

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Androgen receptor (Homo sapiens (Human)) | BDBM29321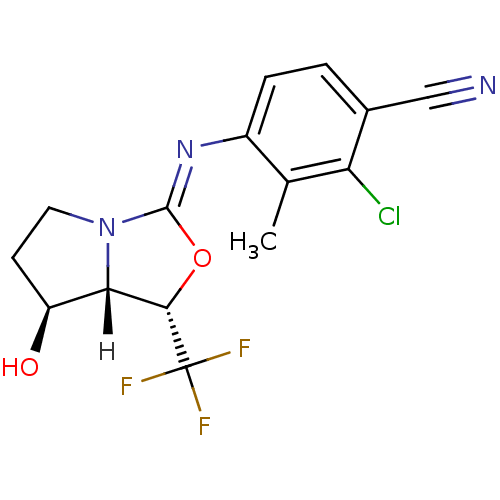 (oxazolidin-2-imine, 6d) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | -54.8 | n/a | n/a | 19 | n/a | n/a | 7.6 | 22 |
Bristol-Myers Squibb Company | Assay Description Binding assays were conducted by incubating test compound at various concentrations with [3H]DHT with human cancer epithelial breast cell lines MDAMB... | J Med Chem 52: 2794-8 (2009) Article DOI: 10.1021/jm801583j BindingDB Entry DOI: 10.7270/Q2W66J30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50215709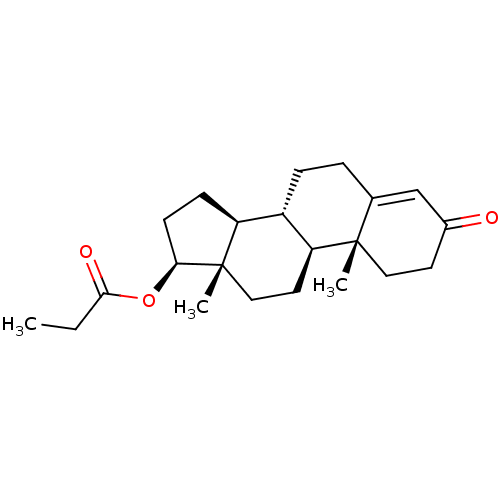 (CHEMBL1170 | Propionic acid (8R,9S,10R,13S,14S,17S...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]DHT from human androgen receptor in MDA453 cells | Bioorg Med Chem Lett 17: 4487-90 (2007) Article DOI: 10.1016/j.bmcl.2007.06.007 BindingDB Entry DOI: 10.7270/Q2NV9HZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM29320 (BMS-665139 | oxazolidin-2-imine, 6c) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.300 | -53.8 | n/a | n/a | 0.200 | n/a | n/a | 7.6 | 22 |
Bristol-Myers Squibb Company | Assay Description Binding assays were conducted by incubating test compound at various concentrations with [3H]DHT with human cancer epithelial breast cell lines MDAMB... | J Med Chem 52: 2794-8 (2009) Article DOI: 10.1021/jm801583j BindingDB Entry DOI: 10.7270/Q2W66J30 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM29319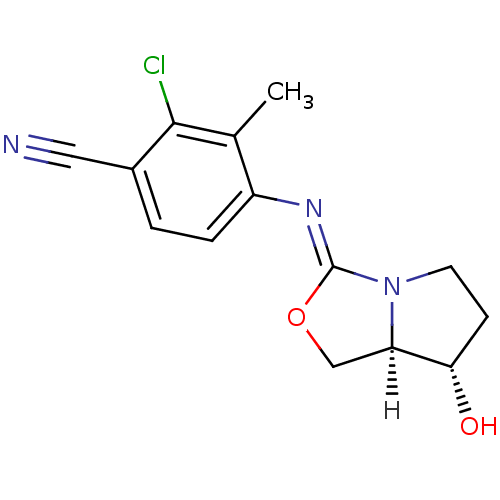 (oxazolidin-2-imine, 6b) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | -53.8 | n/a | n/a | 14 | n/a | n/a | 7.6 | 22 |
Bristol-Myers Squibb Company | Assay Description Binding assays were conducted by incubating test compound at various concentrations with [3H]DHT with human cancer epithelial breast cell lines MDAMB... | J Med Chem 52: 2794-8 (2009) Article DOI: 10.1021/jm801583j BindingDB Entry DOI: 10.7270/Q2W66J30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM29323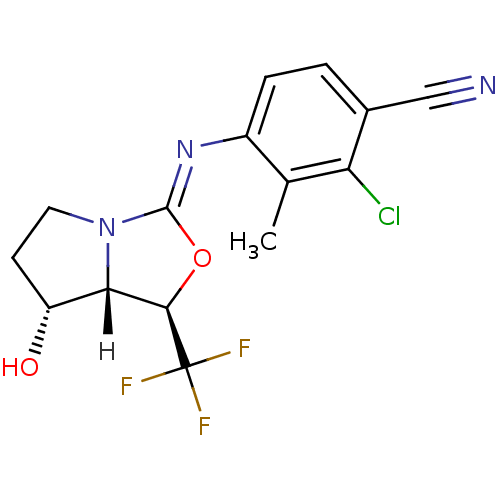 (oxazolidin-2-imine, 6f) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | -53.8 | n/a | n/a | 1.40 | n/a | n/a | 7.6 | 22 |
Bristol-Myers Squibb Company | Assay Description Binding assays were conducted by incubating test compound at various concentrations with [3H]DHT with human cancer epithelial breast cell lines MDAMB... | J Med Chem 52: 2794-8 (2009) Article DOI: 10.1021/jm801583j BindingDB Entry DOI: 10.7270/Q2W66J30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50215713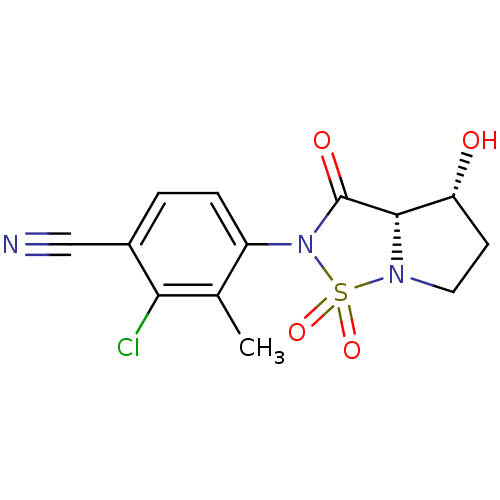 (2-Chloro-4-((3aS,4R)-4-hydroxy-1,1,3-trioxo-tetrah...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]DHT from human androgen receptor in MDA453 cells | Bioorg Med Chem Lett 17: 4487-90 (2007) Article DOI: 10.1016/j.bmcl.2007.06.007 BindingDB Entry DOI: 10.7270/Q2NV9HZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM29324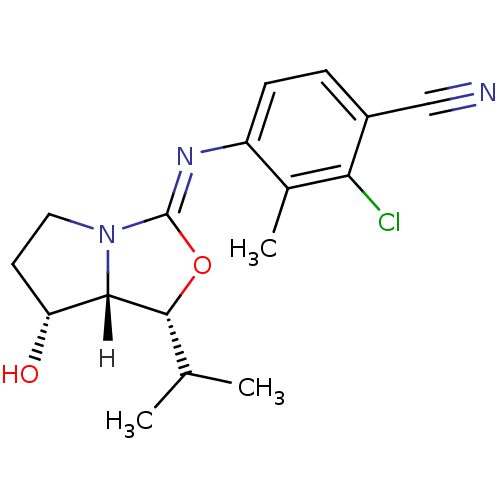 (oxazolidin-2-imine, 6g) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.700 | -51.7 | n/a | n/a | 3.70 | n/a | n/a | 7.6 | 22 |
Bristol-Myers Squibb Company | Assay Description Binding assays were conducted by incubating test compound at various concentrations with [3H]DHT with human cancer epithelial breast cell lines MDAMB... | J Med Chem 52: 2794-8 (2009) Article DOI: 10.1021/jm801583j BindingDB Entry DOI: 10.7270/Q2W66J30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM29318 (oxazolidin-2-imine, 6a) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.800 | -51.4 | n/a | n/a | 4.80 | n/a | n/a | 7.6 | 22 |
Bristol-Myers Squibb Company | Assay Description Binding assays were conducted by incubating test compound at various concentrations with [3H]DHT with human cancer epithelial breast cell lines MDAMB... | J Med Chem 52: 2794-8 (2009) Article DOI: 10.1021/jm801583j BindingDB Entry DOI: 10.7270/Q2W66J30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18176 (4-[(1S,7S,7aR)-7-hydroxy-1-methyl-3-oxo-hexahydro-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.60 | -49.7 | n/a | n/a | 5.40 | n/a | n/a | 7.6 | 22 |
Bristol-Myers Squibb Company | Assay Description Binding assays were conducted by incubating test compound at various concentrations with [3H]DHT with human cancer epithelial breast cell lines MDAMB... | J Med Chem 52: 2794-8 (2009) Article DOI: 10.1021/jm801583j BindingDB Entry DOI: 10.7270/Q2W66J30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM29326 (guanidine derivative, 12) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.90 | -49.3 | n/a | n/a | 44 | n/a | n/a | 7.6 | 22 |
Bristol-Myers Squibb Company | Assay Description Binding assays were conducted by incubating test compound at various concentrations with [3H]DHT with human cancer epithelial breast cell lines MDAMB... | J Med Chem 52: 2794-8 (2009) Article DOI: 10.1021/jm801583j BindingDB Entry DOI: 10.7270/Q2W66J30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18173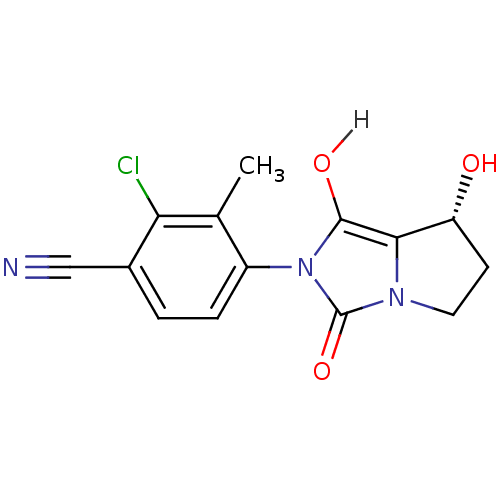 (4-[(7R,7aS)-7-hydroxy-1,3-dioxo-hexahydro-1H-pyrro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 2.11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]DHT from human androgen receptor in MDA453 cells | Bioorg Med Chem Lett 17: 4487-90 (2007) Article DOI: 10.1016/j.bmcl.2007.06.007 BindingDB Entry DOI: 10.7270/Q2NV9HZ9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM29322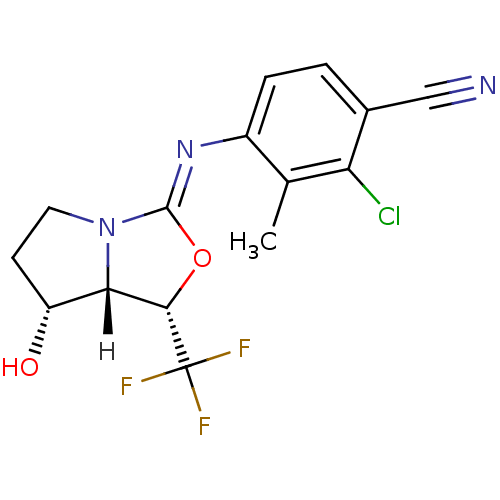 (oxazolidin-2-imine, 6e) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2.30 | -48.8 | n/a | n/a | 1.10 | n/a | n/a | 7.6 | 22 |
Bristol-Myers Squibb Company | Assay Description Binding assays were conducted by incubating test compound at various concentrations with [3H]DHT with human cancer epithelial breast cell lines MDAMB... | J Med Chem 52: 2794-8 (2009) Article DOI: 10.1021/jm801583j BindingDB Entry DOI: 10.7270/Q2W66J30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18171 (4-[(7R,7aS)-7-hydroxy-1,3-dioxo-hexahydro-1H-pyrro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]DHT from human androgen receptor in MDA453 cells | Bioorg Med Chem Lett 17: 4487-90 (2007) Article DOI: 10.1016/j.bmcl.2007.06.007 BindingDB Entry DOI: 10.7270/Q2NV9HZ9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18174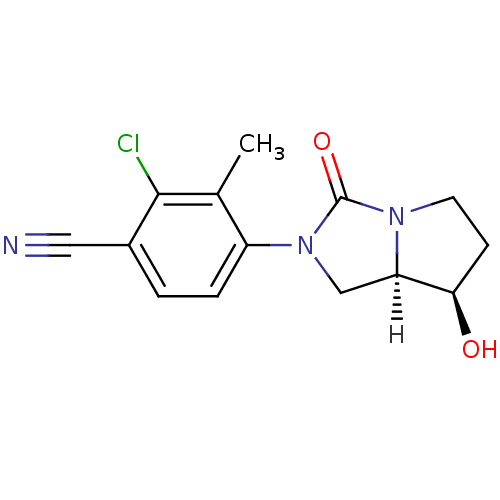 (4-[(7R,7aR)-7-hydroxy-3-oxo-hexahydro-1H-pyrrolo[1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 6 | -46.5 | n/a | n/a | 6.40 | n/a | n/a | 7.6 | 22 |
Bristol-Myers Squibb Company | Assay Description Binding assays were conducted by incubating test compound at various concentrations with [3H]DHT with human cancer epithelial breast cell lines MDAMB... | J Med Chem 52: 2794-8 (2009) Article DOI: 10.1021/jm801583j BindingDB Entry DOI: 10.7270/Q2W66J30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50215716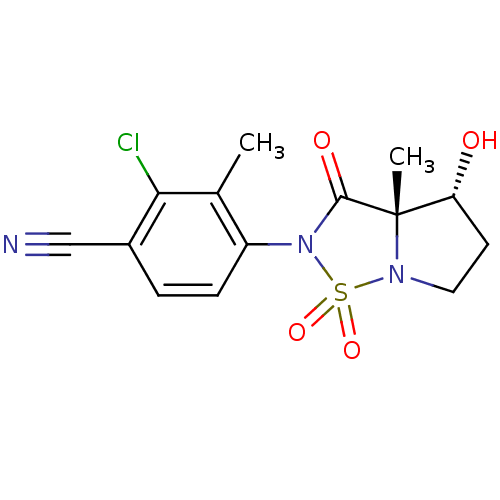 (2-chloro-4-((3aS,4R)-4-hydroxy-3a-methyl-1,1,3-tri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]DHT from human androgen receptor in MDA453 cells | Bioorg Med Chem Lett 17: 4487-90 (2007) Article DOI: 10.1016/j.bmcl.2007.06.007 BindingDB Entry DOI: 10.7270/Q2NV9HZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM29325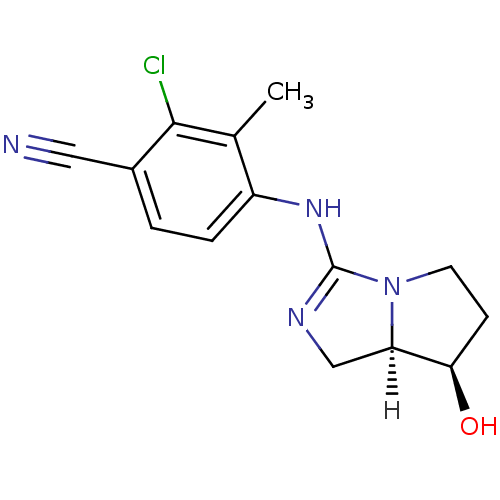 (guanidine derivative, 11) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 10 | -45.2 | n/a | n/a | 1.80E+3 | n/a | n/a | 7.6 | 22 |
Bristol-Myers Squibb Company | Assay Description Binding assays were conducted by incubating test compound at various concentrations with [3H]DHT with human cancer epithelial breast cell lines MDAMB... | J Med Chem 52: 2794-8 (2009) Article DOI: 10.1021/jm801583j BindingDB Entry DOI: 10.7270/Q2W66J30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18173 (4-[(7R,7aS)-7-hydroxy-1,3-dioxo-hexahydro-1H-pyrro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 14 | -44.4 | n/a | n/a | 7.80 | n/a | n/a | 7.6 | 22 |
Bristol-Myers Squibb Company | Assay Description Binding assays were conducted by incubating test compound at various concentrations with [3H]DHT with human cancer epithelial breast cell lines MDAMB... | J Med Chem 52: 2794-8 (2009) Article DOI: 10.1021/jm801583j BindingDB Entry DOI: 10.7270/Q2W66J30 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50215714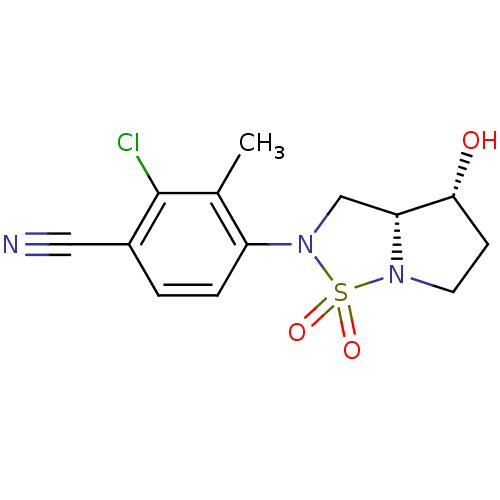 (2-chloro-4-((3aR,4R)-4-hydroxy-1,1-dioxo-tetrahydr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]DHT from human androgen receptor in MDA453 cells | Bioorg Med Chem Lett 17: 4487-90 (2007) Article DOI: 10.1016/j.bmcl.2007.06.007 BindingDB Entry DOI: 10.7270/Q2NV9HZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM29328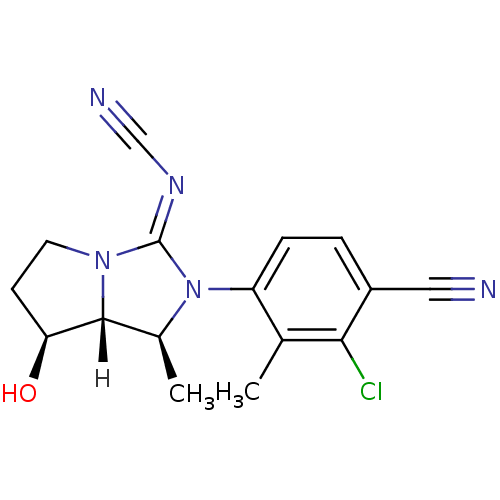 (cyanoguanidine, 15) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 17 | -43.9 | n/a | n/a | 1.11E+3 | n/a | n/a | 7.6 | 22 |
Bristol-Myers Squibb Company | Assay Description Binding assays were conducted by incubating test compound at various concentrations with [3H]DHT with human cancer epithelial breast cell lines MDAMB... | J Med Chem 52: 2794-8 (2009) Article DOI: 10.1021/jm801583j BindingDB Entry DOI: 10.7270/Q2W66J30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM29327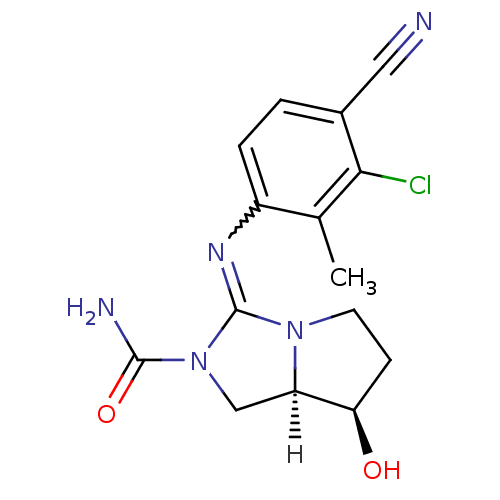 (guanidine derivative, 13) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 19 | -43.6 | n/a | n/a | n/a | n/a | n/a | 7.6 | 22 |
Bristol-Myers Squibb Company | Assay Description Binding assays were conducted by incubating test compound at various concentrations with [3H]DHT with human cancer epithelial breast cell lines MDAMB... | J Med Chem 52: 2794-8 (2009) Article DOI: 10.1021/jm801583j BindingDB Entry DOI: 10.7270/Q2W66J30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50215710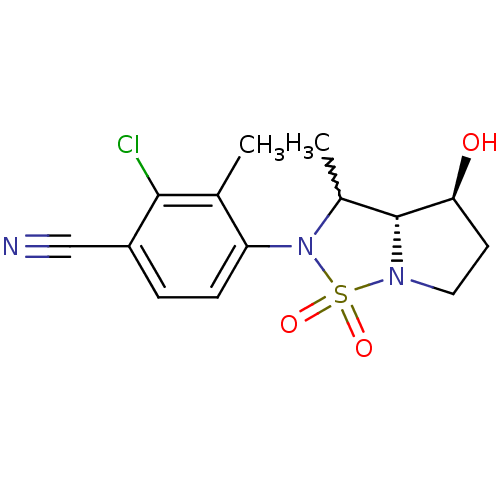 (2-chloro-4-((3aR,4S)-4-hydroxy-3-methyl-1,1-dioxo-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]DHT from human androgen receptor in MDA453 cells | Bioorg Med Chem Lett 17: 4487-90 (2007) Article DOI: 10.1016/j.bmcl.2007.06.007 BindingDB Entry DOI: 10.7270/Q2NV9HZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50215712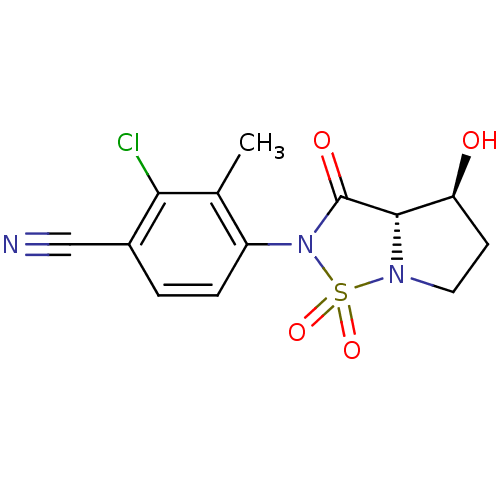 (2-chloro-4-((3aS,4S)-4-hydroxy-1,1,3-trioxo-tetrah...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]DHT from human androgen receptor in MDA453 cells | Bioorg Med Chem Lett 17: 4487-90 (2007) Article DOI: 10.1016/j.bmcl.2007.06.007 BindingDB Entry DOI: 10.7270/Q2NV9HZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50215718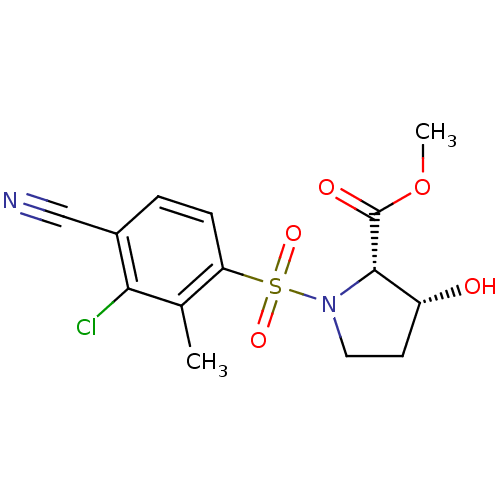 ((2S,3R)-methyl 1-(3-chloro-4-cyano-2-methylphenyls...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]DHT from human androgen receptor in MDA453 cells | Bioorg Med Chem Lett 17: 4487-90 (2007) Article DOI: 10.1016/j.bmcl.2007.06.007 BindingDB Entry DOI: 10.7270/Q2NV9HZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50215711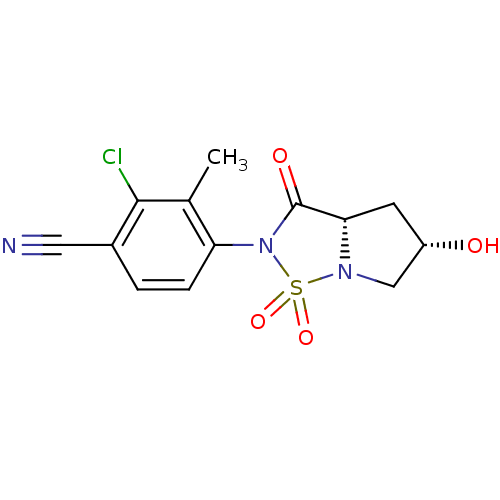 (2-chloro-4-((3aS,5S)-5-hydroxy-1,1,3-trioxo-tetrah...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]DHT from human androgen receptor in MDA453 cells | Bioorg Med Chem Lett 17: 4487-90 (2007) Article DOI: 10.1016/j.bmcl.2007.06.007 BindingDB Entry DOI: 10.7270/Q2NV9HZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50215719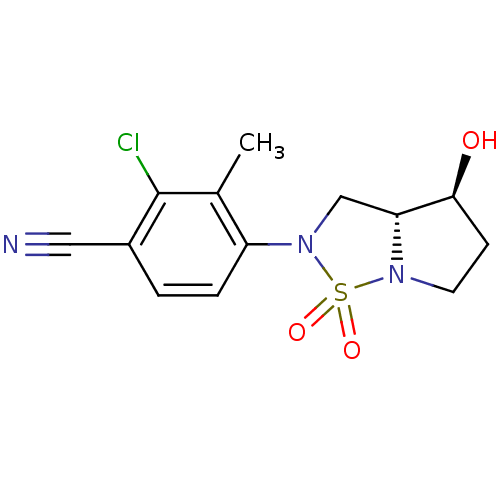 (2-chloro-4-((3aR,4S)-4-hydroxy-1,1-dioxo-tetrahydr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 189 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]DHT from human androgen receptor in MDA453 cells | Bioorg Med Chem Lett 17: 4487-90 (2007) Article DOI: 10.1016/j.bmcl.2007.06.007 BindingDB Entry DOI: 10.7270/Q2NV9HZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Mus musculus) | BDBM50215718 ((2S,3R)-methyl 1-(3-chloro-4-cyano-2-methylphenyls...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 278 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Antagonist activity at androgen receptor in mouse C2C12 cells by receptor transactivation assay | Bioorg Med Chem Lett 17: 4487-90 (2007) Article DOI: 10.1016/j.bmcl.2007.06.007 BindingDB Entry DOI: 10.7270/Q2NV9HZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Mus musculus) | BDBM50215709 (CHEMBL1170 | Propionic acid (8R,9S,10R,13S,14S,17S...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Agonist activity at androgen receptor in mouse C2C12 cells by receptor transactivation assay | Bioorg Med Chem Lett 17: 4487-90 (2007) Article DOI: 10.1016/j.bmcl.2007.06.007 BindingDB Entry DOI: 10.7270/Q2NV9HZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Mus musculus) | BDBM50215712 (2-chloro-4-((3aS,4S)-4-hydroxy-1,1,3-trioxo-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.96E+3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Agonist activity at androgen receptor in mouse C2C12 cells by receptor transactivation assay | Bioorg Med Chem Lett 17: 4487-90 (2007) Article DOI: 10.1016/j.bmcl.2007.06.007 BindingDB Entry DOI: 10.7270/Q2NV9HZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM20878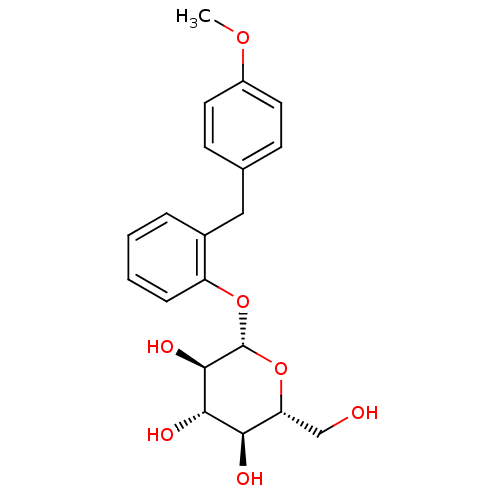 ((2R,3S,4S,5R,6S)-2-(hydroxymethyl)-6-{2-[(4-methox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >8.00E+3 | n/a | n/a | 7.2 | 22 |
Bristol-Myers Squibb Company | Assay Description Inhibitors were assayed for the ability to inhibit [14C]AMG uptake in a protein-free buffer over a 2 h incubation period. The response curve was fitt... | J Med Chem 51: 1145-9 (2008) Article DOI: 10.1021/jm701272q BindingDB Entry DOI: 10.7270/Q2PN93X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Mus musculus) | BDBM18173 (4-[(7R,7aS)-7-hydroxy-1,3-dioxo-hexahydro-1H-pyrro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Agonist activity at androgen receptor in mouse C2C12 cells by receptor transactivation assay | Bioorg Med Chem Lett 17: 4487-90 (2007) Article DOI: 10.1016/j.bmcl.2007.06.007 BindingDB Entry DOI: 10.7270/Q2NV9HZ9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM20877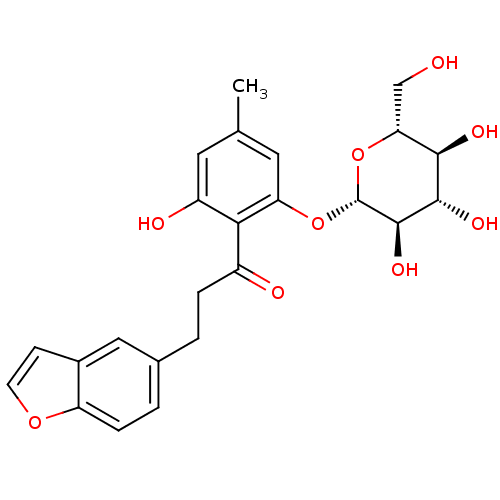 (3-(1-benzofuran-5-yl)-1-(2-hydroxy-4-methyl-6-{[(2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 211 | n/a | n/a | 7.2 | 22 |
Bristol-Myers Squibb Company | Assay Description Inhibitors were assayed for the ability to inhibit [14C]AMG uptake in a protein-free buffer over a 2 h incubation period. The response curve was fitt... | J Med Chem 51: 1145-9 (2008) Article DOI: 10.1021/jm701272q BindingDB Entry DOI: 10.7270/Q2PN93X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Mus musculus) | BDBM50215711 (2-chloro-4-((3aS,5S)-5-hydroxy-1,1,3-trioxo-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.52E+3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Agonist activity at androgen receptor in mouse C2C12 cells by receptor transactivation assay | Bioorg Med Chem Lett 17: 4487-90 (2007) Article DOI: 10.1016/j.bmcl.2007.06.007 BindingDB Entry DOI: 10.7270/Q2NV9HZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Mus musculus) | BDBM50215710 (2-chloro-4-((3aR,4S)-4-hydroxy-3-methyl-1,1-dioxo-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 8.96E+3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Agonist activity at androgen receptor in mouse C2C12 cells by receptor transactivation assay | Bioorg Med Chem Lett 17: 4487-90 (2007) Article DOI: 10.1016/j.bmcl.2007.06.007 BindingDB Entry DOI: 10.7270/Q2NV9HZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM20875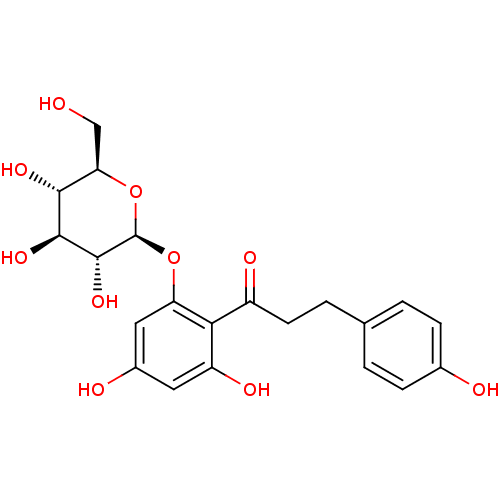 (1-(2,4-dihydroxy-6-{[(2S,3R,4S,5S,6R)-3,4,5-trihyd...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 330 | n/a | n/a | 7.2 | 22 |
Bristol-Myers Squibb Company | Assay Description Inhibitors were assayed for the ability to inhibit [14C]AMG uptake in a protein-free buffer over a 2 h incubation period. The response curve was fitt... | J Med Chem 51: 1145-9 (2008) Article DOI: 10.1021/jm701272q BindingDB Entry DOI: 10.7270/Q2PN93X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM20880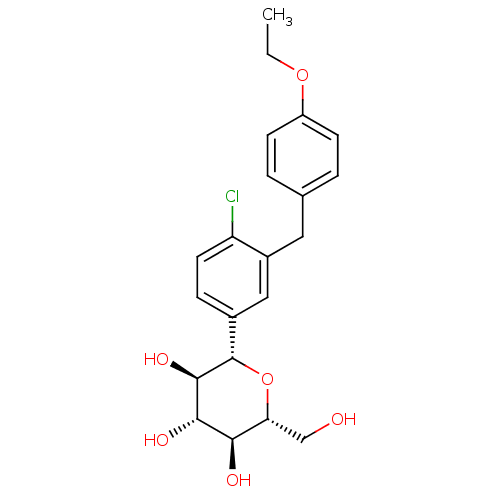 ((2S,3R,4R,5S,6R)-2-{4-chloro-3-[(4-ethoxyphenyl)me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 1.10 | n/a | n/a | 7.2 | 22 |
Bristol-Myers Squibb Company | Assay Description Inhibitors were assayed for the ability to inhibit [14C]AMG uptake in a protein-free buffer over a 2 h incubation period. The response curve was fitt... | J Med Chem 51: 1145-9 (2008) Article DOI: 10.1021/jm701272q BindingDB Entry DOI: 10.7270/Q2PN93X4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM20879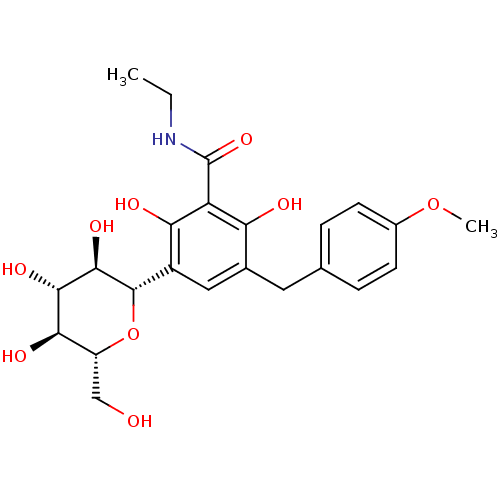 (C-aryl glucoside, 5 | CHEMBL429911 | N-ethyl-2,6-d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | n/a | n/a | 1.30E+3 | n/a | n/a | 7.2 | 22 |
Bristol-Myers Squibb Company | Assay Description Inhibitors were assayed for the ability to inhibit [14C]AMG uptake in a protein-free buffer over a 2 h incubation period. The response curve was fitt... | J Med Chem 51: 1145-9 (2008) Article DOI: 10.1021/jm701272q BindingDB Entry DOI: 10.7270/Q2PN93X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Mus musculus) | BDBM18171 (4-[(7R,7aS)-7-hydroxy-1,3-dioxo-hexahydro-1H-pyrro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Agonist activity at androgen receptor in mouse C2C12 cells by receptor transactivation assay | Bioorg Med Chem Lett 17: 4487-90 (2007) Article DOI: 10.1016/j.bmcl.2007.06.007 BindingDB Entry DOI: 10.7270/Q2NV9HZ9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Androgen receptor (Mus musculus) | BDBM50215713 (2-Chloro-4-((3aS,4R)-4-hydroxy-1,1,3-trioxo-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 11.9 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Agonist activity at androgen receptor in mouse C2C12 cells by receptor transactivation assay | Bioorg Med Chem Lett 17: 4487-90 (2007) Article DOI: 10.1016/j.bmcl.2007.06.007 BindingDB Entry DOI: 10.7270/Q2NV9HZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Mus musculus) | BDBM50215715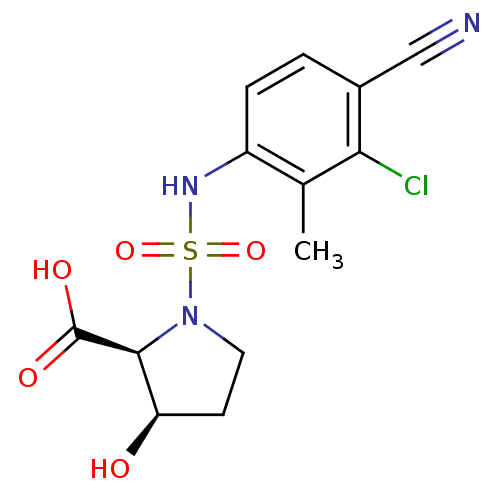 ((2S,3R)-1-(3-chloro-4-cyano-2-methyl-phenylsulfamo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Agonist activity at androgen receptor in mouse C2C12 cells by receptor transactivation assay | Bioorg Med Chem Lett 17: 4487-90 (2007) Article DOI: 10.1016/j.bmcl.2007.06.007 BindingDB Entry DOI: 10.7270/Q2NV9HZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM20878 ((2R,3S,4S,5R,6S)-2-(hydroxymethyl)-6-{2-[(4-methox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 9.20 | n/a | n/a | 7.2 | 22 |
Bristol-Myers Squibb Company | Assay Description Inhibitors were assayed for the ability to inhibit [14C]AMG uptake in a protein-free buffer over a 2 h incubation period. The response curve was fitt... | J Med Chem 51: 1145-9 (2008) Article DOI: 10.1021/jm701272q BindingDB Entry DOI: 10.7270/Q2PN93X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Mus musculus) | BDBM50215716 (2-chloro-4-((3aS,4R)-4-hydroxy-3a-methyl-1,1,3-tri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.25E+3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Agonist activity at androgen receptor in mouse C2C12 cells by receptor transactivation assay | Bioorg Med Chem Lett 17: 4487-90 (2007) Article DOI: 10.1016/j.bmcl.2007.06.007 BindingDB Entry DOI: 10.7270/Q2NV9HZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Mus musculus) | BDBM50215717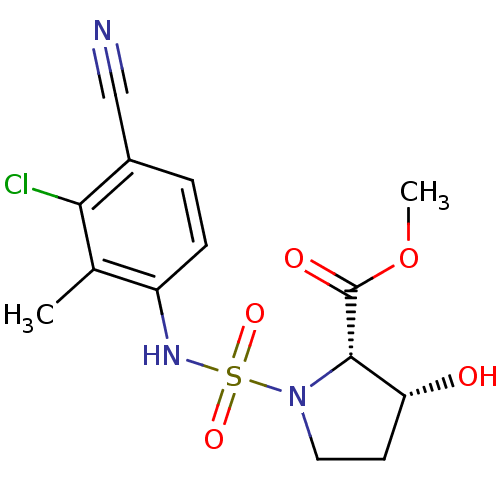 ((2S,3R)-1-(3-chloro-4-cyano-2-methyl-phenylsulfamo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.46E+3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Agonist activity at androgen receptor in mouse C2C12 cells by receptor transactivation assay | Bioorg Med Chem Lett 17: 4487-90 (2007) Article DOI: 10.1016/j.bmcl.2007.06.007 BindingDB Entry DOI: 10.7270/Q2NV9HZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM20877 (3-(1-benzofuran-5-yl)-1-(2-hydroxy-4-methyl-6-{[(2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 6.60 | n/a | n/a | 7.2 | 22 |
Bristol-Myers Squibb Company | Assay Description Inhibitors were assayed for the ability to inhibit [14C]AMG uptake in a protein-free buffer over a 2 h incubation period. The response curve was fitt... | J Med Chem 51: 1145-9 (2008) Article DOI: 10.1021/jm701272q BindingDB Entry DOI: 10.7270/Q2PN93X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Mus musculus) | BDBM50215718 ((2S,3R)-methyl 1-(3-chloro-4-cyano-2-methylphenyls...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Agonist activity at androgen receptor in mouse C2C12 cells by receptor transactivation assay | Bioorg Med Chem Lett 17: 4487-90 (2007) Article DOI: 10.1016/j.bmcl.2007.06.007 BindingDB Entry DOI: 10.7270/Q2NV9HZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM20875 (1-(2,4-dihydroxy-6-{[(2S,3R,4S,5S,6R)-3,4,5-trihyd...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 35.6 | n/a | n/a | 7.2 | 22 |
Bristol-Myers Squibb Company | Assay Description Inhibitors were assayed for the ability to inhibit [14C]AMG uptake in a protein-free buffer over a 2 h incubation period. The response curve was fitt... | J Med Chem 51: 1145-9 (2008) Article DOI: 10.1021/jm701272q BindingDB Entry DOI: 10.7270/Q2PN93X4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Androgen receptor (Mus musculus) | BDBM50215714 (2-chloro-4-((3aR,4R)-4-hydroxy-1,1-dioxo-tetrahydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.73E+3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Agonist activity at androgen receptor in mouse C2C12 cells by receptor transactivation assay | Bioorg Med Chem Lett 17: 4487-90 (2007) Article DOI: 10.1016/j.bmcl.2007.06.007 BindingDB Entry DOI: 10.7270/Q2NV9HZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM20880 ((2S,3R,4R,5S,6R)-2-{4-chloro-3-[(4-ethoxyphenyl)me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.39E+3 | n/a | n/a | 7.2 | 22 |
Bristol-Myers Squibb Company | Assay Description Inhibitors were assayed for the ability to inhibit [14C]AMG uptake in a protein-free buffer over a 2 h incubation period. The response curve was fitt... | J Med Chem 51: 1145-9 (2008) Article DOI: 10.1021/jm701272q BindingDB Entry DOI: 10.7270/Q2PN93X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM20879 (C-aryl glucoside, 5 | CHEMBL429911 | N-ethyl-2,6-d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | n/a | n/a | >8.00E+3 | n/a | n/a | 7.2 | 22 |
Bristol-Myers Squibb Company | Assay Description Inhibitors were assayed for the ability to inhibit [14C]AMG uptake in a protein-free buffer over a 2 h incubation period. The response curve was fitt... | J Med Chem 51: 1145-9 (2008) Article DOI: 10.1021/jm701272q BindingDB Entry DOI: 10.7270/Q2PN93X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||