 Found 202 hits with Last Name = 'nicolaou' and Initial = 'i'
Found 202 hits with Last Name = 'nicolaou' and Initial = 'i' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM21400
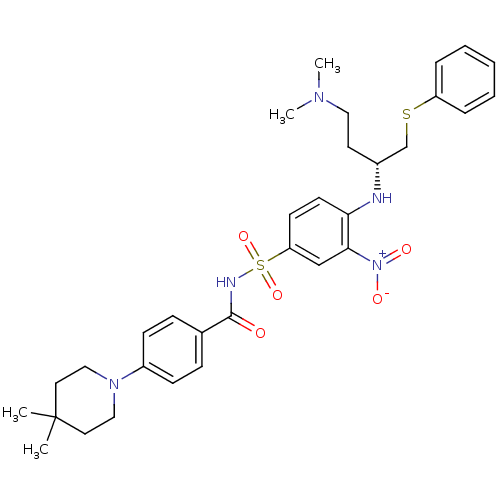
(CHEMBL192571 | N-[(4-{[(2R)-4-(dimethylamino)-1-(p...)Show SMILES CN(C)CC[C@H](CSc1ccccc1)Nc1ccc(cc1[N+]([O-])=O)S(=O)(=O)NC(=O)c1ccc(cc1)N1CCC(C)(C)CC1 |r| Show InChI InChI=1S/C32H41N5O5S2/c1-32(2)17-20-36(21-18-32)26-12-10-24(11-13-26)31(38)34-44(41,42)28-14-15-29(30(22-28)37(39)40)33-25(16-19-35(3)4)23-43-27-8-6-5-7-9-27/h5-15,22,25,33H,16-21,23H2,1-4H3,(H,34,38)/t25-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aristotle University of Thessaloniki
Curated by ChEMBL
| Assay Description
Binding affinity to Bcl-xl |
J Med Chem 53: 6779-810 (2010)
Article DOI: 10.1021/jm100189a
BindingDB Entry DOI: 10.7270/Q29C6XPM |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM13997
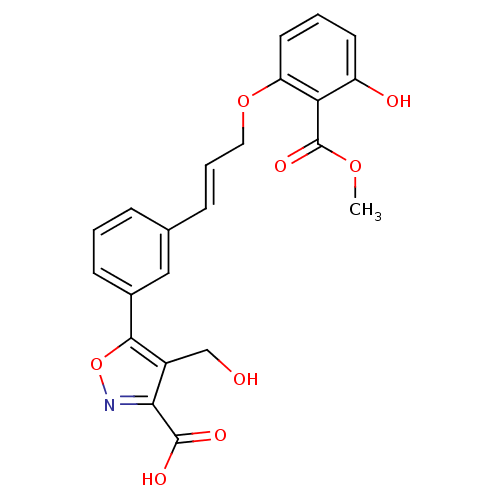
(5-{3-[(1E)-3-[3-hydroxy-2-(methoxycarbonyl)phenoxy...)Show SMILES COC(=O)c1c(O)cccc1OC\C=C\c1cccc(c1)-c1onc(C(O)=O)c1CO Show InChI InChI=1S/C22H19NO8/c1-29-22(28)18-16(25)8-3-9-17(18)30-10-4-6-13-5-2-7-14(11-13)20-15(12-24)19(21(26)27)23-31-20/h2-9,11,24-25H,10,12H2,1H3,(H,26,27)/b6-4+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
| 0.920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM13996
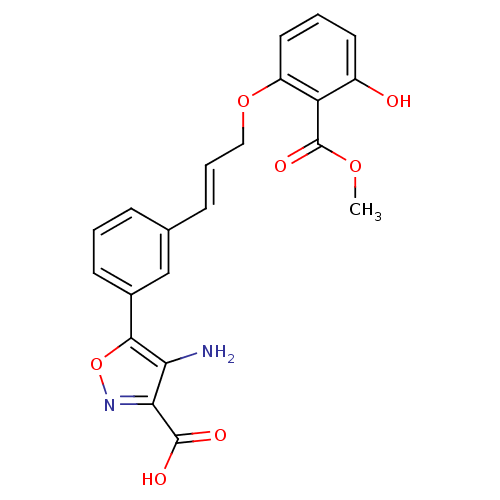
(4-amino-5-{3-[(1E)-3-[3-hydroxy-2-(methoxycarbonyl...)Show SMILES COC(=O)c1c(O)cccc1OC\C=C\c1cccc(c1)-c1onc(C(O)=O)c1N Show InChI InChI=1S/C21H18N2O7/c1-28-21(27)16-14(24)8-3-9-15(16)29-10-4-6-12-5-2-7-13(11-12)19-17(22)18(20(25)26)23-30-19/h2-9,11,24H,10,22H2,1H3,(H,25,26)/b6-4+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | CHEMBL5279725

| PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM13990
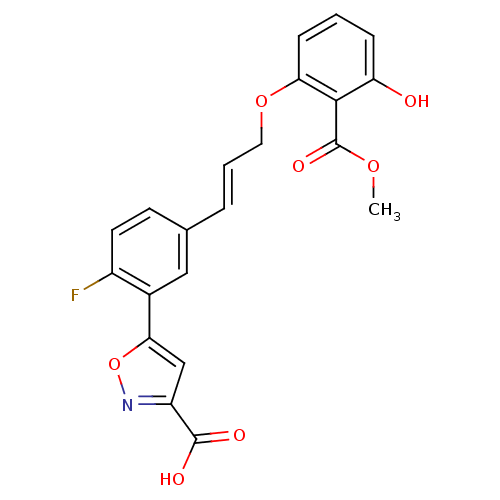
(5-{2-fluoro-5-[(1E)-3-[3-hydroxy-2-(methoxycarbony...)Show SMILES COC(=O)c1c(O)cccc1OC\C=C\c1ccc(F)c(c1)-c1cc(no1)C(O)=O Show InChI InChI=1S/C21H16FNO7/c1-28-21(27)19-16(24)5-2-6-17(19)29-9-3-4-12-7-8-14(22)13(10-12)18-11-15(20(25)26)23-30-18/h2-8,10-11,24H,9H2,1H3,(H,25,26)/b4-3+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
| 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM21447

(4-(4-{[2-(4-chlorophenyl)phenyl]methyl}piperazin-1...)Show SMILES CN(C)CC[C@H](CSc1ccccc1)Nc1ccc(cc1[N+]([O-])=O)S(=O)(=O)NC(=O)c1ccc(cc1)N1CCN(Cc2ccccc2-c2ccc(Cl)cc2)CC1 |r| Show InChI InChI=1S/C42H45ClN6O5S2/c1-46(2)23-22-35(30-55-37-9-4-3-5-10-37)44-40-21-20-38(28-41(40)49(51)52)56(53,54)45-42(50)32-14-18-36(19-15-32)48-26-24-47(25-27-48)29-33-8-6-7-11-39(33)31-12-16-34(43)17-13-31/h3-21,28,35,44H,22-27,29-30H2,1-2H3,(H,45,50)/t35-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aristotle University of Thessaloniki
Curated by ChEMBL
| Assay Description
Binding affinity to BCl2 |
J Med Chem 53: 6779-810 (2010)
Article DOI: 10.1021/jm100189a
BindingDB Entry DOI: 10.7270/Q29C6XPM |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM13954

(3-({5-[(2S)-3-{4-[(2-carboxyphenyl)amidoformic aci...)Show SMILES CC(=O)N[C@@H](Cc1ccc(N(C(=O)C(O)=O)c2ccccc2C(O)=O)c2ccccc12)C(=O)NCCCCCOc1cc2ccccc2cc1C(O)=O |r| Show InChI InChI=1S/C40H37N3O10/c1-24(44)42-32(36(45)41-19-9-2-10-20-53-35-23-26-12-4-3-11-25(26)21-31(35)39(49)50)22-27-17-18-34(29-14-6-5-13-28(27)29)43(37(46)40(51)52)33-16-8-7-15-30(33)38(47)48/h3-8,11-18,21,23,32H,2,9-10,19-20,22H2,1H3,(H,41,45)(H,42,44)(H,47,48)(H,49,50)(H,51,52)/t32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50087883

(CHEMBL3426930)Show SMILES CCCCCCCCCCCCCCC(=O)N[C@@H](Cc1ccc(cc1)C(F)(F)P(O)(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccc(cc1)C(F)(F)P(O)(O)=O)C(N)=O |r| Show InChI InChI=1S/C39H56F4N4O12P2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-33(48)45-31(24-27-17-21-29(22-18-27)39(42,43)61(57,58)59)36(52)47-32(25-34(49)50)37(53)46-30(35(44)51)23-26-15-19-28(20-16-26)38(40,41)60(54,55)56/h15-22,30-32H,2-14,23-25H2,1H3,(H2,44,51)(H,45,48)(H,46,53)(H,47,52)(H,49,50)(H2,54,55,56)(H2,57,58,59)/t30-,31-,32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM21447

(4-(4-{[2-(4-chlorophenyl)phenyl]methyl}piperazin-1...)Show SMILES CN(C)CC[C@H](CSc1ccccc1)Nc1ccc(cc1[N+]([O-])=O)S(=O)(=O)NC(=O)c1ccc(cc1)N1CCN(Cc2ccccc2-c2ccc(Cl)cc2)CC1 |r| Show InChI InChI=1S/C42H45ClN6O5S2/c1-46(2)23-22-35(30-55-37-9-4-3-5-10-37)44-40-21-20-38(28-41(40)49(51)52)56(53,54)45-42(50)32-14-18-36(19-15-32)48-26-24-47(25-27-48)29-33-8-6-7-11-39(33)31-12-16-34(43)17-13-31/h3-21,28,35,44H,22-27,29-30H2,1-2H3,(H,45,50)/t35-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aristotle University of Thessaloniki
Curated by ChEMBL
| Assay Description
Binding affinity to Bcl-xl |
J Med Chem 53: 6779-810 (2010)
Article DOI: 10.1021/jm100189a
BindingDB Entry DOI: 10.7270/Q29C6XPM |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50270877
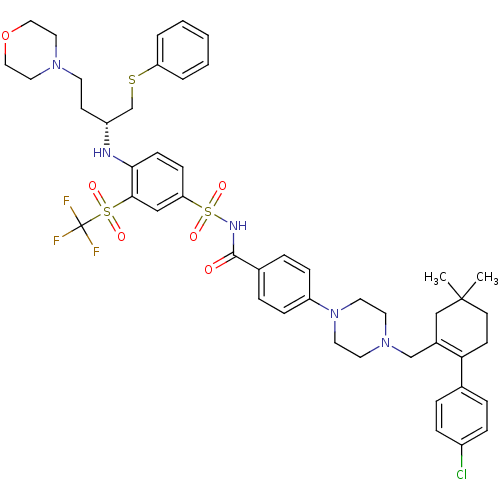
((R)-4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex...)Show SMILES CC1(C)CCC(=C(CN2CCN(CC2)c2ccc(cc2)C(=O)NS(=O)(=O)c2ccc(N[C@H](CCN3CCOCC3)CSc3ccccc3)c(c2)S(=O)(=O)C(F)(F)F)C1)c1ccc(Cl)cc1 |r,t:5| Show InChI InChI=1S/C47H55ClF3N5O6S3/c1-46(2)20-18-42(34-8-12-37(48)13-9-34)36(31-46)32-55-22-24-56(25-23-55)39-14-10-35(11-15-39)45(57)53-65(60,61)41-16-17-43(44(30-41)64(58,59)47(49,50)51)52-38(19-21-54-26-28-62-29-27-54)33-63-40-6-4-3-5-7-40/h3-17,30,38,52H,18-29,31-33H2,1-2H3,(H,53,57)/t38-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aristotle University of Thessaloniki
Curated by ChEMBL
| Assay Description
Binding affinity to Bcl-xl |
J Med Chem 53: 6779-810 (2010)
Article DOI: 10.1021/jm100189a
BindingDB Entry DOI: 10.7270/Q29C6XPM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50131547

(2-(N-(4-(2-acetamido-3-(4-(5-chloro-3-hydroxy-2-(m...)Show SMILES CCc1cc(CC(NC(C)=O)C(=O)NCCCCOc2cc(Cl)cc(O)c2C(=O)OC)ccc1N(C(=O)C(O)=O)c1ccccc1C(O)=O Show InChI InChI=1S/C34H36ClN3O11/c1-4-21-15-20(11-12-25(21)38(31(42)33(45)46)26-10-6-5-9-23(26)32(43)44)16-24(37-19(2)39)30(41)36-13-7-8-14-49-28-18-22(35)17-27(40)29(28)34(47)48-3/h5-6,9-12,15,17-18,24,40H,4,7-8,13-14,16H2,1-3H3,(H,36,41)(H,37,39)(H,43,44)(H,45,46) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM21400

(CHEMBL192571 | N-[(4-{[(2R)-4-(dimethylamino)-1-(p...)Show SMILES CN(C)CC[C@H](CSc1ccccc1)Nc1ccc(cc1[N+]([O-])=O)S(=O)(=O)NC(=O)c1ccc(cc1)N1CCC(C)(C)CC1 |r| Show InChI InChI=1S/C32H41N5O5S2/c1-32(2)17-20-36(21-18-32)26-12-10-24(11-13-26)31(38)34-44(41,42)28-14-15-29(30(22-28)37(39)40)33-25(16-19-35(3)4)23-43-27-8-6-5-7-9-27/h5-15,22,25,33H,16-21,23H2,1-4H3,(H,34,38)/t25-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aristotle University of Thessaloniki
Curated by ChEMBL
| Assay Description
Binding affinity to BCl2 |
J Med Chem 53: 6779-810 (2010)
Article DOI: 10.1021/jm100189a
BindingDB Entry DOI: 10.7270/Q29C6XPM |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Homo sapiens (Human)) | CHEMBL5288197
| PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | 107 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM23223
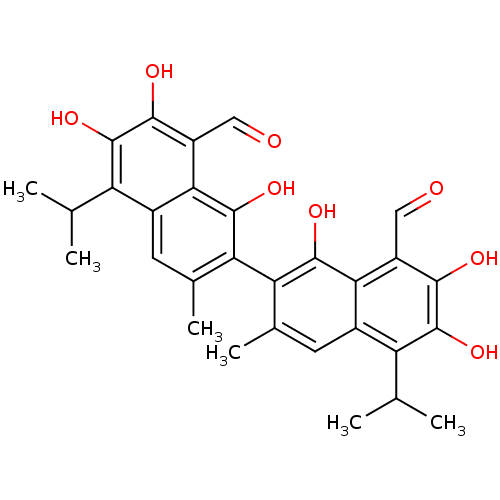
(7-[8-formyl-1,6,7-trihydroxy-3-methyl-5-(propan-2-...)Show SMILES CC(C)c1c(O)c(O)c(C=O)c2c(O)c(c(C)cc12)-c1c(C)cc2c(C(C)C)c(O)c(O)c(C=O)c2c1O |(-4.44,-1.63,;-5.78,-.86,;-7.11,-1.63,;-5.78,.68,;-7.11,1.45,;-8.44,.68,;-7.11,2.99,;-8.44,3.76,;-5.78,3.76,;-5.78,5.3,;-4.44,6.07,;-4.44,2.99,;-3.11,3.76,;-3.11,5.3,;-1.77,2.99,;-1.77,1.45,;-.44,.68,;-3.11,.68,;-4.44,1.45,;-.44,3.76,;-.44,5.3,;-1.77,6.07,;.89,6.07,;2.23,5.3,;3.56,6.07,;3.56,7.61,;4.89,8.38,;2.23,8.38,;4.89,5.3,;6.23,6.07,;4.89,3.76,;6.23,2.99,;3.56,2.99,;3.56,1.45,;4.89,.68,;2.23,3.76,;.89,2.99,;.89,1.45,)| Show InChI InChI=1S/C30H30O8/c1-11(2)19-15-7-13(5)21(27(35)23(15)17(9-31)25(33)29(19)37)22-14(6)8-16-20(12(3)4)30(38)26(34)18(10-32)24(16)28(22)36/h7-12,33-38H,1-6H3 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aristotle University of Thessaloniki
Curated by ChEMBL
| Assay Description
Binding affinity to MCL1 |
J Med Chem 53: 6779-810 (2010)
Article DOI: 10.1021/jm100189a
BindingDB Entry DOI: 10.7270/Q29C6XPM |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM13611
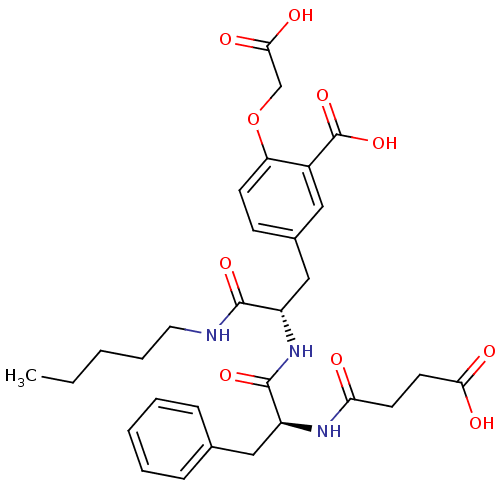
(2-(carboxymethoxy)-5-[(2S)-2-[(2S)-2-(3-formamidop...)Show SMILES CCCCCNC(=O)[C@H](Cc1ccc(OCC(O)=O)c(c1)C(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)CCC(O)=O |r| Show InChI InChI=1S/C30H37N3O10/c1-2-3-7-14-31-28(39)22(17-20-10-11-24(43-18-27(37)38)21(15-20)30(41)42)33-29(40)23(16-19-8-5-4-6-9-19)32-25(34)12-13-26(35)36/h4-6,8-11,15,22-23H,2-3,7,12-14,16-18H2,1H3,(H,31,39)(H,32,34)(H,33,40)(H,35,36)(H,37,38)(H,41,42)/t22-,23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50118750
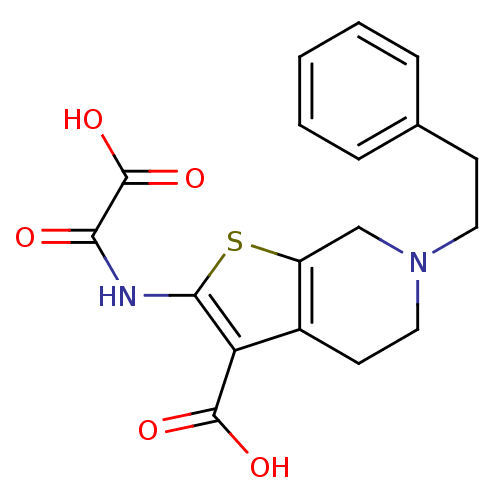
(2-(Oxalyl-amino)-6-phenethyl-4,5,6,7-tetrahydro-th...)Show SMILES OC(=O)C(=O)Nc1sc2CN(CCc3ccccc3)CCc2c1C(O)=O Show InChI InChI=1S/C18H18N2O5S/c21-15(18(24)25)19-16-14(17(22)23)12-7-9-20(10-13(12)26-16)8-6-11-4-2-1-3-5-11/h1-5H,6-10H2,(H,19,21)(H,22,23)(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| | 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
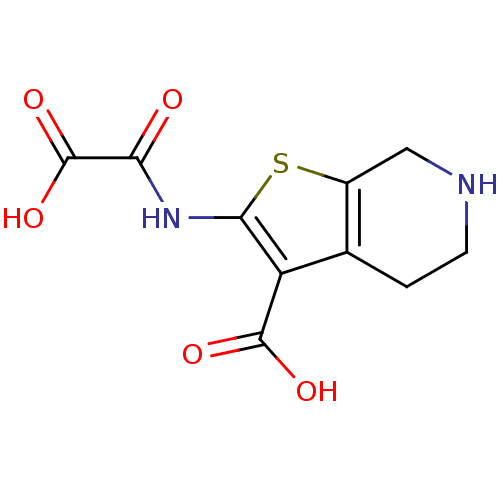
(Homo sapiens (Human)) | BDBM50118792
(2-(OXALYL-AMINO)-4,5,6,7-TETRAHYDRO-THIENO[2,3-C]P...)Show InChI InChI=1S/C10H10N2O5S/c13-7(10(16)17)12-8-6(9(14)15)4-1-2-11-3-5(4)18-8/h11H,1-3H2,(H,12,13)(H,14,15)(H,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
| 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM23223

(7-[8-formyl-1,6,7-trihydroxy-3-methyl-5-(propan-2-...)Show SMILES CC(C)c1c(O)c(O)c(C=O)c2c(O)c(c(C)cc12)-c1c(C)cc2c(C(C)C)c(O)c(O)c(C=O)c2c1O |(-4.44,-1.63,;-5.78,-.86,;-7.11,-1.63,;-5.78,.68,;-7.11,1.45,;-8.44,.68,;-7.11,2.99,;-8.44,3.76,;-5.78,3.76,;-5.78,5.3,;-4.44,6.07,;-4.44,2.99,;-3.11,3.76,;-3.11,5.3,;-1.77,2.99,;-1.77,1.45,;-.44,.68,;-3.11,.68,;-4.44,1.45,;-.44,3.76,;-.44,5.3,;-1.77,6.07,;.89,6.07,;2.23,5.3,;3.56,6.07,;3.56,7.61,;4.89,8.38,;2.23,8.38,;4.89,5.3,;6.23,6.07,;4.89,3.76,;6.23,2.99,;3.56,2.99,;3.56,1.45,;4.89,.68,;2.23,3.76,;.89,2.99,;.89,1.45,)| Show InChI InChI=1S/C30H30O8/c1-11(2)19-15-7-13(5)21(27(35)23(15)17(9-31)25(33)29(19)37)22-14(6)8-16-20(12(3)4)30(38)26(34)18(10-32)24(16)28(22)36/h7-12,33-38H,1-6H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aristotle University of Thessaloniki
Curated by ChEMBL
| Assay Description
Binding affinity to BCl2 |
J Med Chem 53: 6779-810 (2010)
Article DOI: 10.1021/jm100189a
BindingDB Entry DOI: 10.7270/Q29C6XPM |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM23223

(7-[8-formyl-1,6,7-trihydroxy-3-methyl-5-(propan-2-...)Show SMILES CC(C)c1c(O)c(O)c(C=O)c2c(O)c(c(C)cc12)-c1c(C)cc2c(C(C)C)c(O)c(O)c(C=O)c2c1O |(-4.44,-1.63,;-5.78,-.86,;-7.11,-1.63,;-5.78,.68,;-7.11,1.45,;-8.44,.68,;-7.11,2.99,;-8.44,3.76,;-5.78,3.76,;-5.78,5.3,;-4.44,6.07,;-4.44,2.99,;-3.11,3.76,;-3.11,5.3,;-1.77,2.99,;-1.77,1.45,;-.44,.68,;-3.11,.68,;-4.44,1.45,;-.44,3.76,;-.44,5.3,;-1.77,6.07,;.89,6.07,;2.23,5.3,;3.56,6.07,;3.56,7.61,;4.89,8.38,;2.23,8.38,;4.89,5.3,;6.23,6.07,;4.89,3.76,;6.23,2.99,;3.56,2.99,;3.56,1.45,;4.89,.68,;2.23,3.76,;.89,2.99,;.89,1.45,)| Show InChI InChI=1S/C30H30O8/c1-11(2)19-15-7-13(5)21(27(35)23(15)17(9-31)25(33)29(19)37)22-14(6)8-16-20(12(3)4)30(38)26(34)18(10-32)24(16)28(22)36/h7-12,33-38H,1-6H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aristotle University of Thessaloniki
Curated by ChEMBL
| Assay Description
Binding affinity to Bcl-xl |
J Med Chem 53: 6779-810 (2010)
Article DOI: 10.1021/jm100189a
BindingDB Entry DOI: 10.7270/Q29C6XPM |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
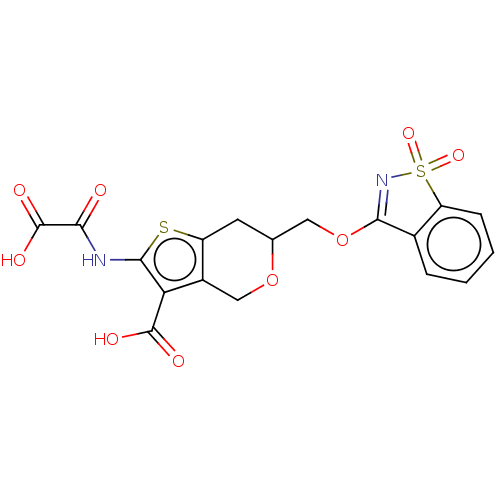
(Homo sapiens (Human)) | CHEMBL5278790
| PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | CHEMBL5269879

| PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | 930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM13609

(2-{4-[(2S)-2-[(2S)-2-(3-formamidopropanoic acid)-3...)Show SMILES CCCCCNC(=O)[C@H](Cc1ccc(OC(C(O)=O)C(O)=O)cc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)CCC(O)=O |r| Show InChI InChI=1S/C30H37N3O10/c1-2-3-7-16-31-27(37)22(18-20-10-12-21(13-11-20)43-26(29(39)40)30(41)42)33-28(38)23(17-19-8-5-4-6-9-19)32-24(34)14-15-25(35)36/h4-6,8-13,22-23,26H,2-3,7,14-18H2,1H3,(H,31,37)(H,32,34)(H,33,38)(H,35,36)(H,39,40)(H,41,42)/t22-,23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50112100
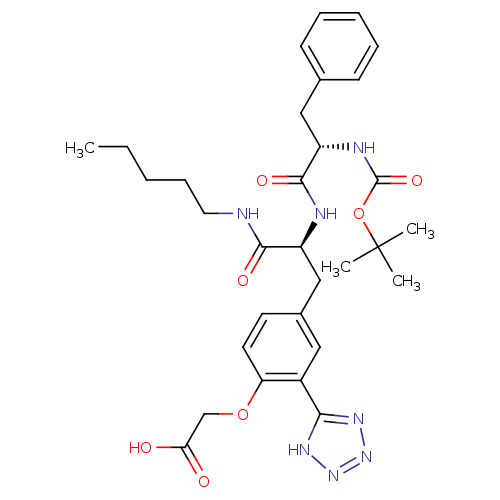
(CHEMBL51202 | [4-[2-(2-tert-Butoxycarbonylamino-3-...)Show SMILES CCCCCNC(=O)[C@H](Cc1ccc(OCC(O)=O)c(c1)-c1nnn[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C Show InChI InChI=1S/C31H41N7O7/c1-5-6-10-15-32-28(41)23(18-21-13-14-25(44-19-26(39)40)22(16-21)27-35-37-38-36-27)33-29(42)24(17-20-11-8-7-9-12-20)34-30(43)45-31(2,3)4/h7-9,11-14,16,23-24H,5-6,10,15,17-19H2,1-4H3,(H,32,41)(H,33,42)(H,34,43)(H,39,40)(H,35,36,37,38)/t23-,24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Homo sapiens (Human)) | CHEMBL5280965

| PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50118765
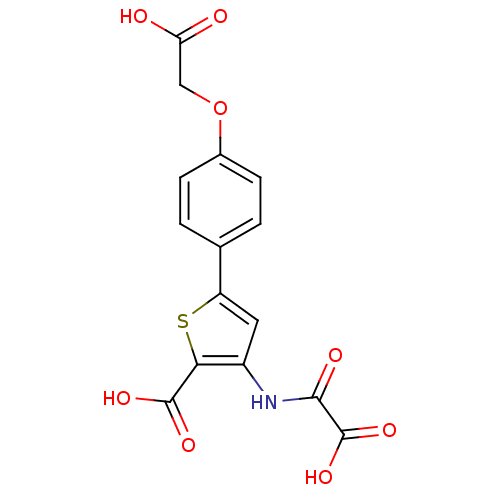
(3-(carboxyformamido)-5-(4-(carboxymethoxy)phenyl)t...)Show SMILES OC(=O)COc1ccc(cc1)-c1cc(NC(=O)C(O)=O)c(s1)C(O)=O Show InChI InChI=1S/C15H11NO8S/c17-11(18)6-24-8-3-1-7(2-4-8)10-5-9(12(25-10)14(20)21)16-13(19)15(22)23/h1-5H,6H2,(H,16,19)(H,17,18)(H,20,21)(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Rattus norvegicus (rat)) | BDBM13606
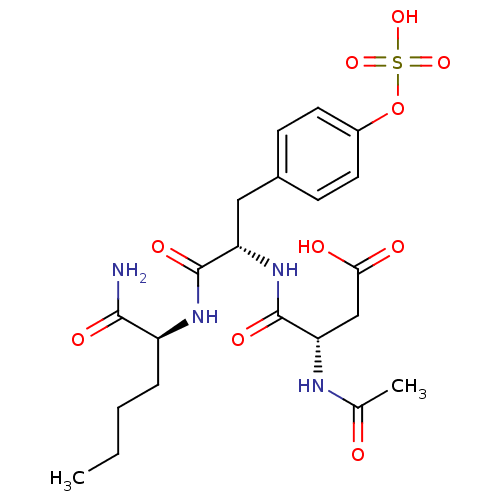
((3S)-3-{[(1S)-1-{[(1S)-1-carbamoylpentyl]carbamoyl...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@H](CC(O)=O)NC(C)=O)C(N)=O |r| Show InChI InChI=1S/C21H30N4O10S/c1-3-4-5-15(19(22)29)24-20(30)16(25-21(31)17(11-18(27)28)23-12(2)26)10-13-6-8-14(9-7-13)35-36(32,33)34/h6-9,15-17H,3-5,10-11H2,1-2H3,(H2,22,29)(H,23,26)(H,24,30)(H,25,31)(H,27,28)(H,32,33,34)/t15-,16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50118796

(6-(OXALYL-AMINO)-1H-INDOLE-5-CARBOXYLIC ACID | 6-(...)Show InChI InChI=1S/C11H8N2O5/c14-9(11(17)18)13-8-4-7-5(1-2-12-7)3-6(8)10(15)16/h1-4,12H,(H,13,14)(H,15,16)(H,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
| 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50118779
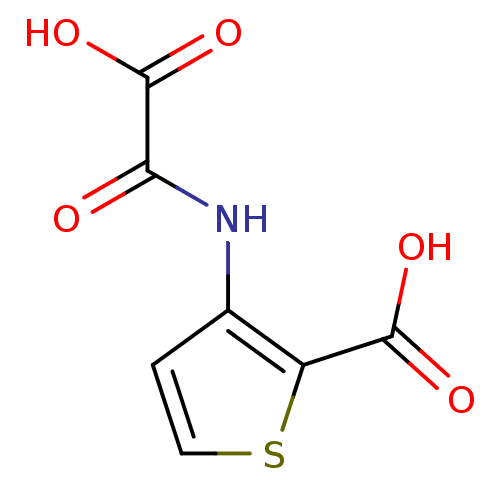
(3-(Oxalyl-amino)-thiophene-2-carboxylic acid | 3-(...)Show InChI InChI=1S/C7H5NO5S/c9-5(7(12)13)8-3-1-2-14-4(3)6(10)11/h1-2H,(H,8,9)(H,10,11)(H,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| | 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein phosphatase F
(Rattus norvegicus) | CHEMBL5269879

| Reactome pathway
KEGG
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein phosphatase alpha
(Homo sapiens (Human)) | CHEMBL5269879

| PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | 1.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein phosphatase alpha
(Homo sapiens (Human)) | BDBM50118796

(6-(OXALYL-AMINO)-1H-INDOLE-5-CARBOXYLIC ACID | 6-(...)Show InChI InChI=1S/C11H8N2O5/c14-9(11(17)18)13-8-4-7-5(1-2-12-7)3-6(8)10(15)16/h1-4,12H,(H,13,14)(H,15,16)(H,17,18) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| | 1.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein phosphatase F
(Homo sapiens (Human)) | BDBM50118796

(6-(OXALYL-AMINO)-1H-INDOLE-5-CARBOXYLIC ACID | 6-(...)Show InChI InChI=1S/C11H8N2O5/c14-9(11(17)18)13-8-4-7-5(1-2-12-7)3-6(8)10(15)16/h1-4,12H,(H,13,14)(H,15,16)(H,17,18) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| | 4.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein phosphatase F
(Homo sapiens (Human)) | BDBM50118779

(3-(Oxalyl-amino)-thiophene-2-carboxylic acid | 3-(...)Show InChI InChI=1S/C7H5NO5S/c9-5(7(12)13)8-3-1-2-14-4(3)6(10)11/h1-2H,(H,8,9)(H,10,11)(H,12,13) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| | 2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein phosphatase alpha
(Homo sapiens (Human)) | BDBM50118779

(3-(Oxalyl-amino)-thiophene-2-carboxylic acid | 3-(...)Show InChI InChI=1S/C7H5NO5S/c9-5(7(12)13)8-3-1-2-14-4(3)6(10)11/h1-2H,(H,8,9)(H,10,11)(H,12,13) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| | 2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Dual specificity protein phosphatase 3
(Homo sapiens (Human)) | CHEMBL5269879

| PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | 3.60E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Homo sapiens (Human)) | BDBM16315
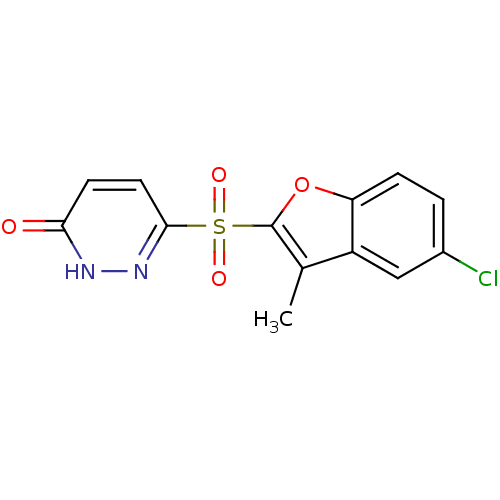
(6-[(5-chloro-3-methyl-1-benzofuran-2-)sulfonyl]-2,...)Show SMILES Cc1c(oc2ccc(Cl)cc12)S(=O)(=O)c1ccc(=O)[nH]n1 Show InChI InChI=1S/C13H9ClN2O4S/c1-7-9-6-8(14)2-3-10(9)20-13(7)21(18,19)12-5-4-11(17)15-16-12/h2-6H,1H3,(H,15,17) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aldo-keto reductase family 1 member B1
(Rattus norvegicus) | BDBM50122956
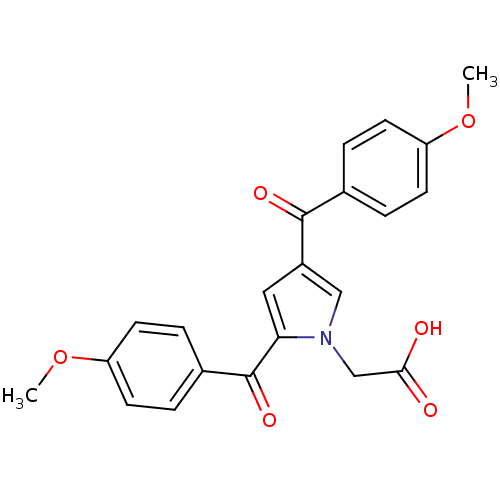
(CHEMBL138065 | [2,4-Bis-(4-methoxy-benzoyl)-pyrrol...)Show SMILES COc1ccc(cc1)C(=O)c1cc(C(=O)c2ccc(OC)cc2)n(CC(O)=O)c1 Show InChI InChI=1S/C22H19NO6/c1-28-17-7-3-14(4-8-17)21(26)16-11-19(23(12-16)13-20(24)25)22(27)15-5-9-18(29-2)10-6-15/h3-12H,13H2,1-2H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.36 | n/a | n/a | n/a | n/a | n/a | n/a |
Aristotle University of Thessaloniki
Curated by ChEMBL
| Assay Description
Inhibitory activity against aldose reductase enzyme |
J Med Chem 46: 417-26 (2003)
Article DOI: 10.1021/jm0209477
BindingDB Entry DOI: 10.7270/Q2PG1SGT |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Rattus norvegicus) | BDBM50122956

(CHEMBL138065 | [2,4-Bis-(4-methoxy-benzoyl)-pyrrol...)Show SMILES COc1ccc(cc1)C(=O)c1cc(C(=O)c2ccc(OC)cc2)n(CC(O)=O)c1 Show InChI InChI=1S/C22H19NO6/c1-28-17-7-3-14(4-8-17)21(26)16-11-19(23(12-16)13-20(24)25)22(27)15-5-9-18(29-2)10-6-15/h3-12H,13H2,1-2H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Homo sapiens (Human)) | BDBM16452

((4-oxo-3-{[5-(trifluoromethyl)-1,3-benzothiazol-2-...)Show SMILES OC(=O)Cc1nn(Cc2nc3cc(ccc3s2)C(F)(F)F)c(=O)c2ccccc12 Show InChI InChI=1S/C19H12F3N3O3S/c20-19(21,22)10-5-6-15-14(7-10)23-16(29-15)9-25-18(28)12-4-2-1-3-11(12)13(24-25)8-17(26)27/h1-7H,8-9H2,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Aristotle University of Thessaloniki
Curated by ChEMBL
| Assay Description
Inhibitory activity against aldose reductase enzyme |
J Med Chem 46: 417-26 (2003)
Article DOI: 10.1021/jm0209477
BindingDB Entry DOI: 10.7270/Q2PG1SGT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | CHEMBL5290902

| PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | CHEMBL5275241

| PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50142318

(CHEMBL428651 | {[2-Chloro-4-(3-oxo-2,3-diphenyl-pr...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(C(=O)c2ccccc2)c2ccccc2)cc1Cl Show InChI InChI=1S/C22H18ClF2O4P/c23-20-14-15(11-12-19(20)22(24,25)30(27,28)29)13-18(16-7-3-1-4-8-16)21(26)17-9-5-2-6-10-17/h1-12,14,18H,13H2,(H2,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
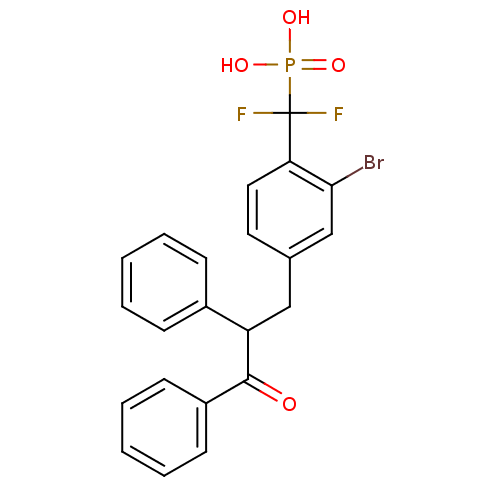
(Homo sapiens (Human)) | BDBM50142317
((2-bromo-4-(3-oxo-2,3-diphenylpropyl)phenyl)difluo...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(C(=O)c2ccccc2)c2ccccc2)cc1Br Show InChI InChI=1S/C22H18BrF2O4P/c23-20-14-15(11-12-19(20)22(24,25)30(27,28)29)13-18(16-7-3-1-4-8-16)21(26)17-9-5-2-6-10-17/h1-12,14,18H,13H2,(H2,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
| PDB
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aldo-keto reductase family 1 member B1
(Homo sapiens (Human)) | CHEMBL5286261
| PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | CHEMBL5285288

| PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
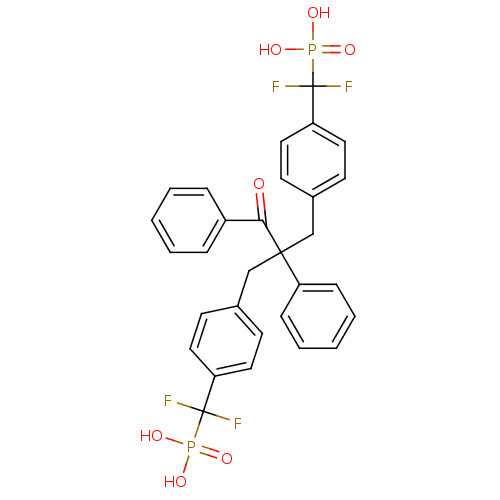
(Homo sapiens (Human)) | BDBM50142311
(CHEMBL266056 | [(4-{2-[4-(Difluoro-phosphono-methy...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(C(=O)c2ccccc2)c2ccccc2)cc1 Show InChI InChI=1S/C30H26F4O7P2/c31-29(32,42(36,37)38)25-15-11-21(12-16-25)19-28(24-9-5-2-6-10-24,27(35)23-7-3-1-4-8-23)20-22-13-17-26(18-14-22)30(33,34)43(39,40)41/h1-18H,19-20H2,(H2,36,37,38)(H2,39,40,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Aldo-keto reductase family 1 member B1
(Homo sapiens (Human)) | BDBM50049730
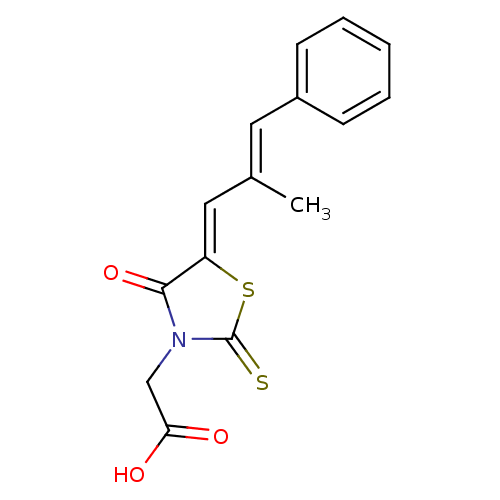
(2-(5-(2-methyl-3-phenylallylidene)-4-oxo-2-thioxot...)Show InChI InChI=1S/C15H13NO3S2/c1-10(7-11-5-3-2-4-6-11)8-12-14(19)16(9-13(17)18)15(20)21-12/h2-8H,9H2,1H3,(H,17,18)/b10-7+,12-8- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Aristotle University of Thessaloniki
Curated by ChEMBL
| Assay Description
Inhibitory activity against aldose reductase enzyme |
J Med Chem 46: 417-26 (2003)
Article DOI: 10.1021/jm0209477
BindingDB Entry DOI: 10.7270/Q2PG1SGT |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4
(Homo sapiens (Human)) | BDBM6309
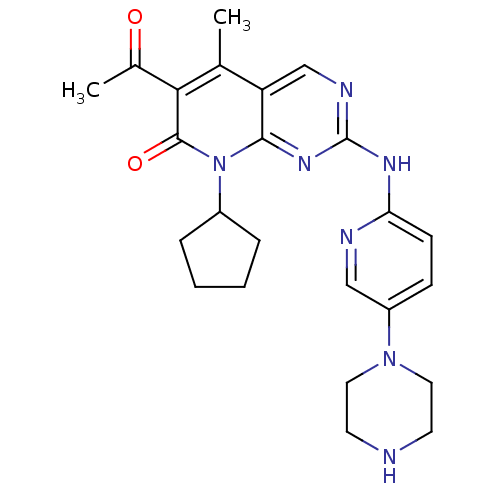
(6-Acetyl-8-cyclopentyl-5-methyl-2-(5-piperazin-1-y...)Show SMILES CC(=O)c1c(C)c2cnc(Nc3ccc(cn3)N3CCNCC3)nc2n(C2CCCC2)c1=O Show InChI InChI=1S/C24H29N7O2/c1-15-19-14-27-24(28-20-8-7-18(13-26-20)30-11-9-25-10-12-30)29-22(19)31(17-5-3-4-6-17)23(33)21(15)16(2)32/h7-8,13-14,17,25H,3-6,9-12H2,1-2H3,(H,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Aristotle University of Thessaloniki
Curated by ChEMBL
| Assay Description
Inhibition of CDK4 |
J Med Chem 53: 6779-810 (2010)
Article DOI: 10.1021/jm100189a
BindingDB Entry DOI: 10.7270/Q29C6XPM |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | CHEMBL5273366
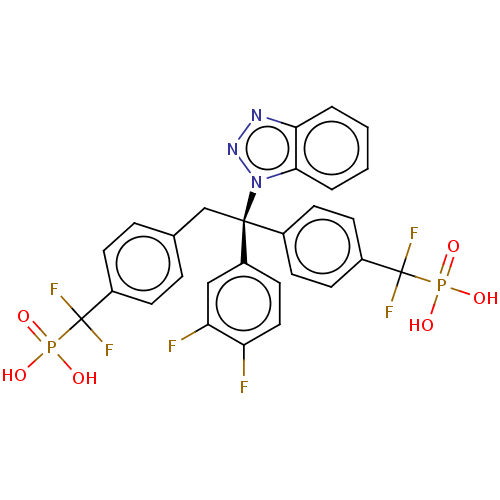
| PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cathepsin G
(Homo sapiens (Human)) | BDBM50329819
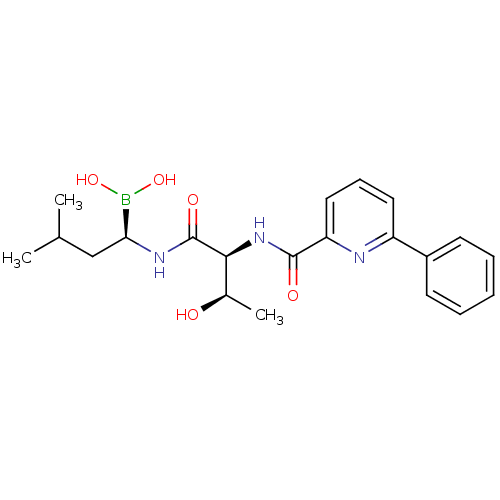
((R)-1-((2S,3R)-3-hydroxy-2-(6-phenylpicolinamido)b...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)c1cccc(n1)-c1ccccc1)[C@@H](C)O)B(O)O Show InChI InChI=1S/C21H28BN3O5/c1-13(2)12-18(22(29)30)24-21(28)19(14(3)26)25-20(27)17-11-7-10-16(23-17)15-8-5-4-6-9-15/h4-11,13-14,18-19,26,29-30H,12H2,1-3H3,(H,24,28)(H,25,27)/t14-,18+,19+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 13.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Aristotle University of Thessaloniki
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin G |
J Med Chem 53: 6779-810 (2010)
Article DOI: 10.1021/jm100189a
BindingDB Entry DOI: 10.7270/Q29C6XPM |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data