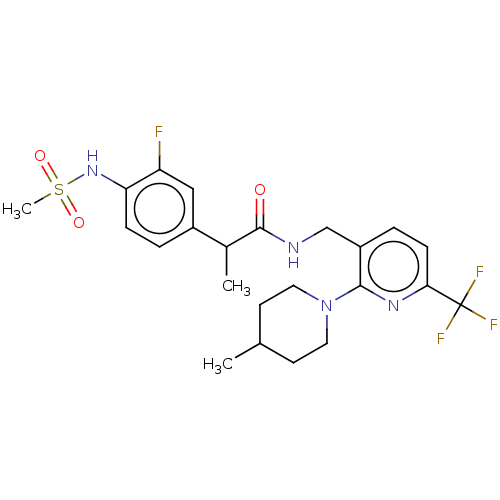
(CHEMBL2177428)Show SMILES CC(C(=O)NCc1ccc(nc1N1CCC(C)CC1)C(F)(F)F)c1ccc(NS(C)(=O)=O)c(F)c1 Show InChI InChI=1S/C23H28F4N4O3S/c1-14-8-10-31(11-9-14)21-17(5-7-20(29-21)23(25,26)27)13-28-22(32)15(2)16-4-6-19(18(24)12-16)30-35(3,33)34/h4-7,12,14-15,30H,8-11,13H2,1-3H3,(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of N-arachidonoyl dopamine-induced activation by FLIPR assay |
Bioorg Med Chem Lett 25: 2326-30 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.024
BindingDB Entry DOI: 10.7270/Q2Z60QSC |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50088020
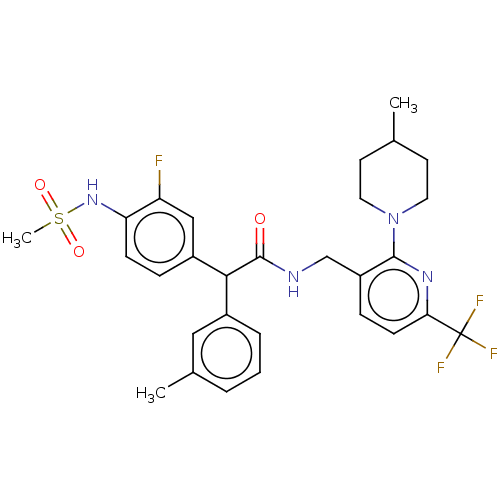
(CHEMBL3427109)Show SMILES CC1CCN(CC1)c1nc(ccc1CNC(=O)C(c1cccc(C)c1)c1ccc(NS(C)(=O)=O)c(F)c1)C(F)(F)F Show InChI InChI=1S/C29H32F4N4O3S/c1-18-11-13-37(14-12-18)27-22(8-10-25(35-27)29(31,32)33)17-34-28(38)26(20-6-4-5-19(2)15-20)21-7-9-24(23(30)16-21)36-41(3,39)40/h4-10,15-16,18,26,36H,11-14,17H2,1-3H3,(H,34,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced activation by FLIPR assay |
Bioorg Med Chem Lett 25: 2326-30 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.024
BindingDB Entry DOI: 10.7270/Q2Z60QSC |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50088020

(CHEMBL3427109)Show SMILES CC1CCN(CC1)c1nc(ccc1CNC(=O)C(c1cccc(C)c1)c1ccc(NS(C)(=O)=O)c(F)c1)C(F)(F)F Show InChI InChI=1S/C29H32F4N4O3S/c1-18-11-13-37(14-12-18)27-22(8-10-25(35-27)29(31,32)33)17-34-28(38)26(20-6-4-5-19(2)15-20)21-7-9-24(23(30)16-21)36-41(3,39)40/h4-10,15-16,18,26,36H,11-14,17H2,1-3H3,(H,34,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of N-arachidonoyl dopamine-induced activation by FLIPR assay |
Bioorg Med Chem Lett 25: 2326-30 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.024
BindingDB Entry DOI: 10.7270/Q2Z60QSC |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50049553

(CHEMBL2177428)Show SMILES CC(C(=O)NCc1ccc(nc1N1CCC(C)CC1)C(F)(F)F)c1ccc(NS(C)(=O)=O)c(F)c1 Show InChI InChI=1S/C23H28F4N4O3S/c1-14-8-10-31(11-9-14)21-17(5-7-20(29-21)23(25,26)27)13-28-22(32)15(2)16-4-6-19(18(24)12-16)30-35(3,33)34/h4-7,12,14-15,30H,8-11,13H2,1-3H3,(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced activation by FLIPR assay |
Bioorg Med Chem Lett 25: 2326-30 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.024
BindingDB Entry DOI: 10.7270/Q2Z60QSC |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50088041
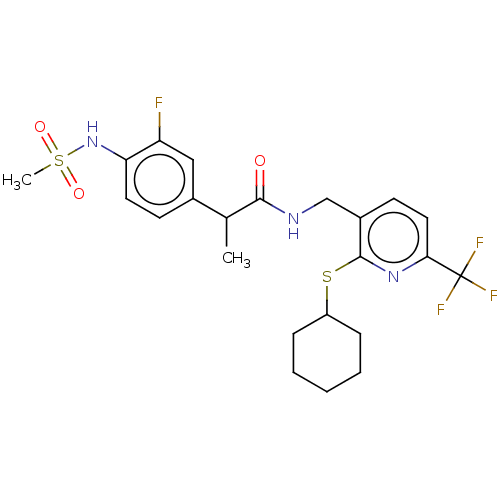
(CHEMBL2442911)Show SMILES CC(C(=O)NCc1ccc(nc1SC1CCCCC1)C(F)(F)F)c1ccc(NS(C)(=O)=O)c(F)c1 Show InChI InChI=1S/C23H27F4N3O3S2/c1-14(15-8-10-19(18(24)12-15)30-35(2,32)33)21(31)28-13-16-9-11-20(23(25,26)27)29-22(16)34-17-6-4-3-5-7-17/h8-12,14,17,30H,3-7,13H2,1-2H3,(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced activation by FLIPR assay |
Bioorg Med Chem Lett 25: 2326-30 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.024
BindingDB Entry DOI: 10.7270/Q2Z60QSC |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50088028

(CHEMBL3425527)Show SMILES CC1CCN(CC1)c1nc(ccc1CNC(=O)C(c1ccc(C)cc1)c1ccc(NS(C)(=O)=O)c(F)c1)C(F)(F)F Show InChI InChI=1S/C29H32F4N4O3S/c1-18-4-6-20(7-5-18)26(21-8-10-24(23(30)16-21)36-41(3,39)40)28(38)34-17-22-9-11-25(29(31,32)33)35-27(22)37-14-12-19(2)13-15-37/h4-11,16,19,26,36H,12-15,17H2,1-3H3,(H,34,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced activation by FLIPR assay |
Bioorg Med Chem Lett 25: 2326-30 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.024
BindingDB Entry DOI: 10.7270/Q2Z60QSC |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50088036
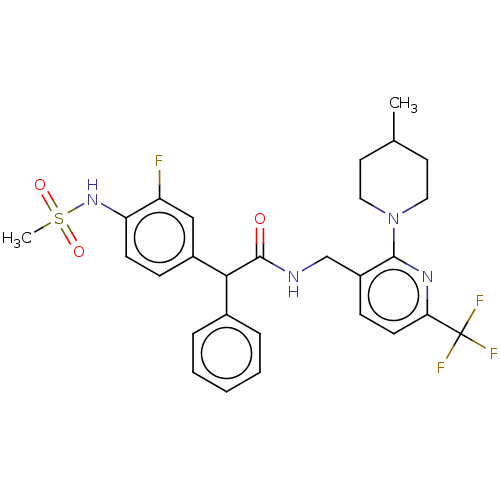
(CHEMBL3427101)Show SMILES CC1CCN(CC1)c1nc(ccc1CNC(=O)C(c1ccccc1)c1ccc(NS(C)(=O)=O)c(F)c1)C(F)(F)F Show InChI InChI=1S/C28H30F4N4O3S/c1-18-12-14-36(15-13-18)26-21(9-11-24(34-26)28(30,31)32)17-33-27(37)25(19-6-4-3-5-7-19)20-8-10-23(22(29)16-20)35-40(2,38)39/h3-11,16,18,25,35H,12-15,17H2,1-2H3,(H,33,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced activation by FLIPR assay |
Bioorg Med Chem Lett 25: 2326-30 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.024
BindingDB Entry DOI: 10.7270/Q2Z60QSC |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50088040

(CHEMBL3427096)Show SMILES CCC(C(=O)NCc1ccc(nc1N1CCC(C)CC1)C(F)(F)F)c1ccc(NS(C)(=O)=O)c(F)c1 Show InChI InChI=1S/C24H30F4N4O3S/c1-4-18(16-5-7-20(19(25)13-16)31-36(3,34)35)23(33)29-14-17-6-8-21(24(26,27)28)30-22(17)32-11-9-15(2)10-12-32/h5-8,13,15,18,31H,4,9-12,14H2,1-3H3,(H,29,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced activation by FLIPR assay |
Bioorg Med Chem Lett 25: 2326-30 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.024
BindingDB Entry DOI: 10.7270/Q2Z60QSC |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50088034
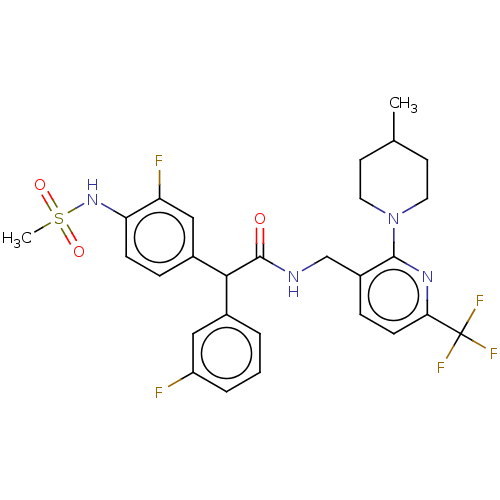
(CHEMBL3427103)Show SMILES CC1CCN(CC1)c1nc(ccc1CNC(=O)C(c1cccc(F)c1)c1ccc(NS(C)(=O)=O)c(F)c1)C(F)(F)F Show InChI InChI=1S/C28H29F5N4O3S/c1-17-10-12-37(13-11-17)26-20(7-9-24(35-26)28(31,32)33)16-34-27(38)25(18-4-3-5-21(29)14-18)19-6-8-23(22(30)15-19)36-41(2,39)40/h3-9,14-15,17,25,36H,10-13,16H2,1-2H3,(H,34,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced activation by FLIPR assay |
Bioorg Med Chem Lett 25: 2326-30 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.024
BindingDB Entry DOI: 10.7270/Q2Z60QSC |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50088032

(CHEMBL3427105)Show SMILES CC1CCN(CC1)c1nc(ccc1CNC(=O)C(c1ccc(F)cc1)c1ccc(NS(C)(=O)=O)c(F)c1)C(F)(F)F Show InChI InChI=1S/C28H29F5N4O3S/c1-17-11-13-37(14-12-17)26-20(6-10-24(35-26)28(31,32)33)16-34-27(38)25(18-3-7-21(29)8-4-18)19-5-9-23(22(30)15-19)36-41(2,39)40/h3-10,15,17,25,36H,11-14,16H2,1-2H3,(H,34,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced activation by FLIPR assay |
Bioorg Med Chem Lett 25: 2326-30 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.024
BindingDB Entry DOI: 10.7270/Q2Z60QSC |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50088027
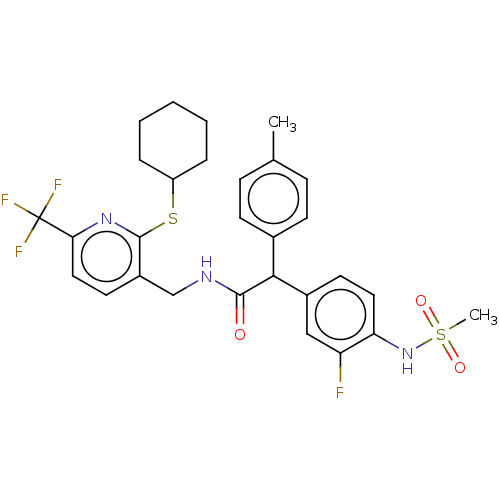
(CHEMBL3427111)Show SMILES Cc1ccc(cc1)C(C(=O)NCc1ccc(nc1SC1CCCCC1)C(F)(F)F)c1ccc(NS(C)(=O)=O)c(F)c1 Show InChI InChI=1S/C29H31F4N3O3S2/c1-18-8-10-19(11-9-18)26(20-12-14-24(23(30)16-20)36-41(2,38)39)27(37)34-17-21-13-15-25(29(31,32)33)35-28(21)40-22-6-4-3-5-7-22/h8-16,22,26,36H,3-7,17H2,1-2H3,(H,34,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced activation by FLIPR assay |
Bioorg Med Chem Lett 25: 2326-30 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.024
BindingDB Entry DOI: 10.7270/Q2Z60QSC |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50088037

(CHEMBL3427099)Show SMILES CC1CCN(CC1)c1nc(ccc1CNC(=O)C(C1CCCCC1)c1ccc(NS(C)(=O)=O)c(F)c1)C(F)(F)F Show InChI InChI=1S/C28H36F4N4O3S/c1-18-12-14-36(15-13-18)26-21(9-11-24(34-26)28(30,31)32)17-33-27(37)25(19-6-4-3-5-7-19)20-8-10-23(22(29)16-20)35-40(2,38)39/h8-11,16,18-19,25,35H,3-7,12-15,17H2,1-2H3,(H,33,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced activation by FLIPR assay |
Bioorg Med Chem Lett 25: 2326-30 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.024
BindingDB Entry DOI: 10.7270/Q2Z60QSC |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50088031
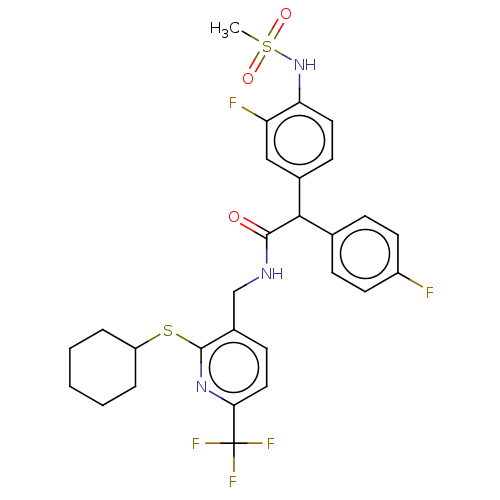
(CHEMBL3427106)Show SMILES CS(=O)(=O)Nc1ccc(cc1F)C(C(=O)NCc1ccc(nc1SC1CCCCC1)C(F)(F)F)c1ccc(F)cc1 Show InChI InChI=1S/C28H28F5N3O3S2/c1-41(38,39)36-23-13-9-18(15-22(23)30)25(17-7-11-20(29)12-8-17)26(37)34-16-19-10-14-24(28(31,32)33)35-27(19)40-21-5-3-2-4-6-21/h7-15,21,25,36H,2-6,16H2,1H3,(H,34,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced activation by FLIPR assay |
Bioorg Med Chem Lett 25: 2326-30 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.024
BindingDB Entry DOI: 10.7270/Q2Z60QSC |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50088024

(CHEMBL3427117)Show SMILES Cc1ccc(CC(C(=O)NCc2ccc(nc2SC2CCCCC2)C(F)(F)F)c2ccc(NS(C)(=O)=O)c(F)c2)cc1 Show InChI InChI=1S/C30H33F4N3O3S2/c1-19-8-10-20(11-9-19)16-24(21-12-14-26(25(31)17-21)37-42(2,39)40)28(38)35-18-22-13-15-27(30(32,33)34)36-29(22)41-23-6-4-3-5-7-23/h8-15,17,23-24,37H,3-7,16,18H2,1-2H3,(H,35,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced activation by FLIPR assay |
Bioorg Med Chem Lett 25: 2326-30 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.024
BindingDB Entry DOI: 10.7270/Q2Z60QSC |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50088030

(CHEMBL3427107)Show SMILES CC1CCN(CC1)c1nc(ccc1CNC(=O)C(c1ccc(NS(C)(=O)=O)c(F)c1)c1ccccc1C)C(F)(F)F Show InChI InChI=1S/C29H32F4N4O3S/c1-18-12-14-37(15-13-18)27-21(9-11-25(35-27)29(31,32)33)17-34-28(38)26(22-7-5-4-6-19(22)2)20-8-10-24(23(30)16-20)36-41(3,39)40/h4-11,16,18,26,36H,12-15,17H2,1-3H3,(H,34,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced activation by FLIPR assay |
Bioorg Med Chem Lett 25: 2326-30 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.024
BindingDB Entry DOI: 10.7270/Q2Z60QSC |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50088025

(CHEMBL3427114)Show SMILES CC1CCN(CC1)c1nc(ccc1CNC(=O)C(Cc1ccc(F)cc1)c1ccc(NS(C)(=O)=O)c(F)c1)C(F)(F)F Show InChI InChI=1S/C29H31F5N4O3S/c1-18-11-13-38(14-12-18)27-21(6-10-26(36-27)29(32,33)34)17-35-28(39)23(15-19-3-7-22(30)8-4-19)20-5-9-25(24(31)16-20)37-42(2,40)41/h3-10,16,18,23,37H,11-15,17H2,1-2H3,(H,35,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced activation by FLIPR assay |
Bioorg Med Chem Lett 25: 2326-30 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.024
BindingDB Entry DOI: 10.7270/Q2Z60QSC |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50088033

(CHEMBL3427104)Show SMILES CS(=O)(=O)Nc1ccc(cc1F)C(C(=O)NCc1ccc(nc1SC1CCCCC1)C(F)(F)F)c1cccc(F)c1 Show InChI InChI=1S/C28H28F5N3O3S2/c1-41(38,39)36-23-12-10-18(15-22(23)30)25(17-6-5-7-20(29)14-17)26(37)34-16-19-11-13-24(28(31,32)33)35-27(19)40-21-8-3-2-4-9-21/h5-7,10-15,21,25,36H,2-4,8-9,16H2,1H3,(H,34,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced activation by FLIPR assay |
Bioorg Med Chem Lett 25: 2326-30 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.024
BindingDB Entry DOI: 10.7270/Q2Z60QSC |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50088023
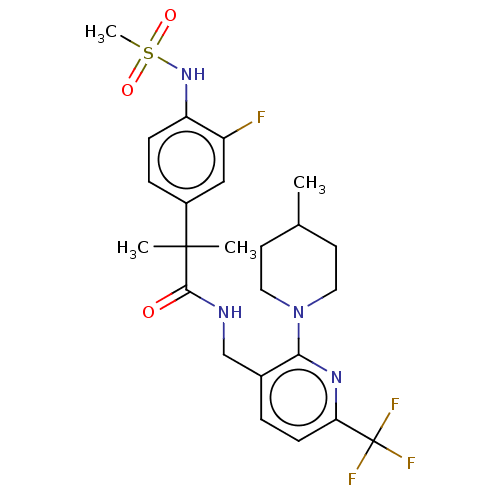
(CHEMBL3427118)Show SMILES CC1CCN(CC1)c1nc(ccc1CNC(=O)C(C)(C)c1ccc(NS(C)(=O)=O)c(F)c1)C(F)(F)F Show InChI InChI=1S/C24H30F4N4O3S/c1-15-9-11-32(12-10-15)21-16(5-8-20(30-21)24(26,27)28)14-29-22(33)23(2,3)17-6-7-19(18(25)13-17)31-36(4,34)35/h5-8,13,15,31H,9-12,14H2,1-4H3,(H,29,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced activation by FLIPR assay |
Bioorg Med Chem Lett 25: 2326-30 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.024
BindingDB Entry DOI: 10.7270/Q2Z60QSC |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50088029

(CHEMBL3427110)Show SMILES Cc1cccc(c1)C(C(=O)NCc1ccc(nc1SC1CCCCC1)C(F)(F)F)c1ccc(NS(C)(=O)=O)c(F)c1 Show InChI InChI=1S/C29H31F4N3O3S2/c1-18-7-6-8-19(15-18)26(20-11-13-24(23(30)16-20)36-41(2,38)39)27(37)34-17-21-12-14-25(29(31,32)33)35-28(21)40-22-9-4-3-5-10-22/h6-8,11-16,22,26,36H,3-5,9-10,17H2,1-2H3,(H,34,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced activation by FLIPR assay |
Bioorg Med Chem Lett 25: 2326-30 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.024
BindingDB Entry DOI: 10.7270/Q2Z60QSC |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50088035

(CHEMBL3427102)Show SMILES CS(=O)(=O)Nc1ccc(cc1F)C(C(=O)NCc1ccc(nc1SC1CCCCC1)C(F)(F)F)c1ccccc1 Show InChI InChI=1S/C28H29F4N3O3S2/c1-40(37,38)35-23-14-12-19(16-22(23)29)25(18-8-4-2-5-9-18)26(36)33-17-20-13-15-24(28(30,31)32)34-27(20)39-21-10-6-3-7-11-21/h2,4-5,8-9,12-16,21,25,35H,3,6-7,10-11,17H2,1H3,(H,33,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced activation by FLIPR assay |
Bioorg Med Chem Lett 25: 2326-30 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.024
BindingDB Entry DOI: 10.7270/Q2Z60QSC |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50088022
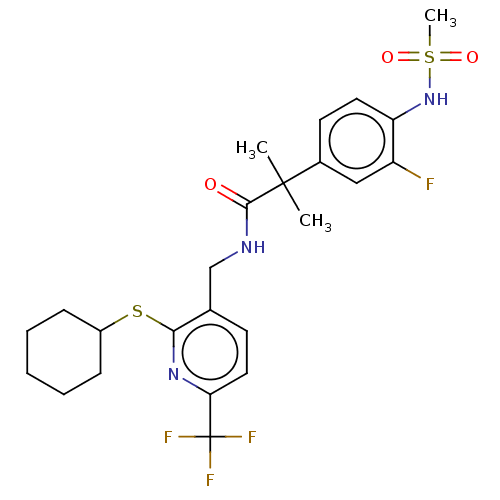
(CHEMBL3427119)Show SMILES CC(C)(C(=O)NCc1ccc(nc1SC1CCCCC1)C(F)(F)F)c1ccc(NS(C)(=O)=O)c(F)c1 Show InChI InChI=1S/C24H29F4N3O3S2/c1-23(2,16-10-11-19(18(25)13-16)31-36(3,33)34)22(32)29-14-15-9-12-20(24(26,27)28)30-21(15)35-17-7-5-4-6-8-17/h9-13,17,31H,4-8,14H2,1-3H3,(H,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced activation by FLIPR assay |
Bioorg Med Chem Lett 25: 2326-30 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.024
BindingDB Entry DOI: 10.7270/Q2Z60QSC |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50088026

(CHEMBL3427112)Show SMILES CC1CCN(CC1)c1nc(ccc1CNC(=O)C(Cc1ccccc1)c1ccc(NS(C)(=O)=O)c(F)c1)C(F)(F)F Show InChI InChI=1S/C29H32F4N4O3S/c1-19-12-14-37(15-13-19)27-22(9-11-26(35-27)29(31,32)33)18-34-28(38)23(16-20-6-4-3-5-7-20)21-8-10-25(24(30)17-21)36-41(2,39)40/h3-11,17,19,23,36H,12-16,18H2,1-2H3,(H,34,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced activation by FLIPR assay |
Bioorg Med Chem Lett 25: 2326-30 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.024
BindingDB Entry DOI: 10.7270/Q2Z60QSC |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50088039
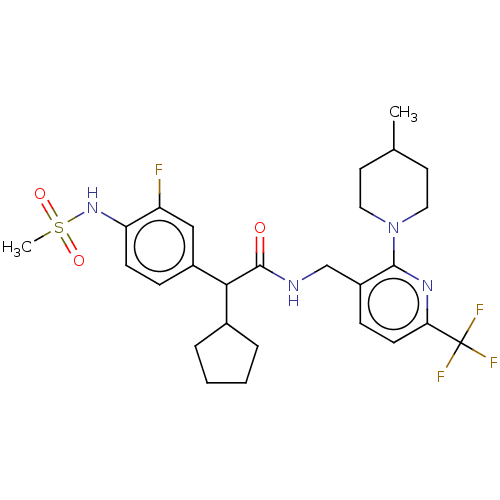
(CHEMBL3427097)Show SMILES CC1CCN(CC1)c1nc(ccc1CNC(=O)C(C1CCCC1)c1ccc(NS(C)(=O)=O)c(F)c1)C(F)(F)F Show InChI InChI=1S/C27H34F4N4O3S/c1-17-11-13-35(14-12-17)25-20(8-10-23(33-25)27(29,30)31)16-32-26(36)24(18-5-3-4-6-18)19-7-9-22(21(28)15-19)34-39(2,37)38/h7-10,15,17-18,24,34H,3-6,11-14,16H2,1-2H3,(H,32,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced activation by FLIPR assay |
Bioorg Med Chem Lett 25: 2326-30 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.024
BindingDB Entry DOI: 10.7270/Q2Z60QSC |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50088038
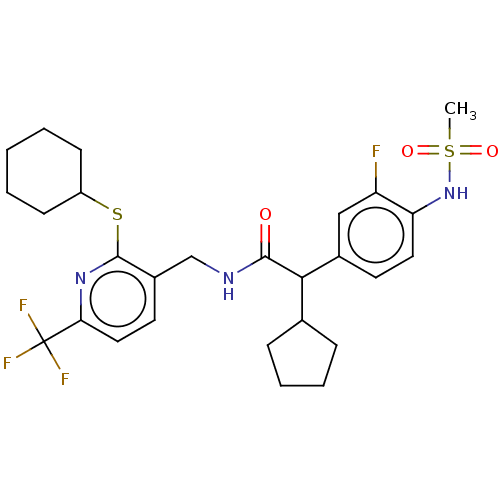
(CHEMBL3427098)Show SMILES CS(=O)(=O)Nc1ccc(cc1F)C(C1CCCC1)C(=O)NCc1ccc(nc1SC1CCCCC1)C(F)(F)F Show InChI InChI=1S/C27H33F4N3O3S2/c1-39(36,37)34-22-13-11-18(15-21(22)28)24(17-7-5-6-8-17)25(35)32-16-19-12-14-23(27(29,30)31)33-26(19)38-20-9-3-2-4-10-20/h11-15,17,20,24,34H,2-10,16H2,1H3,(H,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 103 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced activation by FLIPR assay |
Bioorg Med Chem Lett 25: 2326-30 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.024
BindingDB Entry DOI: 10.7270/Q2Z60QSC |
More data for this
Ligand-Target Pair | |
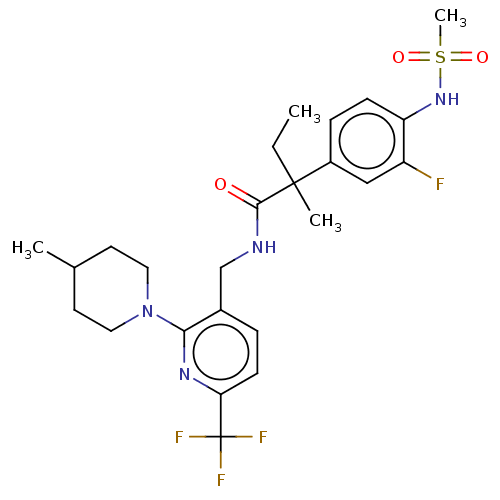
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50088021
(CHEMBL3427120)Show SMILES CCC(C)(C(=O)NCc1ccc(nc1N1CCC(C)CC1)C(F)(F)F)c1ccc(NS(C)(=O)=O)c(F)c1 Show InChI InChI=1S/C25H32F4N4O3S/c1-5-24(3,18-7-8-20(19(26)14-18)32-37(4,35)36)23(34)30-15-17-6-9-21(25(27,28)29)31-22(17)33-12-10-16(2)11-13-33/h6-9,14,16,32H,5,10-13,15H2,1-4H3,(H,30,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 124 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced activation by FLIPR assay |
Bioorg Med Chem Lett 25: 2326-30 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.024
BindingDB Entry DOI: 10.7270/Q2Z60QSC |
More data for this
Ligand-Target Pair | |
Glutaminyl-peptide cyclotransferase
(Homo sapiens (Human)) | BDBM50581458

(CHEMBL5092802)Show SMILES COc1cc(ccc1OCCN1CCOCC1)N(CC1CCCC1)C(=O)NCCCn1cncc1C | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant glutaminyl cyclase using H-Gln-AMC hydrobromide as substrate measured after 15 mins by fluorimetry |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113819
BindingDB Entry DOI: 10.7270/Q2M61Q44 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutaminyl-peptide cyclotransferase
(Homo sapiens (Human)) | BDBM50581397
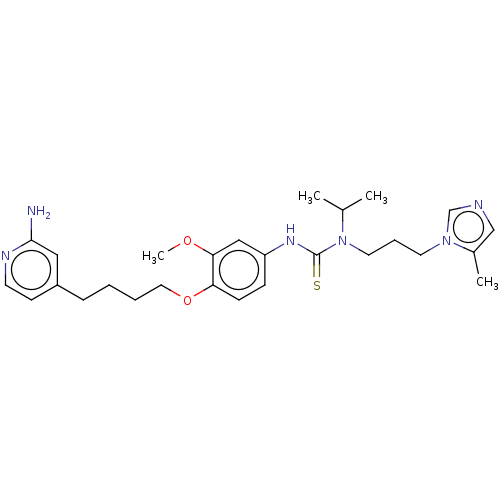
(CHEMBL5085353)Show SMILES COc1cc(NC(=S)N(CCCn2cncc2C)C(C)C)ccc1OCCCCc1ccnc(N)c1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant glutaminyl cyclase using H-Gln-AMC hydrobromide as substrate measured after 15 mins by fluorimetry |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113819
BindingDB Entry DOI: 10.7270/Q2M61Q44 |
More data for this
Ligand-Target Pair | |
Glutaminyl-peptide cyclotransferase
(Homo sapiens (Human)) | BDBM50581442

(CHEMBL5086776)Show SMILES COc1cc(ccc1OCCN1CCNCC1)N(CC1CCCC1)C(=O)NCCCn1cncc1C | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant glutaminyl cyclase using H-Gln-AMC hydrobromide as substrate measured after 15 mins by fluorimetry |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113819
BindingDB Entry DOI: 10.7270/Q2M61Q44 |
More data for this
Ligand-Target Pair | |
Glutaminyl-peptide cyclotransferase
(Homo sapiens (Human)) | BDBM50581399

(CHEMBL5085226)Show SMILES COc1cc(ccc1OCCCCc1ccnc(N)c1)N(C1CCCC1)C(=S)NCCCn1cncc1C | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant glutaminyl cyclase using H-Gln-AMC hydrobromide as substrate measured after 15 mins by fluorimetry |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113819
BindingDB Entry DOI: 10.7270/Q2M61Q44 |
More data for this
Ligand-Target Pair | |
Glutaminyl-peptide cyclotransferase
(Homo sapiens (Human)) | BDBM50581400
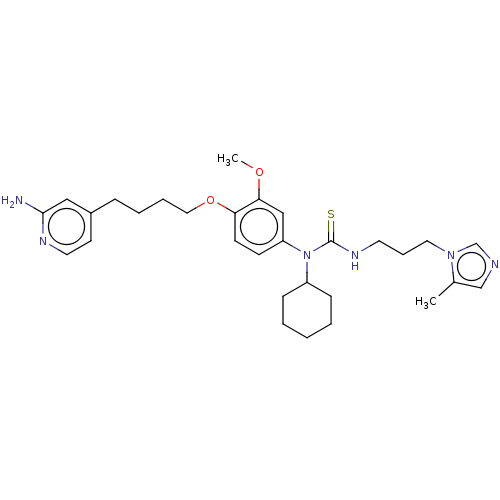
(CHEMBL5075575)Show SMILES COc1cc(ccc1OCCCCc1ccnc(N)c1)N(C1CCCCC1)C(=S)NCCCn1cncc1C | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant glutaminyl cyclase using H-Gln-AMC hydrobromide as substrate measured after 15 mins by fluorimetry |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113819
BindingDB Entry DOI: 10.7270/Q2M61Q44 |
More data for this
Ligand-Target Pair | |
Glutaminyl-peptide cyclotransferase
(Homo sapiens (Human)) | BDBM50237486
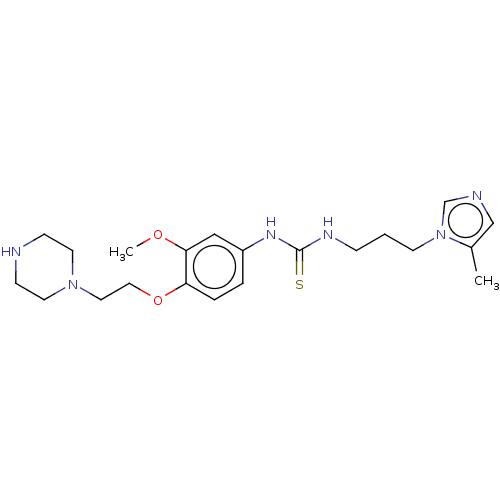
(CHEMBL4077433)Show InChI InChI=1S/C21H32N6O2S/c1-17-15-23-16-27(17)9-3-6-24-21(30)25-18-4-5-19(20(14-18)28-2)29-13-12-26-10-7-22-8-11-26/h4-5,14-16,22H,3,6-13H2,1-2H3,(H2,24,25,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Inhibition of human glutaminyl cyclase assessed as reduction in conversion of H-Gln-AMC hydrobromide to pGlu-AMC preincubated with substrate for 10 m... |
J Med Chem 60: 2573-2590 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00098
BindingDB Entry DOI: 10.7270/Q23X88X9 |
More data for this
Ligand-Target Pair | |
Glutaminyl-peptide cyclotransferase
(Homo sapiens (Human)) | BDBM50581426

(CHEMBL5089771)Show SMILES COc1cc(ccc1OCCCCN(C)C)N(C1CCN(C)CC1)C(=O)NCCCn1cncc1C | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant glutaminyl cyclase using H-Gln-AMC hydrobromide as substrate measured after 15 mins by fluorimetry |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113819
BindingDB Entry DOI: 10.7270/Q2M61Q44 |
More data for this
Ligand-Target Pair | |
Glutaminyl-peptide cyclotransferase
(Homo sapiens (Human)) | BDBM50581398
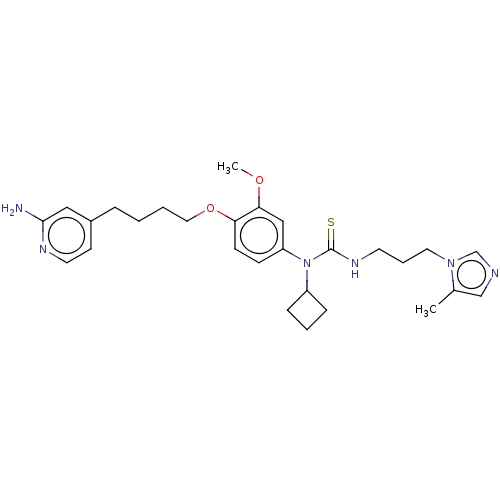
(CHEMBL5083519)Show SMILES COc1cc(ccc1OCCCCc1ccnc(N)c1)N(C1CCC1)C(=S)NCCCn1cncc1C | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant glutaminyl cyclase using H-Gln-AMC hydrobromide as substrate measured after 15 mins by fluorimetry |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113819
BindingDB Entry DOI: 10.7270/Q2M61Q44 |
More data for this
Ligand-Target Pair | |
Glutaminyl-peptide cyclotransferase
(Homo sapiens (Human)) | BDBM50581412

(CHEMBL5088732)Show SMILES COc1cc(ccc1OCCCCc1ccnc(N)c1)N(Cc1ccccc1)C(=O)NCCCn1cncc1C | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant glutaminyl cyclase using H-Gln-AMC hydrobromide as substrate measured after 15 mins by fluorimetry |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113819
BindingDB Entry DOI: 10.7270/Q2M61Q44 |
More data for this
Ligand-Target Pair | |
Glutaminyl-peptide cyclotransferase
(Homo sapiens (Human)) | BDBM50581450

(CHEMBL5085670)Show SMILES COc1cc(ccc1OCCN1CCN(C)CC1)N(CC1CCCC1)C(=O)NCCCn1cncc1C | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant glutaminyl cyclase using H-Gln-AMC hydrobromide as substrate measured after 15 mins by fluorimetry |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113819
BindingDB Entry DOI: 10.7270/Q2M61Q44 |
More data for this
Ligand-Target Pair | |
Glutaminyl-peptide cyclotransferase
(Homo sapiens (Human)) | BDBM50519290
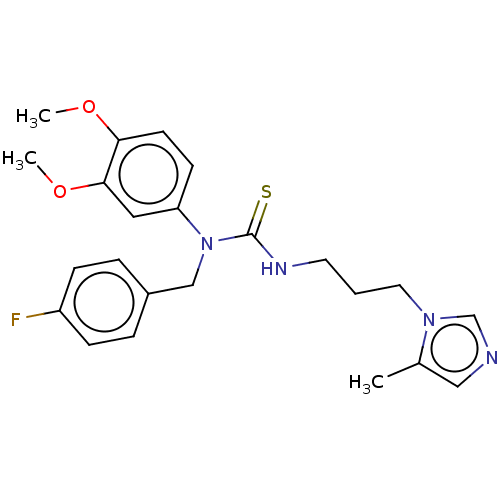
(CHEMBL4525926)Show SMILES COc1ccc(cc1OC)N(Cc1ccc(F)cc1)C(=S)NCCCn1cncc1C Show InChI InChI=1S/C23H27FN4O2S/c1-17-14-25-16-27(17)12-4-11-26-23(31)28(15-18-5-7-19(24)8-6-18)20-9-10-21(29-2)22(13-20)30-3/h5-10,13-14,16H,4,11-12,15H2,1-3H3,(H,26,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal His6-tagged glutaminyl cyclase (Ala33 to Leu361 residues) expressed in baculovirus infected Sf21 insect ce... |
J Med Chem 62: 8011-8027 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00751
BindingDB Entry DOI: 10.7270/Q2W0999V |
More data for this
Ligand-Target Pair | |
Glutaminyl-peptide cyclotransferase
(Homo sapiens (Human)) | BDBM50581445

(CHEMBL5089285)Show SMILES COc1cc(ccc1OCCN1CCNCC1)N(Cc1ccc(F)cc1)C(=O)NCCCn1cncc1C | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant glutaminyl cyclase using H-Gln-AMC hydrobromide as substrate measured after 15 mins by fluorimetry |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113819
BindingDB Entry DOI: 10.7270/Q2M61Q44 |
More data for this
Ligand-Target Pair | |
Glutaminyl-peptide cyclotransferase
(Homo sapiens (Human)) | BDBM50581441
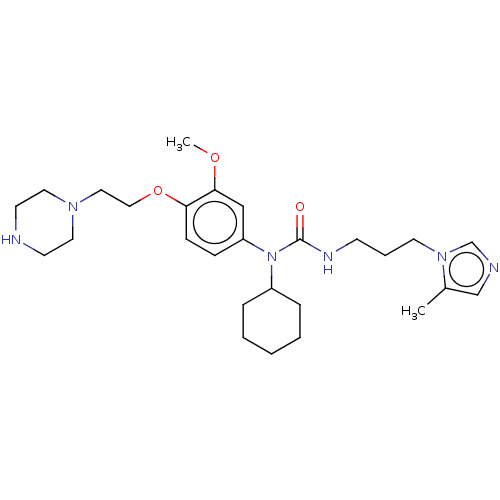
(CHEMBL5081653)Show SMILES COc1cc(ccc1OCCN1CCNCC1)N(C1CCCCC1)C(=O)NCCCn1cncc1C | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant glutaminyl cyclase using H-Gln-AMC hydrobromide as substrate measured after 15 mins by fluorimetry |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113819
BindingDB Entry DOI: 10.7270/Q2M61Q44 |
More data for this
Ligand-Target Pair | |
Glutaminyl-peptide cyclotransferase
(Homo sapiens (Human)) | BDBM50581436

(CHEMBL5081891)Show SMILES COc1cc(ccc1OCCc1ccnc(N)c1)N(Cc1ccc(F)cc1)C(=O)NCCCn1cncc1C | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant glutaminyl cyclase using H-Gln-AMC hydrobromide as substrate measured after 15 mins by fluorimetry |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113819
BindingDB Entry DOI: 10.7270/Q2M61Q44 |
More data for this
Ligand-Target Pair | |
Glutaminyl-peptide cyclotransferase
(Homo sapiens (Human)) | BDBM50581443
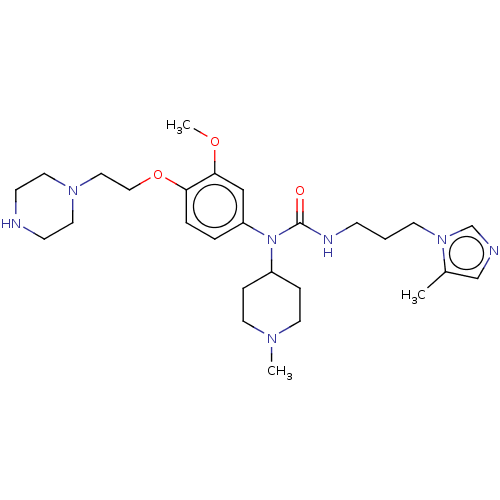
(CHEMBL5083687)Show SMILES COc1cc(ccc1OCCN1CCNCC1)N(C1CCN(C)CC1)C(=O)NCCCn1cncc1C | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant glutaminyl cyclase using H-Gln-AMC hydrobromide as substrate measured after 15 mins by fluorimetry |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113819
BindingDB Entry DOI: 10.7270/Q2M61Q44 |
More data for this
Ligand-Target Pair | |
Glutaminyl-peptide cyclotransferase
(Homo sapiens (Human)) | BDBM50519285
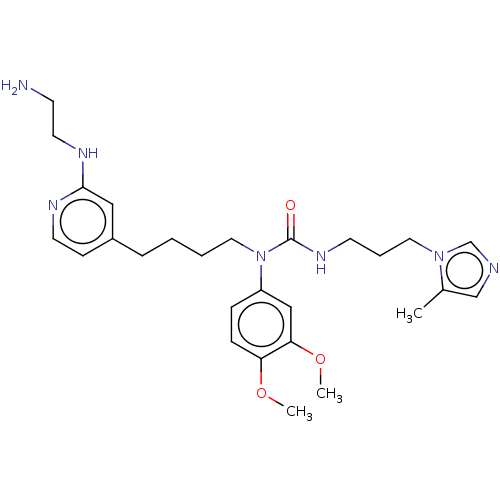
(CHEMBL4445407)Show SMILES COc1ccc(cc1OC)N(CCCCc1ccnc(NCCN)c1)C(=O)NCCCn1cncc1C Show InChI InChI=1S/C27H39N7O3/c1-21-19-29-20-33(21)15-6-12-32-27(35)34(23-8-9-24(36-2)25(18-23)37-3)16-5-4-7-22-10-13-30-26(17-22)31-14-11-28/h8-10,13,17-20H,4-7,11-12,14-16,28H2,1-3H3,(H,30,31)(H,32,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal His6-tagged glutaminyl cyclase (Ala33 to Leu361 residues) expressed in baculovirus infected Sf21 insect ce... |
J Med Chem 62: 8011-8027 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00751
BindingDB Entry DOI: 10.7270/Q2W0999V |
More data for this
Ligand-Target Pair | |
Glutaminyl-peptide cyclotransferase
(Homo sapiens (Human)) | BDBM50581434

(CHEMBL5081537)Show SMILES COc1cc(ccc1OCCc1ccnc(N)c1)N(C1CCCCC1)C(=O)NCCCn1cncc1C | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant glutaminyl cyclase using H-Gln-AMC hydrobromide as substrate measured after 15 mins by fluorimetry |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113819
BindingDB Entry DOI: 10.7270/Q2M61Q44 |
More data for this
Ligand-Target Pair | |
Glutaminyl-peptide cyclotransferase
(Homo sapiens (Human)) | BDBM50454733

(CHEMBL4204093)Show SMILES COc1cc(NC(=S)NCCCn2cncc2C)ccc1OCCCCc1ccnc(NCCN)c1 Show InChI InChI=1S/C26H37N7O2S/c1-20-18-28-19-33(20)14-5-11-31-26(36)32-22-7-8-23(24(17-22)34-2)35-15-4-3-6-21-9-12-29-25(16-21)30-13-10-27/h7-9,12,16-19H,3-6,10-11,13-15,27H2,1-2H3,(H,29,30)(H2,31,32,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Inhibition of human glutaminyl cyclase using Gln-AMC as substrate by pGAPase coupled fluorescence assay |
Bioorg Med Chem 26: 1035-1049 (2018)
Article DOI: 10.1016/j.bmc.2018.01.015
BindingDB Entry DOI: 10.7270/Q2SN0CKG |
More data for this
Ligand-Target Pair | |
Glutaminyl-peptide cyclotransferase
(Homo sapiens (Human)) | BDBM50581451
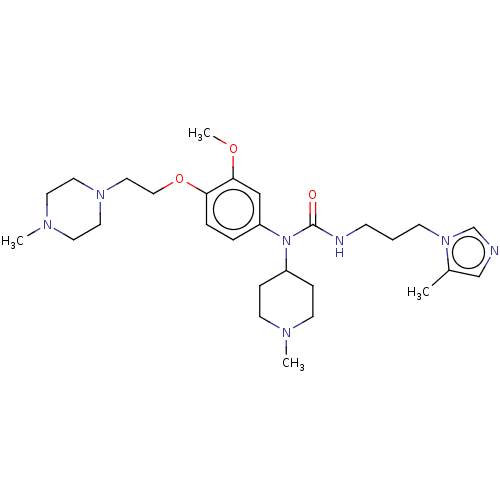
(CHEMBL5092925)Show SMILES COc1cc(ccc1OCCN1CCN(C)CC1)N(C1CCN(C)CC1)C(=O)NCCCn1cncc1C | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant glutaminyl cyclase using H-Gln-AMC hydrobromide as substrate measured after 15 mins by fluorimetry |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113819
BindingDB Entry DOI: 10.7270/Q2M61Q44 |
More data for this
Ligand-Target Pair | |
Glutaminyl-peptide cyclotransferase
(Homo sapiens (Human)) | CHEMBL5279792
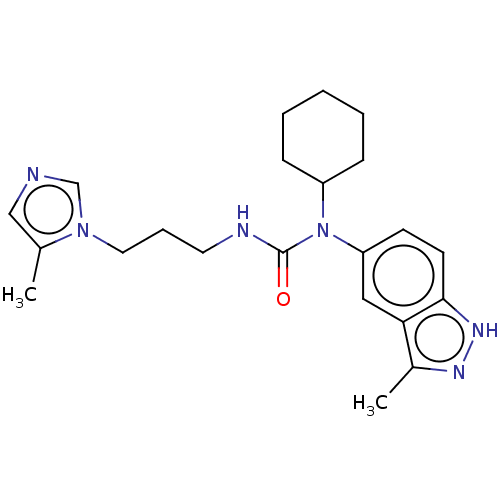
| PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glutaminyl-peptide cyclotransferase
(Homo sapiens (Human)) | CHEMBL5266674
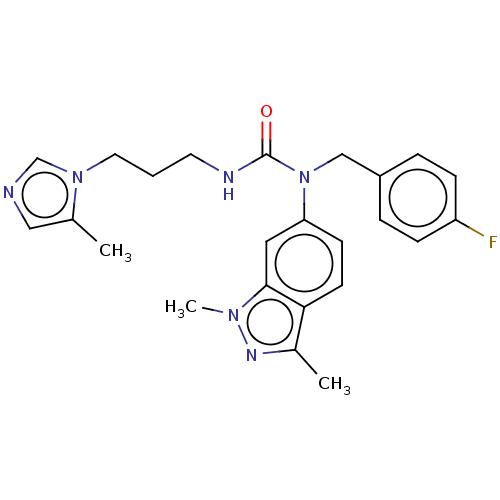
| PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glutaminyl-peptide cyclotransferase
(Homo sapiens (Human)) | BDBM50581460

(CHEMBL5084485)Show SMILES COc1cc(ccc1OCCN1CCOCC1)N(Cc1ccc(F)cc1)C(=O)NCCCn1cncc1C | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant glutaminyl cyclase using H-Gln-AMC hydrobromide as substrate measured after 15 mins by fluorimetry |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113819
BindingDB Entry DOI: 10.7270/Q2M61Q44 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50049553

(CHEMBL2177428)Show SMILES CC(C(=O)NCc1ccc(nc1N1CCC(C)CC1)C(F)(F)F)c1ccc(NS(C)(=O)=O)c(F)c1 Show InChI InChI=1S/C23H28F4N4O3S/c1-14-8-10-31(11-9-14)21-17(5-7-20(29-21)23(25,26)27)13-28-22(32)15(2)16-4-6-19(18(24)12-16)30-35(3,33)34/h4-7,12,14-15,30H,8-11,13H2,1-3H3,(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of 45 degC heat-induced activation by FLIPR assay |
Bioorg Med Chem Lett 25: 2326-30 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.024
BindingDB Entry DOI: 10.7270/Q2Z60QSC |
More data for this
Ligand-Target Pair | |
Glutaminyl-peptide cyclotransferase
(Homo sapiens (Human)) | BDBM50581448

(CHEMBL5094369)Show SMILES COc1cc(ccc1OCCN1CCN(C)CC1)N(C(C)C)C(=O)NCCCn1cncc1C | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant glutaminyl cyclase using H-Gln-AMC hydrobromide as substrate measured after 15 mins by fluorimetry |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113819
BindingDB Entry DOI: 10.7270/Q2M61Q44 |
More data for this
Ligand-Target Pair | |
Glutaminyl-peptide cyclotransferase
(Homo sapiens (Human)) | BDBM50581422
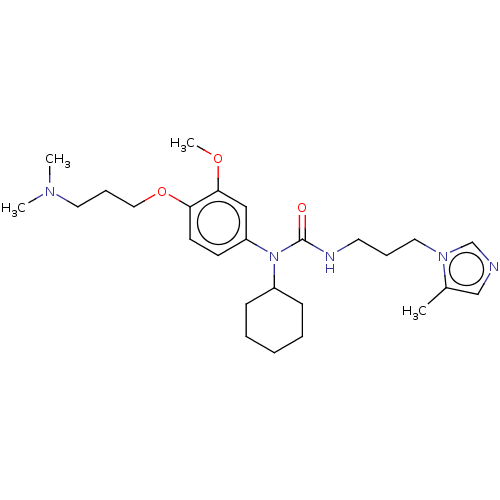
(CHEMBL5079181)Show SMILES COc1cc(ccc1OCCCN(C)C)N(C1CCCCC1)C(=O)NCCCn1cncc1C | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant glutaminyl cyclase using H-Gln-AMC hydrobromide as substrate measured after 15 mins by fluorimetry |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113819
BindingDB Entry DOI: 10.7270/Q2M61Q44 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data