

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Replicase polyprotein 1ab (BtCoV) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PDB UniChem | Article PubMed | 0.0330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. | Assay Description PF-00835231 was evaluated against 3CLpro from a variety of other coronaviruses representing alpha, beta and gamma groups of Coronaviridae, using bioc... | bioRxiv (2020) Article DOI: 10.1101/2020.09.12.293498 BindingDB Entry DOI: 10.7270/Q2VQ352Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (BtCoV) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PDB UniChem | Article PubMed | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. | Assay Description PF-00835231 was evaluated against 3CLpro from a variety of other coronaviruses representing alpha, beta and gamma groups of Coronaviridae, using bioc... | bioRxiv (2020) Article DOI: 10.1101/2020.09.12.293498 BindingDB Entry DOI: 10.7270/Q2VQ352Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Feline coronavirus (strain FIPV WSU-79/1146) (FCoV...) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PDB UniChem | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. | Assay Description PF-00835231 was evaluated against 3CLpro from a variety of other coronaviruses representing alpha, beta and gamma groups of Coronaviridae, using bioc... | bioRxiv (2020) Article DOI: 10.1101/2020.09.12.293498 BindingDB Entry DOI: 10.7270/Q2VQ352Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PDB UniChem | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
Pfizer | Assay Description The respective human coronavirus Mpro in assay buffer (20 mM Tris-HCl, pH 7.3, 100 mM NaCl, 1 mM EDTA, 5 mM TCEP) and 0.1% BSA was added to assay-rea... | Science 374: 1-13 (2021) BindingDB Entry DOI: 10.7270/Q23T9MCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PDB UniChem | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. | Assay Description PF-00835231 was evaluated against 3CLpro from a variety of other coronaviruses representing alpha, beta and gamma groups of Coronaviridae, using bioc... | bioRxiv (2020) Article DOI: 10.1101/2020.09.12.293498 BindingDB Entry DOI: 10.7270/Q2VQ352Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (PEDV) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PDB UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. | Assay Description PF-00835231 was evaluated against 3CLpro from a variety of other coronaviruses representing alpha, beta and gamma groups of Coronaviridae, using bioc... | bioRxiv (2020) Article DOI: 10.1101/2020.09.12.293498 BindingDB Entry DOI: 10.7270/Q2VQ352Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (HCoV-OC43) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PDB UniChem | Article PubMed | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. | Assay Description PF-00835231 was evaluated against 3CLpro from a variety of other coronaviruses representing alpha, beta and gamma groups of Coronaviridae, using bioc... | bioRxiv (2020) Article DOI: 10.1101/2020.09.12.293498 BindingDB Entry DOI: 10.7270/Q2VQ352Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (BtCoV) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PDB UniChem | Article PubMed | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. | Assay Description PF-00835231 was evaluated against 3CLpro from a variety of other coronaviruses representing alpha, beta and gamma groups of Coronaviridae, using bioc... | bioRxiv (2020) Article DOI: 10.1101/2020.09.12.293498 BindingDB Entry DOI: 10.7270/Q2VQ352Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (HCoV-NL63) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PDB UniChem | Article PubMed | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. | Assay Description PF-00835231 was evaluated against 3CLpro from a variety of other coronaviruses representing alpha, beta and gamma groups of Coronaviridae, using bioc... | bioRxiv (2020) Article DOI: 10.1101/2020.09.12.293498 BindingDB Entry DOI: 10.7270/Q2VQ352Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (HCoV-HKU1) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PDB UniChem | Article PubMed | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. | Assay Description PF-00835231 was evaluated against 3CLpro from a variety of other coronaviruses representing alpha, beta and gamma groups of Coronaviridae, using bioc... | bioRxiv (2020) Article DOI: 10.1101/2020.09.12.293498 BindingDB Entry DOI: 10.7270/Q2VQ352Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (MHV-A59) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PDB UniChem | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. | Assay Description PF-00835231 was evaluated against 3CLpro from a variety of other coronaviruses representing alpha, beta and gamma groups of Coronaviridae, using bioc... | bioRxiv (2020) Article DOI: 10.1101/2020.09.12.293498 BindingDB Entry DOI: 10.7270/Q2VQ352Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (HCoV-229E) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB MMDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PDB UniChem | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. | Assay Description PF-00835231 was evaluated against 3CLpro from a variety of other coronaviruses representing alpha, beta and gamma groups of Coronaviridae, using bioc... | bioRxiv (2020) Article DOI: 10.1101/2020.09.12.293498 BindingDB Entry DOI: 10.7270/Q2VQ352Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM496902 (CVD-0018409 | PF-07321332 | US11351149, Example 13...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | 3.11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
Pfizer | Assay Description The respective human coronavirus Mpro in assay buffer (20 mM Tris-HCl, pH 7.3, 100 mM NaCl, 1 mM EDTA, 5 mM TCEP) and 0.1% BSA was added to assay-rea... | Science 374: 1-13 (2021) BindingDB Entry DOI: 10.7270/Q23T9MCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (IBV) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PDB UniChem | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. | Assay Description PF-00835231 was evaluated against 3CLpro from a variety of other coronaviruses representing alpha, beta and gamma groups of Coronaviridae, using bioc... | bioRxiv (2020) Article DOI: 10.1101/2020.09.12.293498 BindingDB Entry DOI: 10.7270/Q2VQ352Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM496900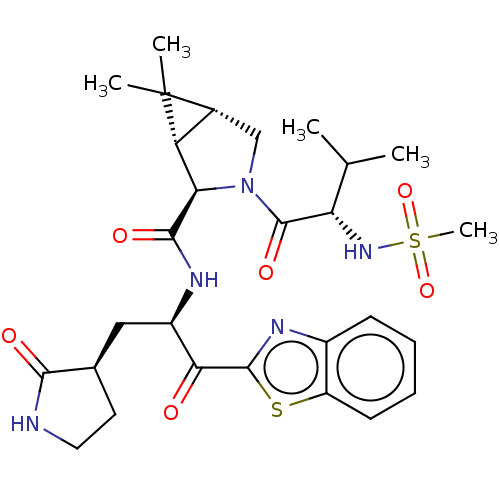 (science.abl4784, 4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | 7.93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
Pfizer | Assay Description The respective human coronavirus Mpro in assay buffer (20 mM Tris-HCl, pH 7.3, 100 mM NaCl, 1 mM EDTA, 5 mM TCEP) and 0.1% BSA was added to assay-rea... | Science 374: 1-13 (2021) BindingDB Entry DOI: 10.7270/Q23T9MCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM496901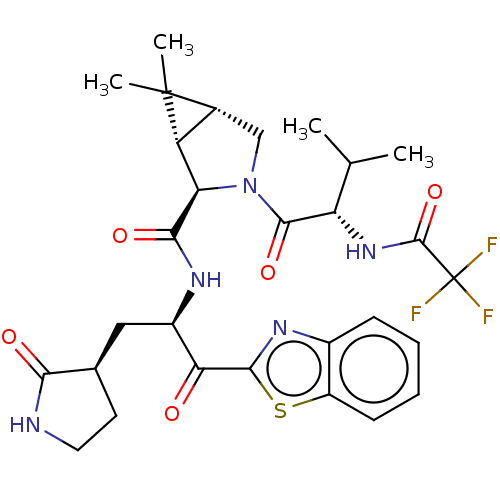 (science.abl4784, 5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
Pfizer | Assay Description The respective human coronavirus Mpro in assay buffer (20 mM Tris-HCl, pH 7.3, 100 mM NaCl, 1 mM EDTA, 5 mM TCEP) and 0.1% BSA was added to assay-rea... | Science 374: 1-13 (2021) BindingDB Entry DOI: 10.7270/Q23T9MCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM496896 (US11312704, Compound 101 | US11351149, Example 49 ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
Pfizer | Assay Description The respective human coronavirus Mpro in assay buffer (20 mM Tris-HCl, pH 7.3, 100 mM NaCl, 1 mM EDTA, 5 mM TCEP) and 0.1% BSA was added to assay-rea... | Science 374: 1-13 (2021) BindingDB Entry DOI: 10.7270/Q23T9MCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM496897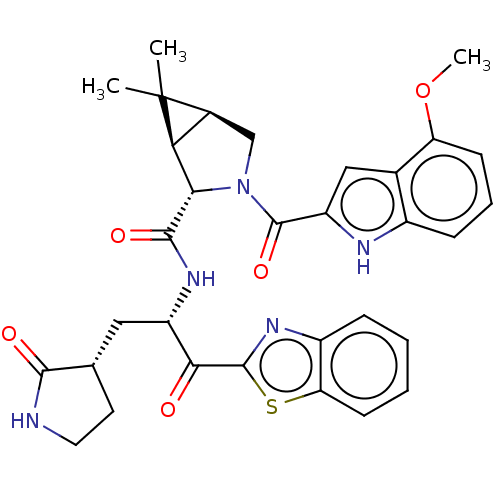 (science.abl4784, 3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
Pfizer | Assay Description The respective human coronavirus Mpro in assay buffer (20 mM Tris-HCl, pH 7.3, 100 mM NaCl, 1 mM EDTA, 5 mM TCEP) and 0.1% BSA was added to assay-rea... | Science 374: 1-13 (2021) BindingDB Entry DOI: 10.7270/Q23T9MCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| m7GpppX diphosphatase (Homo sapiens (Human)) | BDBM50232526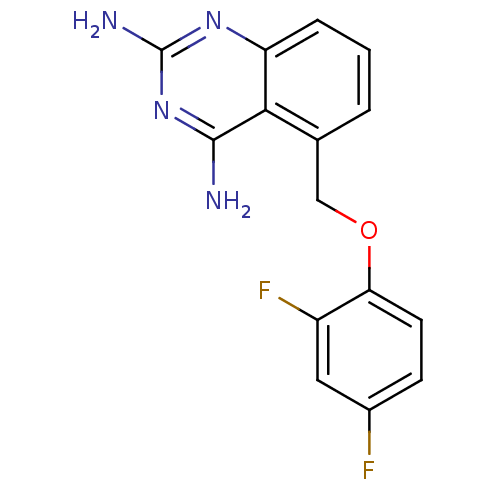 (5-((2,4-difluorophenoxy)methyl)quinazoline-2,4-dia...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human DcpS assessed as increase in SMN2 promoter activity | J Med Chem 60: 3094-3108 (2017) Article DOI: 10.1021/acs.jmedchem.7b00124 BindingDB Entry DOI: 10.7270/Q2251MG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| m7GpppX diphosphatase (Homo sapiens (Human)) | BDBM50232534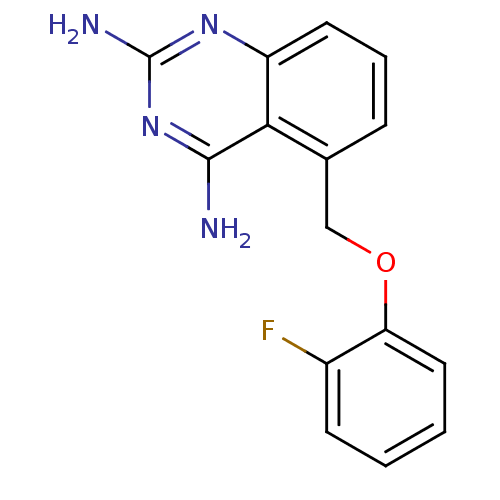 (5-((2-fluorophenoxy)methyl)quinazoline-2,4-diamine...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human DcpS assessed as increase in SMN2 promoter activity | J Med Chem 60: 3094-3108 (2017) Article DOI: 10.1021/acs.jmedchem.7b00124 BindingDB Entry DOI: 10.7270/Q2251MG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| m7GpppX diphosphatase (Homo sapiens (Human)) | BDBM50232538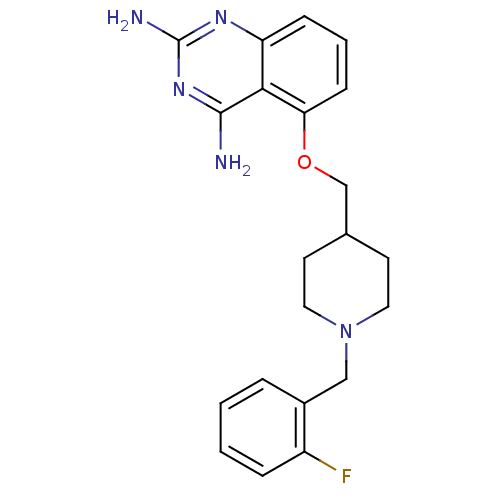 (5-((1-(2-fluorobenzyl)piperidin-4-yl)methoxy)quina...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human DcpS assessed as increase in SMN2 promoter activity | J Med Chem 60: 3094-3108 (2017) Article DOI: 10.1021/acs.jmedchem.7b00124 BindingDB Entry DOI: 10.7270/Q2251MG2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| m7GpppX diphosphatase (Homo sapiens (Human)) | BDBM50237216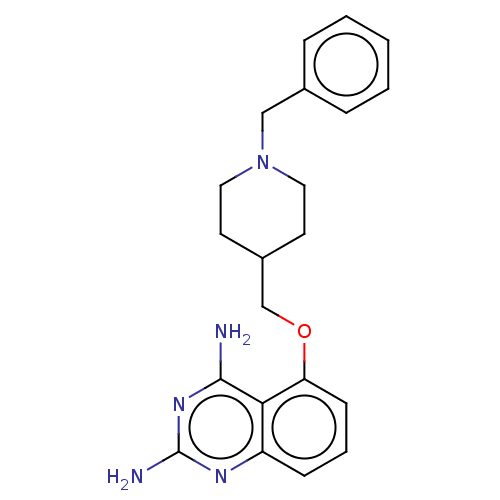 (CHEMBL4080254) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human DcpS assessed as increase in SMN2 promoter activity | J Med Chem 60: 3094-3108 (2017) Article DOI: 10.1021/acs.jmedchem.7b00124 BindingDB Entry DOI: 10.7270/Q2251MG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| m7GpppX diphosphatase (Homo sapiens (Human)) | BDBM50237201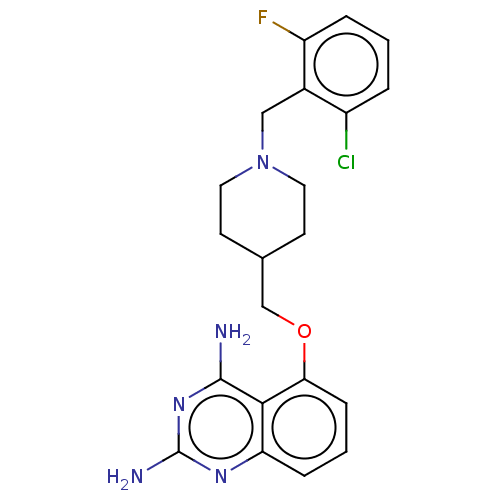 (CHEMBL4082618) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human DcpS assessed as increase in SMN2 promoter activity | J Med Chem 60: 3094-3108 (2017) Article DOI: 10.1021/acs.jmedchem.7b00124 BindingDB Entry DOI: 10.7270/Q2251MG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| m7GpppX diphosphatase (Homo sapiens (Human)) | BDBM50232589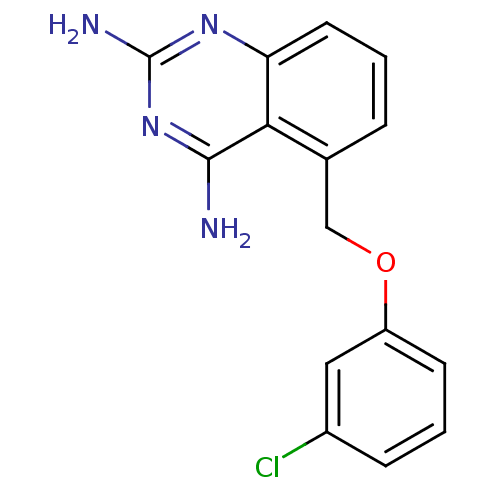 (5-((3-chlorophenoxy)methyl)quinazoline-2,4-diamine...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human DcpS assessed as increase in SMN2 promoter activity | J Med Chem 60: 3094-3108 (2017) Article DOI: 10.1021/acs.jmedchem.7b00124 BindingDB Entry DOI: 10.7270/Q2251MG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| m7GpppX diphosphatase (Homo sapiens (Human)) | BDBM50237200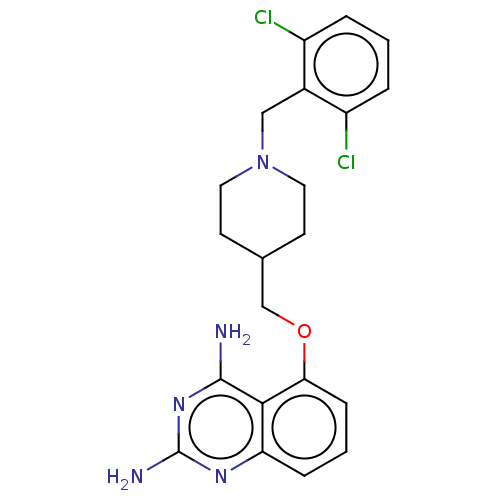 (CHEMBL4072132) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0690 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human DcpS assessed as increase in SMN2 promoter activity | J Med Chem 60: 3094-3108 (2017) Article DOI: 10.1021/acs.jmedchem.7b00124 BindingDB Entry DOI: 10.7270/Q2251MG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| m7GpppX diphosphatase (Homo sapiens (Human)) | BDBM36530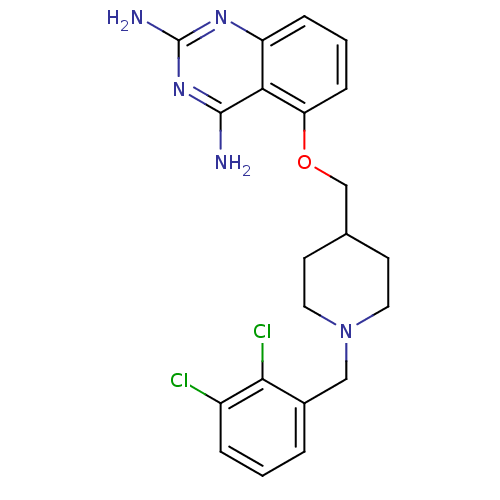 (D157493) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Binding affinity towards 5-HT3 receptor in rat was evaluated | J Med Chem 60: 3094-3108 (2017) Article DOI: 10.1021/acs.jmedchem.7b00124 BindingDB Entry DOI: 10.7270/Q2251MG2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| m7GpppX diphosphatase (Homo sapiens (Human)) | BDBM50237199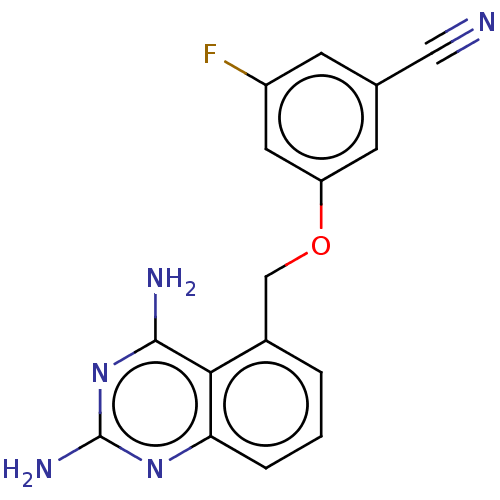 (CHEMBL4077061) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description In vitro reversal of vecuronium-induced block in isolated guinea pig hemi-diaphragm. | J Med Chem 60: 3094-3108 (2017) Article DOI: 10.1021/acs.jmedchem.7b00124 BindingDB Entry DOI: 10.7270/Q2251MG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| m7GpppX diphosphatase (Homo sapiens (Human)) | BDBM50237203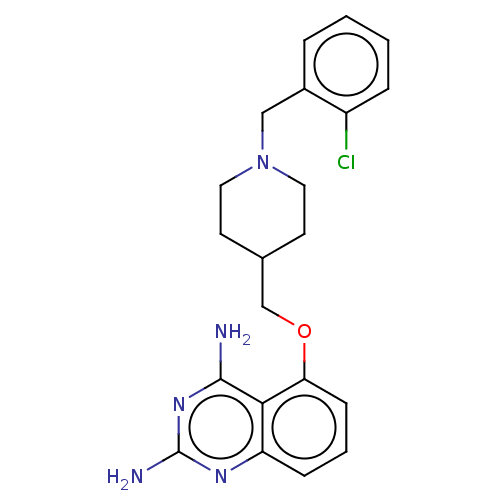 (CHEMBL250072) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human DcpS assessed as increase in SMN2 promoter activity | J Med Chem 60: 3094-3108 (2017) Article DOI: 10.1021/acs.jmedchem.7b00124 BindingDB Entry DOI: 10.7270/Q2251MG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50250852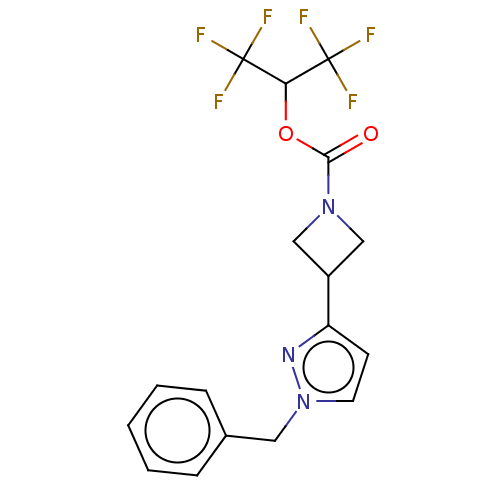 (CHEMBL4078217) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant FAAH using arachidonoyl-AMC as substrate preincubated for 30 mins followed by substrate addition measured after 60 mi... | J Med Chem 60: 9860-9873 (2017) Article DOI: 10.1021/acs.jmedchem.7b01531 BindingDB Entry DOI: 10.7270/Q2N300CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50250859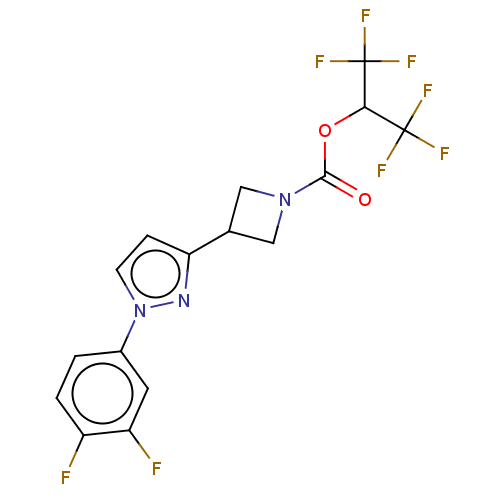 (CHEMBL4097203) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of MAGL in human brain vascular pericytes after 1 hr by TAMRA azide-based In-gel fluorescence method | J Med Chem 60: 9860-9873 (2017) Article DOI: 10.1021/acs.jmedchem.7b01531 BindingDB Entry DOI: 10.7270/Q2N300CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50250848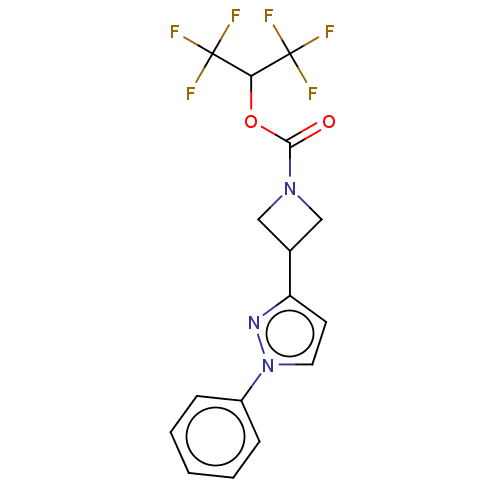 (CHEMBL4059676) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of MAGL in human brain vascular pericytes after 1 hr by TAMRA azide-based In-gel fluorescence method | J Med Chem 60: 9860-9873 (2017) Article DOI: 10.1021/acs.jmedchem.7b01531 BindingDB Entry DOI: 10.7270/Q2N300CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| m7GpppX diphosphatase (Homo sapiens (Human)) | BDBM50237205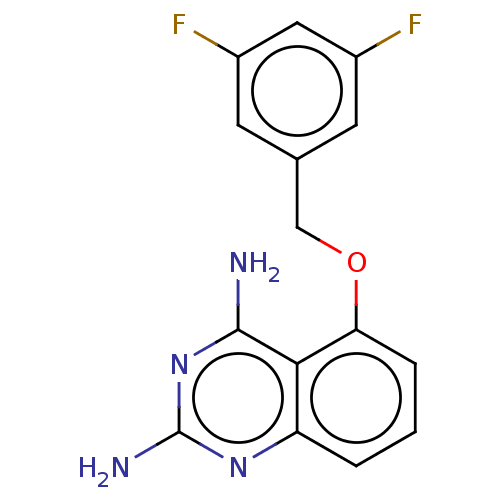 (CHEMBL398675) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human DcpS assessed as increase in SMN2 promoter activity | J Med Chem 60: 3094-3108 (2017) Article DOI: 10.1021/acs.jmedchem.7b00124 BindingDB Entry DOI: 10.7270/Q2251MG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| m7GpppX diphosphatase (Homo sapiens (Human)) | BDBM50237209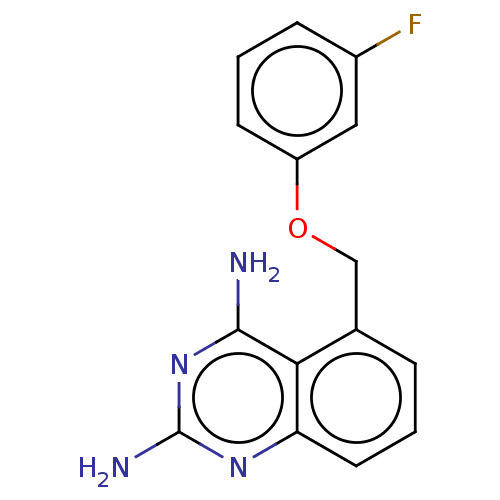 (CHEMBL4062544) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human DcpS assessed as increase in SMN2 promoter activity | J Med Chem 60: 3094-3108 (2017) Article DOI: 10.1021/acs.jmedchem.7b00124 BindingDB Entry DOI: 10.7270/Q2251MG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| m7GpppX diphosphatase (Homo sapiens (Human)) | BDBM50237211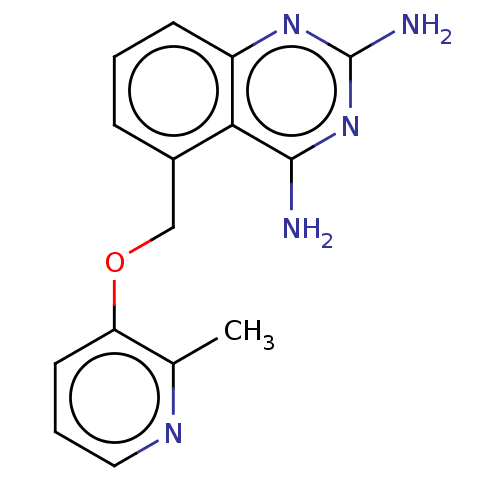 (CHEMBL4061457) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human DcpS assessed as increase in SMN2 promoter activity | J Med Chem 60: 3094-3108 (2017) Article DOI: 10.1021/acs.jmedchem.7b00124 BindingDB Entry DOI: 10.7270/Q2251MG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| m7GpppX diphosphatase (Homo sapiens (Human)) | BDBM50237210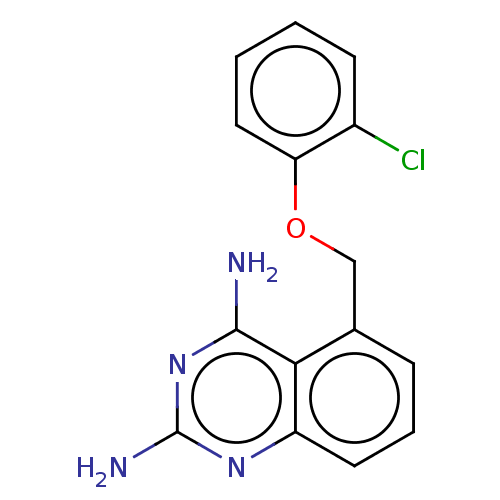 (CHEMBL399673) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human DcpS assessed as increase in SMN2 promoter activity | J Med Chem 60: 3094-3108 (2017) Article DOI: 10.1021/acs.jmedchem.7b00124 BindingDB Entry DOI: 10.7270/Q2251MG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50250855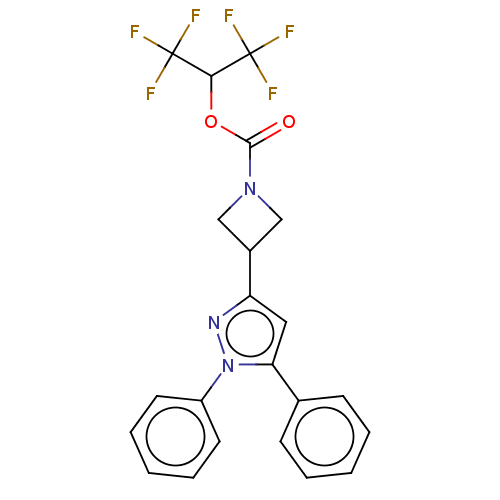 (CHEMBL4077745) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of MAGL in human brain vascular pericytes after 1 hr by TAMRA azide-based In-gel fluorescence method | J Med Chem 60: 9860-9873 (2017) Article DOI: 10.1021/acs.jmedchem.7b01531 BindingDB Entry DOI: 10.7270/Q2N300CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| m7GpppX diphosphatase (Homo sapiens (Human)) | BDBM36534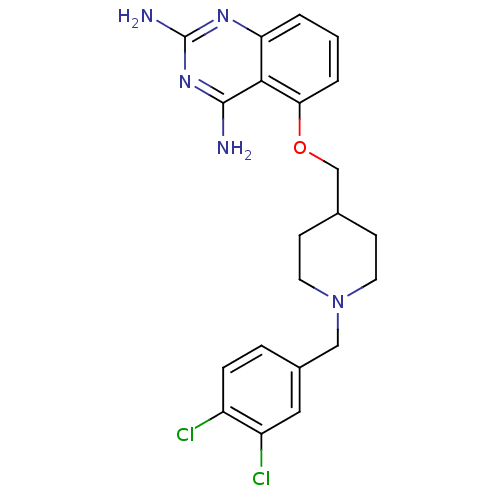 (D156095) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human DcpS assessed as increase in SMN2 promoter activity | J Med Chem 60: 3094-3108 (2017) Article DOI: 10.1021/acs.jmedchem.7b00124 BindingDB Entry DOI: 10.7270/Q2251MG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50250850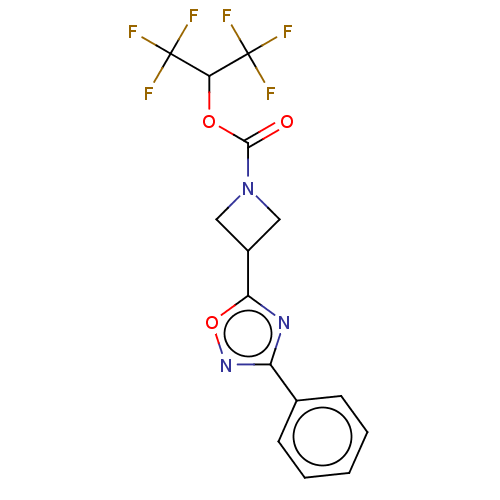 (CHEMBL4089505) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of MAGL in human brain vascular pericytes after 1 hr by TAMRA azide-based In-gel fluorescence method | J Med Chem 60: 9860-9873 (2017) Article DOI: 10.1021/acs.jmedchem.7b01531 BindingDB Entry DOI: 10.7270/Q2N300CG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50250852 (CHEMBL4078217) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of MAGL in human brain vascular pericytes after 1 hr by TAMRA azide-based In-gel fluorescence method | J Med Chem 60: 9860-9873 (2017) Article DOI: 10.1021/acs.jmedchem.7b01531 BindingDB Entry DOI: 10.7270/Q2N300CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50250855 (CHEMBL4077745) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant FAAH using arachidonoyl-AMC as substrate preincubated for 30 mins followed by substrate addition measured after 60 mi... | J Med Chem 60: 9860-9873 (2017) Article DOI: 10.1021/acs.jmedchem.7b01531 BindingDB Entry DOI: 10.7270/Q2N300CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50250859 (CHEMBL4097203) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant FAAH using arachidonoyl-AMC as substrate preincubated for 30 mins followed by substrate addition measured after 60 mi... | J Med Chem 60: 9860-9873 (2017) Article DOI: 10.1021/acs.jmedchem.7b01531 BindingDB Entry DOI: 10.7270/Q2N300CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50250854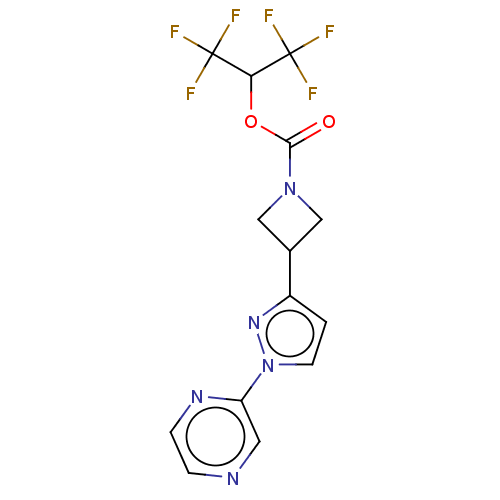 (CHEMBL4079190) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of MAGL in human brain vascular pericytes after 1 hr by TAMRA azide-based In-gel fluorescence method | J Med Chem 60: 9860-9873 (2017) Article DOI: 10.1021/acs.jmedchem.7b01531 BindingDB Entry DOI: 10.7270/Q2N300CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM19130 ((2E,4E,6R)-7-[4-(dimethylamino)phenyl]-N-hydroxy-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of HDAC6 by in vitro deacetylation assay | Nat Chem Biol 6: 25-33 (2009) Article DOI: 10.1038/nchembio.275 BindingDB Entry DOI: 10.7270/Q2FF3SKD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| m7GpppX diphosphatase (Homo sapiens (Human)) | BDBM50237217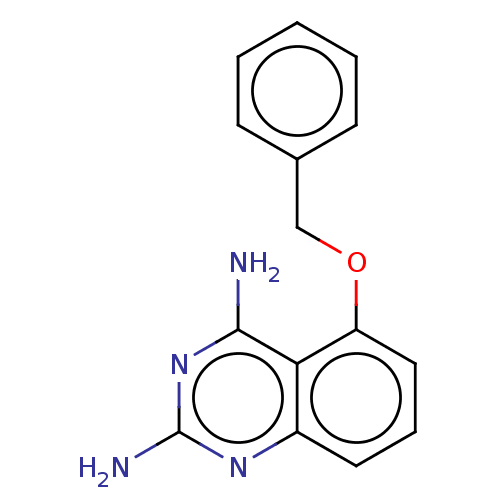 (CHEMBL342595) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Binding affinity towards rat 5-hydroxytryptamine 3 receptor was evaluated | J Med Chem 60: 3094-3108 (2017) Article DOI: 10.1021/acs.jmedchem.7b00124 BindingDB Entry DOI: 10.7270/Q2251MG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50255776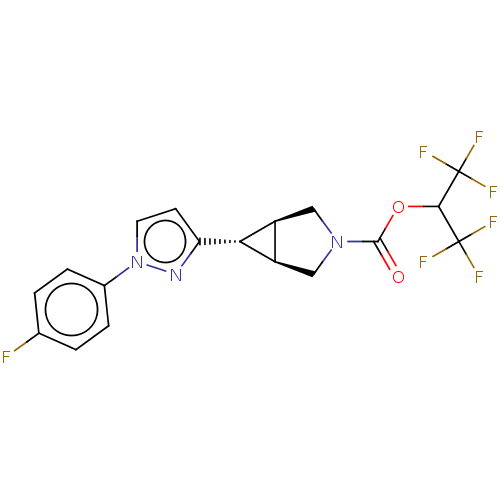 (CHEMBL4075310) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Worldwide Research and Development , 610 Main Street , Cambridge , Massachusetts 02139 , United States. Curated by ChEMBL | Assay Description Inhibition of recombinant human MAGL using fluorogenic-7HCA as substrate preincubated for 30 mins followed by substrate addition measured after 60 mi... | J Med Chem 61: 3008-3026 (2018) Article DOI: 10.1021/acs.jmedchem.8b00070 BindingDB Entry DOI: 10.7270/Q2S46VD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50250854 (CHEMBL4079190) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant FAAH using arachidonoyl-AMC as substrate preincubated for 30 mins followed by substrate addition measured after 60 mi... | J Med Chem 60: 9860-9873 (2017) Article DOI: 10.1021/acs.jmedchem.7b01531 BindingDB Entry DOI: 10.7270/Q2N300CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| m7GpppX diphosphatase (Homo sapiens (Human)) | BDBM50237202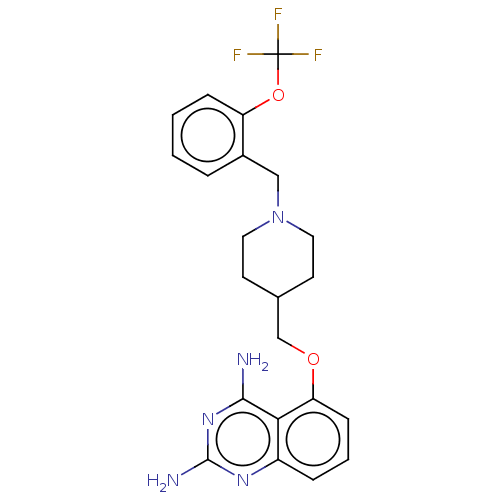 (CHEMBL4103454) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human DcpS assessed as increase in SMN2 promoter activity | J Med Chem 60: 3094-3108 (2017) Article DOI: 10.1021/acs.jmedchem.7b00124 BindingDB Entry DOI: 10.7270/Q2251MG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| m7GpppX diphosphatase (Homo sapiens (Human)) | BDBM50237198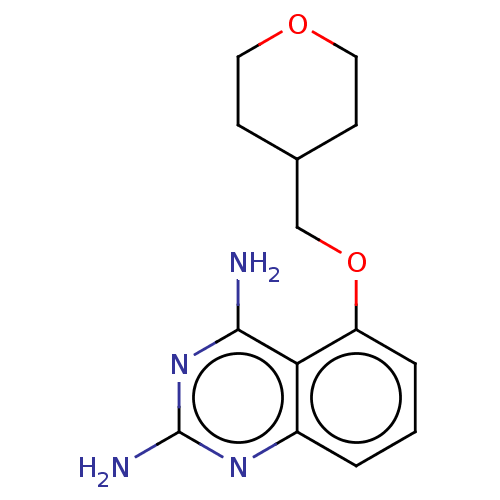 (CHEMBL4068466) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human DcpS assessed as increase in SMN2 promoter activity | J Med Chem 60: 3094-3108 (2017) Article DOI: 10.1021/acs.jmedchem.7b00124 BindingDB Entry DOI: 10.7270/Q2251MG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50250853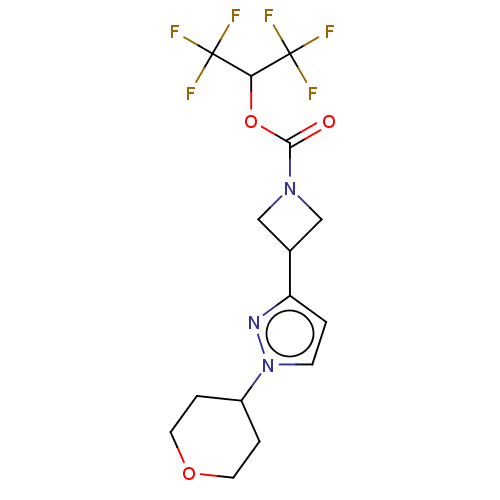 (CHEMBL4102496) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of MAGL in human brain vascular pericytes after 1 hr by TAMRA azide-based In-gel fluorescence method | J Med Chem 60: 9860-9873 (2017) Article DOI: 10.1021/acs.jmedchem.7b01531 BindingDB Entry DOI: 10.7270/Q2N300CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50250849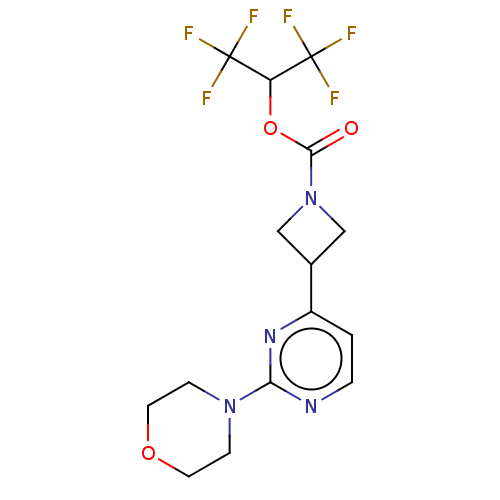 (CHEMBL4068332) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of MAGL in human brain vascular pericytes after 1 hr by TAMRA azide-based In-gel fluorescence method | J Med Chem 60: 9860-9873 (2017) Article DOI: 10.1021/acs.jmedchem.7b01531 BindingDB Entry DOI: 10.7270/Q2N300CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 354 total ) | Next | Last >> |