 Found 408 hits with Last Name = 'nomura' and Initial = 'm'
Found 408 hits with Last Name = 'nomura' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Deoxyuridine 5'-triphosphate nucleotidohydrolase, mitochondrial
(Homo sapiens (Human)) | BDBM50386618

(CHEMBL2048443)Show SMILES O[C@H]1C[C@@H](O[C@@H]1COP(O)(=O)CP(O)(=O)OP(O)(=O)OCc1ccccc1)n1ccc(=O)[nH]c1=O |r| Show InChI InChI=1S/C17H23N2O13P3/c20-13-8-16(19-7-6-15(21)18-17(19)22)31-14(13)10-29-33(23,24)11-34(25,26)32-35(27,28)30-9-12-4-2-1-3-5-12/h1-7,13-14,16,20H,8-11H2,(H,23,24)(H,25,26)(H,27,28)(H,18,21,22)/t13-,14+,16+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human dUTPase |
J Med Chem 55: 2960-9 (2012)
Article DOI: 10.1021/jm201627n
BindingDB Entry DOI: 10.7270/Q2WQ04VS |
More data for this
Ligand-Target Pair | |
Deoxyuridine 5'-triphosphate nucleotidohydrolase, mitochondrial
(Homo sapiens (Human)) | BDBM50173539
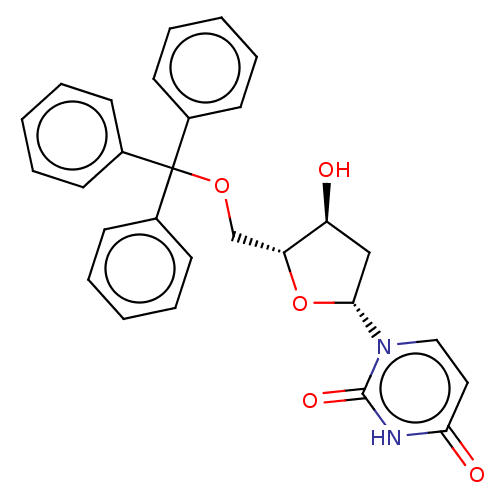
(1-(4-Hydroxy-5-trityloxymethyl-tetrahydro-furan-2-...)Show SMILES O[C@H]1C[C@@H](O[C@@H]1COC(c1ccccc1)(c1ccccc1)c1ccccc1)n1ccc(=O)[nH]c1=O |r| Show InChI InChI=1S/C28H26N2O5/c31-23-18-26(30-17-16-25(32)29-27(30)33)35-24(23)19-34-28(20-10-4-1-5-11-20,21-12-6-2-7-13-21)22-14-8-3-9-15-22/h1-17,23-24,26,31H,18-19H2,(H,29,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human dUTPase assessed as production of [5-3H]dUMP from [5-3H]dUTP after 15 mins measured by HPLC analysis |
J Med Chem 55: 2970-80 (2012)
Article DOI: 10.1021/jm201628y
BindingDB Entry DOI: 10.7270/Q2C82BB5 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50362508

(CHEMBL1940976)Show SMILES C[C@H]1COc2c(NCCNc3ccccn3)c(F)c(N)c3c2n1cc(C#N)c3=O |r| Show InChI InChI=1S/C20H19FN6O2/c1-11-10-29-20-17(26-7-6-25-13-4-2-3-5-24-13)15(21)16(23)14-18(20)27(11)9-12(8-22)19(14)28/h2-5,9,11,26H,6-7,10,23H2,1H3,(H,24,25)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
ActivX Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GSK3-beta |
Bioorg Med Chem Lett 22: 1005-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.006
BindingDB Entry DOI: 10.7270/Q2930TMF |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50362522

(CHEMBL1940990)Show SMILES C[C@H]1COc2c(NCCCc3ccccn3)c(F)c(N)c3c2n1cc(C#N)c3=O |r| Show InChI InChI=1S/C21H20FN5O2/c1-12-11-29-21-18(26-8-4-6-14-5-2-3-7-25-14)16(22)17(24)15-19(21)27(12)10-13(9-23)20(15)28/h2-3,5,7,10,12,26H,4,6,8,11,24H2,1H3/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
ActivX Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GSK3-beta |
Bioorg Med Chem Lett 22: 1005-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.006
BindingDB Entry DOI: 10.7270/Q2930TMF |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50362513
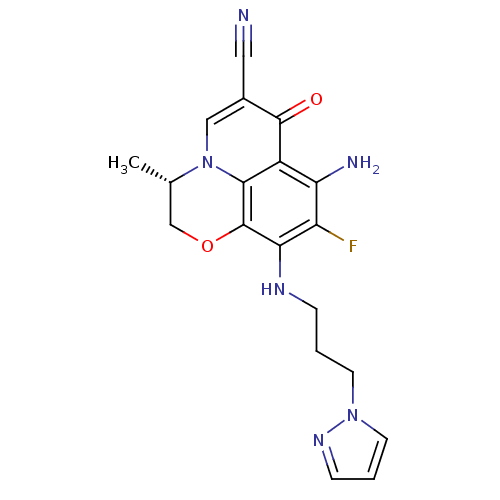
(CHEMBL1940981)Show SMILES C[C@H]1COc2c(NCCCn3cccn3)c(F)c(N)c3c2n1cc(C#N)c3=O |r| Show InChI InChI=1S/C19H19FN6O2/c1-11-10-28-19-16(23-4-2-6-25-7-3-5-24-25)14(20)15(22)13-17(19)26(11)9-12(8-21)18(13)27/h3,5,7,9,11,23H,2,4,6,10,22H2,1H3/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a |
ActivX Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GSK3-beta |
Bioorg Med Chem Lett 22: 1005-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.006
BindingDB Entry DOI: 10.7270/Q2930TMF |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50362518
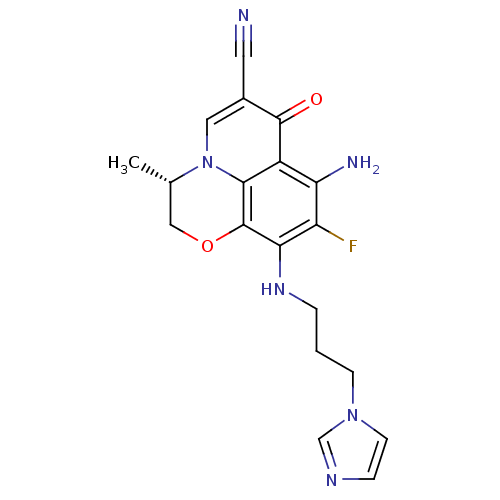
(CHEMBL1940986)Show SMILES C[C@H]1COc2c(NCCCn3ccnc3)c(F)c(N)c3c2n1cc(C#N)c3=O |r| Show InChI InChI=1S/C19H19FN6O2/c1-11-9-28-19-16(24-3-2-5-25-6-4-23-10-25)14(20)15(22)13-17(19)26(11)8-12(7-21)18(13)27/h4,6,8,10-11,24H,2-3,5,9,22H2,1H3/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
ActivX Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GSK3-beta |
Bioorg Med Chem Lett 22: 1005-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.006
BindingDB Entry DOI: 10.7270/Q2930TMF |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50362511

(CHEMBL1940979)Show SMILES C[C@H]1COc2c(NCCCc3ccccc3)c(F)c(N)c3c2n1cc(C#N)c3=O |r| Show InChI InChI=1S/C22H21FN4O2/c1-13-12-29-22-19(26-9-5-8-14-6-3-2-4-7-14)17(23)18(25)16-20(22)27(13)11-15(10-24)21(16)28/h2-4,6-7,11,13,26H,5,8-9,12,25H2,1H3/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
ActivX Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GSK3-beta |
Bioorg Med Chem Lett 22: 1005-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.006
BindingDB Entry DOI: 10.7270/Q2930TMF |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50362521

(CHEMBL1940989)Show SMILES C[C@H]1COc2c(NCCCc3ccccn3)c(F)c(N)c3c2n1cc(-c1nnn[nH]1)c3=O |r| Show InChI InChI=1S/C21H21FN8O2/c1-11-10-32-20-17(25-8-4-6-12-5-2-3-7-24-12)15(22)16(23)14-18(20)30(11)9-13(19(14)31)21-26-28-29-27-21/h2-3,5,7,9,11,25H,4,6,8,10,23H2,1H3,(H,26,27,28,29)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
ActivX Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GSK3-beta |
Bioorg Med Chem Lett 22: 1005-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.006
BindingDB Entry DOI: 10.7270/Q2930TMF |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50200120

(CHEMBL260091 | CHIR-090 | US10875832, Compound ChI...)Show SMILES C[C@@H](O)[C@H](NC(=O)c1ccc(cc1)C#Cc1ccc(CN2CCOCC2)cc1)C(=O)NO |r| Show InChI InChI=1S/C24H27N3O5/c1-17(28)22(24(30)26-31)25-23(29)21-10-8-19(9-11-21)3-2-18-4-6-20(7-5-18)16-27-12-14-32-15-13-27/h4-11,17,22,28,31H,12-16H2,1H3,(H,25,29)(H,26,30)/t17-,22+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LpxC using UDP-3-O-(R-3-hydroxymyristoyl)GlcNAc as substrate measured after 60 mins by fluorescence analysis |
ACS Med Chem Lett 7: 623-8 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00057
BindingDB Entry DOI: 10.7270/Q2DV1NWV |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50501133

(CHEMBL3827314)Show SMILES ONC(=O)[C@@H]1CN(C(=O)O1)c1ccc(C#CC#CC2CC2)c(F)c1 |r| Show InChI InChI=1S/C17H13FN2O4/c18-14-9-13(20-10-15(16(21)19-23)24-17(20)22)8-7-12(14)4-2-1-3-11-5-6-11/h7-9,11,15,23H,5-6,10H2,(H,19,21)/t15-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LpxC using UDP-3-O-(R-3-hydroxymyristoyl)GlcNAc as substrate measured after 60 mins by fluorescence analysis |
ACS Med Chem Lett 7: 623-8 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00057
BindingDB Entry DOI: 10.7270/Q2DV1NWV |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50362519

(CHEMBL1940987)Show SMILES C[C@H]1COc2c(NCCCc3ccccn3)c(F)c(N)c3c2n1cc(C(O)=O)c3=O |r| Show InChI InChI=1S/C21H21FN4O4/c1-11-10-30-20-17(25-8-4-6-12-5-2-3-7-24-12)15(22)16(23)14-18(20)26(11)9-13(19(14)27)21(28)29/h2-3,5,7,9,11,25H,4,6,8,10,23H2,1H3,(H,28,29)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
ActivX Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GSK3-beta |
Bioorg Med Chem Lett 22: 1005-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.006
BindingDB Entry DOI: 10.7270/Q2930TMF |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50354779
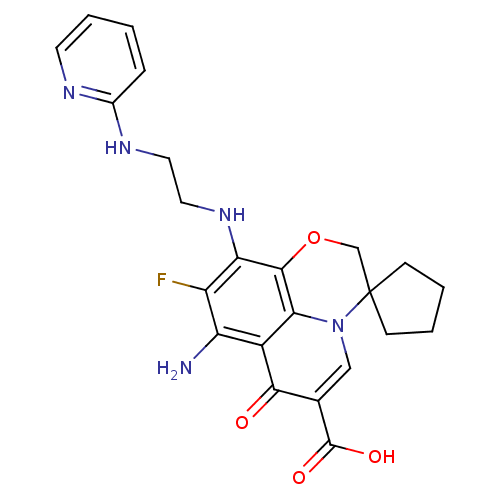
(CHEMBL1834330)Show SMILES Nc1c(F)c(NCCNc2ccccn2)c2OCC3(CCCC3)n3cc(C(O)=O)c(=O)c1c23 Show InChI InChI=1S/C23H24FN5O4/c24-16-17(25)15-19-21(18(16)28-10-9-27-14-5-1-4-8-26-14)33-12-23(6-2-3-7-23)29(19)11-13(20(15)30)22(31)32/h1,4-5,8,11,28H,2-3,6-7,9-10,12,25H2,(H,26,27)(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
ActivX Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length GSK3beta after 1 hrs by luminescence assay |
Bioorg Med Chem Lett 21: 5948-51 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.073
BindingDB Entry DOI: 10.7270/Q23T9HMH |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50354765
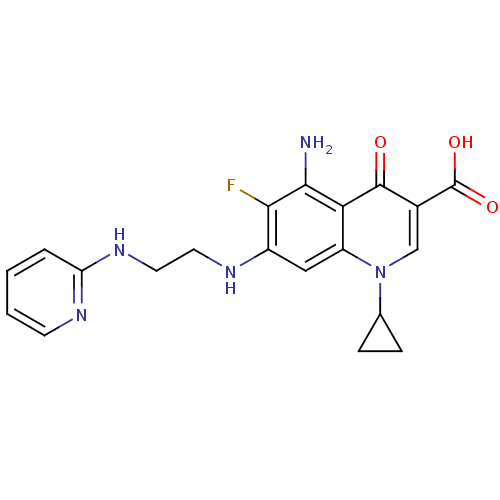
(CHEMBL1834315)Show SMILES Nc1c(F)c(NCCNc2ccccn2)cc2n(cc(C(O)=O)c(=O)c12)C1CC1 Show InChI InChI=1S/C20H20FN5O3/c21-17-13(23-7-8-25-15-3-1-2-6-24-15)9-14-16(18(17)22)19(27)12(20(28)29)10-26(14)11-4-5-11/h1-3,6,9-11,23H,4-5,7-8,22H2,(H,24,25)(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
ActivX Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length GSK3beta after 1 hrs by luminescence assay |
Bioorg Med Chem Lett 21: 5948-51 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.073
BindingDB Entry DOI: 10.7270/Q23T9HMH |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50362512

(CHEMBL1940980)Show SMILES C[C@H]1COc2c(NCCCn3cccn3)c(F)c(N)c3c2n1cc(C(O)=O)c3=O |r| Show InChI InChI=1S/C19H20FN5O4/c1-10-9-29-18-15(22-4-2-6-24-7-3-5-23-24)13(20)14(21)12-16(18)25(10)8-11(17(12)26)19(27)28/h3,5,7-8,10,22H,2,4,6,9,21H2,1H3,(H,27,28)/t10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
ActivX Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GSK3-beta |
Bioorg Med Chem Lett 22: 1005-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.006
BindingDB Entry DOI: 10.7270/Q2930TMF |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50501144

(CHEMBL3827965)Show SMILES ONC(=O)[C@@H]1CN(C(=O)O1)c1ccc(cc1)C#CC#CC1CC1 |r| Show InChI InChI=1S/C17H14N2O4/c20-16(18-22)15-11-19(17(21)23-15)14-9-7-13(8-10-14)4-2-1-3-12-5-6-12/h7-10,12,15,22H,5-6,11H2,(H,18,20)/t15-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LpxC using UDP-3-O-(R-3-hydroxymyristoyl)GlcNAc as substrate measured after 60 mins by fluorescence analysis |
ACS Med Chem Lett 7: 623-8 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00057
BindingDB Entry DOI: 10.7270/Q2DV1NWV |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50362517

(CHEMBL1940985)Show SMILES C[C@H]1COc2c(NCCCn3ccnc3)c(F)c(N)c3c2n1cc(-c1nnn[nH]1)c3=O |r| Show InChI InChI=1S/C19H20FN9O2/c1-10-8-31-18-15(23-3-2-5-28-6-4-22-9-28)13(20)14(21)12-16(18)29(10)7-11(17(12)30)19-24-26-27-25-19/h4,6-7,9-10,23H,2-3,5,8,21H2,1H3,(H,24,25,26,27)/t10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
ActivX Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GSK3-beta |
Bioorg Med Chem Lett 22: 1005-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.006
BindingDB Entry DOI: 10.7270/Q2930TMF |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50501136
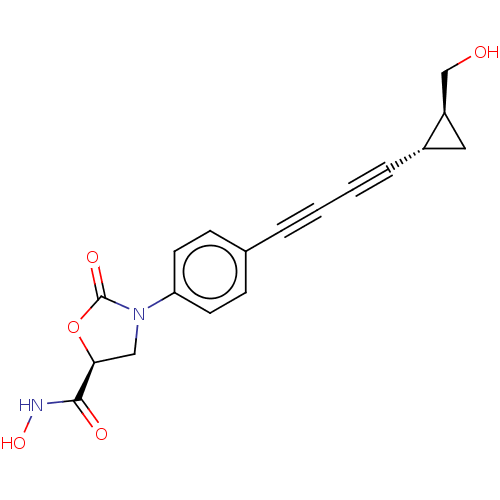
(CHEMBL3827345)Show SMILES OC[C@H]1C[C@@H]1C#CC#Cc1ccc(cc1)N1C[C@H](OC1=O)C(=O)NO |r| Show InChI InChI=1S/C18H16N2O5/c21-11-14-9-13(14)4-2-1-3-12-5-7-15(8-6-12)20-10-16(17(22)19-24)25-18(20)23/h5-8,13-14,16,21,24H,9-11H2,(H,19,22)/t13-,14+,16-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LpxC using UDP-3-O-(R-3-hydroxymyristoyl)GlcNAc as substrate measured after 60 mins by fluorescence analysis |
ACS Med Chem Lett 7: 623-8 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00057
BindingDB Entry DOI: 10.7270/Q2DV1NWV |
More data for this
Ligand-Target Pair | |
Deoxyuridine 5'-triphosphate nucleotidohydrolase, mitochondrial
(Homo sapiens (Human)) | BDBM101748
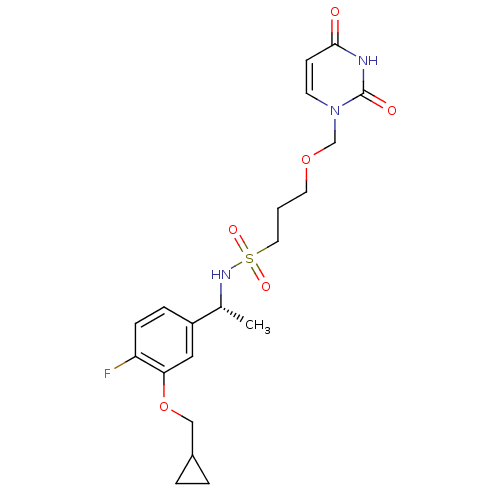
(CHEMBL2057911 | US8530490, 8)Show SMILES C[C@@H](NS(=O)(=O)CCCOCn1ccc(=O)[nH]c1=O)c1ccc(F)c(OCC2CC2)c1 |r| Show InChI InChI=1S/C20H26FN3O6S/c1-14(16-5-6-17(21)18(11-16)30-12-15-3-4-15)23-31(27,28)10-2-9-29-13-24-8-7-19(25)22-20(24)26/h5-8,11,14-15,23H,2-4,9-10,12-13H2,1H3,(H,22,25,26)/t14-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human dUTPase assessed as production of [5-3H]dUMP from [5-3H]dUTP after 15 mins measured by HPLC analysis |
J Med Chem 55: 2970-80 (2012)
Article DOI: 10.1021/jm201628y
BindingDB Entry DOI: 10.7270/Q2C82BB5 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50354767
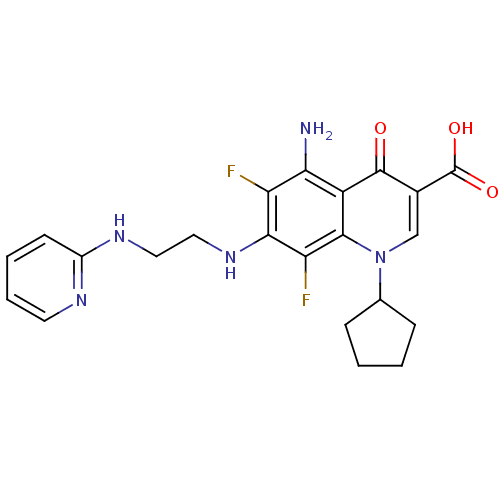
(CHEMBL1834318)Show SMILES Nc1c(F)c(NCCNc2ccccn2)c(F)c2n(cc(C(O)=O)c(=O)c12)C1CCCC1 Show InChI InChI=1S/C22H23F2N5O3/c23-16-18(25)15-20(17(24)19(16)28-10-9-27-14-7-3-4-8-26-14)29(12-5-1-2-6-12)11-13(21(15)30)22(31)32/h3-4,7-8,11-12,28H,1-2,5-6,9-10,25H2,(H,26,27)(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
ActivX Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length GSK3beta after 1 hrs by luminescence assay |
Bioorg Med Chem Lett 21: 5948-51 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.073
BindingDB Entry DOI: 10.7270/Q23T9HMH |
More data for this
Ligand-Target Pair | |
Deoxyuridine 5'-triphosphate nucleotidohydrolase, mitochondrial
(Homo sapiens (Human)) | BDBM50391352
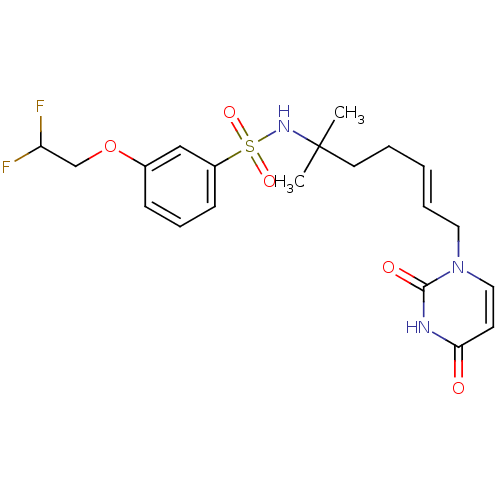
(CHEMBL2147981)Show SMILES CC(C)(CC\C=C\Cn1ccc(=O)[nH]c1=O)NS(=O)(=O)c1cccc(OCC(F)F)c1 Show InChI InChI=1S/C20H25F2N3O5S/c1-20(2,10-4-3-5-11-25-12-9-18(26)23-19(25)27)24-31(28,29)16-8-6-7-15(13-16)30-14-17(21)22/h3,5-9,12-13,17,24H,4,10-11,14H2,1-2H3,(H,23,26,27)/b5-3+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human dUTPase-mediated formation of [5-3H]dUMP expressed in Escherichia coli BL21 (DE3) after 15 mins by HPLC analysis |
J Med Chem 55: 5483-96 (2012)
Article DOI: 10.1021/jm300416h
BindingDB Entry DOI: 10.7270/Q2C53MZW |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50501139
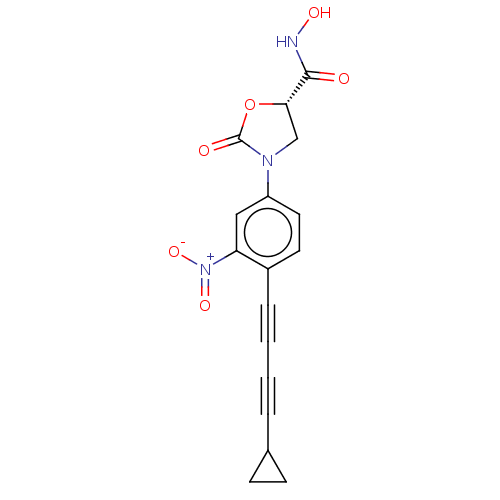
(CHEMBL3827962)Show SMILES ONC(=O)[C@@H]1CN(C(=O)O1)c1ccc(C#CC#CC2CC2)c(c1)[N+]([O-])=O |r| Show InChI InChI=1S/C17H13N3O6/c21-16(18-23)15-10-19(17(22)26-15)13-8-7-12(14(9-13)20(24)25)4-2-1-3-11-5-6-11/h7-9,11,15,23H,5-6,10H2,(H,18,21)/t15-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LpxC using UDP-3-O-(R-3-hydroxymyristoyl)GlcNAc as substrate measured after 60 mins by fluorescence analysis |
ACS Med Chem Lett 7: 623-8 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00057
BindingDB Entry DOI: 10.7270/Q2DV1NWV |
More data for this
Ligand-Target Pair | |
Deoxyuridine 5'-triphosphate nucleotidohydrolase, mitochondrial
(Homo sapiens (Human)) | BDBM50391350
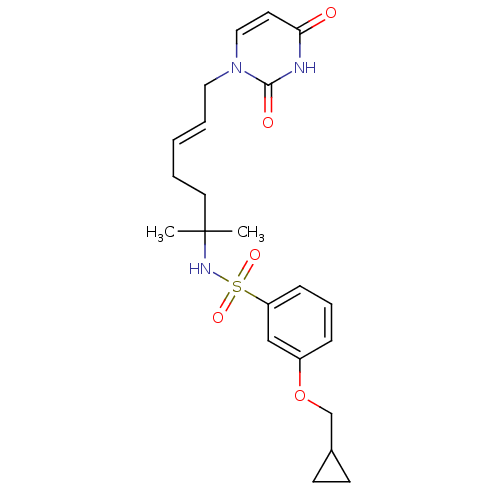
(CHEMBL2147979)Show SMILES CC(C)(CC\C=C\Cn1ccc(=O)[nH]c1=O)NS(=O)(=O)c1cccc(OCC2CC2)c1 Show InChI InChI=1S/C22H29N3O5S/c1-22(2,12-4-3-5-13-25-14-11-20(26)23-21(25)27)24-31(28,29)19-8-6-7-18(15-19)30-16-17-9-10-17/h3,5-8,11,14-15,17,24H,4,9-10,12-13,16H2,1-2H3,(H,23,26,27)/b5-3+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human dUTPase-mediated formation of [5-3H]dUMP expressed in Escherichia coli BL21 (DE3) after 15 mins by HPLC analysis |
J Med Chem 55: 5483-96 (2012)
Article DOI: 10.1021/jm300416h
BindingDB Entry DOI: 10.7270/Q2C53MZW |
More data for this
Ligand-Target Pair | |
Deoxyuridine 5'-triphosphate nucleotidohydrolase, mitochondrial
(Homo sapiens (Human)) | BDBM50395031

(CHEMBL2163854)Show SMILES CC[C@@H](Cn1nncc1CCCCn1ccc(=O)[nH]c1=O)c1cccc(OCC2CC2)c1 |r| Show InChI InChI=1S/C24H31N5O3/c1-2-19(20-6-5-8-22(14-20)32-17-18-9-10-18)16-29-21(15-25-27-29)7-3-4-12-28-13-11-23(30)26-24(28)31/h5-6,8,11,13-15,18-19H,2-4,7,9-10,12,16-17H2,1H3,(H,26,30,31)/t19-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human dUTPase assessed as reduction in [5-3H]dUMP production incubated for 15 mins by HPLC |
J Med Chem 55: 6427-37 (2012)
Article DOI: 10.1021/jm3004174
BindingDB Entry DOI: 10.7270/Q2XS5WJR |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50354764
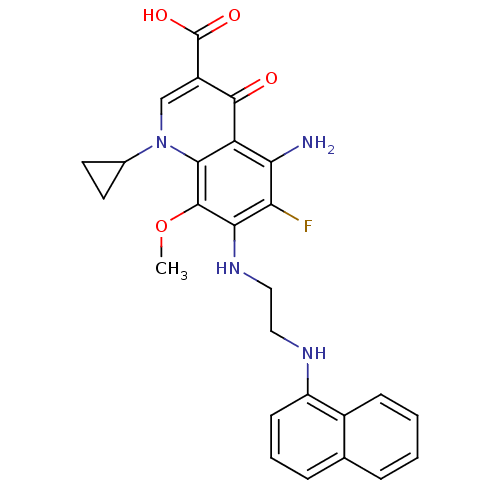
(CHEMBL1834125)Show SMILES COc1c(NCCNc2cccc3ccccc23)c(F)c(N)c2c1n(cc(C(O)=O)c2=O)C1CC1 Show InChI InChI=1S/C26H25FN4O4/c1-35-25-22(30-12-11-29-18-8-4-6-14-5-2-3-7-16(14)18)20(27)21(28)19-23(25)31(15-9-10-15)13-17(24(19)32)26(33)34/h2-8,13,15,29-30H,9-12,28H2,1H3,(H,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
ActivX Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length GSK3beta after 1 hrs by luminescence assay |
Bioorg Med Chem Lett 21: 5948-51 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.073
BindingDB Entry DOI: 10.7270/Q23T9HMH |
More data for this
Ligand-Target Pair | |
Deoxyuridine 5'-triphosphate nucleotidohydrolase, mitochondrial
(Homo sapiens (Human)) | BDBM50391347
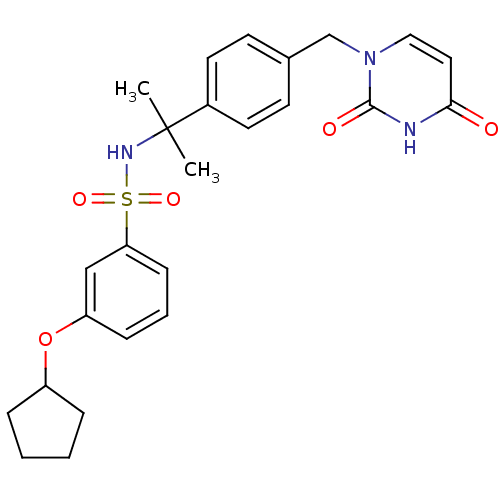
(CHEMBL2147976)Show SMILES CC(C)(NS(=O)(=O)c1cccc(OC2CCCC2)c1)c1ccc(Cn2ccc(=O)[nH]c2=O)cc1 Show InChI InChI=1S/C25H29N3O5S/c1-25(2,19-12-10-18(11-13-19)17-28-15-14-23(29)26-24(28)30)27-34(31,32)22-9-5-8-21(16-22)33-20-6-3-4-7-20/h5,8-16,20,27H,3-4,6-7,17H2,1-2H3,(H,26,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human dUTPase-mediated formation of [5-3H]dUMP expressed in Escherichia coli BL21 (DE3) after 15 mins by HPLC analysis |
J Med Chem 55: 5483-96 (2012)
Article DOI: 10.1021/jm300416h
BindingDB Entry DOI: 10.7270/Q2C53MZW |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50354773
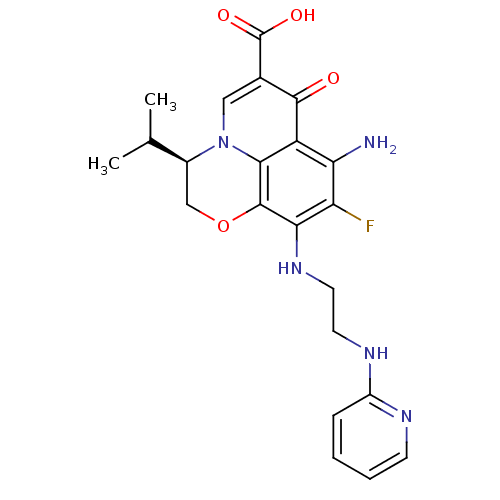
(CHEMBL1834324)Show SMILES CC(C)[C@@H]1COc2c(NCCNc3ccccn3)c(F)c(N)c3c2n1cc(C(O)=O)c3=O |r| Show InChI InChI=1S/C22H24FN5O4/c1-11(2)13-10-32-21-18(27-8-7-26-14-5-3-4-6-25-14)16(23)17(24)15-19(21)28(13)9-12(20(15)29)22(30)31/h3-6,9,11,13,27H,7-8,10,24H2,1-2H3,(H,25,26)(H,30,31)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
ActivX Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length GSK3beta after 1 hrs by luminescence assay |
Bioorg Med Chem Lett 21: 5948-51 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.073
BindingDB Entry DOI: 10.7270/Q23T9HMH |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50362507

(CHEMBL1940900)Show SMILES C[C@H]1COc2c(NCCNc3ccccn3)c(F)c(N)c3c2n1cc(C(N)=O)c3=O |r| Show InChI InChI=1S/C20H21FN6O3/c1-10-9-30-19-16(26-7-6-25-12-4-2-3-5-24-12)14(21)15(22)13-17(19)27(10)8-11(18(13)28)20(23)29/h2-5,8,10,26H,6-7,9,22H2,1H3,(H2,23,29)(H,24,25)/t10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
ActivX Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GSK3-beta |
Bioorg Med Chem Lett 22: 1005-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.006
BindingDB Entry DOI: 10.7270/Q2930TMF |
More data for this
Ligand-Target Pair | |
Deoxyuridine 5'-triphosphate nucleotidohydrolase, mitochondrial
(Homo sapiens (Human)) | BDBM50391351

(CHEMBL2147980)Show SMILES CC(C)(CC\C=C\Cn1ccc(=O)[nH]c1=O)NS(=O)(=O)c1cccc(OC2CCCC2)c1 Show InChI InChI=1S/C23H31N3O5S/c1-23(2,14-6-3-7-15-26-16-13-21(27)24-22(26)28)25-32(29,30)20-12-8-11-19(17-20)31-18-9-4-5-10-18/h3,7-8,11-13,16-18,25H,4-6,9-10,14-15H2,1-2H3,(H,24,27,28)/b7-3+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human dUTPase-mediated formation of [5-3H]dUMP expressed in Escherichia coli BL21 (DE3) after 15 mins by HPLC analysis |
J Med Chem 55: 5483-96 (2012)
Article DOI: 10.1021/jm300416h
BindingDB Entry DOI: 10.7270/Q2C53MZW |
More data for this
Ligand-Target Pair | |
Deoxyuridine 5'-triphosphate nucleotidohydrolase, mitochondrial
(Homo sapiens (Human)) | BDBM101889

(CHEMBL2057599 | US8530490, 159)Show SMILES CC(C)(CCCOCn1ccc(=O)[nH]c1=O)NS(=O)(=O)c1cccc(OCC2CC2)c1 Show InChI InChI=1S/C21H29N3O6S/c1-21(2,10-4-12-29-15-24-11-9-19(25)22-20(24)26)23-31(27,28)18-6-3-5-17(13-18)30-14-16-7-8-16/h3,5-6,9,11,13,16,23H,4,7-8,10,12,14-15H2,1-2H3,(H,22,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human dUTPase assessed as production of [5-3H]dUMP from [5-3H]dUTP after 15 mins measured by HPLC analysis |
J Med Chem 55: 2970-80 (2012)
Article DOI: 10.1021/jm201628y
BindingDB Entry DOI: 10.7270/Q2C82BB5 |
More data for this
Ligand-Target Pair | |
Deoxyuridine 5'-triphosphate nucleotidohydrolase, mitochondrial
(Homo sapiens (Human)) | BDBM101889

(CHEMBL2057599 | US8530490, 159)Show SMILES CC(C)(CCCOCn1ccc(=O)[nH]c1=O)NS(=O)(=O)c1cccc(OCC2CC2)c1 Show InChI InChI=1S/C21H29N3O6S/c1-21(2,10-4-12-29-15-24-11-9-19(25)22-20(24)26)23-31(27,28)18-6-3-5-17(13-18)30-14-16-7-8-16/h3,5-6,9,11,13,16,23H,4,7-8,10,12,14-15H2,1-2H3,(H,22,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human dUTPase-mediated formation of [5-3H]dUMP expressed in Escherichia coli BL21 (DE3) after 15 mins by HPLC analysis |
J Med Chem 55: 5483-96 (2012)
Article DOI: 10.1021/jm300416h
BindingDB Entry DOI: 10.7270/Q2C53MZW |
More data for this
Ligand-Target Pair | |
Deoxyuridine 5'-triphosphate nucleotidohydrolase, mitochondrial
(Homo sapiens (Human)) | BDBM101847

(CHEMBL2147985 | US8530490, 110)Show SMILES C[C@@H](NS(=O)(=O)CC\C=C\Cn1ccc(=O)[nH]c1=O)c1cccc(OCC2CC2)c1 |r| Show InChI InChI=1S/C21H27N3O5S/c1-16(18-6-5-7-19(14-18)29-15-17-8-9-17)23-30(27,28)13-4-2-3-11-24-12-10-20(25)22-21(24)26/h2-3,5-7,10,12,14,16-17,23H,4,8-9,11,13,15H2,1H3,(H,22,25,26)/b3-2+/t16-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human dUTPase-mediated formation of [5-3H]dUMP expressed in Escherichia coli BL21 (DE3) after 15 mins by HPLC analysis |
J Med Chem 55: 5483-96 (2012)
Article DOI: 10.1021/jm300416h
BindingDB Entry DOI: 10.7270/Q2C53MZW |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50362509

(CHEMBL1940977)Show SMILES C[C@H]1COc2c(NCCCc3ccccc3)c(F)c(N)c3c2n1cc(C(O)=O)c3=O |r| Show InChI InChI=1S/C22H22FN3O4/c1-12-11-30-21-18(25-9-5-8-13-6-3-2-4-7-13)16(23)17(24)15-19(21)26(12)10-14(20(15)27)22(28)29/h2-4,6-7,10,12,25H,5,8-9,11,24H2,1H3,(H,28,29)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
ActivX Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GSK3-beta |
Bioorg Med Chem Lett 22: 1005-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.006
BindingDB Entry DOI: 10.7270/Q2930TMF |
More data for this
Ligand-Target Pair | |
Deoxyuridine 5'-triphosphate nucleotidohydrolase, mitochondrial
(Homo sapiens (Human)) | BDBM101763
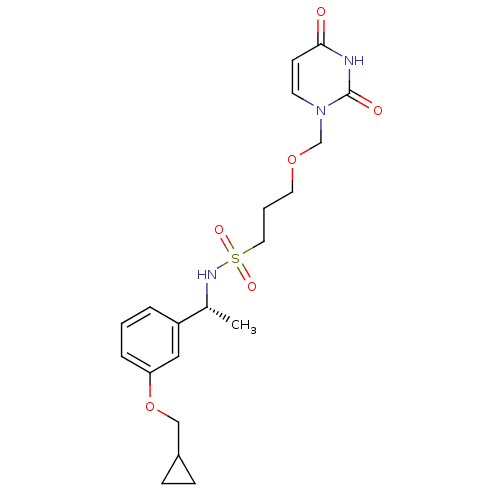
(CHEMBL2057909 | US8530490, 23)Show SMILES C[C@@H](NS(=O)(=O)CCCOCn1ccc(=O)[nH]c1=O)c1cccc(OCC2CC2)c1 |r| Show InChI InChI=1S/C20H27N3O6S/c1-15(17-4-2-5-18(12-17)29-13-16-6-7-16)22-30(26,27)11-3-10-28-14-23-9-8-19(24)21-20(23)25/h2,4-5,8-9,12,15-16,22H,3,6-7,10-11,13-14H2,1H3,(H,21,24,25)/t15-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human dUTPase-mediated formation of [5-3H]dUMP expressed in Escherichia coli BL21 (DE3) after 15 mins by HPLC analysis |
J Med Chem 55: 5483-96 (2012)
Article DOI: 10.1021/jm300416h
BindingDB Entry DOI: 10.7270/Q2C53MZW |
More data for this
Ligand-Target Pair | |
Deoxyuridine 5'-triphosphate nucleotidohydrolase, mitochondrial
(Homo sapiens (Human)) | BDBM101763

(CHEMBL2057909 | US8530490, 23)Show SMILES C[C@@H](NS(=O)(=O)CCCOCn1ccc(=O)[nH]c1=O)c1cccc(OCC2CC2)c1 |r| Show InChI InChI=1S/C20H27N3O6S/c1-15(17-4-2-5-18(12-17)29-13-16-6-7-16)22-30(26,27)11-3-10-28-14-23-9-8-19(24)21-20(23)25/h2,4-5,8-9,12,15-16,22H,3,6-7,10-11,13-14H2,1H3,(H,21,24,25)/t15-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human dUTPase assessed as production of [5-3H]dUMP from [5-3H]dUTP after 15 mins measured by HPLC analysis |
J Med Chem 55: 2970-80 (2012)
Article DOI: 10.1021/jm201628y
BindingDB Entry DOI: 10.7270/Q2C82BB5 |
More data for this
Ligand-Target Pair | |
Deoxyuridine 5'-triphosphate nucleotidohydrolase, mitochondrial
(Homo sapiens (Human)) | BDBM101842
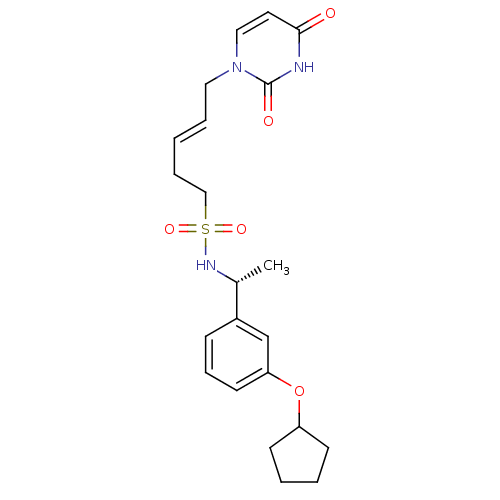
(CHEMBL2147986 | US8530490, 105)Show SMILES C[C@@H](NS(=O)(=O)CC\C=C\Cn1ccc(=O)[nH]c1=O)c1cccc(OC2CCCC2)c1 |r| Show InChI InChI=1S/C22H29N3O5S/c1-17(18-8-7-11-20(16-18)30-19-9-3-4-10-19)24-31(28,29)15-6-2-5-13-25-14-12-21(26)23-22(25)27/h2,5,7-8,11-12,14,16-17,19,24H,3-4,6,9-10,13,15H2,1H3,(H,23,26,27)/b5-2+/t17-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human dUTPase-mediated formation of [5-3H]dUMP expressed in Escherichia coli BL21 (DE3) after 15 mins by HPLC analysis |
J Med Chem 55: 5483-96 (2012)
Article DOI: 10.1021/jm300416h
BindingDB Entry DOI: 10.7270/Q2C53MZW |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50354772
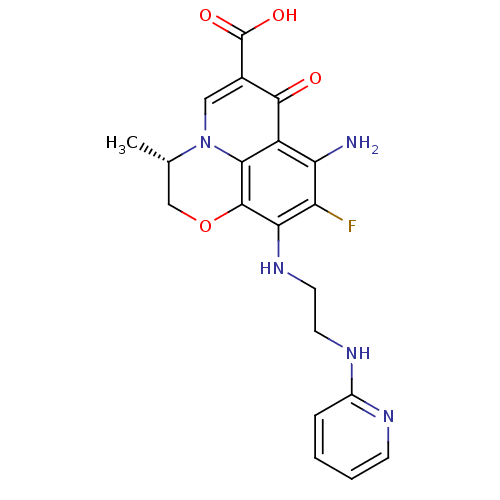
(CHEMBL1834323)Show SMILES C[C@H]1COc2c(NCCNc3ccccn3)c(F)c(N)c3c2n1cc(C(O)=O)c3=O |r| Show InChI InChI=1S/C20H20FN5O4/c1-10-9-30-19-16(25-7-6-24-12-4-2-3-5-23-12)14(21)15(22)13-17(19)26(10)8-11(18(13)27)20(28)29/h2-5,8,10,25H,6-7,9,22H2,1H3,(H,23,24)(H,28,29)/t10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
ActivX Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length GSK3beta after 1 hrs by luminescence assay |
Bioorg Med Chem Lett 21: 5948-51 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.073
BindingDB Entry DOI: 10.7270/Q23T9HMH |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50354772

(CHEMBL1834323)Show SMILES C[C@H]1COc2c(NCCNc3ccccn3)c(F)c(N)c3c2n1cc(C(O)=O)c3=O |r| Show InChI InChI=1S/C20H20FN5O4/c1-10-9-30-19-16(25-7-6-24-12-4-2-3-5-23-12)14(21)15(22)13-17(19)26(10)8-11(18(13)27)20(28)29/h2-5,8,10,25H,6-7,9,22H2,1H3,(H,23,24)(H,28,29)/t10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
ActivX Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GSK3-beta |
Bioorg Med Chem Lett 22: 1005-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.006
BindingDB Entry DOI: 10.7270/Q2930TMF |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50354756
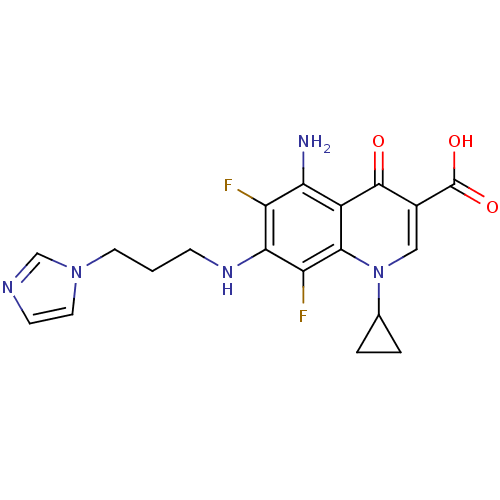
(CHEMBL1834117)Show SMILES Nc1c(F)c(NCCCn2ccnc2)c(F)c2n(cc(C(O)=O)c(=O)c12)C1CC1 Show InChI InChI=1S/C19H19F2N5O3/c20-13-15(22)12-17(26(10-2-3-10)8-11(18(12)27)19(28)29)14(21)16(13)24-4-1-6-25-7-5-23-9-25/h5,7-10,24H,1-4,6,22H2,(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
ActivX Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length GSK3beta after 1 hrs by luminescence assay |
Bioorg Med Chem Lett 21: 5948-51 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.073
BindingDB Entry DOI: 10.7270/Q23T9HMH |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50354753
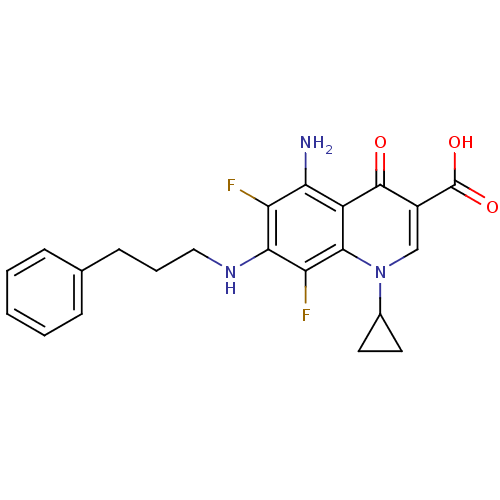
(CHEMBL1834114)Show SMILES Nc1c(F)c(NCCCc2ccccc2)c(F)c2n(cc(C(O)=O)c(=O)c12)C1CC1 Show InChI InChI=1S/C22H21F2N3O3/c23-16-18(25)15-20(27(13-8-9-13)11-14(21(15)28)22(29)30)17(24)19(16)26-10-4-7-12-5-2-1-3-6-12/h1-3,5-6,11,13,26H,4,7-10,25H2,(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
ActivX Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length GSK3beta after 1 hrs by luminescence assay |
Bioorg Med Chem Lett 21: 5948-51 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.073
BindingDB Entry DOI: 10.7270/Q23T9HMH |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50354757
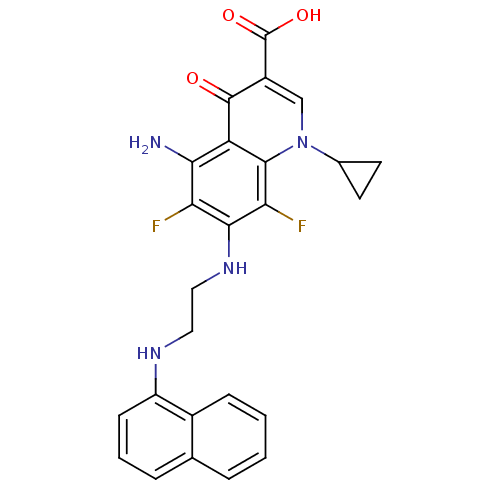
(CHEMBL1834118)Show SMILES Nc1c(F)c(NCCNc2cccc3ccccc23)c(F)c2n(cc(C(O)=O)c(=O)c12)C1CC1 Show InChI InChI=1S/C25H22F2N4O3/c26-19-21(28)18-23(31(14-8-9-14)12-16(24(18)32)25(33)34)20(27)22(19)30-11-10-29-17-7-3-5-13-4-1-2-6-15(13)17/h1-7,12,14,29-30H,8-11,28H2,(H,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
ActivX Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length GSK3beta after 1 hrs by luminescence assay |
Bioorg Med Chem Lett 21: 5948-51 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.073
BindingDB Entry DOI: 10.7270/Q23T9HMH |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50354775
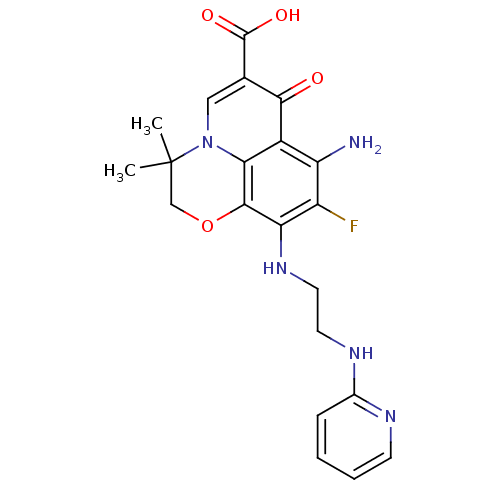
(CHEMBL1834326)Show SMILES CC1(C)COc2c(NCCNc3ccccn3)c(F)c(N)c3c2n1cc(C(O)=O)c3=O Show InChI InChI=1S/C21H22FN5O4/c1-21(2)10-31-19-16(26-8-7-25-12-5-3-4-6-24-12)14(22)15(23)13-17(19)27(21)9-11(18(13)28)20(29)30/h3-6,9,26H,7-8,10,23H2,1-2H3,(H,24,25)(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
ActivX Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length GSK3beta after 1 hrs by luminescence assay |
Bioorg Med Chem Lett 21: 5948-51 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.073
BindingDB Entry DOI: 10.7270/Q23T9HMH |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50354778
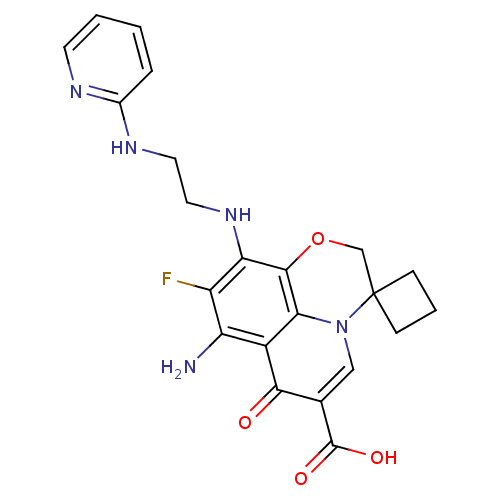
(CHEMBL1834329)Show SMILES Nc1c(F)c(NCCNc2ccccn2)c2OCC3(CCC3)n3cc(C(O)=O)c(=O)c1c23 Show InChI InChI=1S/C22H22FN5O4/c23-15-16(24)14-18-20(17(15)27-9-8-26-13-4-1-2-7-25-13)32-11-22(5-3-6-22)28(18)10-12(19(14)29)21(30)31/h1-2,4,7,10,27H,3,5-6,8-9,11,24H2,(H,25,26)(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
ActivX Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length GSK3beta after 1 hrs by luminescence assay |
Bioorg Med Chem Lett 21: 5948-51 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.073
BindingDB Entry DOI: 10.7270/Q23T9HMH |
More data for this
Ligand-Target Pair | |
Deoxyuridine 5'-triphosphate nucleotidohydrolase, mitochondrial
(Homo sapiens (Human)) | BDBM50395047

(CHEMBL2163852)Show SMILES C[C@@H](Cn1nncc1CCCCn1ccc(=O)[nH]c1=O)c1cccc(OCC2CC2)c1 |r| Show InChI InChI=1S/C23H29N5O3/c1-17(19-5-4-7-21(13-19)31-16-18-8-9-18)15-28-20(14-24-26-28)6-2-3-11-27-12-10-22(29)25-23(27)30/h4-5,7,10,12-14,17-18H,2-3,6,8-9,11,15-16H2,1H3,(H,25,29,30)/t17-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human dUTPase assessed as reduction in [5-3H]dUMP production incubated for 15 mins by HPLC |
J Med Chem 55: 6427-37 (2012)
Article DOI: 10.1021/jm3004174
BindingDB Entry DOI: 10.7270/Q2XS5WJR |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50501135
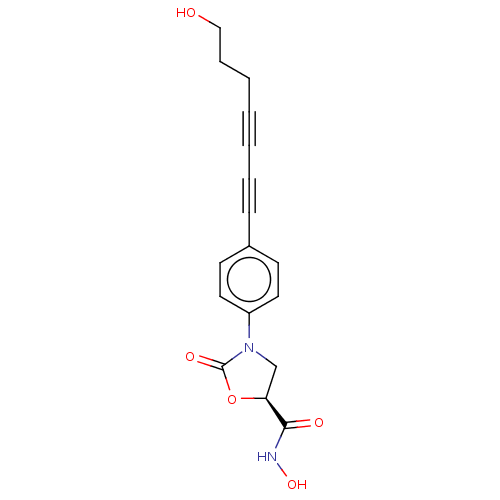
(CHEMBL3827305)Show SMILES OCCCC#CC#Cc1ccc(cc1)N1C[C@H](OC1=O)C(=O)NO |r| Show InChI InChI=1S/C17H16N2O5/c20-11-5-3-1-2-4-6-13-7-9-14(10-8-13)19-12-15(16(21)18-23)24-17(19)22/h7-10,15,20,23H,3,5,11-12H2,(H,18,21)/t15-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LpxC using UDP-3-O-(R-3-hydroxymyristoyl)GlcNAc as substrate measured after 60 mins by fluorescence analysis |
ACS Med Chem Lett 7: 623-8 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00057
BindingDB Entry DOI: 10.7270/Q2DV1NWV |
More data for this
Ligand-Target Pair | |
Deoxyuridine 5'-triphosphate nucleotidohydrolase, mitochondrial
(Homo sapiens (Human)) | BDBM50388166

(CHEMBL2057910)Show SMILES C[C@H](NS(=O)(=O)CCCOCn1ccc(=O)[nH]c1=O)c1cccc(OCC2CC2)c1 |r| Show InChI InChI=1S/C20H27N3O6S/c1-15(17-4-2-5-18(12-17)29-13-16-6-7-16)22-30(26,27)11-3-10-28-14-23-9-8-19(24)21-20(23)25/h2,4-5,8-9,12,15-16,22H,3,6-7,10-11,13-14H2,1H3,(H,21,24,25)/t15-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human dUTPase assessed as production of [5-3H]dUMP from [5-3H]dUTP after 15 mins measured by HPLC analysis |
J Med Chem 55: 2970-80 (2012)
Article DOI: 10.1021/jm201628y
BindingDB Entry DOI: 10.7270/Q2C82BB5 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50354762
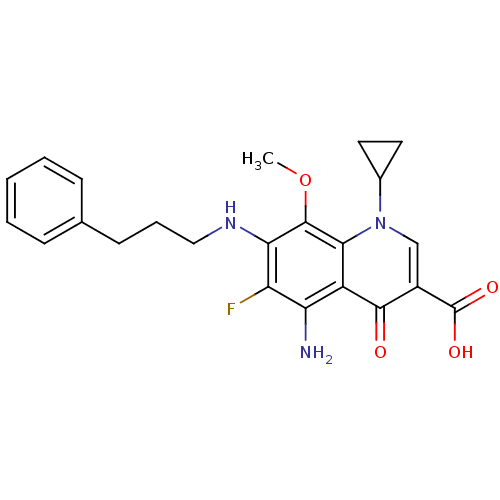
(CHEMBL1834123)Show SMILES COc1c(NCCCc2ccccc2)c(F)c(N)c2c1n(cc(C(O)=O)c2=O)C1CC1 Show InChI InChI=1S/C23H24FN3O4/c1-31-22-19(26-11-5-8-13-6-3-2-4-7-13)17(24)18(25)16-20(22)27(14-9-10-14)12-15(21(16)28)23(29)30/h2-4,6-7,12,14,26H,5,8-11,25H2,1H3,(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
ActivX Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length GSK3beta after 1 hrs by luminescence assay |
Bioorg Med Chem Lett 21: 5948-51 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.073
BindingDB Entry DOI: 10.7270/Q23T9HMH |
More data for this
Ligand-Target Pair | |
Deoxyuridine 5'-triphosphate nucleotidohydrolase, mitochondrial
(Homo sapiens (Human)) | BDBM50395030

(CHEMBL2163866)Show SMILES CC[C@@](O)(Cn1nncc1CCCCn1ccc(=O)[nH]c1=O)c1ccc(F)c(OCC2CC2)c1 |r| Show InChI InChI=1S/C24H30FN5O4/c1-2-24(33,18-8-9-20(25)21(13-18)34-15-17-6-7-17)16-30-19(14-26-28-30)5-3-4-11-29-12-10-22(31)27-23(29)32/h8-10,12-14,17,33H,2-7,11,15-16H2,1H3,(H,27,31,32)/t24-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human dUTPase assessed as reduction in [5-3H]dUMP production incubated for 15 mins by HPLC |
J Med Chem 55: 6427-37 (2012)
Article DOI: 10.1021/jm3004174
BindingDB Entry DOI: 10.7270/Q2XS5WJR |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50362510
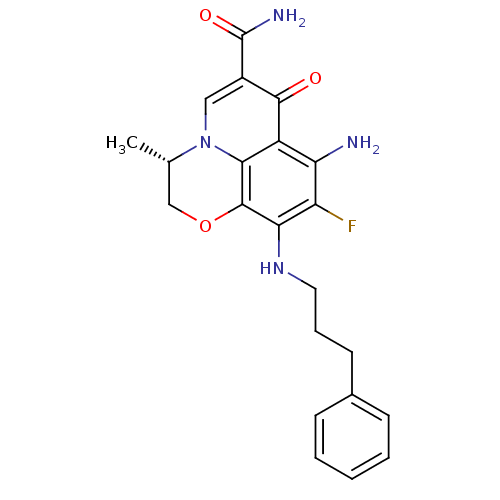
(CHEMBL1940978)Show SMILES C[C@H]1COc2c(NCCCc3ccccc3)c(F)c(N)c3c2n1cc(C(N)=O)c3=O |r| Show InChI InChI=1S/C22H23FN4O3/c1-12-11-30-21-18(26-9-5-8-13-6-3-2-4-7-13)16(23)17(24)15-19(21)27(12)10-14(20(15)28)22(25)29/h2-4,6-7,10,12,26H,5,8-9,11,24H2,1H3,(H2,25,29)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
ActivX Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GSK3-beta |
Bioorg Med Chem Lett 22: 1005-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.006
BindingDB Entry DOI: 10.7270/Q2930TMF |
More data for this
Ligand-Target Pair | |
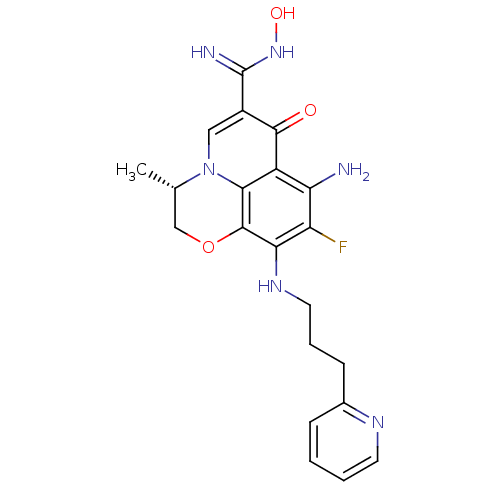
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50362520
(CHEMBL1940988)Show SMILES C[C@H]1COc2c(NCCCc3ccccn3)c(F)c(N)c3c2n1cc(C(=N)NO)c3=O |r| Show InChI InChI=1S/C21H23FN6O3/c1-11-10-31-20-17(26-8-4-6-12-5-2-3-7-25-12)15(22)16(23)14-18(20)28(11)9-13(19(14)29)21(24)27-30/h2-3,5,7,9,11,26,30H,4,6,8,10,23H2,1H3,(H2,24,27)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
ActivX Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GSK3-beta |
Bioorg Med Chem Lett 22: 1005-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.006
BindingDB Entry DOI: 10.7270/Q2930TMF |
More data for this
Ligand-Target Pair | |
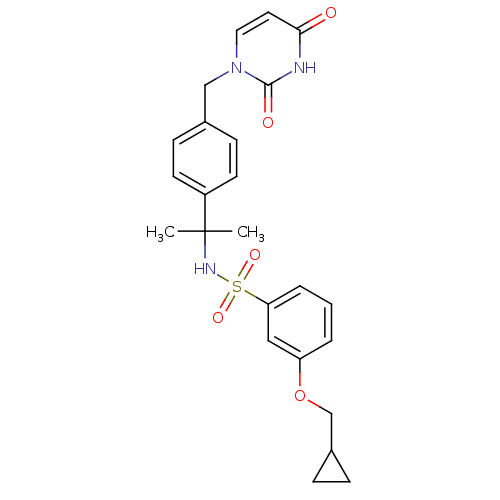
Deoxyuridine 5'-triphosphate nucleotidohydrolase, mitochondrial
(Homo sapiens (Human)) | BDBM101906
(CHEMBL2147975 | US8530490, 178)Show SMILES CC(C)(NS(=O)(=O)c1cccc(OCC2CC2)c1)c1ccc(Cn2ccc(=O)[nH]c2=O)cc1 Show InChI InChI=1S/C24H27N3O5S/c1-24(2,19-10-8-17(9-11-19)15-27-13-12-22(28)25-23(27)29)26-33(30,31)21-5-3-4-20(14-21)32-16-18-6-7-18/h3-5,8-14,18,26H,6-7,15-16H2,1-2H3,(H,25,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human dUTPase-mediated formation of [5-3H]dUMP expressed in Escherichia coli BL21 (DE3) after 15 mins by HPLC analysis |
J Med Chem 55: 5483-96 (2012)
Article DOI: 10.1021/jm300416h
BindingDB Entry DOI: 10.7270/Q2C53MZW |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data