

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM29525 (3H-GR113808 | CHEMBL518682 | GR 113808 | [3H] GR 1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Drug Discovery Laboratory AS Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from human 5-HT4B receptor expressed in HEK293 cells after 1 hr by liquid scintillation counting analysis | Eur J Med Chem 64: 629-37 (2013) Article DOI: 10.1016/j.ejmech.2013.03.060 BindingDB Entry DOI: 10.7270/Q27947NT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM85026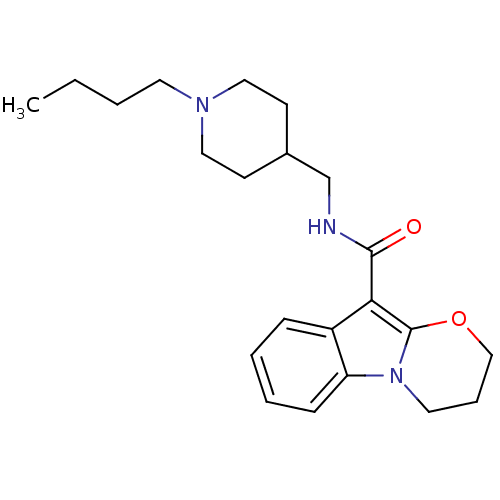 (N-(1-Butylpiperidine-4-ylmethyl)-1,2-(trimethylene...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Drug Discovery Laboratory AS Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from human 5-HT4B receptor expressed in HEK293 cells after 1 hr by liquid scintillation counting analysis | Eur J Med Chem 64: 629-37 (2013) Article DOI: 10.1016/j.ejmech.2013.03.060 BindingDB Entry DOI: 10.7270/Q27947NT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50492064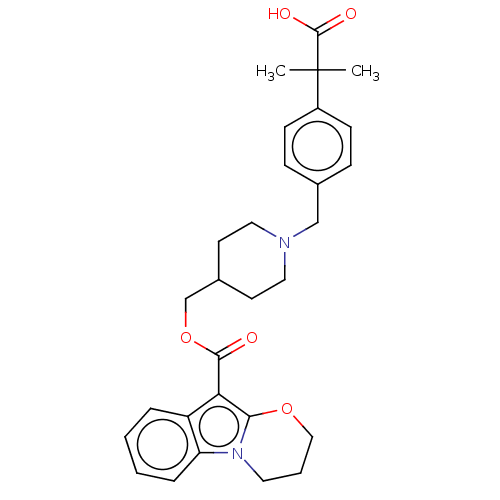 (CHEMBL2391994) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Drug Discovery Laboratory AS Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from human 5-HT4B receptor expressed in HEK293 cells after 1 hr by liquid scintillation counting analysis | Eur J Med Chem 64: 629-37 (2013) Article DOI: 10.1016/j.ejmech.2013.03.060 BindingDB Entry DOI: 10.7270/Q27947NT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16047 ((4S)-4-[(2S)-2-[(2R,4S,5S)-5-[(2S)-2-[(2S)-2-[(4S)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 1.60 | -52.2 | n/a | n/a | n/a | n/a | n/a | 4.5 | 37 |
University of Illinois at Chicago | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Med Chem 44: 2865-8 (2001) Article DOI: 10.1021/jm0101803 BindingDB Entry DOI: 10.7270/Q2BG2M7W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16250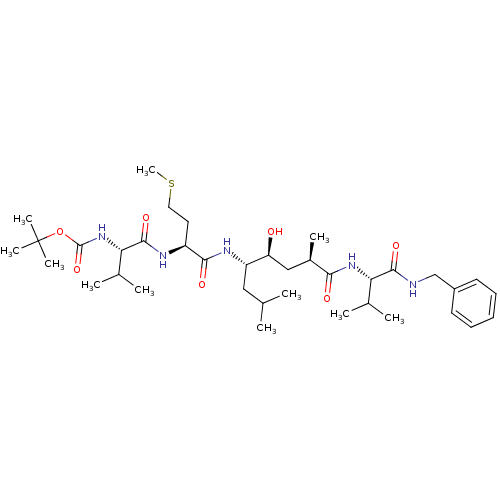 (CHEMBL290001 | N-(tert-butoxycarbonyl)-L-valyl-N-[...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.5 | -51.1 | n/a | n/a | n/a | n/a | n/a | 4.5 | 37 |
University of Illinois at Chicago | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Med Chem 44: 2865-8 (2001) Article DOI: 10.1021/jm0101803 BindingDB Entry DOI: 10.7270/Q2BG2M7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16777 (Substrate-based BACE-1 inhibitor, 16 | tert-butyl ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 5.90 | -48.9 | n/a | n/a | n/a | n/a | n/a | 4.5 | 37 |
University of Illinois at Chicago | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Med Chem 44: 2865-8 (2001) Article DOI: 10.1021/jm0101803 BindingDB Entry DOI: 10.7270/Q2BG2M7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16781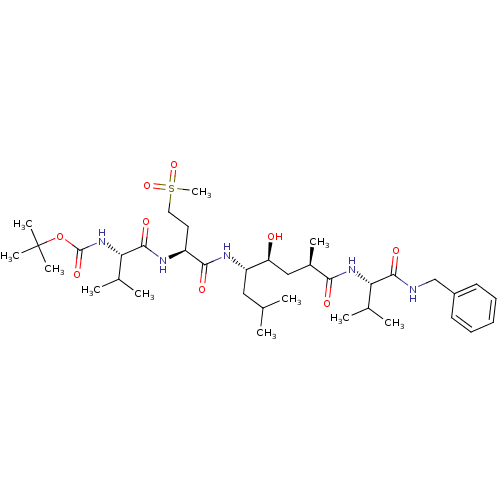 (Substrate-based BACE-1 inhibitor, 23 | tert-butyl ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 8 | -48.1 | n/a | n/a | n/a | n/a | n/a | 4.5 | 37 |
University of Illinois at Chicago | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Med Chem 44: 2865-8 (2001) Article DOI: 10.1021/jm0101803 BindingDB Entry DOI: 10.7270/Q2BG2M7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16779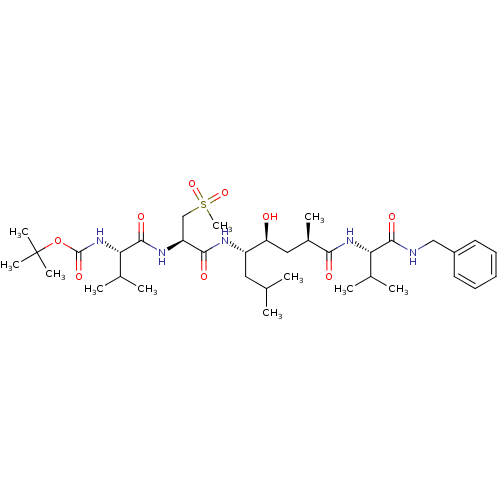 (Substrate-based BACE-1 inhibitor, 18 | tert-butyl ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 9.40 | -47.7 | n/a | n/a | n/a | n/a | n/a | 4.5 | 37 |
University of Illinois at Chicago | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Med Chem 44: 2865-8 (2001) Article DOI: 10.1021/jm0101803 BindingDB Entry DOI: 10.7270/Q2BG2M7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16772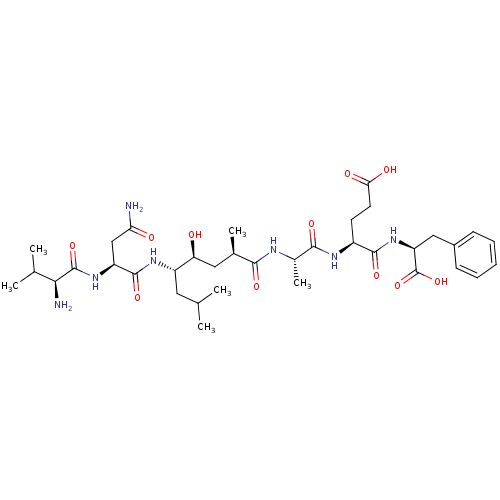 ((4S)-4-[(2S)-2-[(2R,4S,5S)-5-[(2S)-2-[(2S)-2-amino...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 36 | -44.2 | n/a | n/a | n/a | n/a | n/a | 4.5 | 37 |
University of Illinois at Chicago | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Med Chem 44: 2865-8 (2001) Article DOI: 10.1021/jm0101803 BindingDB Entry DOI: 10.7270/Q2BG2M7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16778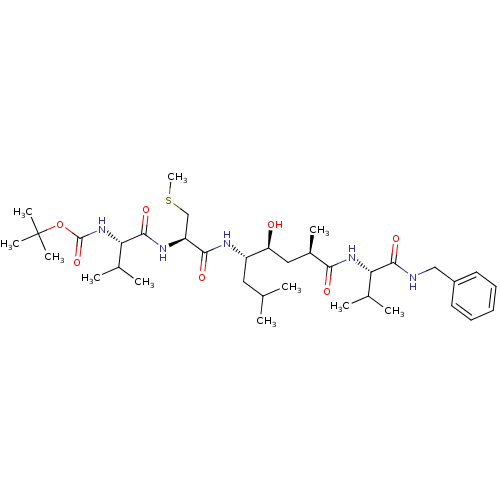 (Substrate-based BACE-1 inhibitor, 17 | tert-butyl ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 50.1 | -43.3 | n/a | n/a | n/a | n/a | n/a | 4.5 | 37 |
University of Illinois at Chicago | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Med Chem 44: 2865-8 (2001) Article DOI: 10.1021/jm0101803 BindingDB Entry DOI: 10.7270/Q2BG2M7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16776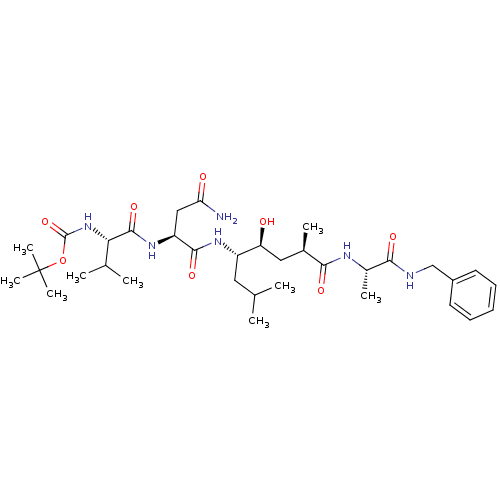 (Substrate-based BACE-1 inhibitor, 15 | tert-butyl ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 61.4 | -42.8 | n/a | n/a | n/a | n/a | n/a | 4.5 | 37 |
University of Illinois at Chicago | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Med Chem 44: 2865-8 (2001) Article DOI: 10.1021/jm0101803 BindingDB Entry DOI: 10.7270/Q2BG2M7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50064657 (CHEMBL277390 | Cyclo(Lys(alphaH2+)-Pro-Aba-[CH2SMe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Versailles Curated by ChEMBL | Assay Description Inhibitory activity against hydrolysis of Gly-Pro-pNa by CD26 (dipeptidylpeptidase 4) purified from CEM H01 cells | J Med Chem 41: 2100-10 (1998) Article DOI: 10.1021/jm970640l BindingDB Entry DOI: 10.7270/Q22N52XM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Deoxyuridine 5'-triphosphate nucleotidohydrolase (Plasmodium falciparum) | BDBM50190543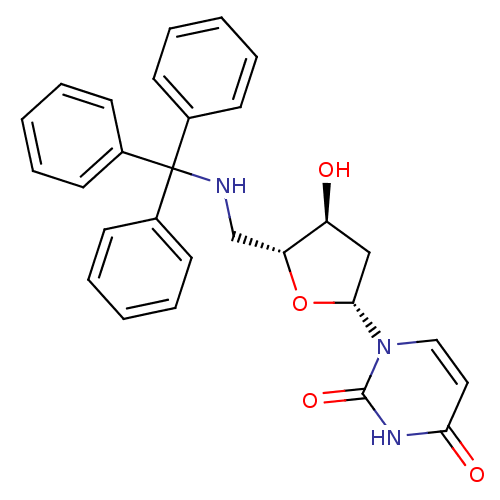 (1-((2R,4S,5R)-4-hydroxy-5-((tritylamino)methyl)-te...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum dUTPase | J Med Chem 49: 4183-95 (2006) Article DOI: 10.1021/jm060126s BindingDB Entry DOI: 10.7270/Q22J6BF0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Deoxyuridine 5'-triphosphate nucleotidohydrolase (Plasmodium falciparum) | BDBM50190556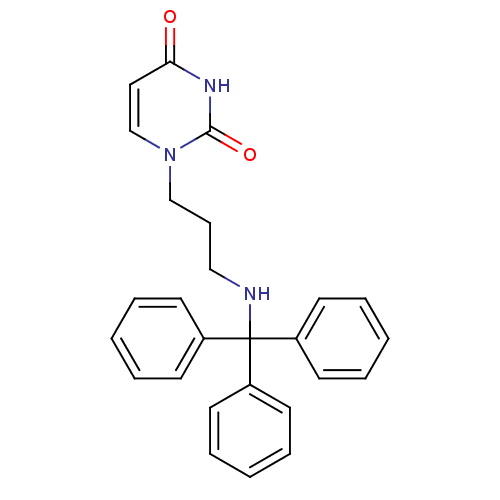 (1-(3-tritylaminopropyl)uracil | CHEMBL211905) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum dUTPase | J Med Chem 49: 4183-95 (2006) Article DOI: 10.1021/jm060126s BindingDB Entry DOI: 10.7270/Q22J6BF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Deoxyuridine 5'-triphosphate nucleotidohydrolase (Plasmodium falciparum) | BDBM50173577 (1-{4-Hydroxy-5-[(trityl-amino)-methyl]-tetrahydro-...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University Curated by ChEMBL | Assay Description Inhibitory constant against Plasmodium falciparum deoxyuridine 5'-triphosphate nucleotidohydrolase expressed in Escherichia coli BL21 (DE3) cells | J Med Chem 48: 5942-54 (2005) Article DOI: 10.1021/jm050111e BindingDB Entry DOI: 10.7270/Q2Z320DG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Deoxyuridine 5'-triphosphate nucleotidohydrolase (Plasmodium falciparum) | BDBM50190549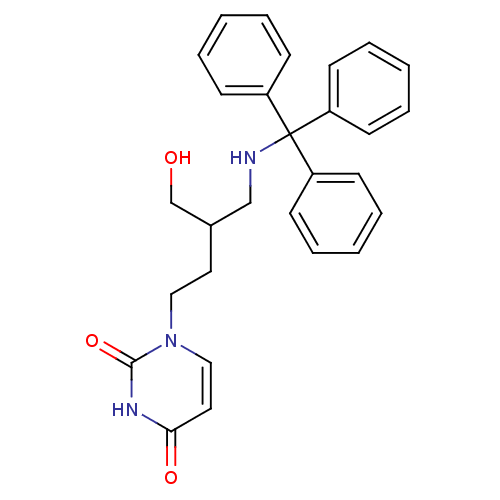 (1-[4-hydroxy-3-(tritylaminomethyl)butyl]uracil | C...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum dUTPase | J Med Chem 49: 4183-95 (2006) Article DOI: 10.1021/jm060126s BindingDB Entry DOI: 10.7270/Q22J6BF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50064657 (CHEMBL277390 | Cyclo(Lys(alphaH2+)-Pro-Aba-[CH2SMe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Versailles Curated by ChEMBL | Assay Description Inhibitory kinetic constant against Dipeptidyl peptidase IV purified from CD26-negative C8166 cells | J Med Chem 41: 2100-10 (1998) Article DOI: 10.1021/jm970640l BindingDB Entry DOI: 10.7270/Q22N52XM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Deoxyuridine 5'-triphosphate nucleotidohydrolase (Plasmodium falciparum) | BDBM50190552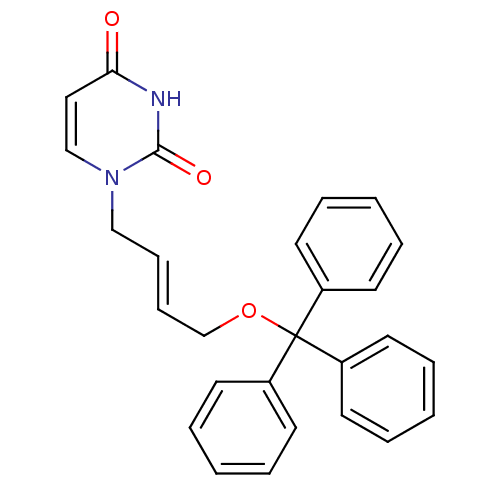 (1-[(E)-4-trityloxy-2-butenyl]uracil | CHEMBL209610) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum dUTPase | J Med Chem 49: 4183-95 (2006) Article DOI: 10.1021/jm060126s BindingDB Entry DOI: 10.7270/Q22J6BF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Deoxyuridine 5'-triphosphate nucleotidohydrolase (Plasmodium falciparum) | BDBM50190553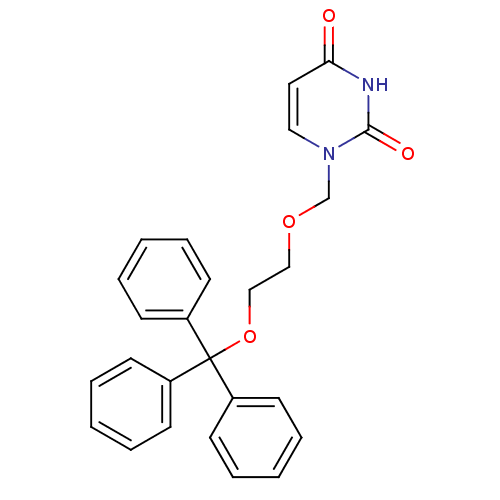 (1-[2-(trityloxy)ethoxymethyl]uracil | CHEMBL424704...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum dUTPase | J Med Chem 49: 4183-95 (2006) Article DOI: 10.1021/jm060126s BindingDB Entry DOI: 10.7270/Q22J6BF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Deoxyuridine 5'-triphosphate nucleotidohydrolase (Plasmodium falciparum) | BDBM50190555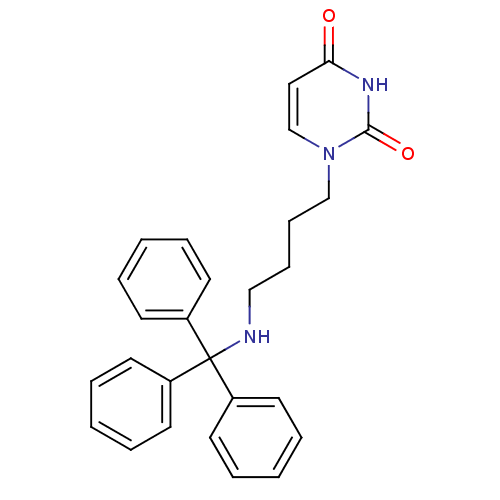 (1-(4-tritylaminobutyl)uracil | CHEMBL211181) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum dUTPase | J Med Chem 49: 4183-95 (2006) Article DOI: 10.1021/jm060126s BindingDB Entry DOI: 10.7270/Q22J6BF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Deoxyuridine 5'-triphosphate nucleotidohydrolase (Plasmodium falciparum) | BDBM50190532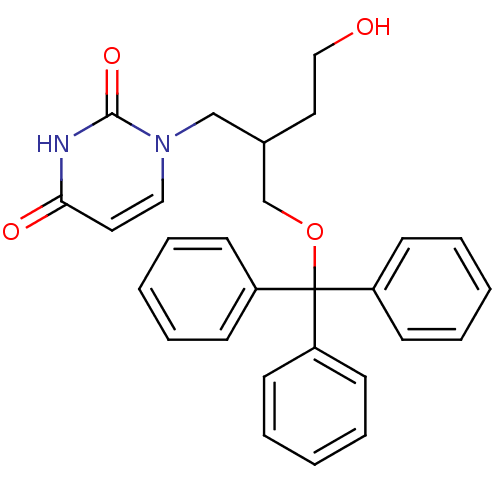 (1-[4-hydroxy-2-(trityloxymethyl)butyl]uracil | CHE...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum dUTPase | J Med Chem 49: 4183-95 (2006) Article DOI: 10.1021/jm060126s BindingDB Entry DOI: 10.7270/Q22J6BF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16775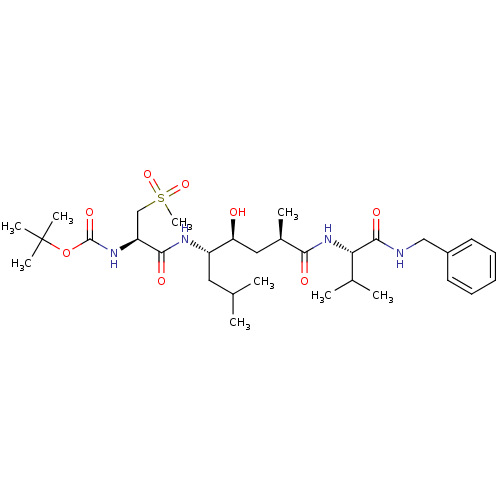 (CHEMBL273916 | Substrate-based BACE-1 inhibitor, 1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.13E+3 | -35.3 | n/a | n/a | n/a | n/a | n/a | 4.5 | 37 |
University of Illinois at Chicago | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Med Chem 44: 2865-8 (2001) Article DOI: 10.1021/jm0101803 BindingDB Entry DOI: 10.7270/Q2BG2M7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Deoxyuridine 5'-triphosphate nucleotidohydrolase (Plasmodium falciparum) | BDBM50173540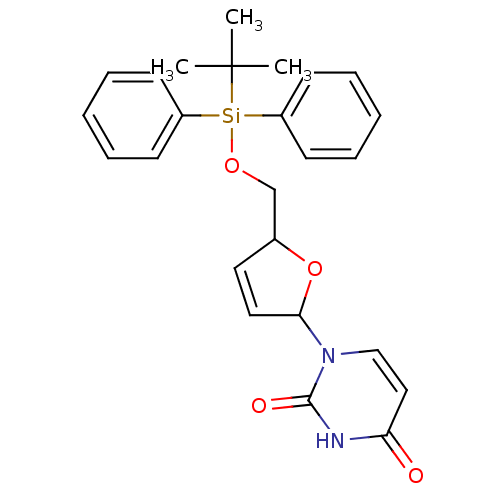 (1-[5-(tert-Butyl-diphenyl-silanyloxymethyl)-2,5-di...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.16E+3 | -33.9 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Instituto de Parastiologia y Biomedicina "Lopez-Neyra" | Assay Description Nucleotide hydrolysis was monitored by mixing enzyme and substrate with a rapid kinetic accessory (Hi-Tech Scientific) attached to a spectrophotomete... | J Enzyme Inhib Med Chem 24: 111-6 (2009) Article DOI: 10.1080/14756360801915476 BindingDB Entry DOI: 10.7270/Q2JH3JST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Deoxyuridine 5'-triphosphate nucleotidohydrolase (Plasmodium falciparum) | BDBM50173540 (1-[5-(tert-Butyl-diphenyl-silanyloxymethyl)-2,5-di...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University Curated by ChEMBL | Assay Description Inhibitory constant against Plasmodium falciparum deoxyuridine 5'-triphosphate nucleotidohydrolase expressed in Escherichia coli BL21 (DE3) cells | J Med Chem 48: 5942-54 (2005) Article DOI: 10.1021/jm050111e BindingDB Entry DOI: 10.7270/Q2Z320DG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Deoxyuridine 5'-triphosphate nucleotidohydrolase (Plasmodium falciparum) | BDBM50190531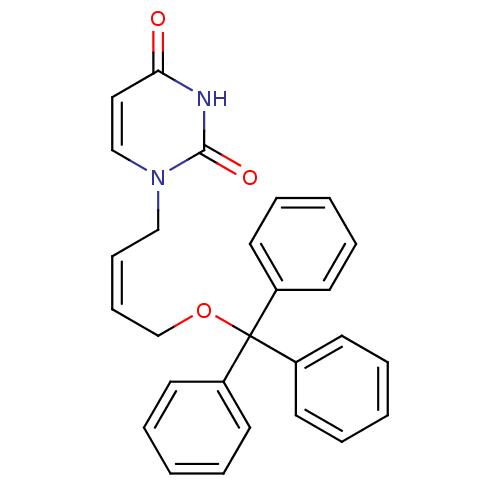 (1-[(Z)-4-trityloxy-2-butenyl]uracil | CHEMBL377199) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum dUTPase | J Med Chem 49: 4183-95 (2006) Article DOI: 10.1021/jm060126s BindingDB Entry DOI: 10.7270/Q22J6BF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Deoxyuridine 5'-triphosphate nucleotidohydrolase (Plasmodium falciparum) | BDBM50190564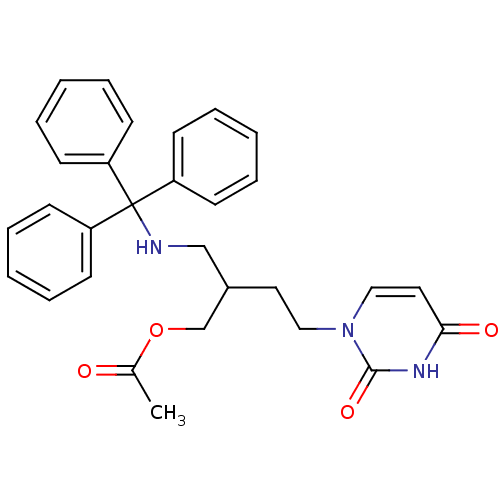 (1-[4-acetyloxy-3-(tritylaminomethyl)butyl]uracil |...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum dUTPase | J Med Chem 49: 4183-95 (2006) Article DOI: 10.1021/jm060126s BindingDB Entry DOI: 10.7270/Q22J6BF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Deoxyuridine 5'-triphosphate nucleotidohydrolase (Plasmodium falciparum) | BDBM50173535 (1-(5-Triphenylsilanyloxymethyl-2,5-dihydro-furan-2...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University Curated by ChEMBL | Assay Description Inhibitory constant against Plasmodium falciparum deoxyuridine 5'-triphosphate nucleotidohydrolase expressed in Escherichia coli BL21 (DE3) cells | J Med Chem 48: 5942-54 (2005) Article DOI: 10.1021/jm050111e BindingDB Entry DOI: 10.7270/Q2Z320DG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Deoxyuridine 5'-triphosphate nucleotidohydrolase, mitochondrial (Homo sapiens (Human)) | BDBM50190556 (1-(3-tritylaminopropyl)uracil | CHEMBL211905) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University Curated by ChEMBL | Assay Description Inhibition of human recombinant dUTPase | J Med Chem 49: 4183-95 (2006) Article DOI: 10.1021/jm060126s BindingDB Entry DOI: 10.7270/Q22J6BF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Deoxyuridine 5'-triphosphate nucleotidohydrolase, mitochondrial (Homo sapiens (Human)) | BDBM50190556 (1-(3-tritylaminopropyl)uracil | CHEMBL211905) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Parasitolog£a y Biomedicina L£pez-Neyra Curated by ChEMBL | Assay Description Inhibition of human dUTPase using dUTP as substrate by spectrophotometric analysis | Eur J Med Chem 46: 3309-14 (2011) Article DOI: 10.1016/j.ejmech.2011.04.052 BindingDB Entry DOI: 10.7270/Q27S7Q1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Deoxyuridine 5'-triphosphate nucleotidohydrolase (Plasmodium falciparum) | BDBM50190547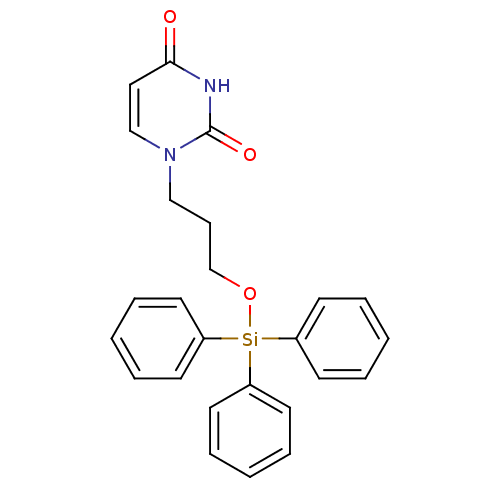 (1-(3-triphenylsilyloxypropyl)uracil | CHEMBL211906) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum dUTPase | J Med Chem 49: 4183-95 (2006) Article DOI: 10.1021/jm060126s BindingDB Entry DOI: 10.7270/Q22J6BF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Deoxyuridine 5'-triphosphate nucleotidohydrolase (Plasmodium falciparum) | BDBM50190530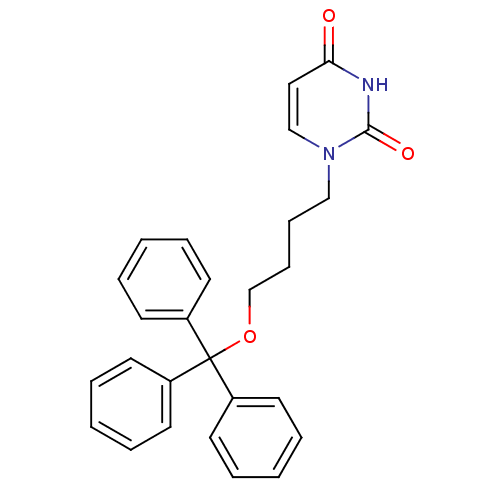 (1-(4-trityloxybutyl)uracil | CHEMBL211067) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum dUTPase | J Med Chem 49: 4183-95 (2006) Article DOI: 10.1021/jm060126s BindingDB Entry DOI: 10.7270/Q22J6BF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Deoxyuridine 5'-triphosphate nucleotidohydrolase (Plasmodium falciparum) | BDBM50190562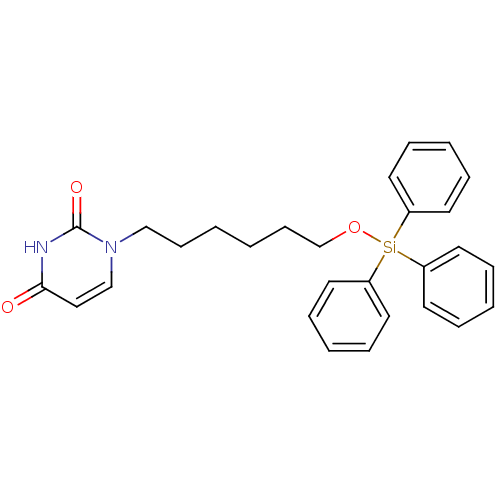 (1-(6-triphenylsilyloxyhexyl)uracil | CHEMBL212690) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum dUTPase | J Med Chem 49: 4183-95 (2006) Article DOI: 10.1021/jm060126s BindingDB Entry DOI: 10.7270/Q22J6BF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Deoxyuridine 5'-triphosphate nucleotidohydrolase (Plasmodium falciparum) | BDBM50190568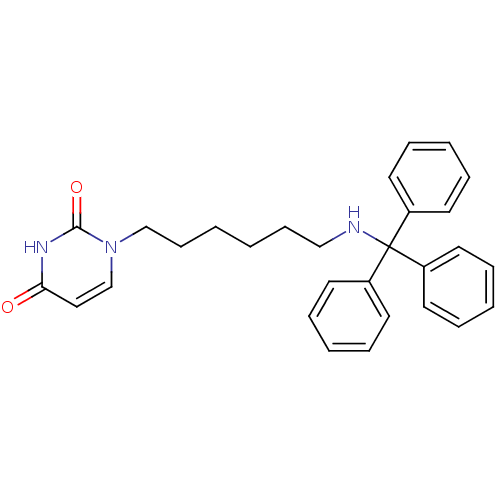 (1-(6-(tritylamino)hexyl)pyrimidine-2,4(1H,3H)-dion...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum dUTPase | J Med Chem 49: 4183-95 (2006) Article DOI: 10.1021/jm060126s BindingDB Entry DOI: 10.7270/Q22J6BF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Deoxyuridine 5'-triphosphate nucleotidohydrolase (Plasmodium falciparum) | BDBM50173539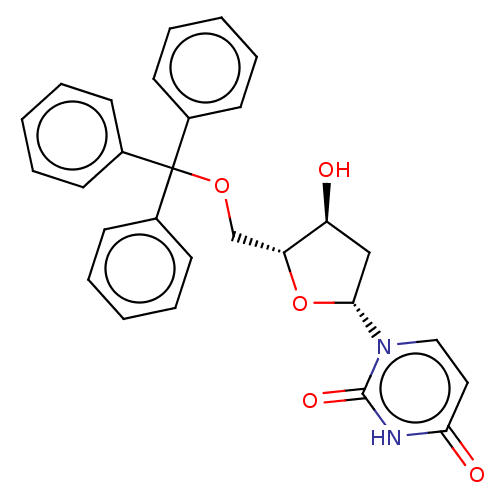 (1-(4-Hydroxy-5-trityloxymethyl-tetrahydro-furan-2-...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University Curated by ChEMBL | Assay Description Inhibitory constant against Plasmodium falciparum deoxyuridine 5'-triphosphate nucleotidohydrolase expressed in Escherichia coli BL21 (DE3) cells | J Med Chem 48: 5942-54 (2005) Article DOI: 10.1021/jm050111e BindingDB Entry DOI: 10.7270/Q2Z320DG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Deoxyuridine 5'-triphosphate nucleotidohydrolase (Plasmodium falciparum) | BDBM50173539 (1-(4-Hydroxy-5-trityloxymethyl-tetrahydro-furan-2-...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum dUTPase | J Med Chem 49: 4183-95 (2006) Article DOI: 10.1021/jm060126s BindingDB Entry DOI: 10.7270/Q22J6BF0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Deoxyuridine 5'-triphosphate nucleotidohydrolase (Plasmodium falciparum) | BDBM50173546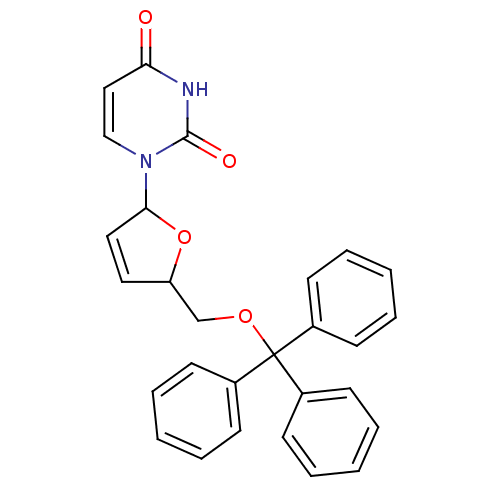 (1-(5-Trityloxymethyl-2,5-dihydro-furan-2-yl)-1H-py...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.90E+3 | -32.7 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Instituto de Parastiologia y Biomedicina "Lopez-Neyra" | Assay Description Nucleotide hydrolysis was monitored by mixing enzyme and substrate with a rapid kinetic accessory (Hi-Tech Scientific) attached to a spectrophotomete... | J Enzyme Inhib Med Chem 24: 111-6 (2009) Article DOI: 10.1080/14756360801915476 BindingDB Entry DOI: 10.7270/Q2JH3JST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Deoxyuridine 5'-triphosphate nucleotidohydrolase (Plasmodium falciparum) | BDBM50173546 (1-(5-Trityloxymethyl-2,5-dihydro-furan-2-yl)-1H-py...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University Curated by ChEMBL | Assay Description Inhibitory constant against Plasmodium falciparum deoxyuridine 5'-triphosphate nucleotidohydrolase expressed in Escherichia coli BL21 (DE3) cells | J Med Chem 48: 5942-54 (2005) Article DOI: 10.1021/jm050111e BindingDB Entry DOI: 10.7270/Q2Z320DG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Deoxyuridine 5'-triphosphate nucleotidohydrolase (Plasmodium falciparum) | BDBM50190550 (1-(5-trityloxypentyl)uracil | CHEMBL377472) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum dUTPase | J Med Chem 49: 4183-95 (2006) Article DOI: 10.1021/jm060126s BindingDB Entry DOI: 10.7270/Q22J6BF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Deoxyuridine 5'-triphosphate nucleotidohydrolase (Plasmodium falciparum) | BDBM50190565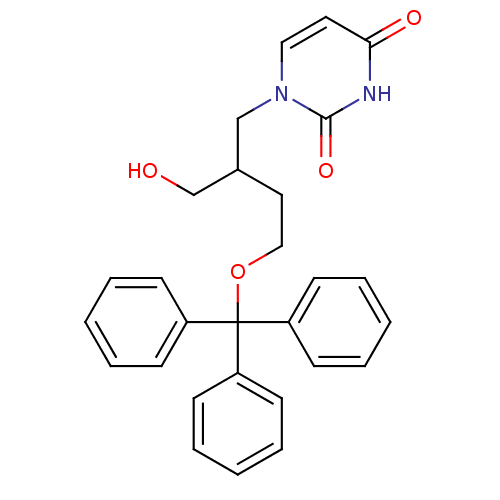 (1-[2-(hydroxymethyl)-4-(trityloxy)butyl]uracil | C...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum dUTPase | J Med Chem 49: 4183-95 (2006) Article DOI: 10.1021/jm060126s BindingDB Entry DOI: 10.7270/Q22J6BF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Deoxyuridine 5'-triphosphate nucleotidohydrolase (Plasmodium falciparum) | BDBM50190567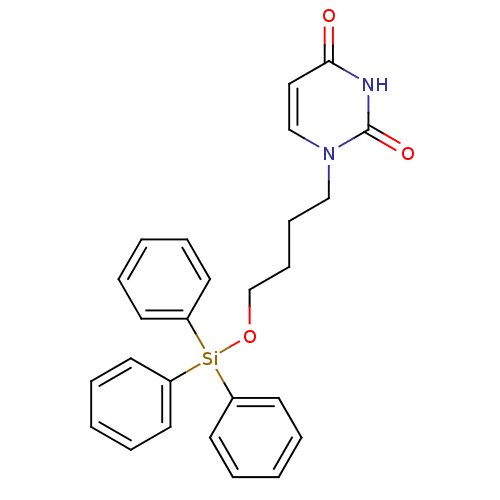 (1-(4-triphenylsilyloxybutyl)uracil | CHEMBL387177) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum dUTPase | J Med Chem 49: 4183-95 (2006) Article DOI: 10.1021/jm060126s BindingDB Entry DOI: 10.7270/Q22J6BF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Deoxyuridine 5'-triphosphate nucleotidohydrolase (Plasmodium falciparum) | BDBM50190558 (1-(6-trityloxyhexyl)uracil | CHEMBL378414) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum dUTPase | J Med Chem 49: 4183-95 (2006) Article DOI: 10.1021/jm060126s BindingDB Entry DOI: 10.7270/Q22J6BF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Deoxyuridine 5'-triphosphate nucleotidohydrolase (Plasmodium falciparum) | BDBM50190533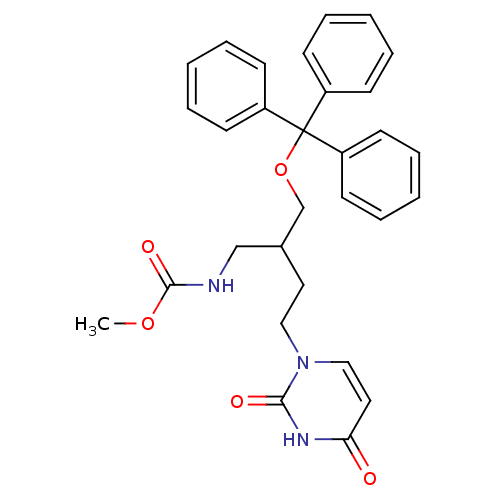 (1-[4-(tert-butoxycarbonylamino)-3-(trityloxymethyl...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum dUTPase | J Med Chem 49: 4183-95 (2006) Article DOI: 10.1021/jm060126s BindingDB Entry DOI: 10.7270/Q22J6BF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Deoxyuridine 5'-triphosphate nucleotidohydrolase (Plasmodium falciparum) | BDBM50190545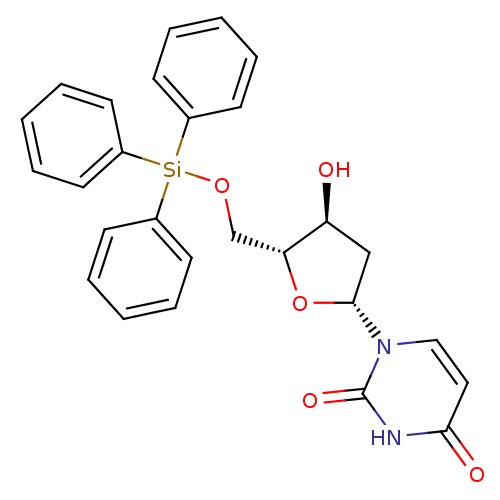 (1-((2R,4S,5R)-4-hydroxy-5-((triphenylsilyloxy)meth...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum dUTPase | J Med Chem 49: 4183-95 (2006) Article DOI: 10.1021/jm060126s BindingDB Entry DOI: 10.7270/Q22J6BF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Deoxyuridine 5'-triphosphate nucleotidohydrolase (Plasmodium falciparum) | BDBM50173531 (1-(4-Hydroxy-5-triphenylsilanyloxymethyl-tetrahydr...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.80E+3 | -31.7 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Instituto de Parastiologia y Biomedicina "Lopez-Neyra" | Assay Description Nucleotide hydrolysis was monitored by mixing enzyme and substrate with a rapid kinetic accessory (Hi-Tech Scientific) attached to a spectrophotomete... | J Enzyme Inhib Med Chem 24: 111-6 (2009) Article DOI: 10.1080/14756360801915476 BindingDB Entry DOI: 10.7270/Q2JH3JST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Deoxyuridine 5'-triphosphate nucleotidohydrolase (Plasmodium falciparum) | BDBM50190545 (1-((2R,4S,5R)-4-hydroxy-5-((triphenylsilyloxy)meth...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University Curated by ChEMBL | Assay Description Inhibitory constant against Plasmodium falciparum deoxyuridine 5'-triphosphate nucleotidohydrolase expressed in Escherichia coli BL21 (DE3) cells | J Med Chem 48: 5942-54 (2005) Article DOI: 10.1021/jm050111e BindingDB Entry DOI: 10.7270/Q2Z320DG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16774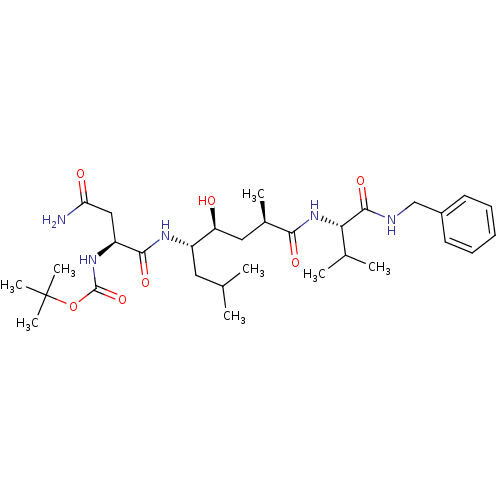 (Substrate-based BACE-1 inhibitor, 13 | tert-butyl ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 3.13E+3 | -32.7 | n/a | n/a | n/a | n/a | n/a | 4.5 | 37 |
University of Illinois at Chicago | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Med Chem 44: 2865-8 (2001) Article DOI: 10.1021/jm0101803 BindingDB Entry DOI: 10.7270/Q2BG2M7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Deoxyuridine 5'-triphosphate nucleotidohydrolase (Plasmodium falciparum) | BDBM50190537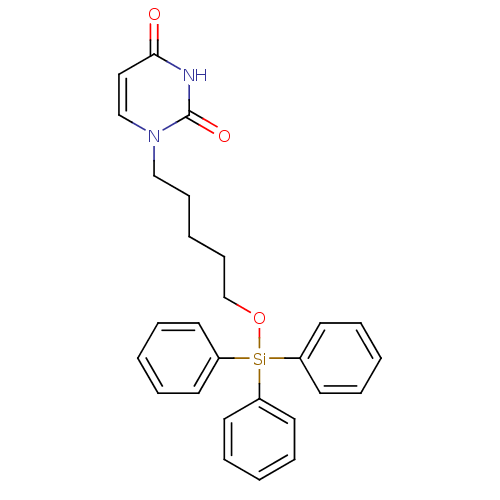 (1-(5-triphenylsilyloxypentyl)uracil | CHEMBL212175) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum dUTPase | J Med Chem 49: 4183-95 (2006) Article DOI: 10.1021/jm060126s BindingDB Entry DOI: 10.7270/Q22J6BF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Deoxyuridine 5'-triphosphate nucleotidohydrolase (Plasmodium falciparum) | BDBM50266744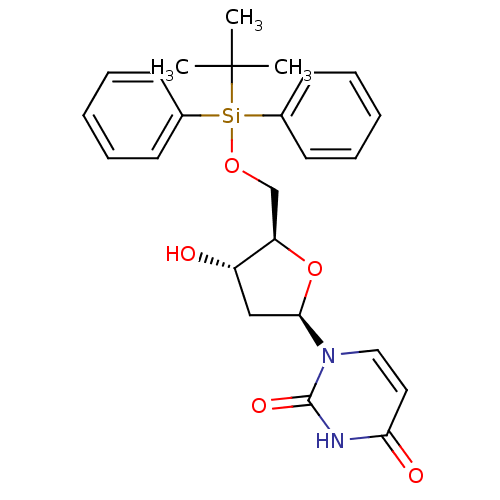 (1-((2R,4S,5R)-5-((tert-butyldiphenylsilyloxy)methy...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University Curated by ChEMBL | Assay Description Inhibitory constant against Plasmodium falciparum deoxyuridine 5'-triphosphate nucleotidohydrolase expressed in Escherichia coli BL21 (DE3) cells | J Med Chem 48: 5942-54 (2005) Article DOI: 10.1021/jm050111e BindingDB Entry DOI: 10.7270/Q2Z320DG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Deoxyuridine 5'-triphosphate nucleotidohydrolase (Plasmodium falciparum) | BDBM50173530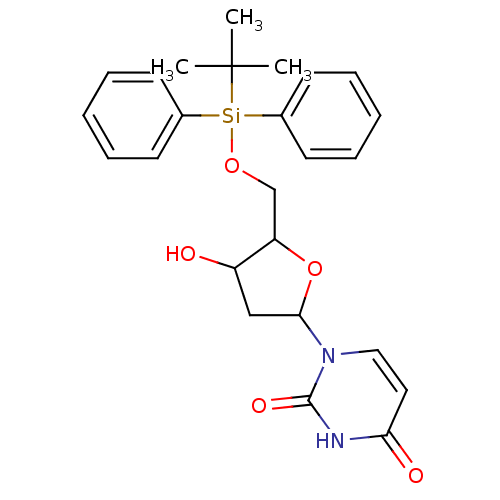 (1-[5-(tert-Butyl-diphenyl-silanyloxymethyl)-4-hydr...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.20E+3 | -30.7 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Instituto de Parastiologia y Biomedicina "Lopez-Neyra" | Assay Description Nucleotide hydrolysis was monitored by mixing enzyme and substrate with a rapid kinetic accessory (Hi-Tech Scientific) attached to a spectrophotomete... | J Enzyme Inhib Med Chem 24: 111-6 (2009) Article DOI: 10.1080/14756360801915476 BindingDB Entry DOI: 10.7270/Q2JH3JST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Deoxyuridine 5'-triphosphate nucleotidohydrolase (Plasmodium falciparum) | BDBM50190557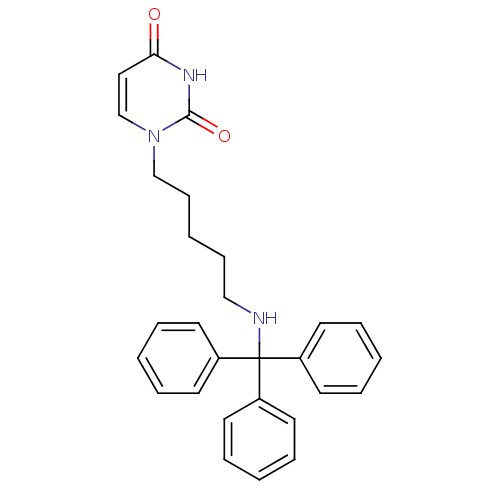 (1-(5-(tritylamino)pentyl)pyrimidine-2,4(1H,3H)-dio...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum dUTPase | J Med Chem 49: 4183-95 (2006) Article DOI: 10.1021/jm060126s BindingDB Entry DOI: 10.7270/Q22J6BF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 830 total ) | Next | Last >> |