

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin D2 receptor (Mus musculus) | BDBM50357626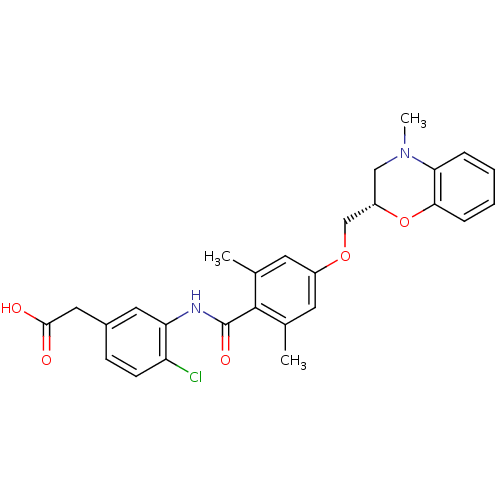 (CHEMBL1915856) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-PGD2 from mouse DP receptor expressed in CHO cells after 20 mins by liquid scintillation counting | Bioorg Med Chem 19: 6935-48 (2011) Article DOI: 10.1016/j.bmc.2011.08.065 BindingDB Entry DOI: 10.7270/Q2K35V22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Mus musculus) | BDBM50351489 (CHEMBL1819614) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-PGD2 from mouse DP receptor expressed in CHO cells after 20 mins by liquid scintillation counting | Bioorg Med Chem 19: 6935-48 (2011) Article DOI: 10.1016/j.bmc.2011.08.065 BindingDB Entry DOI: 10.7270/Q2K35V22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Mus musculus) | BDBM50351483 (CHEMBL1819622) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-PGD2 from mouse DP receptor expressed in CHO cells after 20 mins by liquid scintillation counting | Bioorg Med Chem 19: 6935-48 (2011) Article DOI: 10.1016/j.bmc.2011.08.065 BindingDB Entry DOI: 10.7270/Q2K35V22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Mus musculus) | BDBM50152515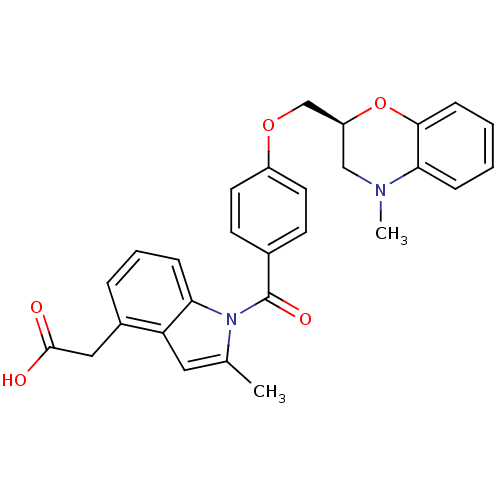 (CHEMBL364841 | {2-Methyl-1-[4-(4-methyl-3,4-dihydr...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-PGD2 from mouse DP receptor expressed in CHO cells after 20 mins by liquid scintillation counting | Bioorg Med Chem 19: 6935-48 (2011) Article DOI: 10.1016/j.bmc.2011.08.065 BindingDB Entry DOI: 10.7270/Q2K35V22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Mus musculus) | BDBM50351486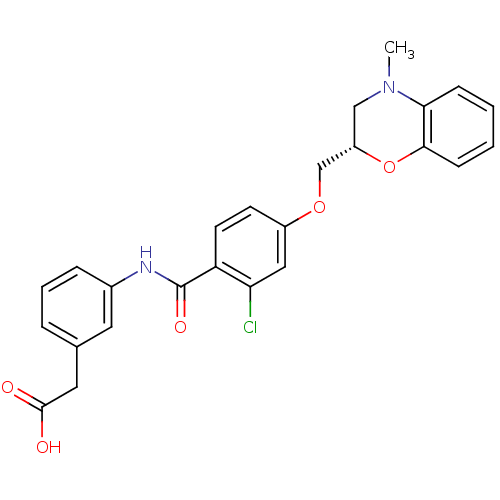 (CHEMBL1819609) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-PGD2 from mouse DP receptor expressed in CHO cells after 20 mins by liquid scintillation counting | Bioorg Med Chem 19: 6935-48 (2011) Article DOI: 10.1016/j.bmc.2011.08.065 BindingDB Entry DOI: 10.7270/Q2K35V22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Mus musculus) | BDBM50351484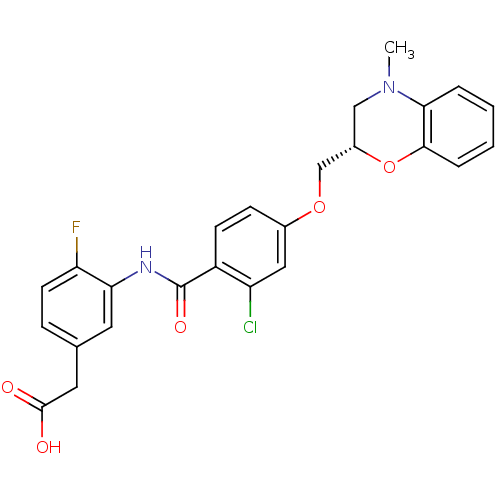 (CHEMBL1819623) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-PGD2 from mouse DP receptor expressed in CHO cells after 20 mins by liquid scintillation counting | Bioorg Med Chem 19: 6935-48 (2011) Article DOI: 10.1016/j.bmc.2011.08.065 BindingDB Entry DOI: 10.7270/Q2K35V22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Mus musculus) | BDBM50351480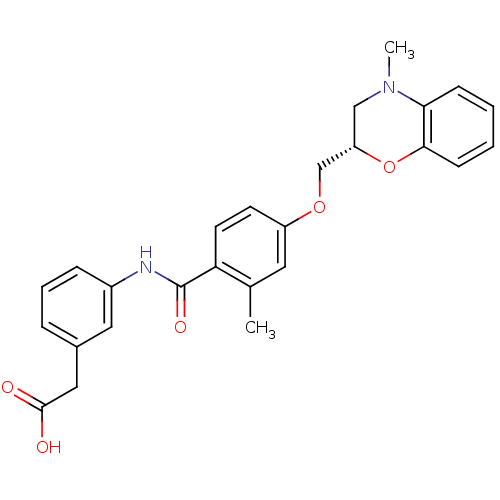 (CHEMBL1819608) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-PGD2 from mouse DP receptor expressed in CHO cells after 20 mins by liquid scintillation counting | Bioorg Med Chem 19: 6935-48 (2011) Article DOI: 10.1016/j.bmc.2011.08.065 BindingDB Entry DOI: 10.7270/Q2K35V22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Mus musculus) | BDBM50351490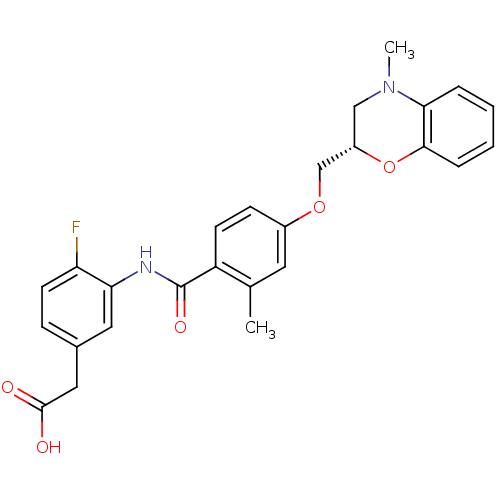 (CHEMBL1819615) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-PGD2 from mouse DP receptor expressed in CHO cells after 20 mins by liquid scintillation counting | Bioorg Med Chem 19: 6935-48 (2011) Article DOI: 10.1016/j.bmc.2011.08.065 BindingDB Entry DOI: 10.7270/Q2K35V22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Mus musculus) | BDBM50351494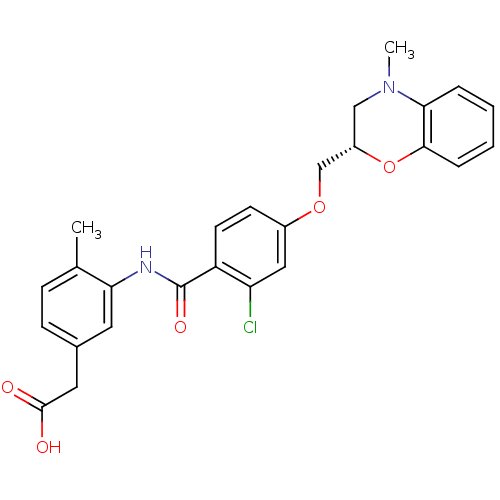 (CHEMBL1819619) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-PGD2 from mouse DP receptor expressed in CHO cells after 20 mins by liquid scintillation counting | Bioorg Med Chem 19: 6935-48 (2011) Article DOI: 10.1016/j.bmc.2011.08.065 BindingDB Entry DOI: 10.7270/Q2K35V22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Mus musculus) | BDBM50357627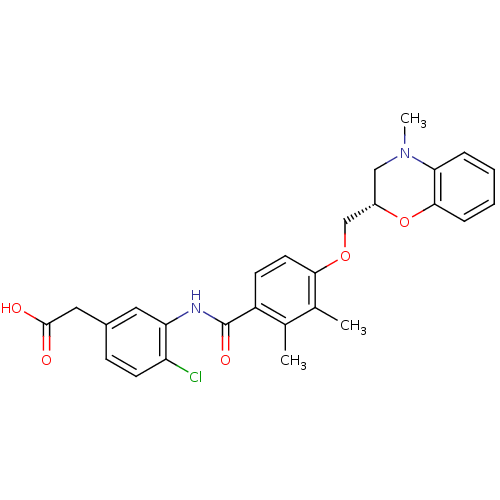 (CHEMBL1915676) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-PGD2 from mouse DP receptor expressed in CHO cells after 20 mins by liquid scintillation counting | Bioorg Med Chem 19: 6935-48 (2011) Article DOI: 10.1016/j.bmc.2011.08.065 BindingDB Entry DOI: 10.7270/Q2K35V22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Mus musculus) | BDBM50351481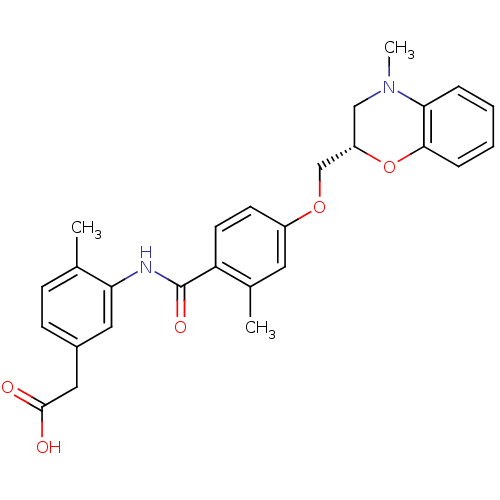 (CHEMBL1819611) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-PGD2 from mouse DP receptor expressed in CHO cells after 20 mins by liquid scintillation counting | Bioorg Med Chem 19: 6935-48 (2011) Article DOI: 10.1016/j.bmc.2011.08.065 BindingDB Entry DOI: 10.7270/Q2K35V22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50152515 (CHEMBL364841 | {2-Methyl-1-[4-(4-methyl-3,4-dihydr...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [3H]Iloprost from human prostanoid IP receptor expressed in CHO cells after 30 mins by liquid scintillation counting | Bioorg Med Chem 19: 6935-48 (2011) Article DOI: 10.1016/j.bmc.2011.08.065 BindingDB Entry DOI: 10.7270/Q2K35V22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Mus musculus) | BDBM50351485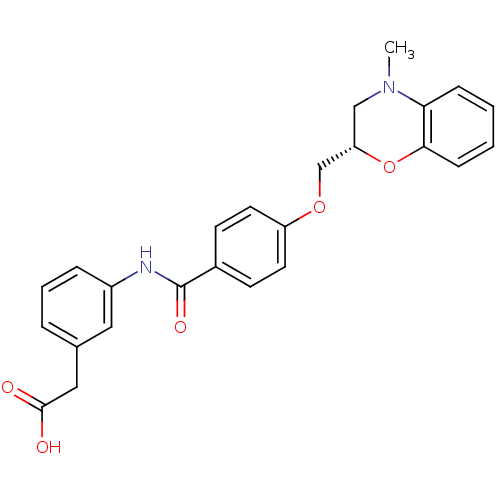 (CHEMBL1819603) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-PGD2 from mouse DP receptor expressed in CHO cells after 20 mins by liquid scintillation counting | Bioorg Med Chem 19: 6935-48 (2011) Article DOI: 10.1016/j.bmc.2011.08.065 BindingDB Entry DOI: 10.7270/Q2K35V22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50357636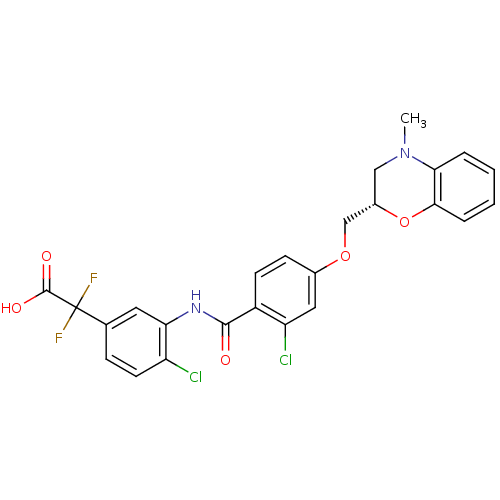 (CHEMBL1915866) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [3H]Iloprost from human prostanoid IP receptor expressed in CHO cells after 30 mins by liquid scintillation counting | Bioorg Med Chem 19: 6935-48 (2011) Article DOI: 10.1016/j.bmc.2011.08.065 BindingDB Entry DOI: 10.7270/Q2K35V22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50351494 (CHEMBL1819619) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [3H]Iloprost from human prostanoid IP receptor expressed in CHO cells after 30 mins by liquid scintillation counting | Bioorg Med Chem 19: 6935-48 (2011) Article DOI: 10.1016/j.bmc.2011.08.065 BindingDB Entry DOI: 10.7270/Q2K35V22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50351483 (CHEMBL1819622) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [3H]Iloprost from human prostanoid IP receptor expressed in CHO cells after 30 mins by liquid scintillation counting | Bioorg Med Chem 19: 6935-48 (2011) Article DOI: 10.1016/j.bmc.2011.08.065 BindingDB Entry DOI: 10.7270/Q2K35V22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50357619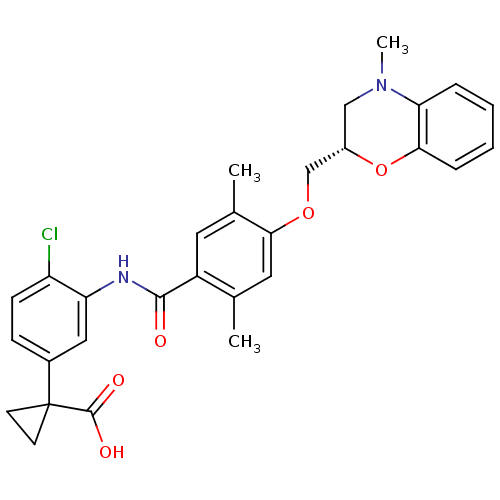 (CHEMBL1915864) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [3H]Iloprost from human prostanoid IP receptor expressed in CHO cells after 30 mins by liquid scintillation counting | Bioorg Med Chem 19: 6935-48 (2011) Article DOI: 10.1016/j.bmc.2011.08.065 BindingDB Entry DOI: 10.7270/Q2K35V22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Mus musculus) | BDBM50357625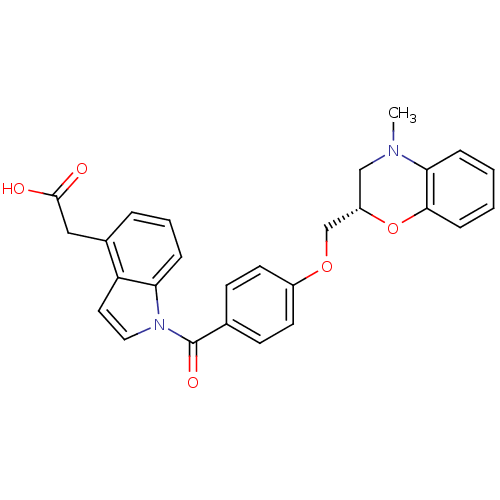 (CHEMBL1915669) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-PGD2 from mouse DP receptor expressed in CHO cells after 20 mins by liquid scintillation counting | Bioorg Med Chem 19: 6935-48 (2011) Article DOI: 10.1016/j.bmc.2011.08.065 BindingDB Entry DOI: 10.7270/Q2K35V22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50351489 (CHEMBL1819614) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [3H]Iloprost from human prostanoid IP receptor expressed in CHO cells after 30 mins by liquid scintillation counting | Bioorg Med Chem 19: 6935-48 (2011) Article DOI: 10.1016/j.bmc.2011.08.065 BindingDB Entry DOI: 10.7270/Q2K35V22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50357637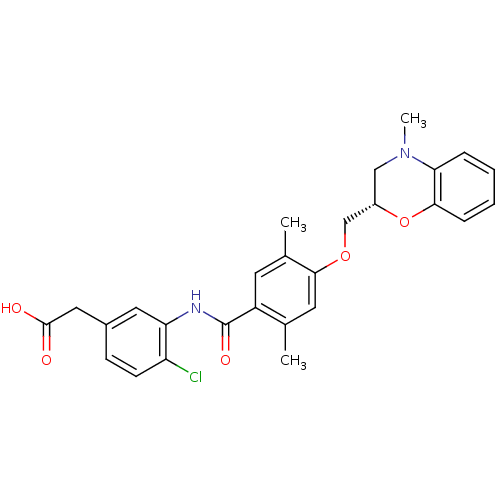 (CHEMBL1915677) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [3H]Iloprost from human prostanoid IP receptor expressed in CHO cells after 30 mins by liquid scintillation counting | Bioorg Med Chem 19: 6935-48 (2011) Article DOI: 10.1016/j.bmc.2011.08.065 BindingDB Entry DOI: 10.7270/Q2K35V22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50357625 (CHEMBL1915669) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [3H]Iloprost from human prostanoid IP receptor expressed in CHO cells after 30 mins by liquid scintillation counting | Bioorg Med Chem 19: 6935-48 (2011) Article DOI: 10.1016/j.bmc.2011.08.065 BindingDB Entry DOI: 10.7270/Q2K35V22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50351481 (CHEMBL1819611) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [3H]Iloprost from human prostanoid IP receptor expressed in CHO cells after 30 mins by liquid scintillation counting | Bioorg Med Chem 19: 6935-48 (2011) Article DOI: 10.1016/j.bmc.2011.08.065 BindingDB Entry DOI: 10.7270/Q2K35V22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50357639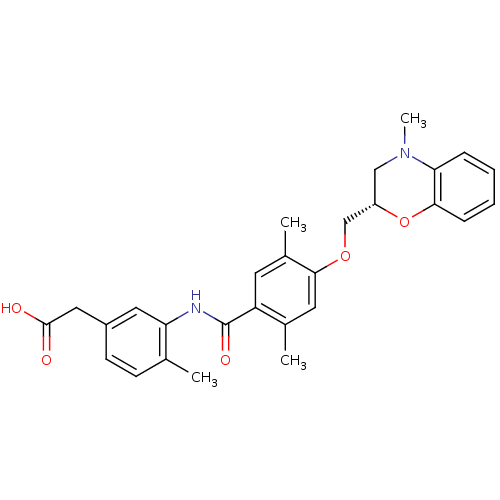 (CHEMBL1915674) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [3H]Iloprost from human prostanoid IP receptor expressed in CHO cells after 30 mins by liquid scintillation counting | Bioorg Med Chem 19: 6935-48 (2011) Article DOI: 10.1016/j.bmc.2011.08.065 BindingDB Entry DOI: 10.7270/Q2K35V22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50351486 (CHEMBL1819609) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [3H]Iloprost from human prostanoid IP receptor expressed in CHO cells after 30 mins by liquid scintillation counting | Bioorg Med Chem 19: 6935-48 (2011) Article DOI: 10.1016/j.bmc.2011.08.065 BindingDB Entry DOI: 10.7270/Q2K35V22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50357633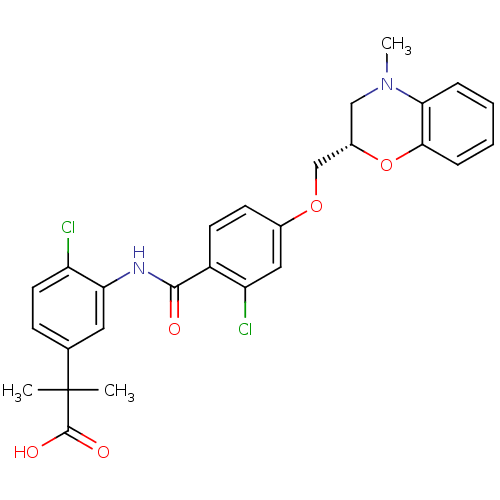 (CHEMBL1915861) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [3H]Iloprost from human prostanoid IP receptor expressed in CHO cells after 30 mins by liquid scintillation counting | Bioorg Med Chem 19: 6935-48 (2011) Article DOI: 10.1016/j.bmc.2011.08.065 BindingDB Entry DOI: 10.7270/Q2K35V22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50357634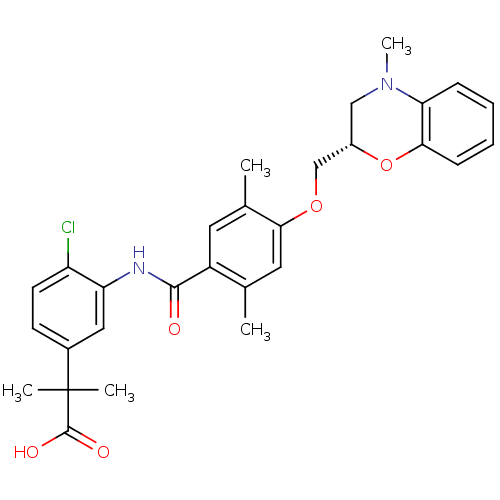 (CHEMBL1915862) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [3H]Iloprost from human prostanoid IP receptor expressed in CHO cells after 30 mins by liquid scintillation counting | Bioorg Med Chem 19: 6935-48 (2011) Article DOI: 10.1016/j.bmc.2011.08.065 BindingDB Entry DOI: 10.7270/Q2K35V22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50357627 (CHEMBL1915676) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [3H]Iloprost from human prostanoid IP receptor expressed in CHO cells after 30 mins by liquid scintillation counting | Bioorg Med Chem 19: 6935-48 (2011) Article DOI: 10.1016/j.bmc.2011.08.065 BindingDB Entry DOI: 10.7270/Q2K35V22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50351480 (CHEMBL1819608) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [3H]Iloprost from human prostanoid IP receptor expressed in CHO cells after 30 mins by liquid scintillation counting | Bioorg Med Chem 19: 6935-48 (2011) Article DOI: 10.1016/j.bmc.2011.08.065 BindingDB Entry DOI: 10.7270/Q2K35V22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50357621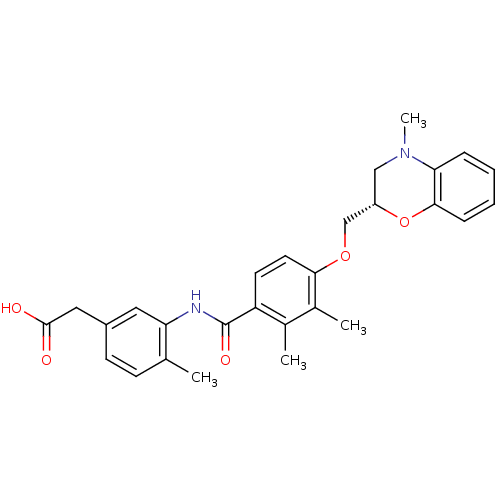 (CHEMBL1915673) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [3H]Iloprost from human prostanoid IP receptor expressed in CHO cells after 30 mins by liquid scintillation counting | Bioorg Med Chem 19: 6935-48 (2011) Article DOI: 10.1016/j.bmc.2011.08.065 BindingDB Entry DOI: 10.7270/Q2K35V22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50351485 (CHEMBL1819603) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [3H]Iloprost from human prostanoid IP receptor expressed in CHO cells after 30 mins by liquid scintillation counting | Bioorg Med Chem 19: 6935-48 (2011) Article DOI: 10.1016/j.bmc.2011.08.065 BindingDB Entry DOI: 10.7270/Q2K35V22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50357629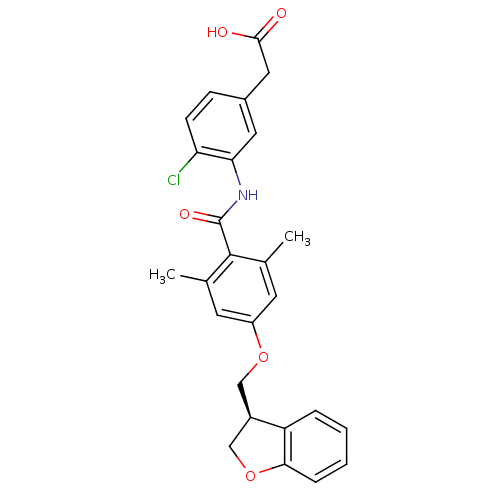 (CHEMBL1915858) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [3H]Iloprost from human prostanoid IP receptor expressed in CHO cells after 30 mins by liquid scintillation counting | Bioorg Med Chem 19: 6935-48 (2011) Article DOI: 10.1016/j.bmc.2011.08.065 BindingDB Entry DOI: 10.7270/Q2K35V22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50357635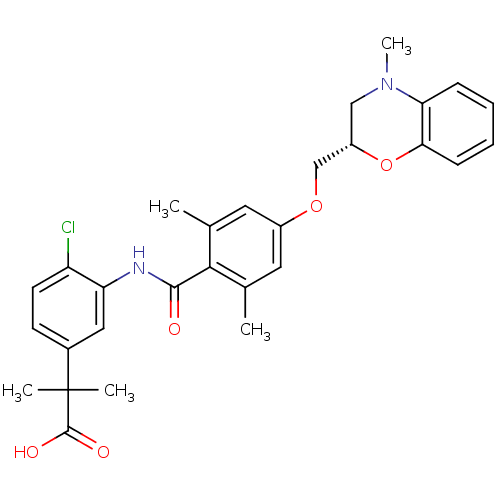 (CHEMBL1915863) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [3H]Iloprost from human prostanoid IP receptor expressed in CHO cells after 30 mins by liquid scintillation counting | Bioorg Med Chem 19: 6935-48 (2011) Article DOI: 10.1016/j.bmc.2011.08.065 BindingDB Entry DOI: 10.7270/Q2K35V22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50357628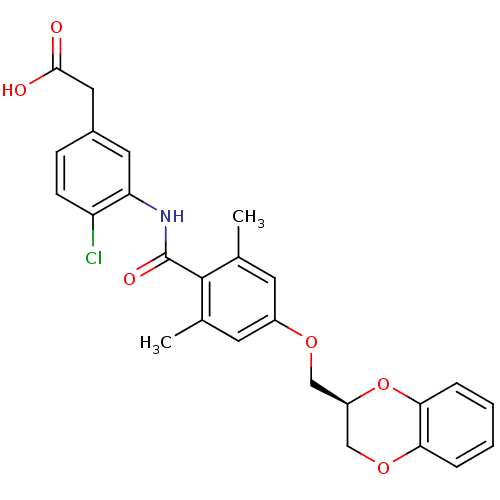 (CHEMBL1915857) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [3H]Iloprost from human prostanoid IP receptor expressed in CHO cells after 30 mins by liquid scintillation counting | Bioorg Med Chem 19: 6935-48 (2011) Article DOI: 10.1016/j.bmc.2011.08.065 BindingDB Entry DOI: 10.7270/Q2K35V22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50357620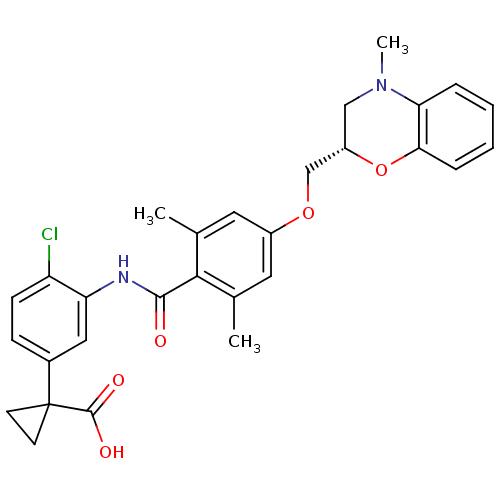 (CHEMBL1915865) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [3H]Iloprost from human prostanoid IP receptor expressed in CHO cells after 30 mins by liquid scintillation counting | Bioorg Med Chem 19: 6935-48 (2011) Article DOI: 10.1016/j.bmc.2011.08.065 BindingDB Entry DOI: 10.7270/Q2K35V22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50357626 (CHEMBL1915856) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [3H]Iloprost from human prostanoid IP receptor expressed in CHO cells after 30 mins by liquid scintillation counting | Bioorg Med Chem 19: 6935-48 (2011) Article DOI: 10.1016/j.bmc.2011.08.065 BindingDB Entry DOI: 10.7270/Q2K35V22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50357622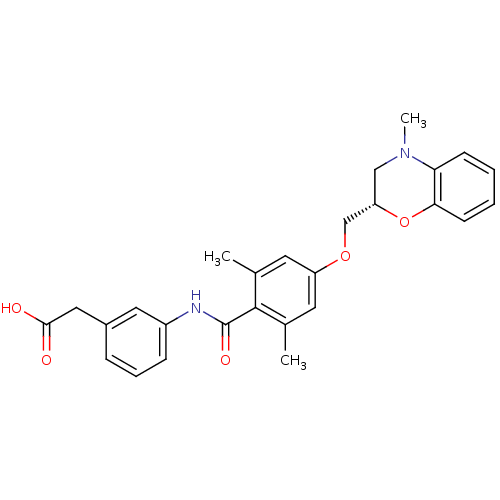 (CHEMBL1915672) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [3H]Iloprost from human prostanoid IP receptor expressed in CHO cells after 30 mins by liquid scintillation counting | Bioorg Med Chem 19: 6935-48 (2011) Article DOI: 10.1016/j.bmc.2011.08.065 BindingDB Entry DOI: 10.7270/Q2K35V22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50357623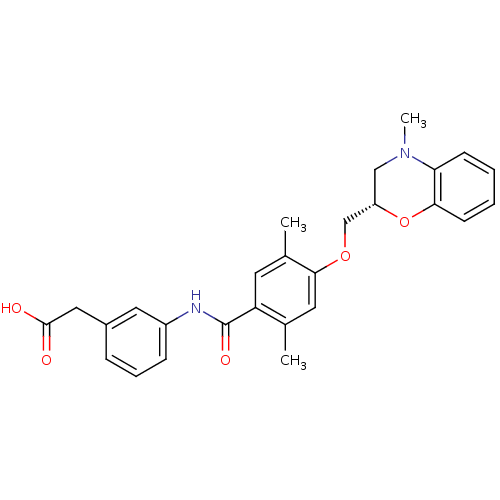 (CHEMBL1915671) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [3H]Iloprost from human prostanoid IP receptor expressed in CHO cells after 30 mins by liquid scintillation counting | Bioorg Med Chem 19: 6935-48 (2011) Article DOI: 10.1016/j.bmc.2011.08.065 BindingDB Entry DOI: 10.7270/Q2K35V22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50357624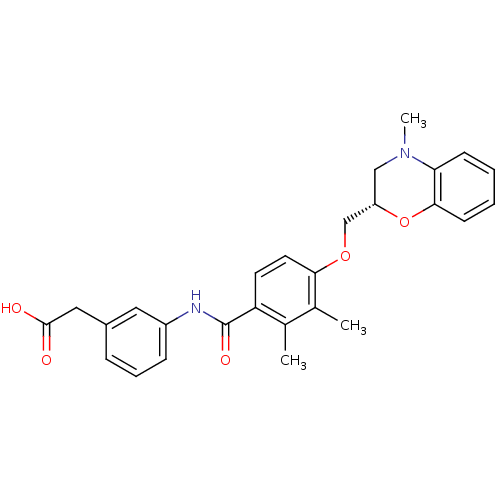 (CHEMBL1915670) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [3H]Iloprost from human prostanoid IP receptor expressed in CHO cells after 30 mins by liquid scintillation counting | Bioorg Med Chem 19: 6935-48 (2011) Article DOI: 10.1016/j.bmc.2011.08.065 BindingDB Entry DOI: 10.7270/Q2K35V22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50351484 (CHEMBL1819623) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [3H]Iloprost from human prostanoid IP receptor expressed in CHO cells after 30 mins by liquid scintillation counting | Bioorg Med Chem 19: 6935-48 (2011) Article DOI: 10.1016/j.bmc.2011.08.065 BindingDB Entry DOI: 10.7270/Q2K35V22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50351490 (CHEMBL1819615) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [3H]Iloprost from human prostanoid IP receptor expressed in CHO cells after 30 mins by liquid scintillation counting | Bioorg Med Chem 19: 6935-48 (2011) Article DOI: 10.1016/j.bmc.2011.08.065 BindingDB Entry DOI: 10.7270/Q2K35V22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50357638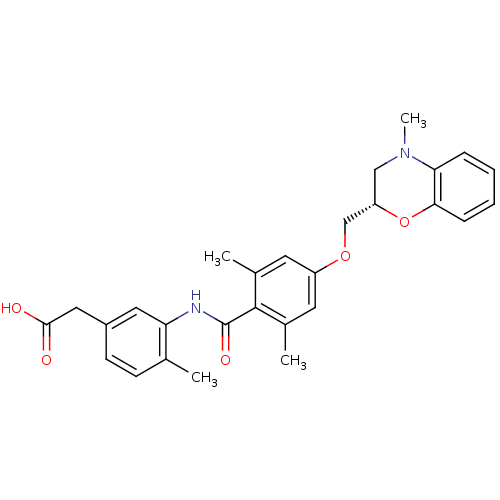 (CHEMBL1915675) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [3H]Iloprost from human prostanoid IP receptor expressed in CHO cells after 30 mins by liquid scintillation counting | Bioorg Med Chem 19: 6935-48 (2011) Article DOI: 10.1016/j.bmc.2011.08.065 BindingDB Entry DOI: 10.7270/Q2K35V22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50357631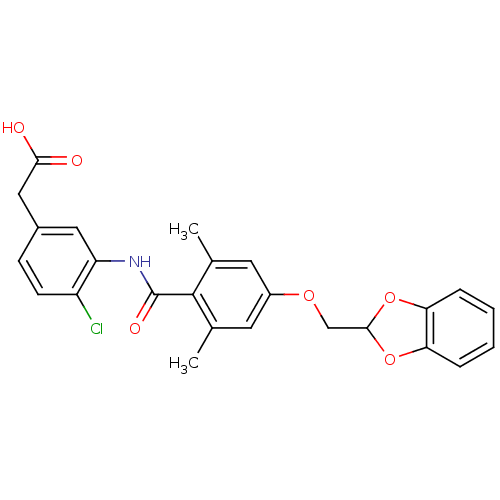 (CHEMBL1915860) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [3H]Iloprost from human prostanoid IP receptor expressed in CHO cells after 30 mins by liquid scintillation counting | Bioorg Med Chem 19: 6935-48 (2011) Article DOI: 10.1016/j.bmc.2011.08.065 BindingDB Entry DOI: 10.7270/Q2K35V22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50357632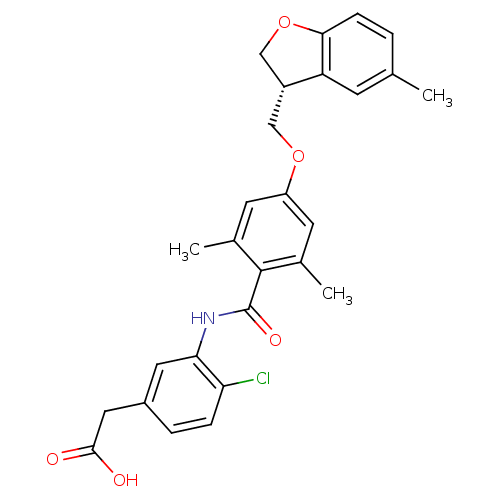 (CHEMBL1915867) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [3H]Iloprost from human prostanoid IP receptor expressed in CHO cells after 30 mins by liquid scintillation counting | Bioorg Med Chem 19: 6935-48 (2011) Article DOI: 10.1016/j.bmc.2011.08.065 BindingDB Entry DOI: 10.7270/Q2K35V22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50357630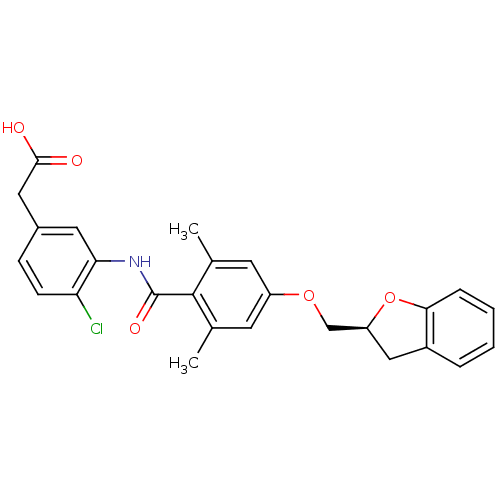 (CHEMBL1915859) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [3H]Iloprost from human prostanoid IP receptor expressed in CHO cells after 30 mins by liquid scintillation counting | Bioorg Med Chem 19: 6935-48 (2011) Article DOI: 10.1016/j.bmc.2011.08.065 BindingDB Entry DOI: 10.7270/Q2K35V22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Mus musculus) | BDBM50357635 (CHEMBL1915863) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Antagonist activity at mouse prostanoid DP receptor expressed in CHO cells assessed as inhibition of PGD2-induced intracellular cAMP production after... | Bioorg Med Chem 19: 6935-48 (2011) Article DOI: 10.1016/j.bmc.2011.08.065 BindingDB Entry DOI: 10.7270/Q2K35V22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Mus musculus) | BDBM50357620 (CHEMBL1915865) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Antagonist activity at mouse prostanoid DP receptor expressed in CHO cells assessed as inhibition of PGD2-induced intracellular cAMP production after... | Bioorg Med Chem 19: 6935-48 (2011) Article DOI: 10.1016/j.bmc.2011.08.065 BindingDB Entry DOI: 10.7270/Q2K35V22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50198936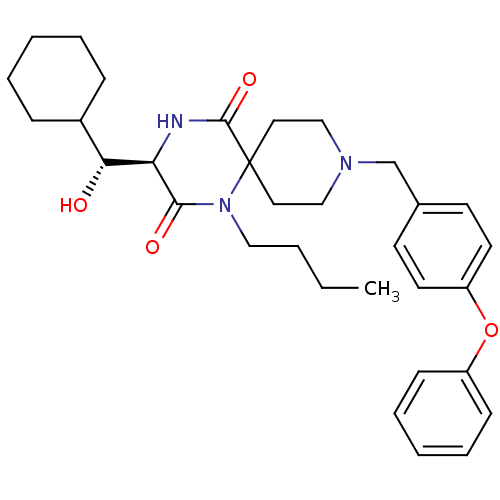 ((R)-1-butyl-3-((R)-cyclohexyl-hydroxy-methyl)-9-(4...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [125I]MIP1-alpha from human CCR5 expressed in CHO cells | Bioorg Med Chem Lett 17: 727-31 (2007) Article DOI: 10.1016/j.bmcl.2006.10.084 BindingDB Entry DOI: 10.7270/Q2XK8GCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM238267 (US9394250, 99) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
ONO PHARMACEUTICAL CO., LTD. US Patent | Assay Description Human Factor XIa (Haematologic Technologies Inc.) activity was measured at an enzyme concentration of 0.1 U/mL in 150 mM NaCl, 5 mM KCl, 1 mg/mL PEG6... | US Patent US9394250 (2016) BindingDB Entry DOI: 10.7270/Q2222SP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM294112 (US10336741, Example 99 | US10882855, Example 99 | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd US Patent | Assay Description Inhibitory activities of compounds of the present invention against factor XIa, Xa, XIIa, IXa, VIIa, plasma kallikrein or thrombin were evaluated usi... | US Patent US10336741 (2019) BindingDB Entry DOI: 10.7270/Q2CR5WQP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM294112 (US10336741, Example 99 | US10882855, Example 99 | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
ONO PHARMACEUTICAL CO., LTD. US Patent | Assay Description Human Factor XIa (Haematologic Technologies Inc.) activity was measured at an enzyme concentration of 0.1 U/mL in 150 mM NaCl, 5 mM KCl, 1 mg/mL PEG6... | US Patent US9585881 (2017) BindingDB Entry DOI: 10.7270/Q2D50Q0R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 676 total ) | Next | Last >> |