

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50230322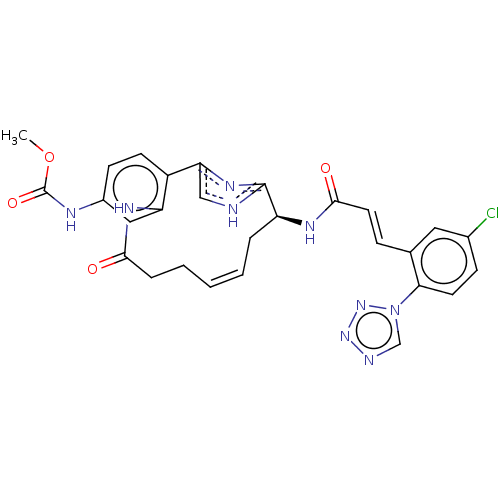 (CHEMBL4071545) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of activated human coagulation factor 11 using pyroGlu-Pro-Arg-pNA as substrate incubated for 10 to 120 mins by spectrofluorometric method | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.08.008 BindingDB Entry DOI: 10.7270/Q23F4T2C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50525762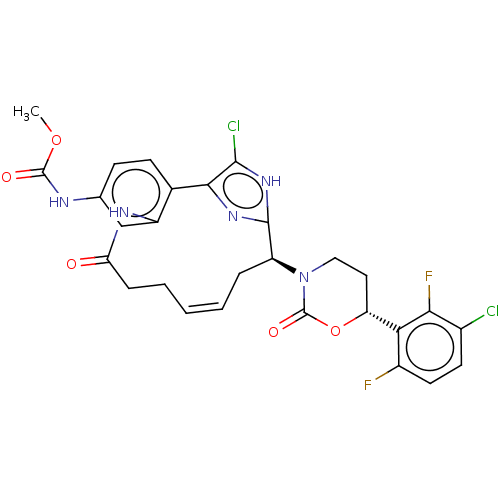 (CHEMBL4467360) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of activated human coagulation factor 11 using pyroGlu-Pro-Arg-pNA as substrate incubated for 10 to 120 mins by spectrofluorometric method | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.08.008 BindingDB Entry DOI: 10.7270/Q23F4T2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50525762 (CHEMBL4467360) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of activated human coagulation factor 11 using pyroGlu-Pro-Arg-pNA as substrate incubated for 10 to 120 mins by spectrofluorometric method | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.08.008 BindingDB Entry DOI: 10.7270/Q23F4T2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50525779 (CHEMBL4550408) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of activated human coagulation factor 11 using pyroGlu-Pro-Arg-pNA as substrate incubated for 10 to 120 mins by spectrofluorometric method | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.08.008 BindingDB Entry DOI: 10.7270/Q23F4T2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50525770 (CHEMBL4452300) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of activated human coagulation factor 11 using pyroGlu-Pro-Arg-pNA as substrate incubated for 10 to 120 mins by spectrofluorometric method | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.08.008 BindingDB Entry DOI: 10.7270/Q23F4T2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50525772 (CHEMBL4475559) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of activated human coagulation factor 11 using pyroGlu-Pro-Arg-pNA as substrate incubated for 10 to 120 mins by spectrofluorometric method | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.08.008 BindingDB Entry DOI: 10.7270/Q23F4T2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50525773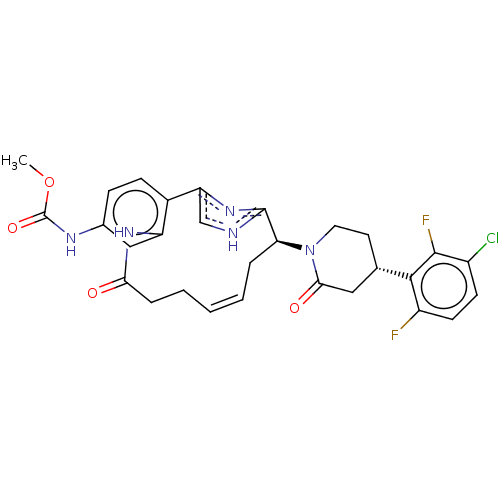 (CHEMBL4460266) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of activated human coagulation factor 11 using pyroGlu-Pro-Arg-pNA as substrate incubated for 10 to 120 mins by spectrofluorometric method | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.08.008 BindingDB Entry DOI: 10.7270/Q23F4T2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50063669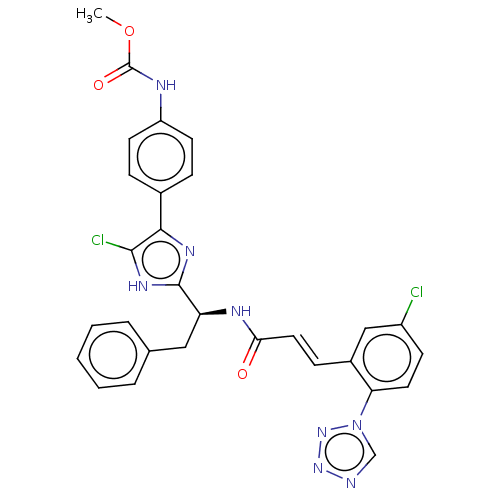 (CHEMBL3398641) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of activated coagulation factor 11 (unknown origin) assessed as inhibitory constant | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.08.008 BindingDB Entry DOI: 10.7270/Q23F4T2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50525771 (CHEMBL4465144) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of activated human coagulation factor 11 using pyroGlu-Pro-Arg-pNA as substrate incubated for 10 to 120 mins by spectrofluorometric method | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.08.008 BindingDB Entry DOI: 10.7270/Q23F4T2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50525769 (CHEMBL4551053) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of activated human coagulation factor 11 using pyroGlu-Pro-Arg-pNA as substrate incubated for 10 to 120 mins by spectrofluorometric method | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.08.008 BindingDB Entry DOI: 10.7270/Q23F4T2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50525761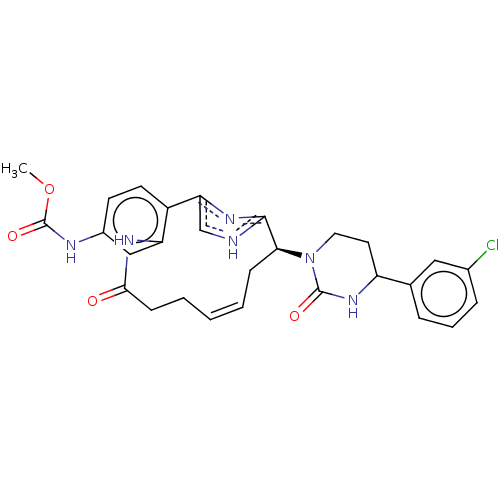 (CHEMBL4463167) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of activated human coagulation factor 11 using pyroGlu-Pro-Arg-pNA as substrate incubated for 10 to 120 mins by spectrofluorometric method | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.08.008 BindingDB Entry DOI: 10.7270/Q23F4T2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50525763 (CHEMBL4438562) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of activated human coagulation factor 11 using pyroGlu-Pro-Arg-pNA as substrate incubated for 10 to 120 mins by spectrofluorometric method | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.08.008 BindingDB Entry DOI: 10.7270/Q23F4T2C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50267028 (CHEMBL4073525) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Agonist activity at human GPR40 expressed in CHO-A12 cells assessed as increase in intracellular calcium flux measured for 100 secs by FLIPR assay | J Med Chem 60: 1417-1431 (2017) Article DOI: 10.1021/acs.jmedchem.6b01559 BindingDB Entry DOI: 10.7270/Q25X2CD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50267031 (CHEMBL4079930) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Agonist activity at human GPR40 expressed in CHO-A12 cells assessed as increase in intracellular calcium flux measured for 100 secs by FLIPR assay | J Med Chem 60: 1417-1431 (2017) Article DOI: 10.1021/acs.jmedchem.6b01559 BindingDB Entry DOI: 10.7270/Q25X2CD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50525764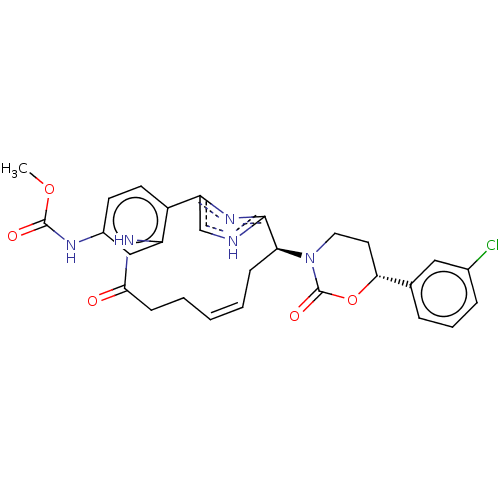 (CHEMBL4515523) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of activated human coagulation factor 11 using pyroGlu-Pro-Arg-pNA as substrate incubated for 10 to 120 mins by spectrofluorometric method | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.08.008 BindingDB Entry DOI: 10.7270/Q23F4T2C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50267009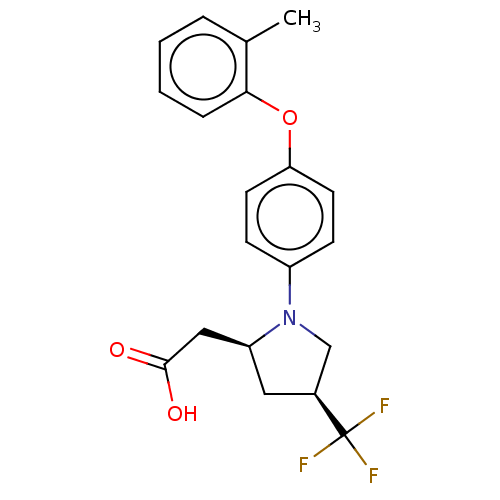 (CHEMBL4089171) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human GPR40 expressed in HEK293 cell membranes after 1 hr by radioligand displacement based scintillation counting method | J Med Chem 60: 1417-1431 (2017) Article DOI: 10.1021/acs.jmedchem.6b01559 BindingDB Entry DOI: 10.7270/Q25X2CD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50267029 (CHEMBL4069191) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human GPR40 expressed in HEK293 cell membranes after 1 hr by radioligand displacement based scintillation counting method | J Med Chem 60: 1417-1431 (2017) Article DOI: 10.1021/acs.jmedchem.6b01559 BindingDB Entry DOI: 10.7270/Q25X2CD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50267010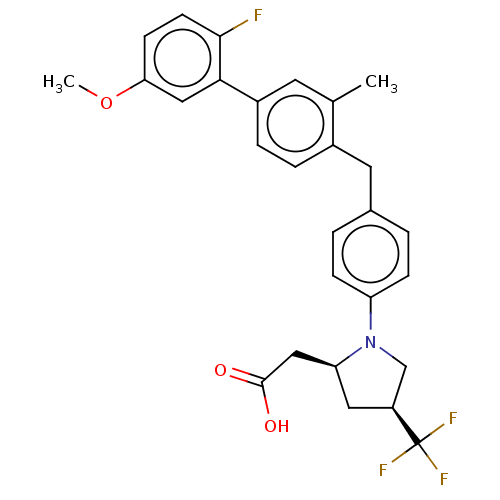 (CHEMBL4082395) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human GPR40 expressed in HEK293 cell membranes after 1 hr by radioligand displacement based scintillation counting method | J Med Chem 60: 1417-1431 (2017) Article DOI: 10.1021/acs.jmedchem.6b01559 BindingDB Entry DOI: 10.7270/Q25X2CD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50267117 (CHEMBL4060499) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human GPR40 expressed in HEK293 cell membranes after 1 hr by radioligand displacement based scintillation counting method | J Med Chem 60: 1417-1431 (2017) Article DOI: 10.1021/acs.jmedchem.6b01559 BindingDB Entry DOI: 10.7270/Q25X2CD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50267030 (CHEMBL4090240) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human GPR40 expressed in HEK293 cell membranes after 1 hr by radioligand displacement based scintillation counting method | J Med Chem 60: 1417-1431 (2017) Article DOI: 10.1021/acs.jmedchem.6b01559 BindingDB Entry DOI: 10.7270/Q25X2CD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50267044 (CHEMBL4069764) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 73 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human GPR40 expressed in HEK293 cell membranes after 1 hr by radioligand displacement based scintillation counting method | J Med Chem 60: 1417-1431 (2017) Article DOI: 10.1021/acs.jmedchem.6b01559 BindingDB Entry DOI: 10.7270/Q25X2CD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50525777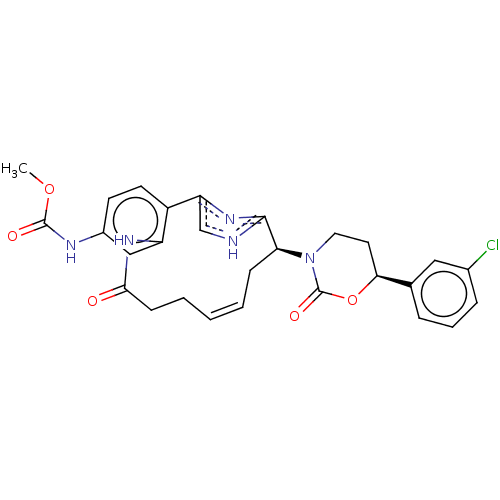 (CHEMBL4552540) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 99 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of activated human coagulation factor 11 using pyroGlu-Pro-Arg-pNA as substrate incubated for 10 to 120 mins by spectrofluorometric method | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.08.008 BindingDB Entry DOI: 10.7270/Q23F4T2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50525767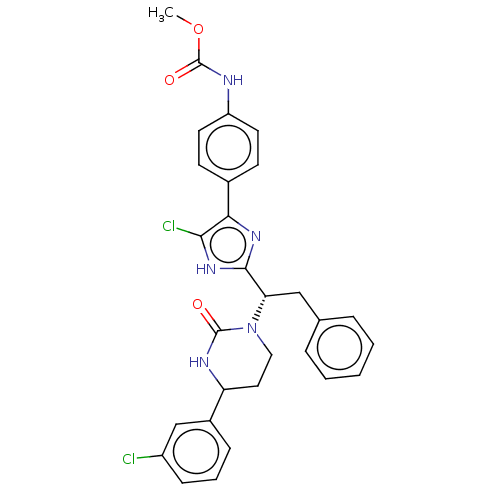 (CHEMBL4544010) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 175 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of activated coagulation factor 11 (unknown origin) assessed as inhibitory constant | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.08.008 BindingDB Entry DOI: 10.7270/Q23F4T2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50267039 (CHEMBL4090766) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human GPR40 expressed in HEK293 cell membranes after 1 hr by radioligand displacement based scintillation counting method | J Med Chem 60: 1417-1431 (2017) Article DOI: 10.1021/acs.jmedchem.6b01559 BindingDB Entry DOI: 10.7270/Q25X2CD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50525775 (CHEMBL4441354) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 189 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of activated coagulation factor 11 (unknown origin) assessed as inhibitory constant | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.08.008 BindingDB Entry DOI: 10.7270/Q23F4T2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50525776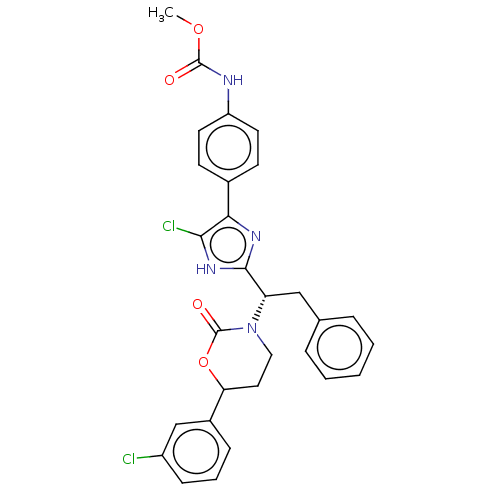 (CHEMBL4514410) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 201 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of activated human coagulation factor 11 using pyroGlu-Pro-Arg-pNA as substrate incubated for 10 to 120 mins by spectrofluorometric method | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.08.008 BindingDB Entry DOI: 10.7270/Q23F4T2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50267035 (CHEMBL4071899) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human GPR40 expressed in HEK293 cell membranes after 1 hr by radioligand displacement based scintillation counting method | J Med Chem 60: 1417-1431 (2017) Article DOI: 10.1021/acs.jmedchem.6b01559 BindingDB Entry DOI: 10.7270/Q25X2CD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50267049 (CHEMBL4095599) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Agonist activity at human GPR40 expressed in CHO-A12 cells assessed as increase in intracellular calcium flux measured for 100 secs by FLIPR assay | J Med Chem 60: 1417-1431 (2017) Article DOI: 10.1021/acs.jmedchem.6b01559 BindingDB Entry DOI: 10.7270/Q25X2CD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50525778 (CHEMBL4558196) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 333 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of activated human coagulation factor 11 using pyroGlu-Pro-Arg-pNA as substrate incubated for 10 to 120 mins by spectrofluorometric method | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.08.008 BindingDB Entry DOI: 10.7270/Q23F4T2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50267038 (CHEMBL4061093) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Agonist activity at human GPR40 expressed in CHO-A12 cells assessed as increase in intracellular calcium flux measured for 100 secs by FLIPR assay | J Med Chem 60: 1417-1431 (2017) Article DOI: 10.1021/acs.jmedchem.6b01559 BindingDB Entry DOI: 10.7270/Q25X2CD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50525765 (CHEMBL4455657) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 372 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of activated coagulation factor 11 (unknown origin) assessed as inhibitory constant | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.08.008 BindingDB Entry DOI: 10.7270/Q23F4T2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50525774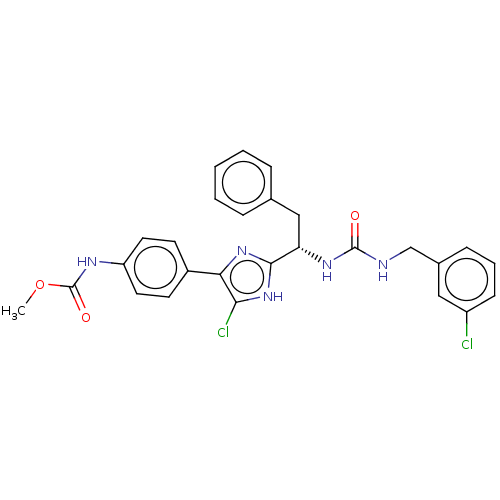 (CHEMBL4535183) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 422 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of activated coagulation factor 11 (unknown origin) assessed as inhibitory constant | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.08.008 BindingDB Entry DOI: 10.7270/Q23F4T2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50525768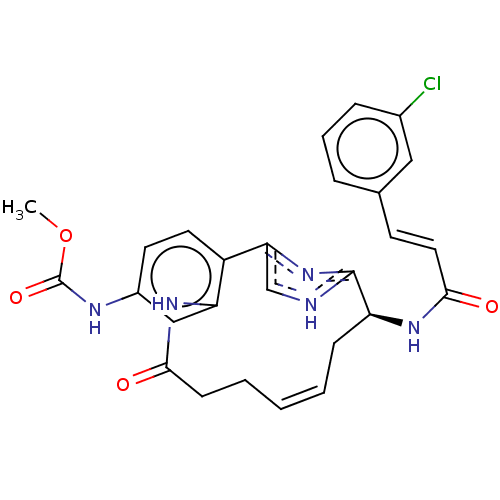 (CHEMBL4454856) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of activated human coagulation factor 11 using pyroGlu-Pro-Arg-pNA as substrate incubated for 10 to 120 mins by spectrofluorometric method | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.08.008 BindingDB Entry DOI: 10.7270/Q23F4T2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50525766 (CHEMBL4438868) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 871 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of activated coagulation factor 11 (unknown origin) assessed as inhibitory constant | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.08.008 BindingDB Entry DOI: 10.7270/Q23F4T2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50267046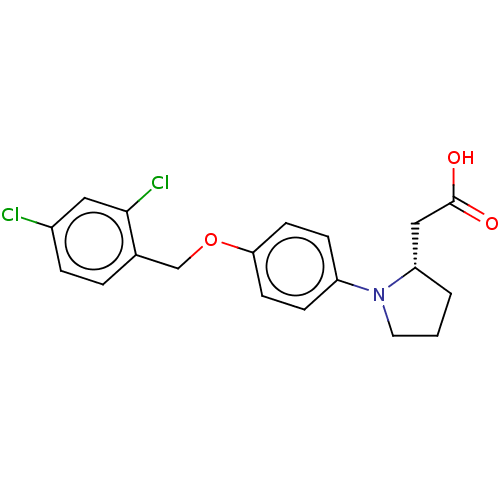 (CHEMBL4103729) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Agonist activity at human GPR40 expressed in CHO-A12 cells assessed as increase in intracellular calcium flux measured for 100 secs by FLIPR assay | J Med Chem 60: 1417-1431 (2017) Article DOI: 10.1021/acs.jmedchem.6b01559 BindingDB Entry DOI: 10.7270/Q25X2CD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50267066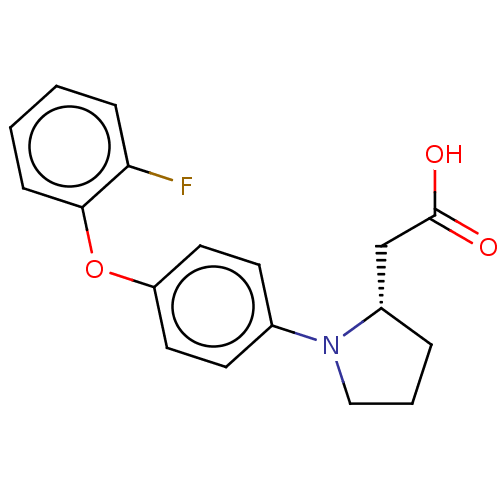 (CHEMBL4067052) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human GPR40 expressed in HEK293 cell membranes after 1 hr by radioligand displacement based scintillation counting method | J Med Chem 60: 1417-1431 (2017) Article DOI: 10.1021/acs.jmedchem.6b01559 BindingDB Entry DOI: 10.7270/Q25X2CD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50267118 (CHEMBL4096887) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human GPR40 expressed in HEK293 cell membranes after 1 hr by radioligand displacement based scintillation counting method | J Med Chem 60: 1417-1431 (2017) Article DOI: 10.1021/acs.jmedchem.6b01559 BindingDB Entry DOI: 10.7270/Q25X2CD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50267059 (CHEMBL4091552) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human GPR40 expressed in HEK293 cell membranes after 1 hr by radioligand displacement based scintillation counting method | J Med Chem 60: 1417-1431 (2017) Article DOI: 10.1021/acs.jmedchem.6b01559 BindingDB Entry DOI: 10.7270/Q25X2CD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XII (Homo sapiens (Human)) | BDBM50525763 (CHEMBL4438562) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | >3.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of activated human coagulation factor 12 | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.08.008 BindingDB Entry DOI: 10.7270/Q23F4T2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XII (Homo sapiens (Human)) | BDBM50525764 (CHEMBL4515523) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | >3.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of activated human coagulation factor 12 | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.08.008 BindingDB Entry DOI: 10.7270/Q23F4T2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50525764 (CHEMBL4515523) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 3.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of activated human coagulation factor 10 | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.08.008 BindingDB Entry DOI: 10.7270/Q23F4T2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50267047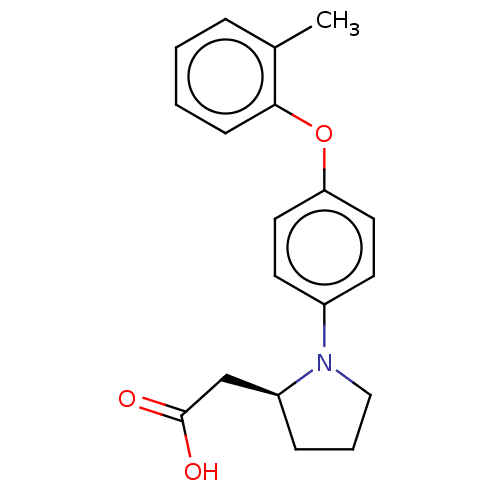 (CHEMBL4078852) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human GPR40 expressed in HEK293 cell membranes after 1 hr by radioligand displacement based scintillation counting method | J Med Chem 60: 1417-1431 (2017) Article DOI: 10.1021/acs.jmedchem.6b01559 BindingDB Entry DOI: 10.7270/Q25X2CD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM50525763 (CHEMBL4438562) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 4.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of activated human coagulation factor 7 | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.08.008 BindingDB Entry DOI: 10.7270/Q23F4T2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM50525764 (CHEMBL4515523) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | >6.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human TPA | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.08.008 BindingDB Entry DOI: 10.7270/Q23F4T2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM50525763 (CHEMBL4438562) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | >6.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human TPA | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.08.008 BindingDB Entry DOI: 10.7270/Q23F4T2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypsin (Homo sapiens (Human)) | BDBM50525763 (CHEMBL4438562) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | >6.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human trypsin | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.08.008 BindingDB Entry DOI: 10.7270/Q23F4T2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypsin (Homo sapiens (Human)) | BDBM50525764 (CHEMBL4515523) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 6.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human trypsin | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.08.008 BindingDB Entry DOI: 10.7270/Q23F4T2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50267064 (CHEMBL4070703) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human GPR40 expressed in HEK293 cell membranes after 1 hr by radioligand displacement based scintillation counting method | J Med Chem 60: 1417-1431 (2017) Article DOI: 10.1021/acs.jmedchem.6b01559 BindingDB Entry DOI: 10.7270/Q25X2CD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin K-dependent protein C (Homo sapiens (Human)) | BDBM50525763 (CHEMBL4438562) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | >7.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human activated protein C | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.08.008 BindingDB Entry DOI: 10.7270/Q23F4T2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50267045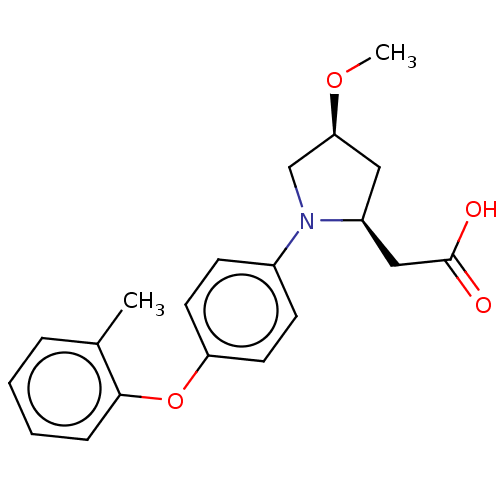 (CHEMBL4063889) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human GPR40 expressed in HEK293 cell membranes after 1 hr by radioligand displacement based scintillation counting method | J Med Chem 60: 1417-1431 (2017) Article DOI: 10.1021/acs.jmedchem.6b01559 BindingDB Entry DOI: 10.7270/Q25X2CD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 829 total ) | Next | Last >> |