

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50203422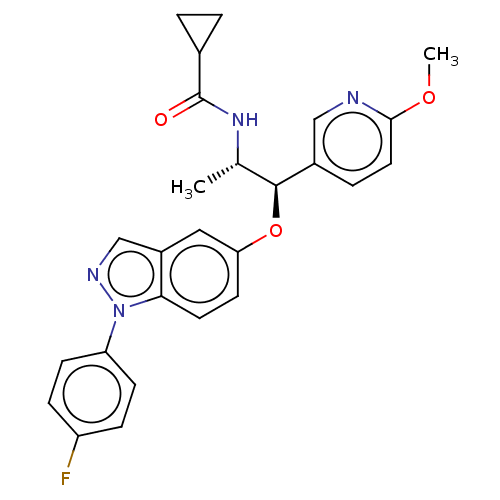 (CHEMBL3937635) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Transrepression activity at glucocorticoid receptor (unknown origin) expressed in human ChaGoK1 cells assessed as inhibition of PMA-stimulated gene e... | J Med Chem 61: 1785-1799 (2018) Article DOI: 10.1021/acs.jmedchem.7b01690 BindingDB Entry DOI: 10.7270/Q2XK8J5R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM223288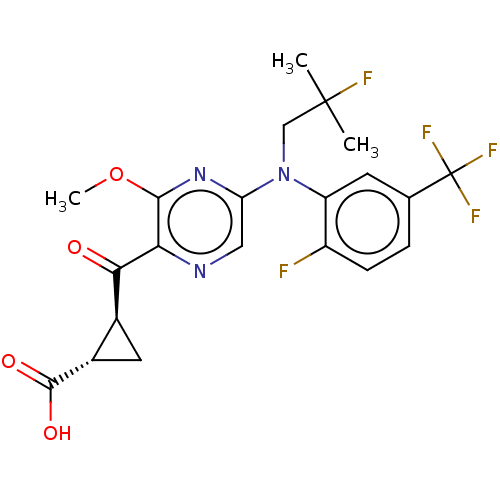 ((1S,2S)-2-[(5-{(2-Fluoro-2-methylpropyl)[2-fluoro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.248 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Astrazeneca Ab US Patent | Assay Description In the assay, LTC4 synthase catalyses the reaction where the substrate LTA4 methyl ester is converted to LTC4 methyl ester. In order to obtain IC50-v... | US Patent US20160326143 (2016) BindingDB Entry DOI: 10.7270/Q2W66JN5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM223288 ((1S,2S)-2-[(5-{(2-Fluoro-2-methylpropyl)[2-fluoro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.248 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTRAZENECA AB US Patent | Assay Description In the assay, LTC4 synthase catalyses the reaction where the substrate LTA4 methyl ester is converted to LTC4 methyl ester.In order to obtain IC50-va... | US Patent US9657001 (2017) BindingDB Entry DOI: 10.7270/Q2SJ1NPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM223288 ((1S,2S)-2-[(5-{(2-Fluoro-2-methylpropyl)[2-fluoro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged LTC4S expressed in Pichia pastoris X33 using LTA4 methyl ester and glutathione as substrate preincubated f... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM50519568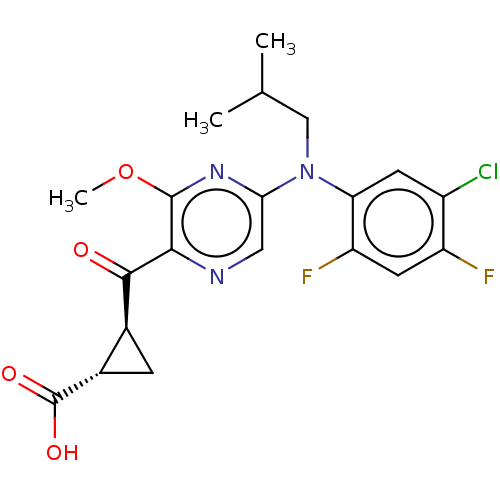 (CHEMBL4549822) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged LTC4S expressed in Pichia pastoris X33 using LTA4 methyl ester and glutathione as substrate preincubated f... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM223287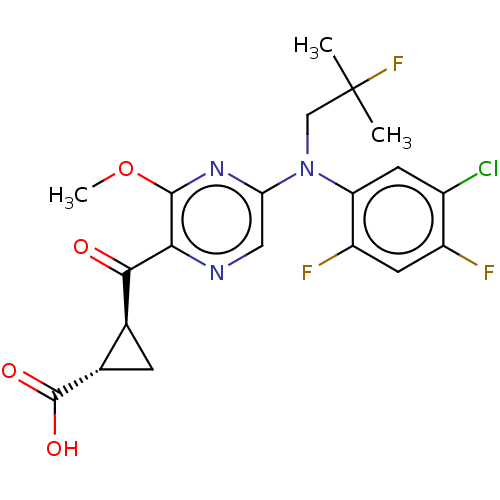 ((1S,2S)-2-({5-[(5-Chloro-2,4-difluorophenyl)(2-flu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged LTC4S expressed in Pichia pastoris X33 using LTA4 methyl ester and glutathione as substrate preincubated f... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM223287 ((1S,2S)-2-({5-[(5-Chloro-2,4-difluorophenyl)(2-flu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 0.289 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Astrazeneca Ab US Patent | Assay Description In the assay, LTC4 synthase catalyses the reaction where the substrate LTA4 methyl ester is converted to LTC4 methyl ester. In order to obtain IC50-v... | US Patent US20160326143 (2016) BindingDB Entry DOI: 10.7270/Q2W66JN5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM223287 ((1S,2S)-2-({5-[(5-Chloro-2,4-difluorophenyl)(2-flu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 0.289 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTRAZENECA AB US Patent | Assay Description In the assay, LTC4 synthase catalyses the reaction where the substrate LTA4 methyl ester is converted to LTC4 methyl ester.In order to obtain IC50-va... | US Patent US9657001 (2017) BindingDB Entry DOI: 10.7270/Q2SJ1NPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM489245 (N-{6-[(5-{2-[(1S)-1-Cyclopropylethyl]-7-(methylsul...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB US Patent | Assay Description The activity of recombinant human PI3Kγ ((aa144-1102)-6His) and PI3Kα, β, δ (6-His(p110-p85α)) was determined by measuring t... | US Patent US10961236 (2021) BindingDB Entry DOI: 10.7270/Q2M90CS5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM489244 (N-{6-[(5-{2-[(1S)-1-Cyclopropylethyl]-7-(methylsul...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB US Patent | Assay Description The activity of recombinant human PI3Kγ ((aa144-1102)-6His) and PI3Kα, β, δ (6-His(p110-p85α)) was determined by measuring t... | US Patent US10961236 (2021) BindingDB Entry DOI: 10.7270/Q2M90CS5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50457410 (CHEMBL4213191) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Transrepression activity at glucocorticoid receptor (unknown origin) expressed in human ChaGoK1 cells assessed as inhibition of PMA-stimulated gene e... | J Med Chem 61: 1785-1799 (2018) Article DOI: 10.1021/acs.jmedchem.7b01690 BindingDB Entry DOI: 10.7270/Q2XK8J5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM50519570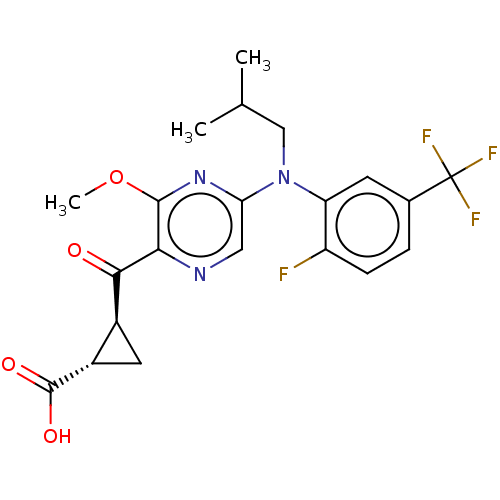 (CHEMBL4567083) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged LTC4S expressed in Pichia pastoris X33 using LTA4 methyl ester and glutathione as substrate preincubated f... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM50519585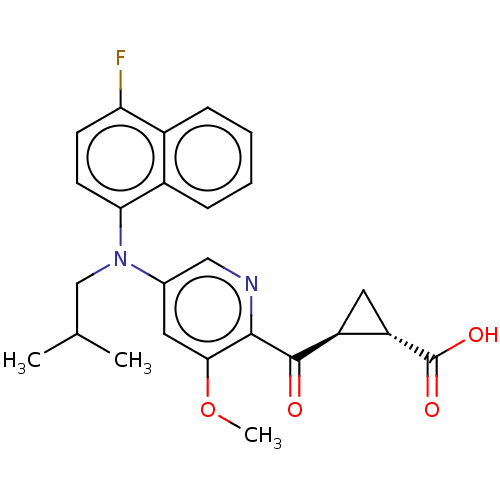 (CHEMBL4464773) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.381 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged LTC4S expressed in Pichia pastoris X33 using LTA4 methyl ester and glutathione as substrate preincubated f... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM489251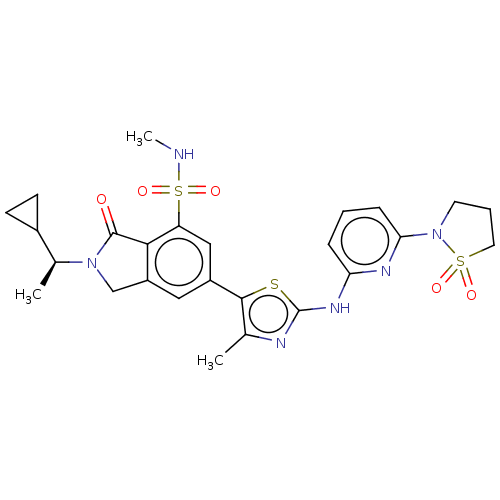 (2-[(1S)-1-Cyclopropylethyl]-6-(2-{[6-(1,1-dioxido-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB US Patent | Assay Description The activity of recombinant human PI3Kγ ((aa144-1102)-6His) and PI3Kα, β, δ (6-His(p110-p85α)) was determined by measuring t... | US Patent US10961236 (2021) BindingDB Entry DOI: 10.7270/Q2M90CS5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM489241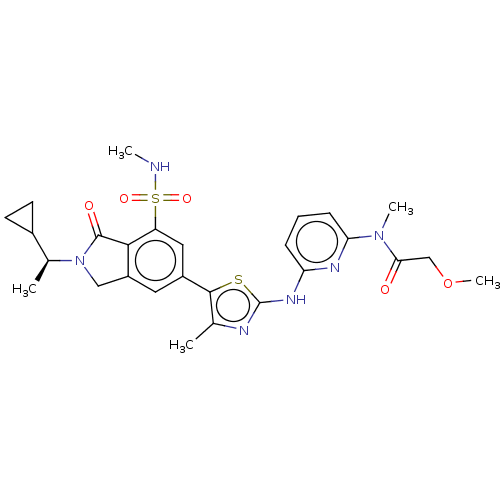 (N-{6-[(5-{2-[(1S)-1-Cyclopropylethyl]-7-(methylsul...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB US Patent | Assay Description The activity of recombinant human PI3Kγ ((aa144-1102)-6His) and PI3Kα, β, δ (6-His(p110-p85α)) was determined by measuring t... | US Patent US10961236 (2021) BindingDB Entry DOI: 10.7270/Q2M90CS5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50457406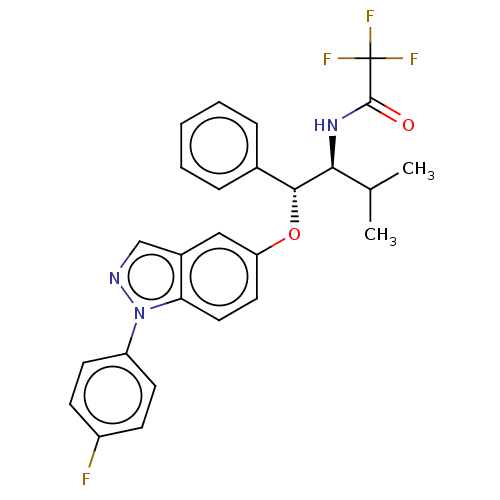 (CHEMBL4208262) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Transrepression activity at glucocorticoid receptor (unknown origin) expressed in human ChaGoK1 cells assessed as inhibition of PMA-stimulated gene e... | J Med Chem 61: 1785-1799 (2018) Article DOI: 10.1021/acs.jmedchem.7b01690 BindingDB Entry DOI: 10.7270/Q2XK8J5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM310035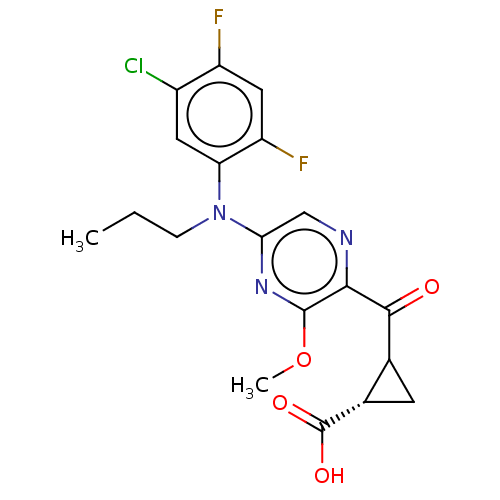 ((1S,2S)-2-({5-[(5-Chloro-2,4-difluorophenyl)(propy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.483 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTRAZENECA AB US Patent | Assay Description In the assay, LTC4 synthase catalyses the reaction where the substrate LTA4 methyl ester is converted to LTC4 methyl ester.In order to obtain IC50-va... | US Patent US9657001 (2017) BindingDB Entry DOI: 10.7270/Q2SJ1NPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM223289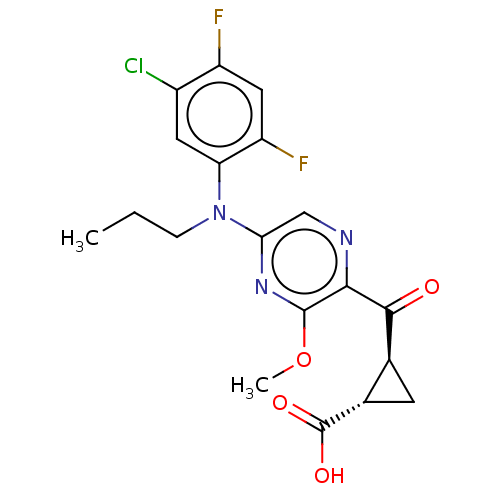 ((1S,2S)-2-({5-[(5-Chloro-2,4-difluorophenyl)(propy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.483 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Astrazeneca Ab US Patent | Assay Description In the assay, LTC4 synthase catalyses the reaction where the substrate LTA4 methyl ester is converted to LTC4 methyl ester. In order to obtain IC50-v... | US Patent US20160326143 (2016) BindingDB Entry DOI: 10.7270/Q2W66JN5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM489257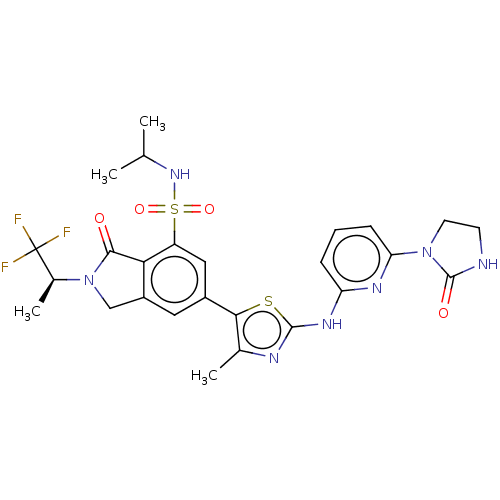 (6-(4-Methyl-2-{[6-(2-oxoimidazolidin-1-yl)pyridin-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB US Patent | Assay Description The activity of recombinant human PI3Kγ ((aa144-1102)-6His) and PI3Kα, β, δ (6-His(p110-p85α)) was determined by measuring t... | US Patent US10961236 (2021) BindingDB Entry DOI: 10.7270/Q2M90CS5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM489256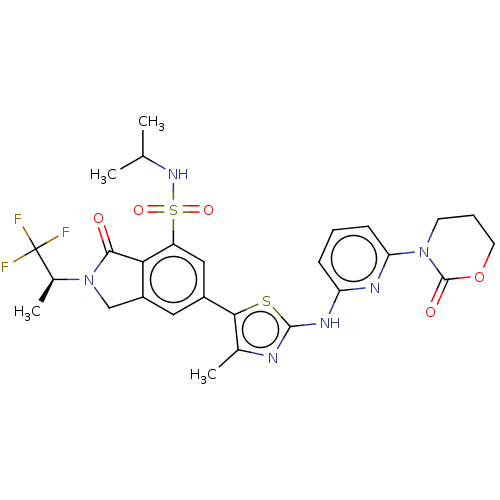 (6-(4-Methyl-2-{[6-(2-oxo-1,3-oxazinan-3-yl)pyridin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB US Patent | Assay Description The activity of recombinant human PI3Kγ ((aa144-1102)-6His) and PI3Kα, β, δ (6-His(p110-p85α)) was determined by measuring t... | US Patent US10961236 (2021) BindingDB Entry DOI: 10.7270/Q2M90CS5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM489287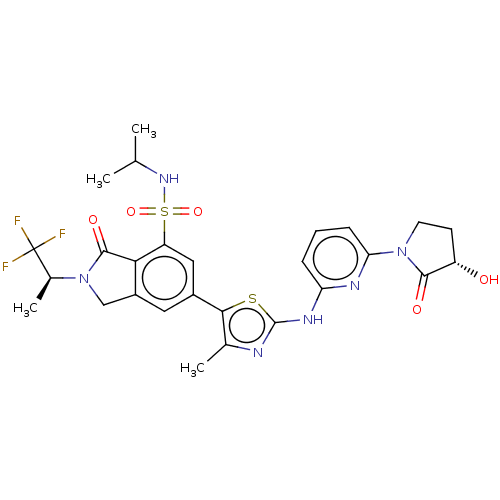 (6-[2-({6-[(3S)-3-Hydroxy-2-oxopyrrolidin-1-yl]pyri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB US Patent | Assay Description The activity of recombinant human PI3Kγ ((aa144-1102)-6His) and PI3Kα, β, δ (6-His(p110-p85α)) was determined by measuring t... | US Patent US10961236 (2021) BindingDB Entry DOI: 10.7270/Q2M90CS5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM489281 (2-[(1S)-1-Cyclopropylethyl]-6-[2-({6-[(3R)-3-hydro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB US Patent | Assay Description The activity of recombinant human PI3Kγ ((aa144-1102)-6His) and PI3Kα, β, δ (6-His(p110-p85α)) was determined by measuring t... | US Patent US10961236 (2021) BindingDB Entry DOI: 10.7270/Q2M90CS5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM489261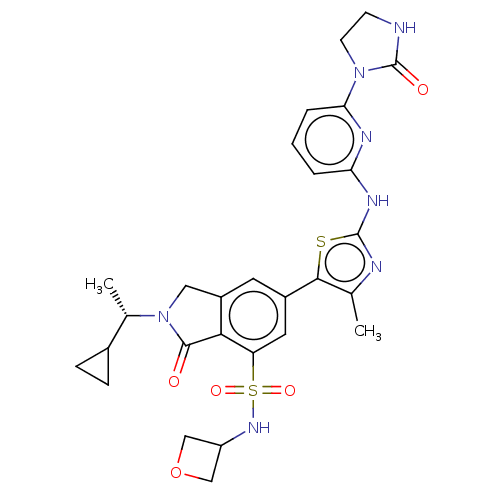 (2-[(1S)-1-Cyclopropylethyl]-6-(4-methyl-2-{[6-(2-o...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB US Patent | Assay Description The activity of recombinant human PI3Kγ ((aa144-1102)-6His) and PI3Kα, β, δ (6-His(p110-p85α)) was determined by measuring t... | US Patent US10961236 (2021) BindingDB Entry DOI: 10.7270/Q2M90CS5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM489283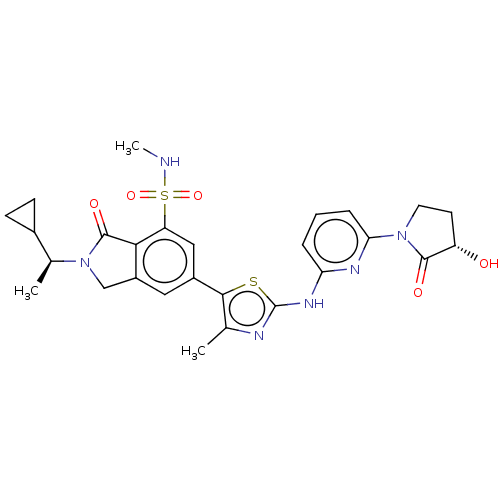 (2-[(1S)-1-Cyclopropylethyl]-6-[2-({6-[(3S)-3-hydro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB US Patent | Assay Description The activity of recombinant human PI3Kγ ((aa144-1102)-6His) and PI3Kα, β, δ (6-His(p110-p85α)) was determined by measuring t... | US Patent US10961236 (2021) BindingDB Entry DOI: 10.7270/Q2M90CS5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM489253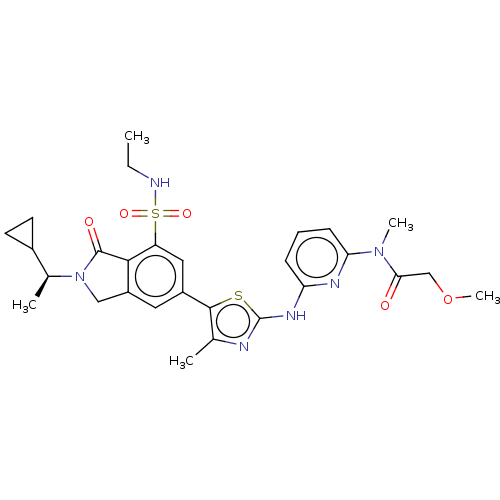 (N-{6-[(5-{2-[(1S)-1-Cyclopropylethyl]-7-(ethylsulf...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB US Patent | Assay Description The activity of recombinant human PI3Kγ ((aa144-1102)-6His) and PI3Kα, β, δ (6-His(p110-p85α)) was determined by measuring t... | US Patent US10961236 (2021) BindingDB Entry DOI: 10.7270/Q2M90CS5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM489281 (2-[(1S)-1-Cyclopropylethyl]-6-[2-({6-[(3R)-3-hydro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human PI3Kdelta assessed as reduction in ADP production using Dic8-PIP2 as substrate pre-treated for 15 mins followed by su... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00434 BindingDB Entry DOI: 10.7270/Q24Q7ZVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50579665 (CHEMBL5082066) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human PI3Kdelta assessed as reduction in ADP production using Dic8-PIP2 as substrate pre-treated for 15 mins followed by su... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00434 BindingDB Entry DOI: 10.7270/Q24Q7ZVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50579666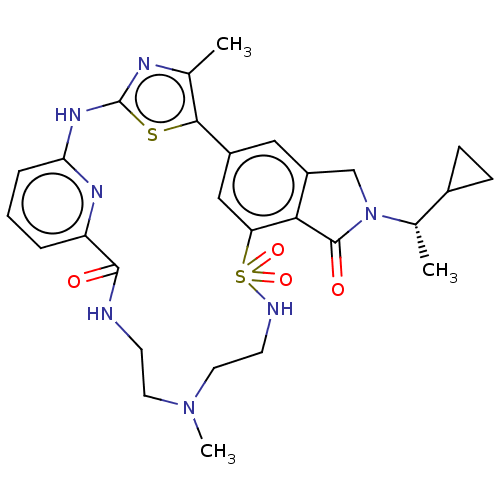 (CHEMBL5081964) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human PI3Kdelta assessed as reduction in ADP production using Dic8-PIP2 as substrate pre-treated for 15 mins followed by su... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00434 BindingDB Entry DOI: 10.7270/Q24Q7ZVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50457403 (CHEMBL4208798) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Transrepression activity at glucocorticoid receptor (unknown origin) expressed in human ChaGoK1 cells assessed as inhibition of PMA-stimulated gene e... | J Med Chem 61: 1785-1799 (2018) Article DOI: 10.1021/acs.jmedchem.7b01690 BindingDB Entry DOI: 10.7270/Q2XK8J5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM50519591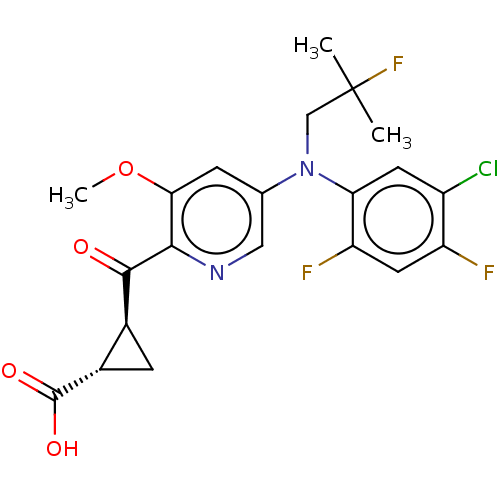 (CHEMBL4562583) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged LTC4S expressed in Pichia pastoris X33 using LTA4 methyl ester and glutathione as substrate preincubated f... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM489264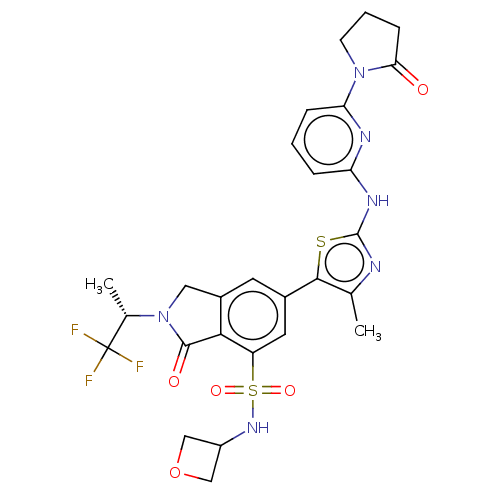 (6-(4-Methyl-2-{[6-(2-oxopyrrolidin-1-yl)pyridin-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB US Patent | Assay Description The activity of recombinant human PI3Kγ ((aa144-1102)-6His) and PI3Kα, β, δ (6-His(p110-p85α)) was determined by measuring t... | US Patent US10961236 (2021) BindingDB Entry DOI: 10.7270/Q2M90CS5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM489248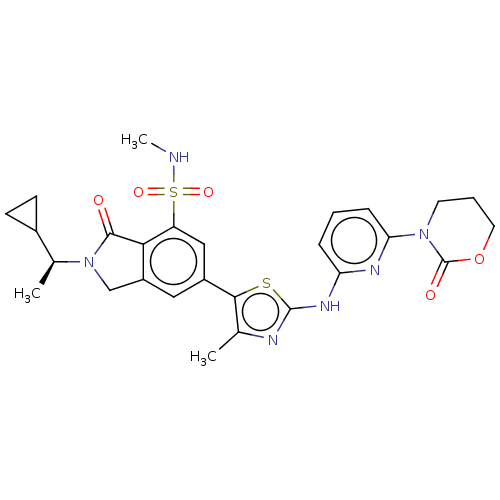 (2-[(1S)-1-Cyclopropylethyl]-N-methyl-6-(4-methyl-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB US Patent | Assay Description The activity of recombinant human PI3Kγ ((aa144-1102)-6His) and PI3Kα, β, δ (6-His(p110-p85α)) was determined by measuring t... | US Patent US10961236 (2021) BindingDB Entry DOI: 10.7270/Q2M90CS5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM489237 (5-(4-Methyl-2-{[6-(2-oxopyrrolidin-1-yl)pyridin-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB US Patent | Assay Description The activity of recombinant human PI3Kγ ((aa144-1102)-6His) and PI3Kα, β, δ (6-His(p110-p85α)) was determined by measuring t... | US Patent US10961236 (2021) BindingDB Entry DOI: 10.7270/Q2M90CS5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM50519574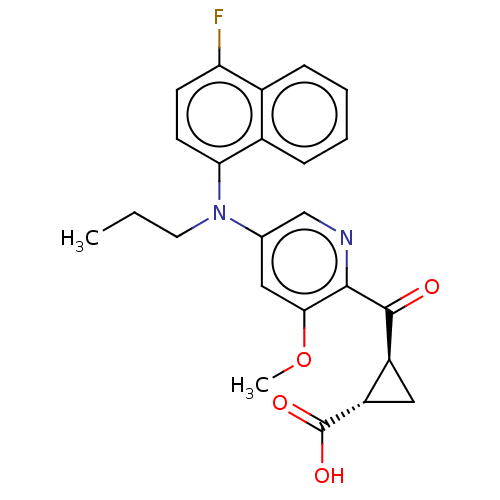 (CHEMBL4563433) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.626 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged LTC4S expressed in Pichia pastoris X33 using LTA4 methyl ester and glutathione as substrate preincubated f... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM50519588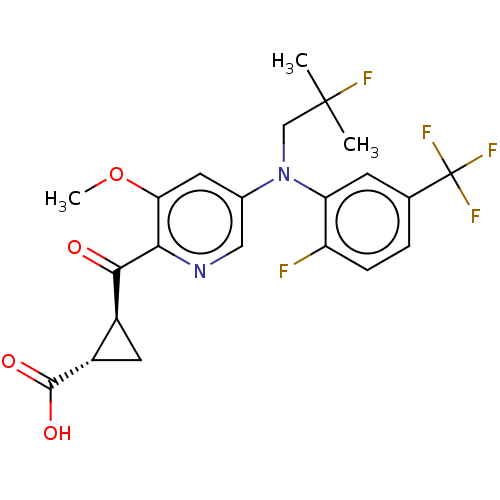 (CHEMBL4584584) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged LTC4S expressed in Pichia pastoris X33 using LTA4 methyl ester and glutathione as substrate preincubated f... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50579667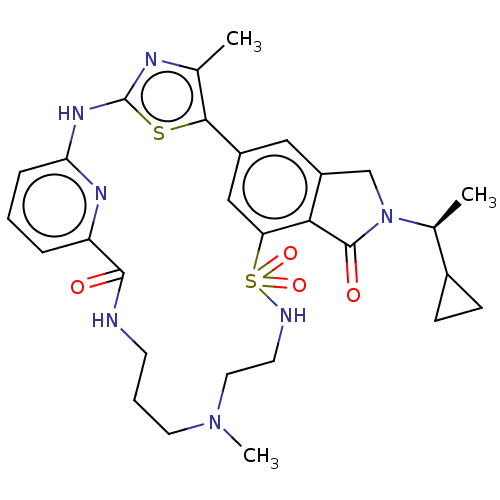 (CHEMBL5090799) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human PI3Kdelta assessed as reduction in ADP production using Dic8-PIP2 as substrate pre-treated for 15 mins followed by su... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00434 BindingDB Entry DOI: 10.7270/Q24Q7ZVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50579668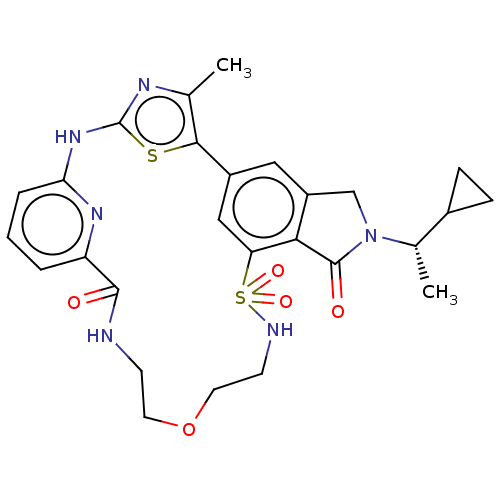 (CHEMBL5091438) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human PI3Kdelta assessed as reduction in ADP production using Dic8-PIP2 as substrate pre-treated for 15 mins followed by su... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00434 BindingDB Entry DOI: 10.7270/Q24Q7ZVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM489261 (2-[(1S)-1-Cyclopropylethyl]-6-(4-methyl-2-{[6-(2-o...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human PI3Kdelta assessed as reduction in ADP production using Dic8-PIP2 as substrate pre-treated for 15 mins followed by su... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00434 BindingDB Entry DOI: 10.7270/Q24Q7ZVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM489247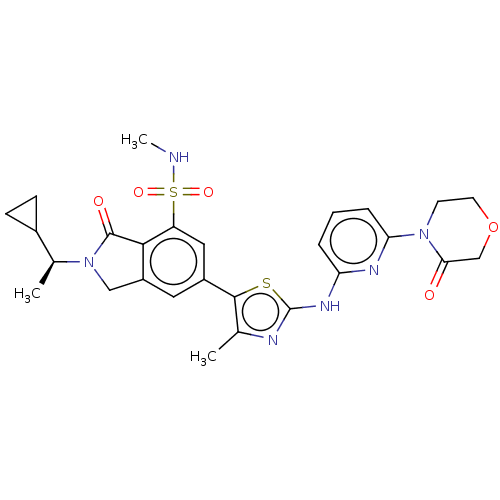 (2-[(1S)-1-Cyclopropylethyl]-N-methyl-6-(4-methyl-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human PI3Kdelta assessed as reduction in ADP production using Dic8-PIP2 as substrate pre-treated for 15 mins followed by su... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00434 BindingDB Entry DOI: 10.7270/Q24Q7ZVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM489248 (2-[(1S)-1-Cyclopropylethyl]-N-methyl-6-(4-methyl-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human PI3Kdelta assessed as reduction in ADP production using Dic8-PIP2 as substrate pre-treated for 15 mins followed by su... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00434 BindingDB Entry DOI: 10.7270/Q24Q7ZVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50579671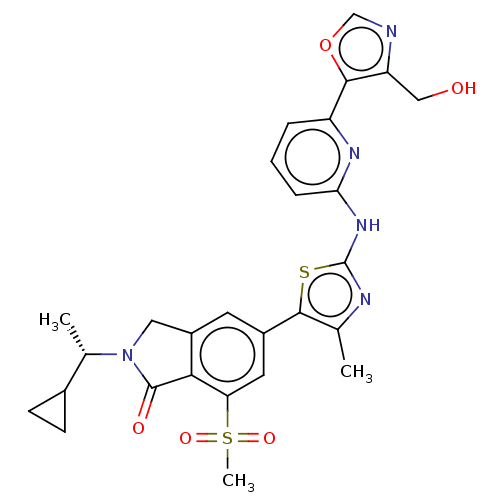 (CHEMBL5090959) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human PI3Kgamma assessed as reduction in ADP production using Dic8-PIP2 as substrate pre-treated for 15 mins followed by su... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00434 BindingDB Entry DOI: 10.7270/Q24Q7ZVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50512861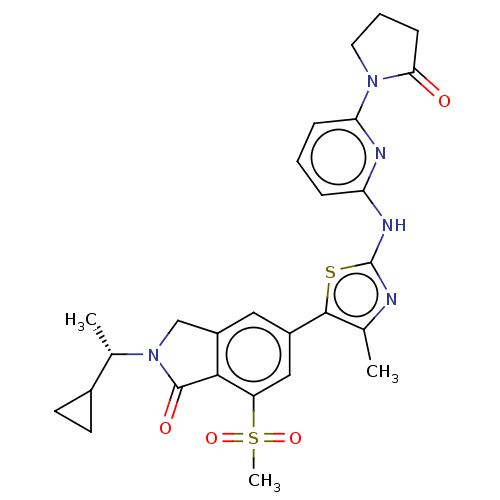 (CHEMBL4558527) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human PI3Kdelta assessed as reduction in ADP production using Dic8-PIP2 as substrate pre-treated for 15 mins followed by su... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00434 BindingDB Entry DOI: 10.7270/Q24Q7ZVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM223290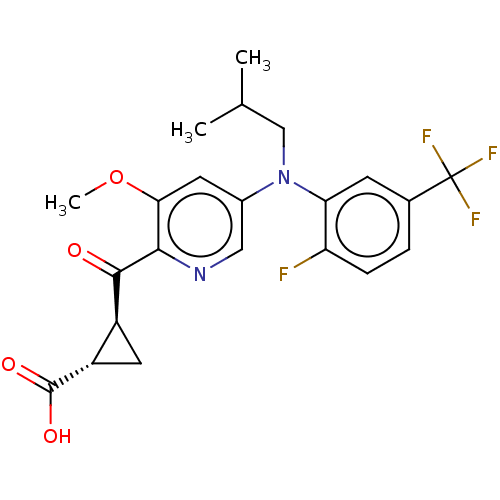 ((1S,2S)-2-[(5-{[2-Fluoro-5-(trifluoromethyl)phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.658 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTRAZENECA AB US Patent | Assay Description In the assay, LTC4 synthase catalyses the reaction where the substrate LTA4 methyl ester is converted to LTC4 methyl ester.In order to obtain IC50-va... | US Patent US9657001 (2017) BindingDB Entry DOI: 10.7270/Q2SJ1NPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM223290 ((1S,2S)-2-[(5-{[2-Fluoro-5-(trifluoromethyl)phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.658 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Astrazeneca Ab US Patent | Assay Description In the assay, LTC4 synthase catalyses the reaction where the substrate LTA4 methyl ester is converted to LTC4 methyl ester. In order to obtain IC50-v... | US Patent US20160326143 (2016) BindingDB Entry DOI: 10.7270/Q2W66JN5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM223290 ((1S,2S)-2-[(5-{[2-Fluoro-5-(trifluoromethyl)phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged LTC4S expressed in Pichia pastoris X33 using LTA4 methyl ester and glutathione as substrate preincubated f... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM140009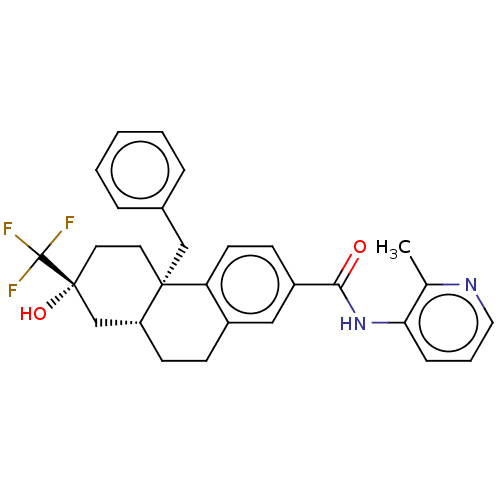 (US8901310, Example 1 ) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Transrepression activity at glucocorticoid receptor (unknown origin) expressed in human ChaGoK1 cells assessed as inhibition of PMA-stimulated gene e... | J Med Chem 61: 1785-1799 (2018) Article DOI: 10.1021/acs.jmedchem.7b01690 BindingDB Entry DOI: 10.7270/Q2XK8J5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM489275 (2-[(1S)-1-Cyclopropylethyl]-6-(2-{[6-(5,5-dimethyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB US Patent | Assay Description The activity of recombinant human PI3Kγ ((aa144-1102)-6His) and PI3Kα, β, δ (6-His(p110-p85α)) was determined by measuring t... | US Patent US10961236 (2021) BindingDB Entry DOI: 10.7270/Q2M90CS5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM489280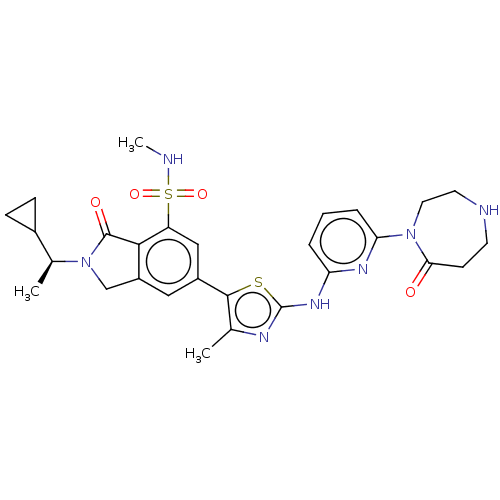 (2-[(1S)-1-Cyclopropylethyl]-N-methyl-6-(4-methyl-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB US Patent | Assay Description The activity of recombinant human PI3Kγ ((aa144-1102)-6His) and PI3Kα, β, δ (6-His(p110-p85α)) was determined by measuring t... | US Patent US10961236 (2021) BindingDB Entry DOI: 10.7270/Q2M90CS5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM489284 (2-[(1S)-1-Cyclopropylethyl]-6-[2-({6-[(2S)-2-(hydr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB US Patent | Assay Description The activity of recombinant human PI3Kγ ((aa144-1102)-6His) and PI3Kα, β, δ (6-His(p110-p85α)) was determined by measuring t... | US Patent US10961236 (2021) BindingDB Entry DOI: 10.7270/Q2M90CS5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM489286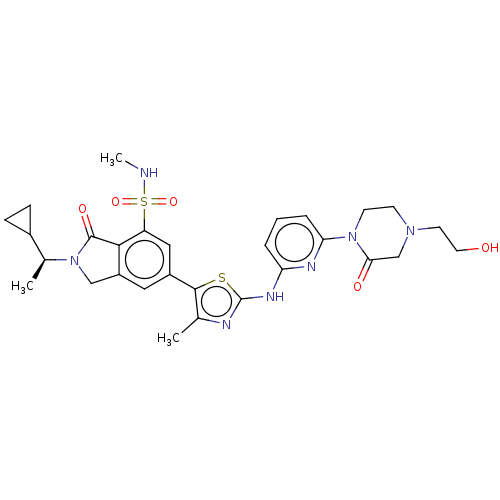 (2-[(1S)-1-Cyclopropylethyl]-6-[2-({6-[4-(2-hydroxy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB US Patent | Assay Description The activity of recombinant human PI3Kγ ((aa144-1102)-6His) and PI3Kα, β, δ (6-His(p110-p85α)) was determined by measuring t... | US Patent US10961236 (2021) BindingDB Entry DOI: 10.7270/Q2M90CS5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 753 total ) | Next | Last >> |