 Found 223 hits with Last Name = 'niedzwiecki' and Initial = 'lm'
Found 223 hits with Last Name = 'niedzwiecki' and Initial = 'lm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Stromelysin-1
(Homo sapiens (Human)) | BDBM50289127
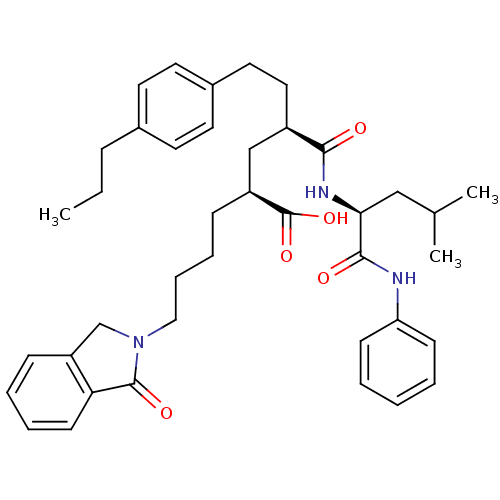
((2S,4R)-4-(3-Methyl-1-phenylcarbamoyl-butylcarbamo...)Show SMILES CCCc1ccc(CC[C@H](C[C@H](CCCCN2Cc3ccccc3C2=O)C(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)Nc2ccccc2)cc1 Show InChI InChI=1S/C40H51N3O5/c1-4-12-29-18-20-30(21-19-29)22-23-31(37(44)42-36(25-28(2)3)38(45)41-34-15-6-5-7-16-34)26-32(40(47)48)13-10-11-24-43-27-33-14-8-9-17-35(33)39(43)46/h5-9,14-21,28,31-32,36H,4,10-13,22-27H2,1-3H3,(H,41,45)(H,42,44)(H,47,48)/t31-,32+,36+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloproteinase-3(MMP-3). |
Bioorg Med Chem Lett 6: 803-806 (1996)
Article DOI: 10.1016/0960-894X(96)00109-6
BindingDB Entry DOI: 10.7270/Q2HM58FH |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50288674
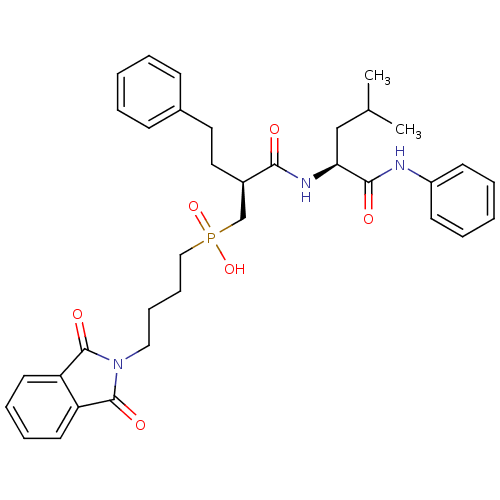
(CHEMBL420674 | [4-(1,3-Dioxo-1,3-dihydro-isoindol-...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCc1ccccc1)CP(O)(=O)CCCCN1C(=O)c2ccccc2C1=O)C(=O)Nc1ccccc1 Show InChI InChI=1S/C35H42N3O6P/c1-25(2)23-31(33(40)36-28-15-7-4-8-16-28)37-32(39)27(20-19-26-13-5-3-6-14-26)24-45(43,44)22-12-11-21-38-34(41)29-17-9-10-18-30(29)35(38)42/h3-10,13-18,25,27,31H,11-12,19-24H2,1-2H3,(H,36,40)(H,37,39)(H,43,44)/t27-,31+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of stromelysin-1 (MMP-3). |
Bioorg Med Chem Lett 6: 323-328 (1996)
Article DOI: 10.1016/0960-894X(96)00023-6
BindingDB Entry DOI: 10.7270/Q2X0671M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50289128
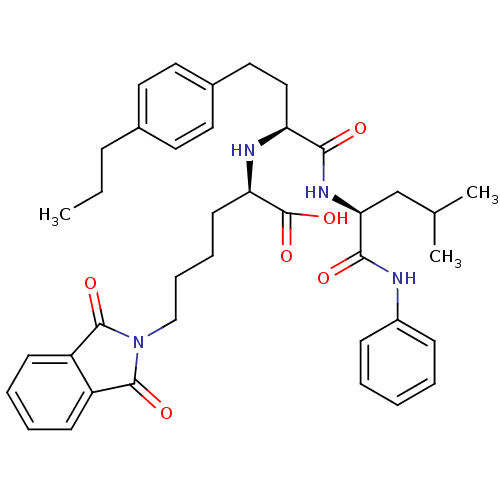
((R)-6-(1,3-Dioxo-1,3-dihydro-isoindol-2-yl)-2-[(S)...)Show SMILES CCCc1ccc(CC[C@H](N[C@H](CCCCN2C(=O)c3ccccc3C2=O)C(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)Nc2ccccc2)cc1 Show InChI InChI=1S/C39H48N4O6/c1-4-12-27-18-20-28(21-19-27)22-23-32(35(44)42-34(25-26(2)3)36(45)40-29-13-6-5-7-14-29)41-33(39(48)49)17-10-11-24-43-37(46)30-15-8-9-16-31(30)38(43)47/h5-9,13-16,18-21,26,32-34,41H,4,10-12,17,22-25H2,1-3H3,(H,40,45)(H,42,44)(H,48,49)/t32-,33+,34-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloproteinase-3(MMP-3). |
Bioorg Med Chem Lett 6: 803-806 (1996)
Article DOI: 10.1016/0960-894X(96)00109-6
BindingDB Entry DOI: 10.7270/Q2HM58FH |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50288673

(CHEMBL264455 | {4-[((S)-1-Benzoyl-pyrrolidine-2-ca...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCc1ccccc1)CP(O)(=O)CCCCNC(=O)[C@@H]1CCCN1C(=O)c1ccccc1)C(=O)Nc1ccccc1 Show InChI InChI=1S/C39H51N4O6P/c1-29(2)27-34(37(45)41-33-19-10-5-11-20-33)42-36(44)32(23-22-30-15-6-3-7-16-30)28-50(48,49)26-13-12-24-40-38(46)35-21-14-25-43(35)39(47)31-17-8-4-9-18-31/h3-11,15-20,29,32,34-35H,12-14,21-28H2,1-2H3,(H,40,46)(H,41,45)(H,42,44)(H,48,49)/t32-,34+,35+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of gelatinase-A (MMP-2). |
Bioorg Med Chem Lett 6: 323-328 (1996)
Article DOI: 10.1016/0960-894X(96)00023-6
BindingDB Entry DOI: 10.7270/Q2X0671M |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50288672

(CHEMBL113362 | [4-(1,3-Dioxo-1,3-dihydro-isoindol-...)Show SMILES COc1cccc(CC[C@H](CP(O)(=O)CCCCN2C(=O)c3ccccc3C2=O)C(=O)N[C@@H](CC(C)C)C(=O)Nc2ccccc2)c1 Show InChI InChI=1S/C36H44N3O7P/c1-25(2)22-32(34(41)37-28-13-5-4-6-14-28)38-33(40)27(19-18-26-12-11-15-29(23-26)46-3)24-47(44,45)21-10-9-20-39-35(42)30-16-7-8-17-31(30)36(39)43/h4-8,11-17,23,25,27,32H,9-10,18-22,24H2,1-3H3,(H,37,41)(H,38,40)(H,44,45)/t27-,32+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of gelatinase-A (MMP-2). |
Bioorg Med Chem Lett 6: 323-328 (1996)
Article DOI: 10.1016/0960-894X(96)00023-6
BindingDB Entry DOI: 10.7270/Q2X0671M |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50285557
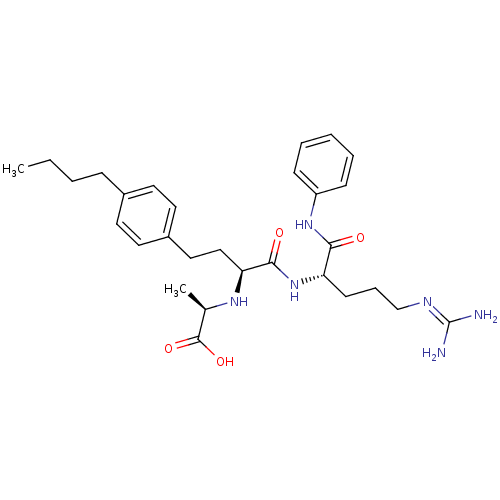
((R)-2-[(S)-3-(4-butyl-phenyl)-1-((S)-4-guanidino-1...)Show SMILES [#6]-[#6]-[#6]-[#6]-c1ccc(-[#6]-[#6]-[#6@H](-[#7]-[#6@H](-[#6])-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-c2ccccc2)cc1 Show InChI InChI=1S/C29H42N6O4/c1-3-4-9-21-13-15-22(16-14-21)17-18-25(33-20(2)28(38)39)27(37)35-24(12-8-19-32-29(30)31)26(36)34-23-10-6-5-7-11-23/h5-7,10-11,13-16,20,24-25,33H,3-4,8-9,12,17-19H2,1-2H3,(H,34,36)(H,35,37)(H,38,39)(H4,30,31,32)/t20-,24+,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human gelatinase-A (MMP-2) |
Bioorg Med Chem Lett 5: 2441-2446 (1995)
Article DOI: 10.1016/0960-894X(95)00425-S
BindingDB Entry DOI: 10.7270/Q2D21XK6 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50288683
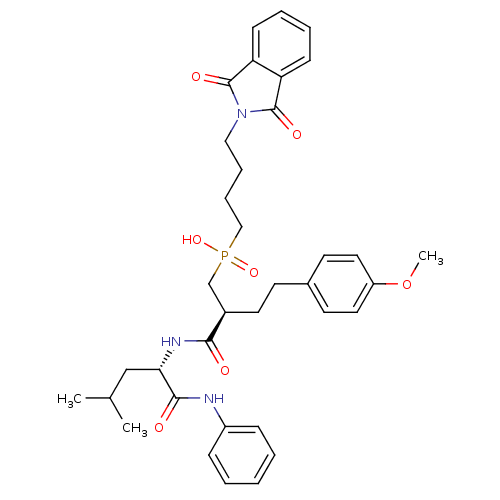
(CHEMBL109438 | [4-(1,3-Dioxo-1,3-dihydro-isoindol-...)Show SMILES COc1ccc(CC[C@H](CP(O)(=O)CCCCN2C(=O)c3ccccc3C2=O)C(=O)N[C@@H](CC(C)C)C(=O)Nc2ccccc2)cc1 Show InChI InChI=1S/C36H44N3O7P/c1-25(2)23-32(34(41)37-28-11-5-4-6-12-28)38-33(40)27(18-15-26-16-19-29(46-3)20-17-26)24-47(44,45)22-10-9-21-39-35(42)30-13-7-8-14-31(30)36(39)43/h4-8,11-14,16-17,19-20,25,27,32H,9-10,15,18,21-24H2,1-3H3,(H,37,41)(H,38,40)(H,44,45)/t27-,32+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of stromelysin-1 (MMP-3). |
Bioorg Med Chem Lett 6: 323-328 (1996)
Article DOI: 10.1016/0960-894X(96)00023-6
BindingDB Entry DOI: 10.7270/Q2X0671M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50288672

(CHEMBL113362 | [4-(1,3-Dioxo-1,3-dihydro-isoindol-...)Show SMILES COc1cccc(CC[C@H](CP(O)(=O)CCCCN2C(=O)c3ccccc3C2=O)C(=O)N[C@@H](CC(C)C)C(=O)Nc2ccccc2)c1 Show InChI InChI=1S/C36H44N3O7P/c1-25(2)22-32(34(41)37-28-13-5-4-6-14-28)38-33(40)27(19-18-26-12-11-15-29(23-26)46-3)24-47(44,45)21-10-9-20-39-35(42)30-16-7-8-17-31(30)36(39)43/h4-8,11-17,23,25,27,32H,9-10,18-22,24H2,1-3H3,(H,37,41)(H,38,40)(H,44,45)/t27-,32+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of stromelysin-1 (MMP-3). |
Bioorg Med Chem Lett 6: 323-328 (1996)
Article DOI: 10.1016/0960-894X(96)00023-6
BindingDB Entry DOI: 10.7270/Q2X0671M |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50289128

((R)-6-(1,3-Dioxo-1,3-dihydro-isoindol-2-yl)-2-[(S)...)Show SMILES CCCc1ccc(CC[C@H](N[C@H](CCCCN2C(=O)c3ccccc3C2=O)C(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)Nc2ccccc2)cc1 Show InChI InChI=1S/C39H48N4O6/c1-4-12-27-18-20-28(21-19-27)22-23-32(35(44)42-34(25-26(2)3)36(45)40-29-13-6-5-7-14-29)41-33(39(48)49)17-10-11-24-43-37(46)30-15-8-9-16-31(30)38(43)47/h5-9,13-16,18-21,26,32-34,41H,4,10-12,17,22-25H2,1-3H3,(H,40,45)(H,42,44)(H,48,49)/t32-,33+,34-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloproteinase-2(MMP-2) |
Bioorg Med Chem Lett 6: 803-806 (1996)
Article DOI: 10.1016/0960-894X(96)00109-6
BindingDB Entry DOI: 10.7270/Q2HM58FH |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50285567
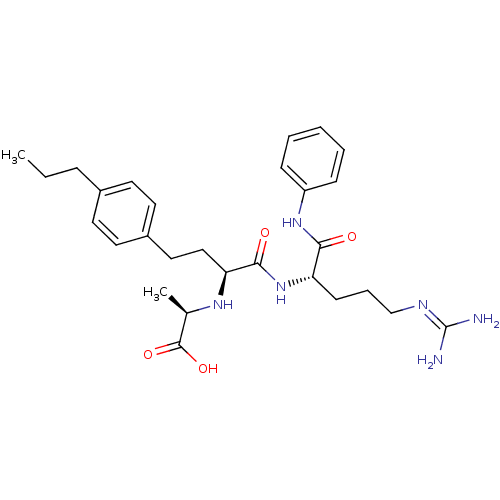
((R)-2-[(S)-1-((S)-4-guanidino-1-phenylcarbamoyl-bu...)Show SMILES [#6]-[#6]-[#6]-c1ccc(-[#6]-[#6]-[#6@H](-[#7]-[#6@H](-[#6])-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-c2ccccc2)cc1 Show InChI InChI=1S/C28H40N6O4/c1-3-8-20-12-14-21(15-13-20)16-17-24(32-19(2)27(37)38)26(36)34-23(11-7-18-31-28(29)30)25(35)33-22-9-5-4-6-10-22/h4-6,9-10,12-15,19,23-24,32H,3,7-8,11,16-18H2,1-2H3,(H,33,35)(H,34,36)(H,37,38)(H4,29,30,31)/t19-,23+,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human gelatinase-A (MMP-2) |
Bioorg Med Chem Lett 5: 2441-2446 (1995)
Article DOI: 10.1016/0960-894X(95)00425-S
BindingDB Entry DOI: 10.7270/Q2D21XK6 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50057090
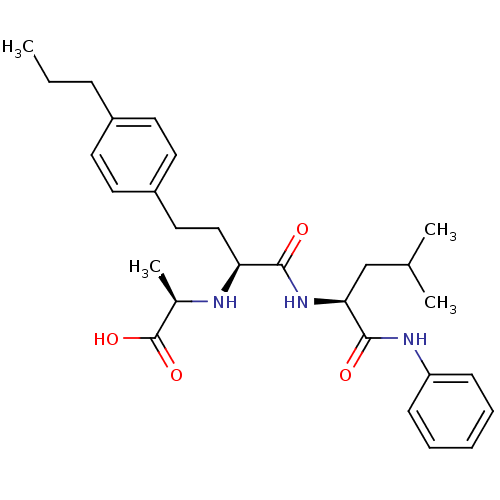
((R)-2-((S)-1-((S)-4-methyl-1-oxo-1-(phenylamino)pe...)Show SMILES CCCc1ccc(CC[C@H](N[C@H](C)C(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)Nc2ccccc2)cc1 Show InChI InChI=1S/C28H39N3O4/c1-5-9-21-12-14-22(15-13-21)16-17-24(29-20(4)28(34)35)26(32)31-25(18-19(2)3)27(33)30-23-10-7-6-8-11-23/h6-8,10-15,19-20,24-25,29H,5,9,16-18H2,1-4H3,(H,30,33)(H,31,32)(H,34,35)/t20-,24+,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloproteinase-2(MMP-2) |
Bioorg Med Chem Lett 6: 803-806 (1996)
Article DOI: 10.1016/0960-894X(96)00109-6
BindingDB Entry DOI: 10.7270/Q2HM58FH |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50057090

((R)-2-((S)-1-((S)-4-methyl-1-oxo-1-(phenylamino)pe...)Show SMILES CCCc1ccc(CC[C@H](N[C@H](C)C(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)Nc2ccccc2)cc1 Show InChI InChI=1S/C28H39N3O4/c1-5-9-21-12-14-22(15-13-21)16-17-24(29-20(4)28(34)35)26(32)31-25(18-19(2)3)27(33)30-23-10-7-6-8-11-23/h6-8,10-15,19-20,24-25,29H,5,9,16-18H2,1-4H3,(H,30,33)(H,31,32)(H,34,35)/t20-,24+,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human gelatinase-A (MMP-2) |
Bioorg Med Chem Lett 5: 2441-2446 (1995)
Article DOI: 10.1016/0960-894X(95)00425-S
BindingDB Entry DOI: 10.7270/Q2D21XK6 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50288677
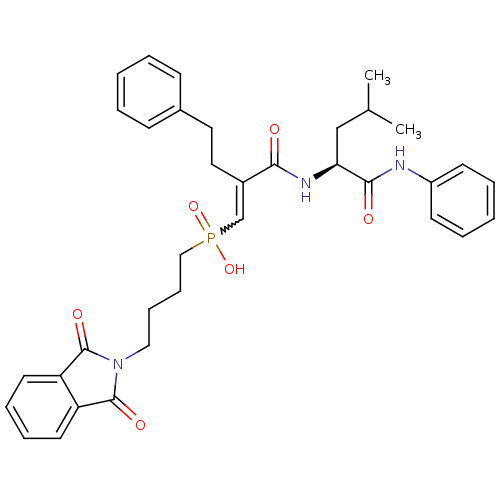
(CHEMBL324691 | [(E)-4-(1,3-Dioxo-1,3-dihydro-isoin...)Show SMILES CC(C)C[C@H](NC(=O)C(CCc1ccccc1)=CP(O)(=O)CCCCN1C(=O)c2ccccc2C1=O)C(=O)Nc1ccccc1 |w:17.18| Show InChI InChI=1S/C35H40N3O6P/c1-25(2)23-31(33(40)36-28-15-7-4-8-16-28)37-32(39)27(20-19-26-13-5-3-6-14-26)24-45(43,44)22-12-11-21-38-34(41)29-17-9-10-18-30(29)35(38)42/h3-10,13-18,24-25,31H,11-12,19-23H2,1-2H3,(H,36,40)(H,37,39)(H,43,44)/t31-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of stromelysin-1 (MMP-3). |
Bioorg Med Chem Lett 6: 323-328 (1996)
Article DOI: 10.1016/0960-894X(96)00023-6
BindingDB Entry DOI: 10.7270/Q2X0671M |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50288677

(CHEMBL324691 | [(E)-4-(1,3-Dioxo-1,3-dihydro-isoin...)Show SMILES CC(C)C[C@H](NC(=O)C(CCc1ccccc1)=CP(O)(=O)CCCCN1C(=O)c2ccccc2C1=O)C(=O)Nc1ccccc1 |w:17.18| Show InChI InChI=1S/C35H40N3O6P/c1-25(2)23-31(33(40)36-28-15-7-4-8-16-28)37-32(39)27(20-19-26-13-5-3-6-14-26)24-45(43,44)22-12-11-21-38-34(41)29-17-9-10-18-30(29)35(38)42/h3-10,13-18,24-25,31H,11-12,19-23H2,1-2H3,(H,36,40)(H,37,39)(H,43,44)/t31-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of gelatinase-A (MMP-2). |
Bioorg Med Chem Lett 6: 323-328 (1996)
Article DOI: 10.1016/0960-894X(96)00023-6
BindingDB Entry DOI: 10.7270/Q2X0671M |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50040594
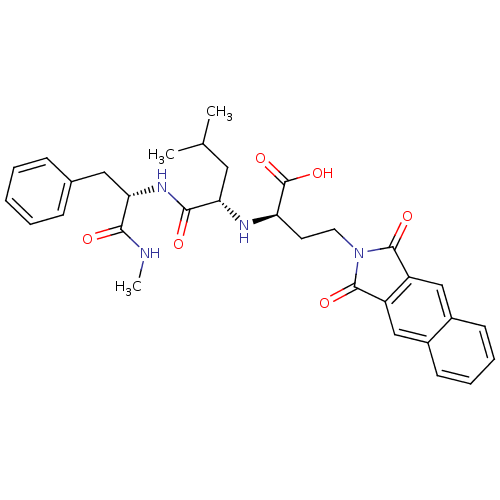
((R)-4-(1,3-Dioxo-1,3-dihydro-benzo[f]isoindol-2-yl...)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)N[C@H](CCN1C(=O)c2cc3ccccc3cc2C1=O)C(O)=O Show InChI InChI=1S/C32H36N4O6/c1-19(2)15-26(29(38)35-27(28(37)33-3)16-20-9-5-4-6-10-20)34-25(32(41)42)13-14-36-30(39)23-17-21-11-7-8-12-22(21)18-24(23)31(36)40/h4-12,17-19,25-27,34H,13-16H2,1-3H3,(H,33,37)(H,35,38)(H,41,42)/t25-,26+,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloproteinase -1 |
Bioorg Med Chem Lett 6: 329-332 (1996)
Article DOI: 10.1016/0960-894X(96)00024-8
BindingDB Entry DOI: 10.7270/Q2S75GBB |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50288678

(CHEMBL320968 | [(S)-2-((S)-3-Methyl-1-phenylcarbam...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCc1ccccc1)CP(O)(=O)CCCCN1Cc2ccccc2C1=O)C(=O)Nc1ccccc1 Show InChI InChI=1S/C35H44N3O5P/c1-26(2)23-32(34(40)36-30-16-7-4-8-17-30)37-33(39)29(20-19-27-13-5-3-6-14-27)25-44(42,43)22-12-11-21-38-24-28-15-9-10-18-31(28)35(38)41/h3-10,13-18,26,29,32H,11-12,19-25H2,1-2H3,(H,36,40)(H,37,39)(H,42,43)/t29-,32+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of stromelysin-1 (MMP-3). |
Bioorg Med Chem Lett 6: 323-328 (1996)
Article DOI: 10.1016/0960-894X(96)00023-6
BindingDB Entry DOI: 10.7270/Q2X0671M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50288681
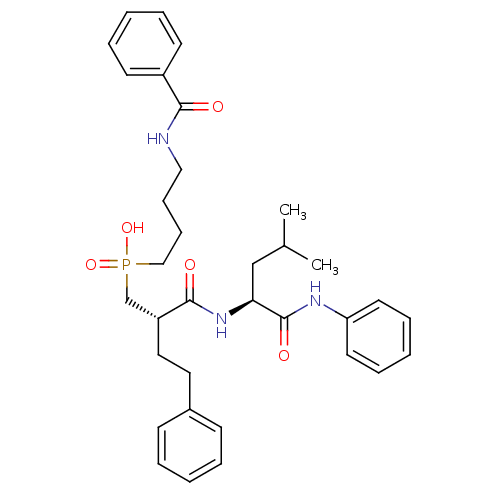
((4-Benzoylamino-butyl)-[(S)-2-((S)-3-methyl-1-phen...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCc1ccccc1)CP(O)(=O)CCCCNC(=O)c1ccccc1)C(=O)Nc1ccccc1 Show InChI InChI=1S/C34H44N3O5P/c1-26(2)24-31(34(40)36-30-18-10-5-11-19-30)37-33(39)29(21-20-27-14-6-3-7-15-27)25-43(41,42)23-13-12-22-35-32(38)28-16-8-4-9-17-28/h3-11,14-19,26,29,31H,12-13,20-25H2,1-2H3,(H,35,38)(H,36,40)(H,37,39)(H,41,42)/t29-,31+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of stromelysin-1 (MMP-3). |
Bioorg Med Chem Lett 6: 323-328 (1996)
Article DOI: 10.1016/0960-894X(96)00023-6
BindingDB Entry DOI: 10.7270/Q2X0671M |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50289127

((2S,4R)-4-(3-Methyl-1-phenylcarbamoyl-butylcarbamo...)Show SMILES CCCc1ccc(CC[C@H](C[C@H](CCCCN2Cc3ccccc3C2=O)C(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)Nc2ccccc2)cc1 Show InChI InChI=1S/C40H51N3O5/c1-4-12-29-18-20-30(21-19-29)22-23-31(37(44)42-36(25-28(2)3)38(45)41-34-15-6-5-7-16-34)26-32(40(47)48)13-10-11-24-43-27-33-14-8-9-17-35(33)39(43)46/h5-9,14-21,28,31-32,36H,4,10-13,22-27H2,1-3H3,(H,41,45)(H,42,44)(H,47,48)/t31-,32+,36+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloproteinase-2(MMP-2) |
Bioorg Med Chem Lett 6: 803-806 (1996)
Article DOI: 10.1016/0960-894X(96)00109-6
BindingDB Entry DOI: 10.7270/Q2HM58FH |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50288687

(CHEMBL111975 | {4-[((S)-1-Acetyl-pyrrolidine-2-car...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCc1ccccc1)CP(O)(=O)CCCCNC(=O)[C@@H]1CCCN1C(C)=O)C(=O)Nc1ccccc1 Show InChI InChI=1S/C34H49N4O6P/c1-25(2)23-30(33(41)36-29-15-8-5-9-16-29)37-32(40)28(19-18-27-13-6-4-7-14-27)24-45(43,44)22-11-10-20-35-34(42)31-17-12-21-38(31)26(3)39/h4-9,13-16,25,28,30-31H,10-12,17-24H2,1-3H3,(H,35,42)(H,36,41)(H,37,40)(H,43,44)/t28-,30+,31+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of gelatinase-A (MMP-2). |
Bioorg Med Chem Lett 6: 323-328 (1996)
Article DOI: 10.1016/0960-894X(96)00023-6
BindingDB Entry DOI: 10.7270/Q2X0671M |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50285573
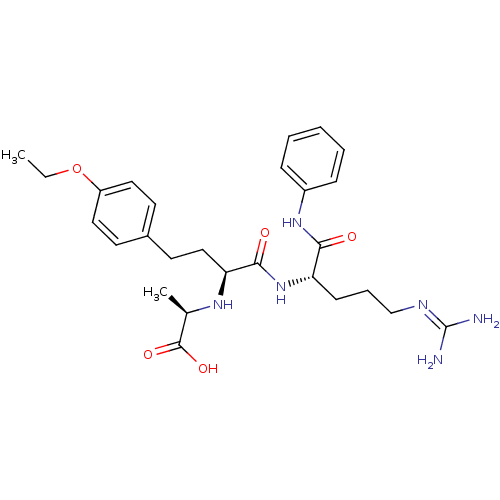
((R)-2-[(S)-3-(4-ethoxy-phenyl)-1-((S)-4-guanidino-...)Show SMILES [#6]-[#6]-[#8]-c1ccc(-[#6]-[#6]-[#6@H](-[#7]-[#6@H](-[#6])-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-c2ccccc2)cc1 Show InChI InChI=1S/C27H38N6O5/c1-3-38-21-14-11-19(12-15-21)13-16-23(31-18(2)26(36)37)25(35)33-22(10-7-17-30-27(28)29)24(34)32-20-8-5-4-6-9-20/h4-6,8-9,11-12,14-15,18,22-23,31H,3,7,10,13,16-17H2,1-2H3,(H,32,34)(H,33,35)(H,36,37)(H4,28,29,30)/t18-,22+,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human gelatinase-A (MMP-2) |
Bioorg Med Chem Lett 5: 2441-2446 (1995)
Article DOI: 10.1016/0960-894X(95)00425-S
BindingDB Entry DOI: 10.7270/Q2D21XK6 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50288678

(CHEMBL320968 | [(S)-2-((S)-3-Methyl-1-phenylcarbam...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCc1ccccc1)CP(O)(=O)CCCCN1Cc2ccccc2C1=O)C(=O)Nc1ccccc1 Show InChI InChI=1S/C35H44N3O5P/c1-26(2)23-32(34(40)36-30-16-7-4-8-17-30)37-33(39)29(20-19-27-13-5-3-6-14-27)25-44(42,43)22-12-11-21-38-24-28-15-9-10-18-31(28)35(38)41/h3-10,13-18,26,29,32H,11-12,19-25H2,1-2H3,(H,36,40)(H,37,39)(H,42,43)/t29-,32+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of gelatinase-A (MMP-2). |
Bioorg Med Chem Lett 6: 323-328 (1996)
Article DOI: 10.1016/0960-894X(96)00023-6
BindingDB Entry DOI: 10.7270/Q2X0671M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50288702
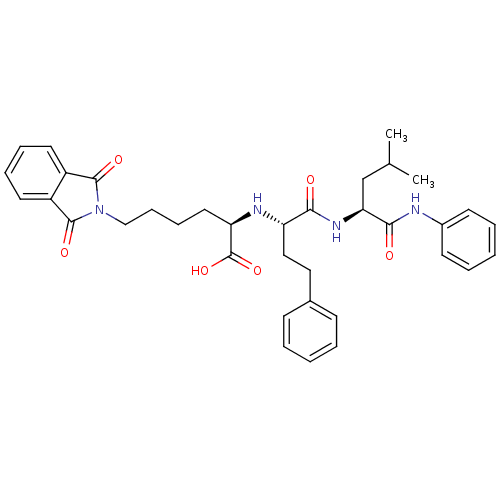
((R)-6-(1,3-Dioxo-1,3-dihydro-isoindol-2-yl)-2-[(S)...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCc1ccccc1)N[C@H](CCCCN1C(=O)c2ccccc2C1=O)C(O)=O)C(=O)Nc1ccccc1 Show InChI InChI=1S/C36H42N4O6/c1-24(2)23-31(33(42)37-26-15-7-4-8-16-26)39-32(41)29(21-20-25-13-5-3-6-14-25)38-30(36(45)46)19-11-12-22-40-34(43)27-17-9-10-18-28(27)35(40)44/h3-10,13-18,24,29-31,38H,11-12,19-23H2,1-2H3,(H,37,42)(H,39,41)(H,45,46)/t29-,30+,31-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloproteinase-3(MMP-3). |
Bioorg Med Chem Lett 6: 803-806 (1996)
Article DOI: 10.1016/0960-894X(96)00109-6
BindingDB Entry DOI: 10.7270/Q2HM58FH |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50288702

((R)-6-(1,3-Dioxo-1,3-dihydro-isoindol-2-yl)-2-[(S)...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCc1ccccc1)N[C@H](CCCCN1C(=O)c2ccccc2C1=O)C(O)=O)C(=O)Nc1ccccc1 Show InChI InChI=1S/C36H42N4O6/c1-24(2)23-31(33(42)37-26-15-7-4-8-16-26)39-32(41)29(21-20-25-13-5-3-6-14-25)38-30(36(45)46)19-11-12-22-40-34(43)27-17-9-10-18-28(27)35(40)44/h3-10,13-18,24,29-31,38H,11-12,19-23H2,1-2H3,(H,37,42)(H,39,41)(H,45,46)/t29-,30+,31-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MMP-3. |
Bioorg Med Chem Lett 6: 329-332 (1996)
Article DOI: 10.1016/0960-894X(96)00024-8
BindingDB Entry DOI: 10.7270/Q2S75GBB |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50289123

((R)-2-[(S)-1-(3-Methyl-1-phenylcarbamoyl-butylcarb...)Show SMILES CCCc1ccc(CC[C@H](N[C@H](CCCCN2Cc3ccccc3C2=O)C(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)Nc2ccccc2)cc1 Show InChI InChI=1S/C39H50N4O5/c1-4-12-28-18-20-29(21-19-28)22-23-33(36(44)42-35(25-27(2)3)37(45)40-31-14-6-5-7-15-31)41-34(39(47)48)17-10-11-24-43-26-30-13-8-9-16-32(30)38(43)46/h5-9,13-16,18-21,27,33-35,41H,4,10-12,17,22-26H2,1-3H3,(H,40,45)(H,42,44)(H,47,48)/t33-,34+,35-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 9.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloproteinase-3(MMP-3). |
Bioorg Med Chem Lett 6: 803-806 (1996)
Article DOI: 10.1016/0960-894X(96)00109-6
BindingDB Entry DOI: 10.7270/Q2HM58FH |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50288710
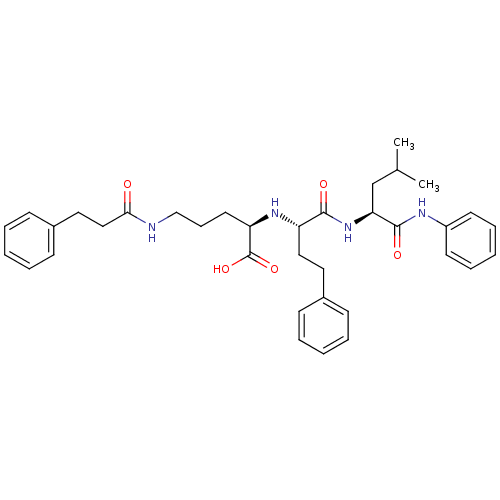
((R)-2-[(S)-1-((S)-3-Methyl-1-phenylcarbamoyl-butyl...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCc1ccccc1)N[C@H](CCCNC(=O)CCc1ccccc1)C(O)=O)C(=O)Nc1ccccc1 Show InChI InChI=1S/C36H46N4O5/c1-26(2)25-32(35(43)38-29-17-10-5-11-18-29)40-34(42)30(22-20-27-13-6-3-7-14-27)39-31(36(44)45)19-12-24-37-33(41)23-21-28-15-8-4-9-16-28/h3-11,13-18,26,30-32,39H,12,19-25H2,1-2H3,(H,37,41)(H,38,43)(H,40,42)(H,44,45)/t30-,31+,32-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 9.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MMP-3. |
Bioorg Med Chem Lett 6: 329-332 (1996)
Article DOI: 10.1016/0960-894X(96)00024-8
BindingDB Entry DOI: 10.7270/Q2S75GBB |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50288705
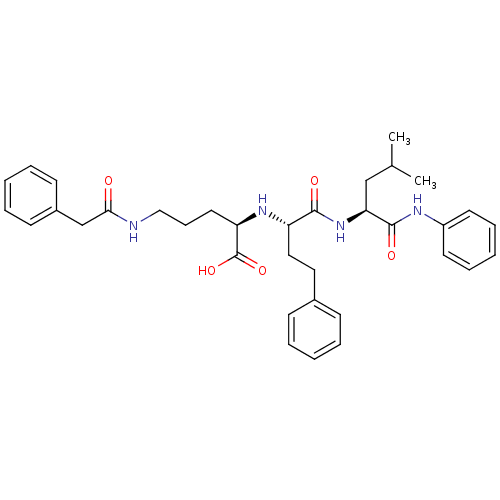
((R)-2-[(S)-1-((S)-3-Methyl-1-phenylcarbamoyl-butyl...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCc1ccccc1)N[C@H](CCCNC(=O)Cc1ccccc1)C(O)=O)C(=O)Nc1ccccc1 Show InChI InChI=1S/C35H44N4O5/c1-25(2)23-31(34(42)37-28-17-10-5-11-18-28)39-33(41)29(21-20-26-13-6-3-7-14-26)38-30(35(43)44)19-12-22-36-32(40)24-27-15-8-4-9-16-27/h3-11,13-18,25,29-31,38H,12,19-24H2,1-2H3,(H,36,40)(H,37,42)(H,39,41)(H,43,44)/t29-,30+,31-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MMP-3. |
Bioorg Med Chem Lett 6: 329-332 (1996)
Article DOI: 10.1016/0960-894X(96)00024-8
BindingDB Entry DOI: 10.7270/Q2S75GBB |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50285559
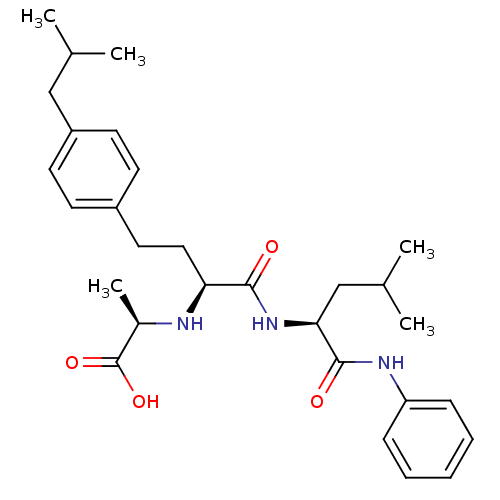
((R)-2-((S)-4-(4-isobutylphenyl)-1-((S)-4-methyl-1-...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCc1ccc(CC(C)C)cc1)N[C@H](C)C(O)=O)C(=O)Nc1ccccc1 Show InChI InChI=1S/C29H41N3O4/c1-19(2)17-23-13-11-22(12-14-23)15-16-25(30-21(5)29(35)36)27(33)32-26(18-20(3)4)28(34)31-24-9-7-6-8-10-24/h6-14,19-21,25-26,30H,15-18H2,1-5H3,(H,31,34)(H,32,33)(H,35,36)/t21-,25+,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human gelatinase-A (MMP-2) |
Bioorg Med Chem Lett 5: 2441-2446 (1995)
Article DOI: 10.1016/0960-894X(95)00425-S
BindingDB Entry DOI: 10.7270/Q2D21XK6 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50288708
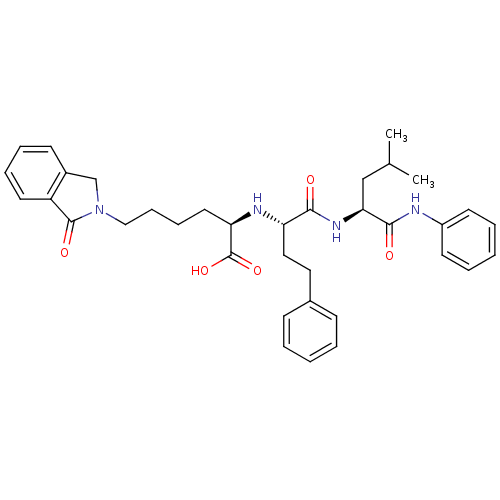
((R)-2-[(S)-1-((S)-3-Methyl-1-phenylcarbamoyl-butyl...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCc1ccccc1)N[C@H](CCCCN1Cc2ccccc2C1=O)C(O)=O)C(=O)Nc1ccccc1 Show InChI InChI=1S/C36H44N4O5/c1-25(2)23-32(34(42)37-28-16-7-4-8-17-28)39-33(41)30(21-20-26-13-5-3-6-14-26)38-31(36(44)45)19-11-12-22-40-24-27-15-9-10-18-29(27)35(40)43/h3-10,13-18,25,30-32,38H,11-12,19-24H2,1-2H3,(H,37,42)(H,39,41)(H,44,45)/t30-,31+,32-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MMP-3. |
Bioorg Med Chem Lett 6: 329-332 (1996)
Article DOI: 10.1016/0960-894X(96)00024-8
BindingDB Entry DOI: 10.7270/Q2S75GBB |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50285564
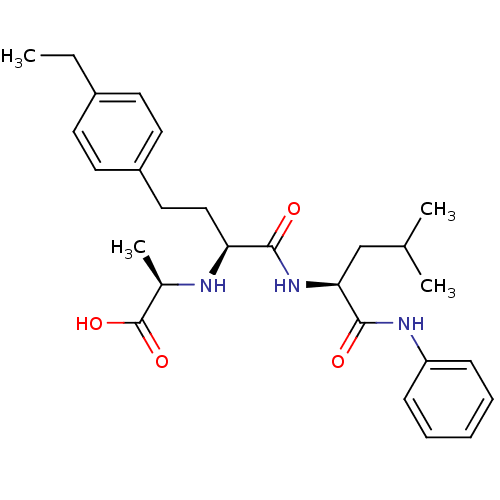
((R)-2-((S)-4-(4-ethylphenyl)-1-((S)-4-methyl-1-oxo...)Show SMILES CCc1ccc(CC[C@H](N[C@H](C)C(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)Nc2ccccc2)cc1 Show InChI InChI=1S/C27H37N3O4/c1-5-20-11-13-21(14-12-20)15-16-23(28-19(4)27(33)34)25(31)30-24(17-18(2)3)26(32)29-22-9-7-6-8-10-22/h6-14,18-19,23-24,28H,5,15-17H2,1-4H3,(H,29,32)(H,30,31)(H,33,34)/t19-,23+,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human gelatinase-A (MMP-2) |
Bioorg Med Chem Lett 5: 2441-2446 (1995)
Article DOI: 10.1016/0960-894X(95)00425-S
BindingDB Entry DOI: 10.7270/Q2D21XK6 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50288683

(CHEMBL109438 | [4-(1,3-Dioxo-1,3-dihydro-isoindol-...)Show SMILES COc1ccc(CC[C@H](CP(O)(=O)CCCCN2C(=O)c3ccccc3C2=O)C(=O)N[C@@H](CC(C)C)C(=O)Nc2ccccc2)cc1 Show InChI InChI=1S/C36H44N3O7P/c1-25(2)23-32(34(41)37-28-11-5-4-6-12-28)38-33(40)27(18-15-26-16-19-29(46-3)20-17-26)24-47(44,45)22-10-9-21-39-35(42)30-13-7-8-14-31(30)36(39)43/h4-8,11-14,16-17,19-20,25,27,32H,9-10,15,18,21-24H2,1-3H3,(H,37,41)(H,38,40)(H,44,45)/t27-,32+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of gelatinase-A (MMP-2). |
Bioorg Med Chem Lett 6: 323-328 (1996)
Article DOI: 10.1016/0960-894X(96)00023-6
BindingDB Entry DOI: 10.7270/Q2X0671M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50288673

(CHEMBL264455 | {4-[((S)-1-Benzoyl-pyrrolidine-2-ca...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCc1ccccc1)CP(O)(=O)CCCCNC(=O)[C@@H]1CCCN1C(=O)c1ccccc1)C(=O)Nc1ccccc1 Show InChI InChI=1S/C39H51N4O6P/c1-29(2)27-34(37(45)41-33-19-10-5-11-20-33)42-36(44)32(23-22-30-15-6-3-7-16-30)28-50(48,49)26-13-12-24-40-38(46)35-21-14-25-43(35)39(47)31-17-8-4-9-18-31/h3-11,15-20,29,32,34-35H,12-14,21-28H2,1-2H3,(H,40,46)(H,41,45)(H,42,44)(H,48,49)/t32-,34+,35+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of stromelysin-1 (MMP-3). |
Bioorg Med Chem Lett 6: 323-328 (1996)
Article DOI: 10.1016/0960-894X(96)00023-6
BindingDB Entry DOI: 10.7270/Q2X0671M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50288699
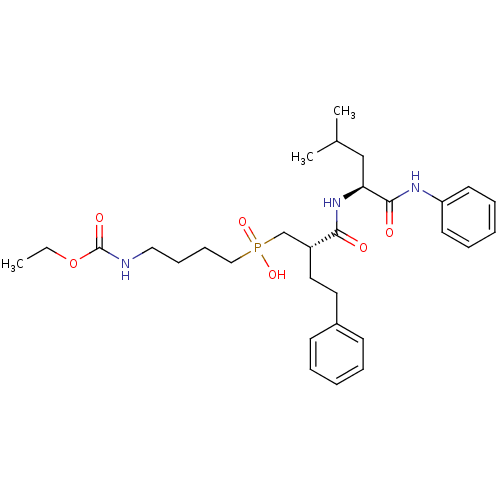
((4-Ethoxycarbonylamino-butyl)-[(S)-2-((S)-3-methyl...)Show SMILES CCOC(=O)NCCCCP(O)(=O)C[C@@H](CCc1ccccc1)C(=O)N[C@@H](CC(C)C)C(=O)Nc1ccccc1 Show InChI InChI=1S/C30H44N3O6P/c1-4-39-30(36)31-19-11-12-20-40(37,38)22-25(18-17-24-13-7-5-8-14-24)28(34)33-27(21-23(2)3)29(35)32-26-15-9-6-10-16-26/h5-10,13-16,23,25,27H,4,11-12,17-22H2,1-3H3,(H,31,36)(H,32,35)(H,33,34)(H,37,38)/t25-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of stromelysin-1 (MMP-3). |
Bioorg Med Chem Lett 6: 323-328 (1996)
Article DOI: 10.1016/0960-894X(96)00023-6
BindingDB Entry DOI: 10.7270/Q2X0671M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50288668
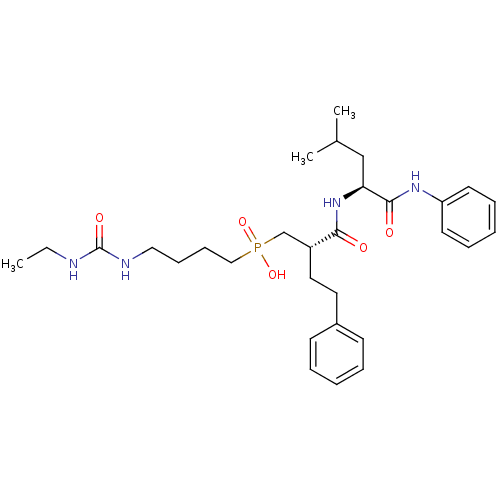
(CHEMBL109829 | [4-(3-Ethyl-ureido)-butyl]-[(S)-2-(...)Show SMILES CCNC(=O)NCCCCP(O)(=O)C[C@@H](CCc1ccccc1)C(=O)N[C@@H](CC(C)C)C(=O)Nc1ccccc1 Show InChI InChI=1S/C30H45N4O5P/c1-4-31-30(37)32-19-11-12-20-40(38,39)22-25(18-17-24-13-7-5-8-14-24)28(35)34-27(21-23(2)3)29(36)33-26-15-9-6-10-16-26/h5-10,13-16,23,25,27H,4,11-12,17-22H2,1-3H3,(H,33,36)(H,34,35)(H,38,39)(H2,31,32,37)/t25-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of stromelysin-1 (MMP-3). |
Bioorg Med Chem Lett 6: 323-328 (1996)
Article DOI: 10.1016/0960-894X(96)00023-6
BindingDB Entry DOI: 10.7270/Q2X0671M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50057090

((R)-2-((S)-1-((S)-4-methyl-1-oxo-1-(phenylamino)pe...)Show SMILES CCCc1ccc(CC[C@H](N[C@H](C)C(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)Nc2ccccc2)cc1 Show InChI InChI=1S/C28H39N3O4/c1-5-9-21-12-14-22(15-13-21)16-17-24(29-20(4)28(34)35)26(32)31-25(18-19(2)3)27(33)30-23-10-7-6-8-11-23/h6-8,10-15,19-20,24-25,29H,5,9,16-18H2,1-4H3,(H,30,33)(H,31,32)(H,34,35)/t20-,24+,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human stromelysin-3 (MMP-3) |
Bioorg Med Chem Lett 5: 2441-2446 (1995)
Article DOI: 10.1016/0960-894X(95)00425-S
BindingDB Entry DOI: 10.7270/Q2D21XK6 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50057090

((R)-2-((S)-1-((S)-4-methyl-1-oxo-1-(phenylamino)pe...)Show SMILES CCCc1ccc(CC[C@H](N[C@H](C)C(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)Nc2ccccc2)cc1 Show InChI InChI=1S/C28H39N3O4/c1-5-9-21-12-14-22(15-13-21)16-17-24(29-20(4)28(34)35)26(32)31-25(18-19(2)3)27(33)30-23-10-7-6-8-11-23/h6-8,10-15,19-20,24-25,29H,5,9,16-18H2,1-4H3,(H,30,33)(H,31,32)(H,34,35)/t20-,24+,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloproteinase-3(MMP-3). |
Bioorg Med Chem Lett 6: 803-806 (1996)
Article DOI: 10.1016/0960-894X(96)00109-6
BindingDB Entry DOI: 10.7270/Q2HM58FH |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50288668

(CHEMBL109829 | [4-(3-Ethyl-ureido)-butyl]-[(S)-2-(...)Show SMILES CCNC(=O)NCCCCP(O)(=O)C[C@@H](CCc1ccccc1)C(=O)N[C@@H](CC(C)C)C(=O)Nc1ccccc1 Show InChI InChI=1S/C30H45N4O5P/c1-4-31-30(37)32-19-11-12-20-40(38,39)22-25(18-17-24-13-7-5-8-14-24)28(35)34-27(21-23(2)3)29(36)33-26-15-9-6-10-16-26/h5-10,13-16,23,25,27H,4,11-12,17-22H2,1-3H3,(H,33,36)(H,34,35)(H,38,39)(H2,31,32,37)/t25-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of gelatinase-A (MMP-2) |
Bioorg Med Chem Lett 6: 323-328 (1996)
Article DOI: 10.1016/0960-894X(96)00023-6
BindingDB Entry DOI: 10.7270/Q2X0671M |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50288674

(CHEMBL420674 | [4-(1,3-Dioxo-1,3-dihydro-isoindol-...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCc1ccccc1)CP(O)(=O)CCCCN1C(=O)c2ccccc2C1=O)C(=O)Nc1ccccc1 Show InChI InChI=1S/C35H42N3O6P/c1-25(2)23-31(33(40)36-28-15-7-4-8-16-28)37-32(39)27(20-19-26-13-5-3-6-14-26)24-45(43,44)22-12-11-21-38-34(41)29-17-9-10-18-30(29)35(38)42/h3-10,13-18,25,27,31H,11-12,19-24H2,1-2H3,(H,36,40)(H,37,39)(H,43,44)/t27-,31+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of gelatinase-A (MMP-2). |
Bioorg Med Chem Lett 6: 323-328 (1996)
Article DOI: 10.1016/0960-894X(96)00023-6
BindingDB Entry DOI: 10.7270/Q2X0671M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50040594

((R)-4-(1,3-Dioxo-1,3-dihydro-benzo[f]isoindol-2-yl...)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)N[C@H](CCN1C(=O)c2cc3ccccc3cc2C1=O)C(O)=O Show InChI InChI=1S/C32H36N4O6/c1-19(2)15-26(29(38)35-27(28(37)33-3)16-20-9-5-4-6-10-20)34-25(32(41)42)13-14-36-30(39)23-17-21-11-7-8-12-22(21)18-24(23)31(36)40/h4-12,17-19,25-27,34H,13-16H2,1-3H3,(H,33,37)(H,35,38)(H,41,42)/t25-,26+,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloproteinase -3 |
Bioorg Med Chem Lett 6: 329-332 (1996)
Article DOI: 10.1016/0960-894X(96)00024-8
BindingDB Entry DOI: 10.7270/Q2S75GBB |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50288716
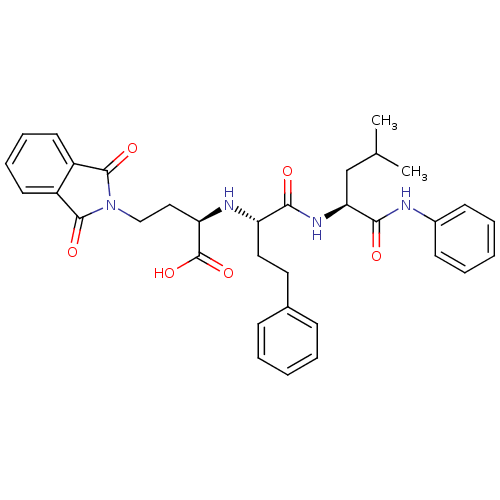
((R)-4-(1,3-Dioxo-1,3-dihydro-isoindol-2-yl)-2-[(S)...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCc1ccccc1)N[C@H](CCN1C(=O)c2ccccc2C1=O)C(O)=O)C(=O)Nc1ccccc1 Show InChI InChI=1S/C34H38N4O6/c1-22(2)21-29(31(40)35-24-13-7-4-8-14-24)37-30(39)27(18-17-23-11-5-3-6-12-23)36-28(34(43)44)19-20-38-32(41)25-15-9-10-16-26(25)33(38)42/h3-16,22,27-29,36H,17-21H2,1-2H3,(H,35,40)(H,37,39)(H,43,44)/t27-,28+,29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MMP-2. |
Bioorg Med Chem Lett 6: 329-332 (1996)
Article DOI: 10.1016/0960-894X(96)00024-8
BindingDB Entry DOI: 10.7270/Q2S75GBB |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50288704

((R)-7-Benzoylamino-2-[(S)-1-((S)-3-methyl-1-phenyl...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCc1ccccc1)N[C@H](CCCCCNC(=O)c1ccccc1)C(O)=O)C(=O)Nc1ccccc1 Show InChI InChI=1S/C36H46N4O5/c1-26(2)25-32(35(43)38-29-19-11-5-12-20-29)40-34(42)30(23-22-27-15-7-3-8-16-27)39-31(36(44)45)21-13-6-14-24-37-33(41)28-17-9-4-10-18-28/h3-5,7-12,15-20,26,30-32,39H,6,13-14,21-25H2,1-2H3,(H,37,41)(H,38,43)(H,40,42)(H,44,45)/t30-,31+,32-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MMP-3. |
Bioorg Med Chem Lett 6: 329-332 (1996)
Article DOI: 10.1016/0960-894X(96)00024-8
BindingDB Entry DOI: 10.7270/Q2S75GBB |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50288682
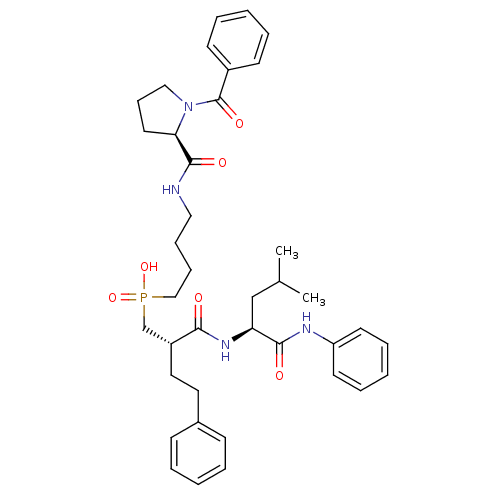
(CHEMBL110991 | {4-[((R)-1-Benzoyl-pyrrolidine-2-ca...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCc1ccccc1)CP(O)(=O)CCCCNC(=O)[C@H]1CCCN1C(=O)c1ccccc1)C(=O)Nc1ccccc1 Show InChI InChI=1S/C39H51N4O6P/c1-29(2)27-34(37(45)41-33-19-10-5-11-20-33)42-36(44)32(23-22-30-15-6-3-7-16-30)28-50(48,49)26-13-12-24-40-38(46)35-21-14-25-43(35)39(47)31-17-8-4-9-18-31/h3-11,15-20,29,32,34-35H,12-14,21-28H2,1-2H3,(H,40,46)(H,41,45)(H,42,44)(H,48,49)/t32-,34+,35-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of stromelysin-1 (MMP-3). |
Bioorg Med Chem Lett 6: 323-328 (1996)
Article DOI: 10.1016/0960-894X(96)00023-6
BindingDB Entry DOI: 10.7270/Q2X0671M |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50285571
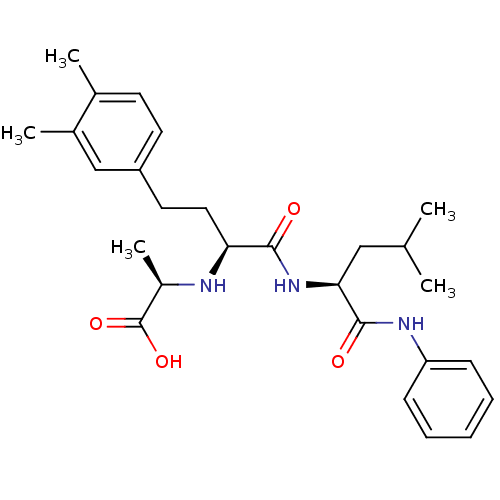
((R)-2-((S)-4-(3,4-dimethylphenyl)-1-((S)-4-methyl-...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCc1ccc(C)c(C)c1)N[C@H](C)C(O)=O)C(=O)Nc1ccccc1 Show InChI InChI=1S/C27H37N3O4/c1-17(2)15-24(26(32)29-22-9-7-6-8-10-22)30-25(31)23(28-20(5)27(33)34)14-13-21-12-11-18(3)19(4)16-21/h6-12,16-17,20,23-24,28H,13-15H2,1-5H3,(H,29,32)(H,30,31)(H,33,34)/t20-,23+,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human gelatinase-A (MMP-2) |
Bioorg Med Chem Lett 5: 2441-2446 (1995)
Article DOI: 10.1016/0960-894X(95)00425-S
BindingDB Entry DOI: 10.7270/Q2D21XK6 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50288681

((4-Benzoylamino-butyl)-[(S)-2-((S)-3-methyl-1-phen...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCc1ccccc1)CP(O)(=O)CCCCNC(=O)c1ccccc1)C(=O)Nc1ccccc1 Show InChI InChI=1S/C34H44N3O5P/c1-26(2)24-31(34(40)36-30-18-10-5-11-19-30)37-33(39)29(21-20-27-14-6-3-7-15-27)25-43(41,42)23-13-12-22-35-32(38)28-16-8-4-9-17-28/h3-11,14-19,26,29,31H,12-13,20-25H2,1-2H3,(H,35,38)(H,36,40)(H,37,39)(H,41,42)/t29-,31+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of gelatinase-A (MMP-2). |
Bioorg Med Chem Lett 6: 323-328 (1996)
Article DOI: 10.1016/0960-894X(96)00023-6
BindingDB Entry DOI: 10.7270/Q2X0671M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50288675
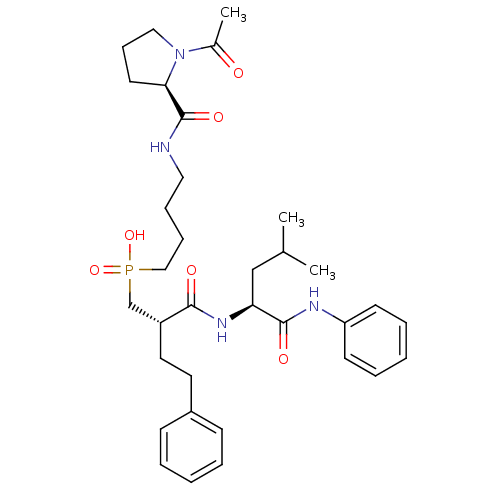
(CHEMBL113234 | {4-[((R)-1-Acetyl-pyrrolidine-2-car...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCc1ccccc1)CP(O)(=O)CCCCNC(=O)[C@H]1CCCN1C(C)=O)C(=O)Nc1ccccc1 Show InChI InChI=1S/C34H49N4O6P/c1-25(2)23-30(33(41)36-29-15-8-5-9-16-29)37-32(40)28(19-18-27-13-6-4-7-14-27)24-45(43,44)22-11-10-20-35-34(42)31-17-12-21-38(31)26(3)39/h4-9,13-16,25,28,30-31H,10-12,17-24H2,1-3H3,(H,35,42)(H,36,41)(H,37,40)(H,43,44)/t28-,30+,31-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of stromelysin-1 (MMP-3). |
Bioorg Med Chem Lett 6: 323-328 (1996)
Article DOI: 10.1016/0960-894X(96)00023-6
BindingDB Entry DOI: 10.7270/Q2X0671M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50288707

((R)-2-[(S)-1-((S)-3-Methyl-1-phenylcarbamoyl-butyl...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCc1ccccc1)N[C@H](CCCCNC(=O)Cc1ccccc1)C(O)=O)C(=O)Nc1ccccc1 Show InChI InChI=1S/C36H46N4O5/c1-26(2)24-32(35(43)38-29-18-10-5-11-19-29)40-34(42)30(22-21-27-14-6-3-7-15-27)39-31(36(44)45)20-12-13-23-37-33(41)25-28-16-8-4-9-17-28/h3-11,14-19,26,30-32,39H,12-13,20-25H2,1-2H3,(H,37,41)(H,38,43)(H,40,42)(H,44,45)/t30-,31+,32-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MMP-3. |
Bioorg Med Chem Lett 6: 329-332 (1996)
Article DOI: 10.1016/0960-894X(96)00024-8
BindingDB Entry DOI: 10.7270/Q2S75GBB |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50288679

(CHEMBL325835 | [(S)-2-((S)-3-Methyl-1-phenylcarbam...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCc1ccccc1)CP(O)(=O)CCCCNC(=O)Cc1ccccc1)C(=O)Nc1ccccc1 Show InChI InChI=1S/C35H46N3O5P/c1-27(2)24-32(35(41)37-31-18-10-5-11-19-31)38-34(40)30(21-20-28-14-6-3-7-15-28)26-44(42,43)23-13-12-22-36-33(39)25-29-16-8-4-9-17-29/h3-11,14-19,27,30,32H,12-13,20-26H2,1-2H3,(H,36,39)(H,37,41)(H,38,40)(H,42,43)/t30-,32+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of stromelysin-1 (MMP-3). |
Bioorg Med Chem Lett 6: 323-328 (1996)
Article DOI: 10.1016/0960-894X(96)00023-6
BindingDB Entry DOI: 10.7270/Q2X0671M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50288720

((R)-6-Benzoylamino-2-[(S)-1-((S)-3-methyl-1-phenyl...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCc1ccccc1)N[C@H](CCCCNC(=O)c1ccccc1)C(O)=O)C(=O)Nc1ccccc1 Show InChI InChI=1S/C35H44N4O5/c1-25(2)24-31(34(42)37-28-18-10-5-11-19-28)39-33(41)29(22-21-26-14-6-3-7-15-26)38-30(35(43)44)20-12-13-23-36-32(40)27-16-8-4-9-17-27/h3-11,14-19,25,29-31,38H,12-13,20-24H2,1-2H3,(H,36,40)(H,37,42)(H,39,41)(H,43,44)/t29-,30+,31-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MMP-3. |
Bioorg Med Chem Lett 6: 329-332 (1996)
Article DOI: 10.1016/0960-894X(96)00024-8
BindingDB Entry DOI: 10.7270/Q2S75GBB |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50288716

((R)-4-(1,3-Dioxo-1,3-dihydro-isoindol-2-yl)-2-[(S)...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCc1ccccc1)N[C@H](CCN1C(=O)c2ccccc2C1=O)C(O)=O)C(=O)Nc1ccccc1 Show InChI InChI=1S/C34H38N4O6/c1-22(2)21-29(31(40)35-24-13-7-4-8-14-24)37-30(39)27(18-17-23-11-5-3-6-12-23)36-28(34(43)44)19-20-38-32(41)25-15-9-10-16-26(25)33(38)42/h3-16,22,27-29,36H,17-21H2,1-2H3,(H,35,40)(H,37,39)(H,43,44)/t27-,28+,29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MMP-3. |
Bioorg Med Chem Lett 6: 329-332 (1996)
Article DOI: 10.1016/0960-894X(96)00024-8
BindingDB Entry DOI: 10.7270/Q2S75GBB |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50285567

((R)-2-[(S)-1-((S)-4-guanidino-1-phenylcarbamoyl-bu...)Show SMILES [#6]-[#6]-[#6]-c1ccc(-[#6]-[#6]-[#6@H](-[#7]-[#6@H](-[#6])-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-c2ccccc2)cc1 Show InChI InChI=1S/C28H40N6O4/c1-3-8-20-12-14-21(15-13-20)16-17-24(32-19(2)27(37)38)26(36)34-23(11-7-18-31-28(29)30)25(35)33-22-9-5-4-6-10-22/h4-6,9-10,12-15,19,23-24,32H,3,7-8,11,16-18H2,1-2H3,(H,33,35)(H,34,36)(H,37,38)(H4,29,30,31)/t19-,23+,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human stromelysin-3 (MMP-3) |
Bioorg Med Chem Lett 5: 2441-2446 (1995)
Article DOI: 10.1016/0960-894X(95)00425-S
BindingDB Entry DOI: 10.7270/Q2D21XK6 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50285557

((R)-2-[(S)-3-(4-butyl-phenyl)-1-((S)-4-guanidino-1...)Show SMILES [#6]-[#6]-[#6]-[#6]-c1ccc(-[#6]-[#6]-[#6@H](-[#7]-[#6@H](-[#6])-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-c2ccccc2)cc1 Show InChI InChI=1S/C29H42N6O4/c1-3-4-9-21-13-15-22(16-14-21)17-18-25(33-20(2)28(38)39)27(37)35-24(12-8-19-32-29(30)31)26(36)34-23-10-6-5-7-11-23/h5-7,10-11,13-16,20,24-25,33H,3-4,8-9,12,17-19H2,1-2H3,(H,34,36)(H,35,37)(H,38,39)(H4,30,31,32)/t20-,24+,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human stromelysin-3 (MMP-3) |
Bioorg Med Chem Lett 5: 2441-2446 (1995)
Article DOI: 10.1016/0960-894X(95)00425-S
BindingDB Entry DOI: 10.7270/Q2D21XK6 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data