

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type-1 angiotensin II receptor B (RAT) | BDBM50282324 (2-Cyclopropyl-3-[2'-(2H-tetrazol-5-yl)-biphenyl-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125I]Sar1,Ile8 angiotensin II binding to Angiotensin II receptor type 1 in rat adrenocortical membrane | Bioorg Med Chem Lett 4: 195-200 (1994) Article DOI: 10.1016/S0960-894X(01)81146-X BindingDB Entry DOI: 10.7270/Q2Z60P13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50049201 (2-Cyclopropyl-3-[2'-(1H-tetrazol-5-yl)-biphenyl-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125I]Sar1,Ile8 angiotensin II binding to Angiotensin II receptor type 1 in rat adrenocortical membrane | Bioorg Med Chem Lett 4: 195-200 (1994) Article DOI: 10.1016/S0960-894X(01)81146-X BindingDB Entry DOI: 10.7270/Q2Z60P13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50282318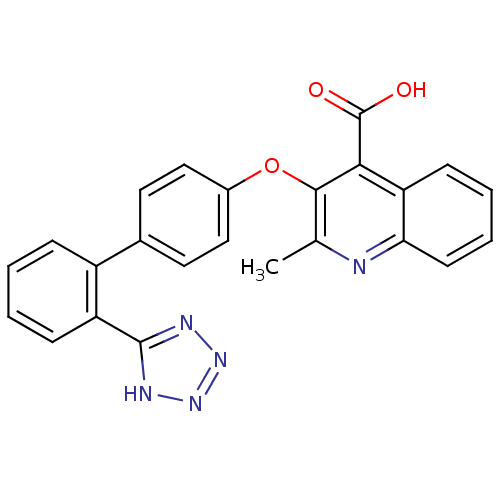 (2-Methyl-3-[2'-(2H-tetrazol-5-yl)-biphenyl-4-yloxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125I]Sar1,Ile8 angiotensin II binding to Angiotensin II receptor type 1 in rat adrenocortical membrane | Bioorg Med Chem Lett 4: 195-200 (1994) Article DOI: 10.1016/S0960-894X(01)81146-X BindingDB Entry DOI: 10.7270/Q2Z60P13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50282322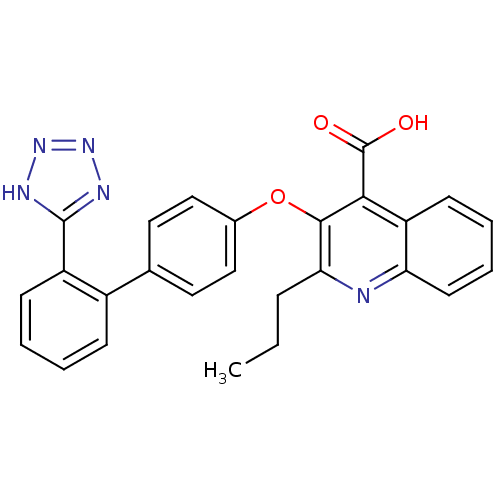 (2-Propyl-3-[2'-(2H-tetrazol-5-yl)-biphenyl-4-yloxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125I]Sar1,Ile8 angiotensin II binding to Angiotensin II receptor type 1 in rat adrenocortical membrane | Bioorg Med Chem Lett 4: 195-200 (1994) Article DOI: 10.1016/S0960-894X(01)81146-X BindingDB Entry DOI: 10.7270/Q2Z60P13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50282316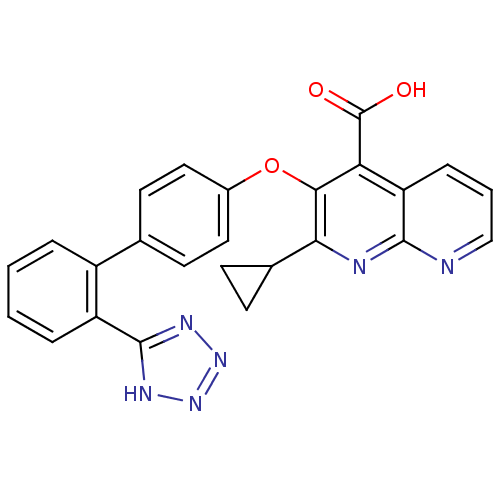 (2-Cyclopropyl-3-[2'-(2H-tetrazol-5-yl)-biphenyl-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125I]Sar1,Ile8 angiotensin II binding to Angiotensin II receptor type 1 in rat adrenocortical membrane | Bioorg Med Chem Lett 4: 195-200 (1994) Article DOI: 10.1016/S0960-894X(01)81146-X BindingDB Entry DOI: 10.7270/Q2Z60P13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50282315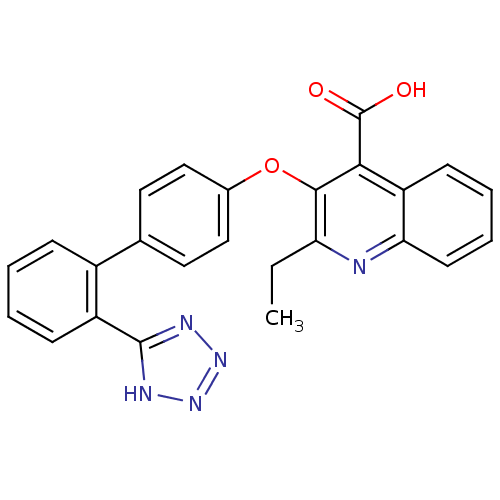 (2-Ethyl-3-[2'-(2H-tetrazol-5-yl)-biphenyl-4-yloxy]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125I]Sar1,Ile8 angiotensin II binding to Angiotensin II receptor type 1 in rat adrenocortical membrane | Bioorg Med Chem Lett 4: 195-200 (1994) Article DOI: 10.1016/S0960-894X(01)81146-X BindingDB Entry DOI: 10.7270/Q2Z60P13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50282317 (2-Cyclopropyl-5-methyl-3-[2'-(2H-tetrazol-5-yl)-bi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125I]Sar1,Ile8 angiotensin II binding to Angiotensin II receptor type 1 in rat adrenocortical membrane | Bioorg Med Chem Lett 4: 195-200 (1994) Article DOI: 10.1016/S0960-894X(01)81146-X BindingDB Entry DOI: 10.7270/Q2Z60P13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50282319 (2-Cyclopropyl-6-fluoro-3-[2'-(2H-tetrazol-5-yl)-bi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125I]Sar1,Ile8 angiotensin II binding to Angiotensin II receptor type 1 in rat adrenocortical membrane | Bioorg Med Chem Lett 4: 195-200 (1994) Article DOI: 10.1016/S0960-894X(01)81146-X BindingDB Entry DOI: 10.7270/Q2Z60P13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-binding cassette sub-family C member 9/ATP-sensitive inward rectifier potassium channel 11 (Homo sapiens (Human)) | BDBM50062397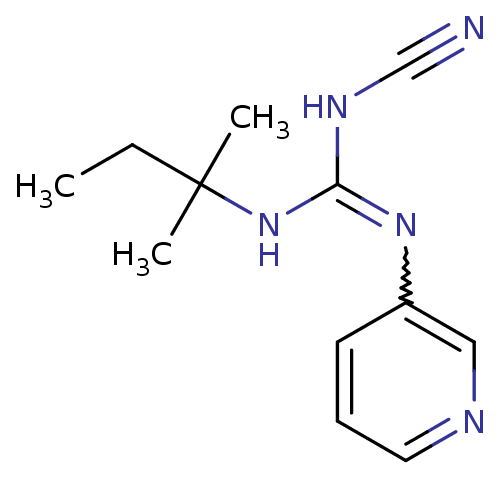 (3N-cyanoimino(tert-pentylamino)methyl-3-pyridinami...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity was determined by displacement of [3H]-P1075 from its binding sites in canine cardiac membranes | J Med Chem 41: 271-5 (1998) Article DOI: 10.1021/jm970762d BindingDB Entry DOI: 10.7270/Q2FN159P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50282321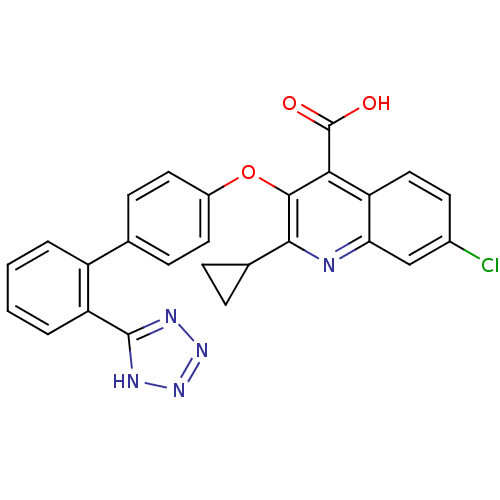 (7-Chloro-2-cyclopropyl-3-[2'-(2H-tetrazol-5-yl)-bi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125I]Sar1,Ile8 angiotensin II binding to Angiotensin II receptor type 1 in rat adrenocortical membrane | Bioorg Med Chem Lett 4: 195-200 (1994) Article DOI: 10.1016/S0960-894X(01)81146-X BindingDB Entry DOI: 10.7270/Q2Z60P13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50282313 (2-Cyclopropyl-6-methoxy-3-[2'-(2H-tetrazol-5-yl)-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125I]Sar1,Ile8 angiotensin II binding to Angiotensin II receptor type 1 in rat adrenocortical membrane | Bioorg Med Chem Lett 4: 195-200 (1994) Article DOI: 10.1016/S0960-894X(01)81146-X BindingDB Entry DOI: 10.7270/Q2Z60P13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-binding cassette sub-family C member 9/ATP-sensitive inward rectifier potassium channel 11 (Homo sapiens (Human)) | BDBM50062402 (1N-cyanoimino(tert-pentylamino)methyl-3-azido-4-io...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity was determined by displacement of [3H]-P1075 from its binding sites in canine cardiac membranes | J Med Chem 41: 271-5 (1998) Article DOI: 10.1021/jm970762d BindingDB Entry DOI: 10.7270/Q2FN159P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-binding cassette sub-family C member 9/ATP-sensitive inward rectifier potassium channel 11 (Homo sapiens (Human)) | BDBM50062394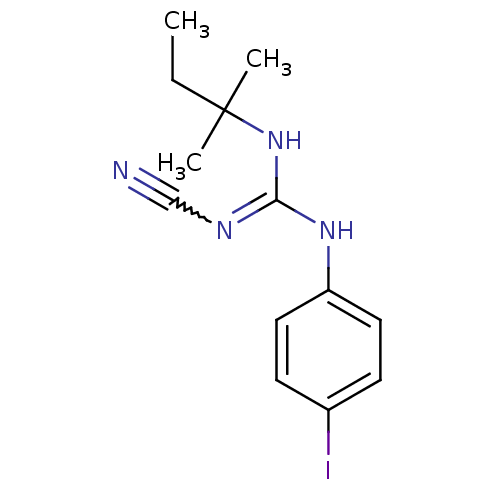 (1N-cyanoimino(tert-pentylamino)methyl-4-iodoanilin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity was determined by displacement of [3H]-P1075 from its binding sites in canine cardiac membranes | J Med Chem 41: 271-5 (1998) Article DOI: 10.1021/jm970762d BindingDB Entry DOI: 10.7270/Q2FN159P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-binding cassette sub-family C member 9/ATP-sensitive inward rectifier potassium channel 11 (Homo sapiens (Human)) | BDBM50062396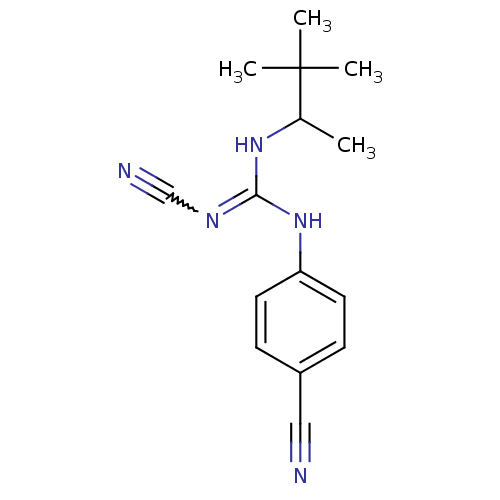 (4-cyanoimino(1,2,2-trimethylpropylamino)methylamin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 99 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity was determined by displacement of [3H]-P1075 from its binding sites in canine cardiac membranes | J Med Chem 41: 271-5 (1998) Article DOI: 10.1021/jm970762d BindingDB Entry DOI: 10.7270/Q2FN159P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50282323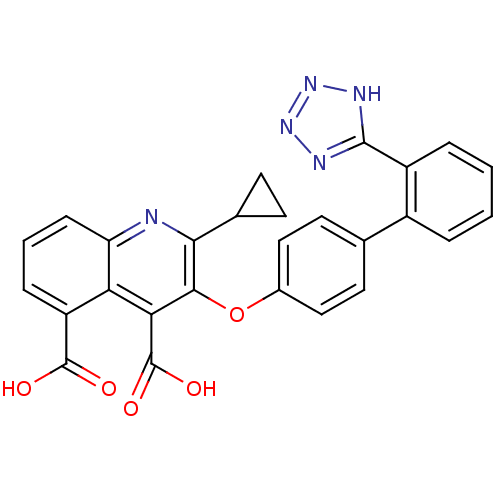 (2-Cyclopropyl-3-[2'-(2H-tetrazol-5-yl)-biphenyl-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125I]Sar1,Ile8 angiotensin II binding to Angiotensin II receptor type 1 in rat adrenocortical membrane | Bioorg Med Chem Lett 4: 195-200 (1994) Article DOI: 10.1016/S0960-894X(01)81146-X BindingDB Entry DOI: 10.7270/Q2Z60P13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-binding cassette sub-family C member 9/ATP-sensitive inward rectifier potassium channel 11 (Homo sapiens (Human)) | BDBM50012957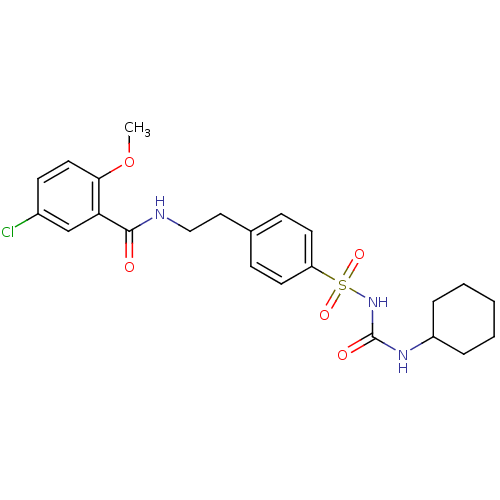 (1-((p-(2-(5-chloro-o-anisamido)ethyl)phenyl)sulfon...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity was determined by displacement of [3H]-P1075 from its binding sites in canine cardiac membranes | J Med Chem 41: 271-5 (1998) Article DOI: 10.1021/jm970762d BindingDB Entry DOI: 10.7270/Q2FN159P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| ATP-binding cassette sub-family C member 9/ATP-sensitive inward rectifier potassium channel 11 (Homo sapiens (Human)) | BDBM50062398 (4-cyanoimino(tert-pentylamino)methylaminobenzonitr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity was determined by displacement of [3H]-P1075 from its binding sites in canine cardiac membranes | J Med Chem 41: 271-5 (1998) Article DOI: 10.1021/jm970762d BindingDB Entry DOI: 10.7270/Q2FN159P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-binding cassette sub-family C member 9/ATP-sensitive inward rectifier potassium channel 11 (Homo sapiens (Human)) | BDBM50240750 (()-N-Cyano-N'-4-pyridinyl-N''-(1,2,2-trimethylprop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity was determined by displacement of [3H]-P1075 from its binding sites in canine cardiac membranes | J Med Chem 41: 271-5 (1998) Article DOI: 10.1021/jm970762d BindingDB Entry DOI: 10.7270/Q2FN159P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-binding cassette sub-family C member 9/ATP-sensitive inward rectifier potassium channel 11 (Homo sapiens (Human)) | BDBM50062393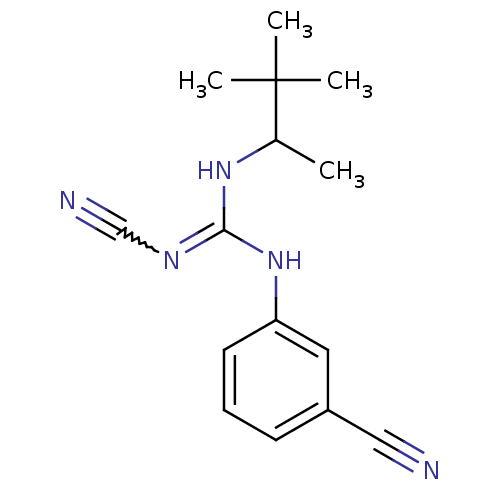 (3-cyanoimino(1,2,2-trimethylpropylamino)methylamin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity was determined by displacement of [3H]-P1075 from its binding sites in canine cardiac membranes | J Med Chem 41: 271-5 (1998) Article DOI: 10.1021/jm970762d BindingDB Entry DOI: 10.7270/Q2FN159P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-binding cassette sub-family C member 9/ATP-sensitive inward rectifier potassium channel 11 (Homo sapiens (Human)) | BDBM50062399 (4-[tert-butylamino(cyanoimino)methylamino]benzonit...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity was determined by displacement of [3H]-P1075 from its binding sites in canine cardiac membranes | J Med Chem 41: 271-5 (1998) Article DOI: 10.1021/jm970762d BindingDB Entry DOI: 10.7270/Q2FN159P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-binding cassette sub-family C member 9/ATP-sensitive inward rectifier potassium channel 11 (Homo sapiens (Human)) | BDBM50062400 (1N-cyanoimino(1,2,2-trimethylpropylamino)methylani...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity was determined by displacement of [3H]-P1075 from its binding sites in canine cardiac membranes | J Med Chem 41: 271-5 (1998) Article DOI: 10.1021/jm970762d BindingDB Entry DOI: 10.7270/Q2FN159P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50282320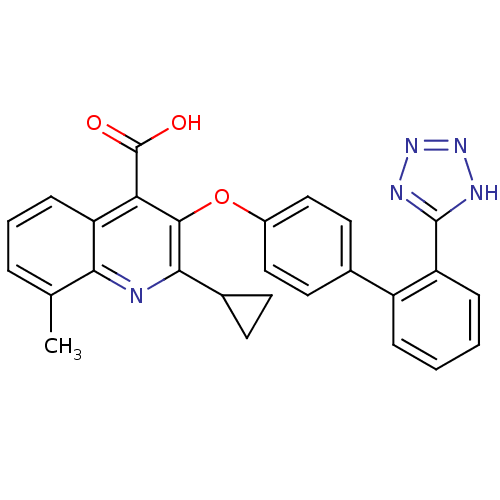 (2-Cyclopropyl-8-methyl-3-[2'-(2H-tetrazol-5-yl)-bi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125I]Sar1,Ile8 angiotensin II binding to Angiotensin II receptor type 1 in rat adrenocortical membrane | Bioorg Med Chem Lett 4: 195-200 (1994) Article DOI: 10.1016/S0960-894X(01)81146-X BindingDB Entry DOI: 10.7270/Q2Z60P13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-binding cassette sub-family C member 9/ATP-sensitive inward rectifier potassium channel 11 (Homo sapiens (Human)) | BDBM50062392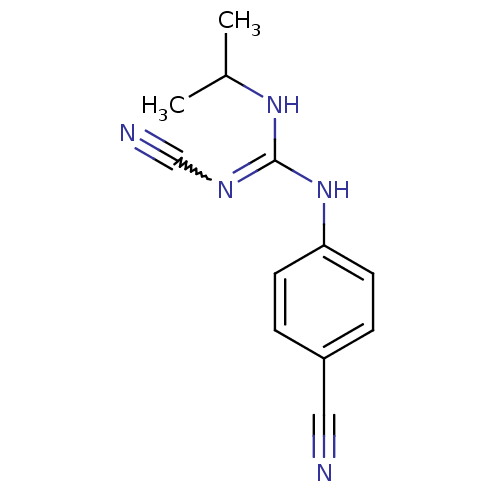 (4-isopropylamino(cyanoimino)methylaminobenzonitril...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity was determined by displacement of [3H]-P1075 from its binding sites in canine cardiac membranes | J Med Chem 41: 271-5 (1998) Article DOI: 10.1021/jm970762d BindingDB Entry DOI: 10.7270/Q2FN159P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-binding cassette sub-family C member 9/ATP-sensitive inward rectifier potassium channel 11 (Homo sapiens (Human)) | BDBM50062391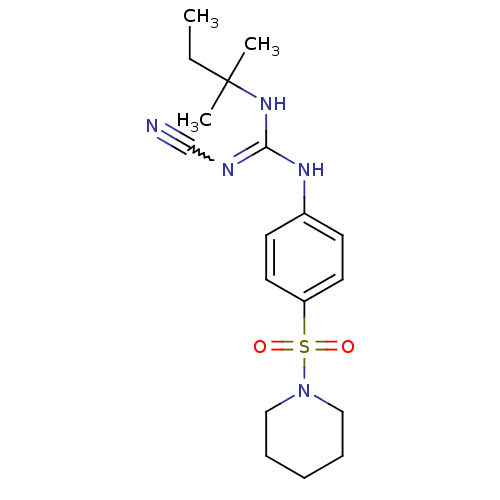 (1N-cyanoimino(tert-pentylamino)methyl-4-hexahydro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity was determined by displacement of [3H]-P1075 from its binding sites in canine cardiac membranes | J Med Chem 41: 271-5 (1998) Article DOI: 10.1021/jm970762d BindingDB Entry DOI: 10.7270/Q2FN159P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-binding cassette sub-family C member 9/ATP-sensitive inward rectifier potassium channel 11 (Homo sapiens (Human)) | BDBM50062395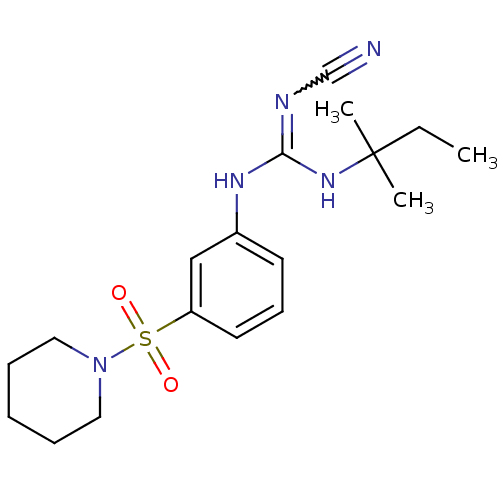 (1N-cyanoimino(tert-pentylamino)methyl-3-hexahydro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity was determined by displacement of [3H]-P1075 from its binding sites in canine cardiac membranes | J Med Chem 41: 271-5 (1998) Article DOI: 10.1021/jm970762d BindingDB Entry DOI: 10.7270/Q2FN159P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-binding cassette sub-family C member 9/ATP-sensitive inward rectifier potassium channel 11 (Homo sapiens (Human)) | BDBM50062401 (4-methylamino(cyanoimino)methylaminobenzonitrile |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity was determined by displacement of [3H]-P1075 from its binding sites in canine cardiac membranes | J Med Chem 41: 271-5 (1998) Article DOI: 10.1021/jm970762d BindingDB Entry DOI: 10.7270/Q2FN159P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-binding cassette sub-family C member 9/ATP-sensitive inward rectifier potassium channel 11 (Homo sapiens (Human)) | BDBM50044253 ((3R,4R)-3-Hydroxy-2,2-dimethyl-4-(2-oxo-pyrrolidin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 7.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity was determined by displacement of [3H]-P1075 from its binding sites in canine cardiac membranes | J Med Chem 41: 271-5 (1998) Article DOI: 10.1021/jm970762d BindingDB Entry DOI: 10.7270/Q2FN159P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-binding cassette sub-family C member 9/ATP-sensitive inward rectifier potassium channel 11 (Homo sapiens (Human)) | BDBM50044253 ((3R,4R)-3-Hydroxy-2,2-dimethyl-4-(2-oxo-pyrrolidin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 7.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity was determined by displacement of [3H]-P1075 from its binding sites in canine cardiac membranes | J Med Chem 41: 271-5 (1998) Article DOI: 10.1021/jm970762d BindingDB Entry DOI: 10.7270/Q2FN159P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily KQT member 1 (Cavia porcellus) | BDBM50106179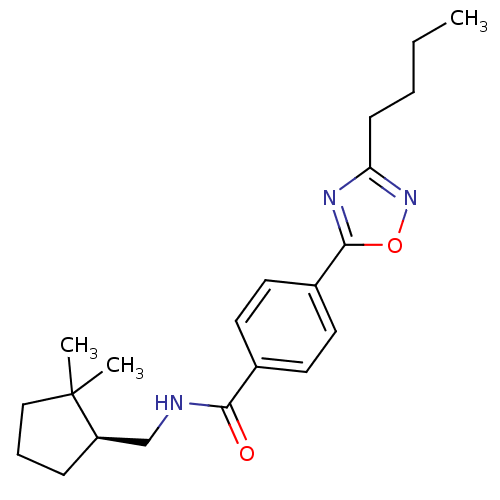 (CHEMBL125327 | Enantiomer-4-(3-Butyl-[1,2,4]oxadia...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Molar concentration required to inhibit 50% of the activating delayed-rectifier K+ current in isolated guinea pig ventricularmyocytes | J Med Chem 44: 3764-7 (2001) BindingDB Entry DOI: 10.7270/Q2G1604C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily KQT member 1 (Cavia porcellus) | BDBM50106169 (CHEMBL338973 | Enantiomer-4-(3-Butyl-[1,2,4]oxadia...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Molar concentration required to inhibit 50% of the activating delayed-rectifier K+ current in isolated guinea pig ventricularmyocytes | J Med Chem 44: 3764-7 (2001) BindingDB Entry DOI: 10.7270/Q2G1604C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily KQT member 1 (Cavia porcellus) | BDBM50106167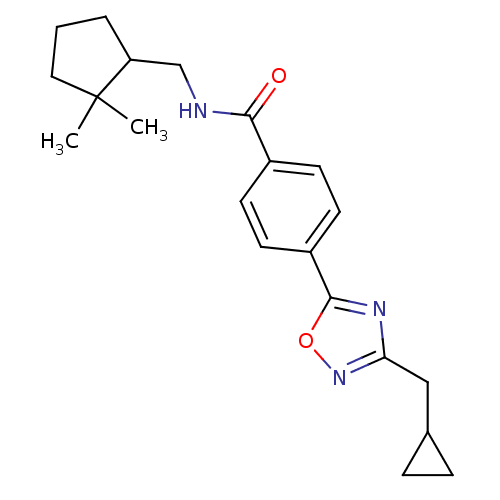 (CHEMBL125000 | Enantiomer-4-(3-Cyclopropylmethyl-[...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Molar concentration required to inhibit 50% of the activating delayed-rectifier K+ current in isolated guinea pig ventricularmyocytes | J Med Chem 44: 3764-7 (2001) BindingDB Entry DOI: 10.7270/Q2G1604C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily KQT member 1 (Cavia porcellus) | BDBM50106167 (CHEMBL125000 | Enantiomer-4-(3-Cyclopropylmethyl-[...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Bindind affinity value obtained by measuring the displacement of radioligand [3H]-(-)-cytisine from whole rat brain Nicotinic acetylcholine receptor | J Med Chem 44: 3764-7 (2001) BindingDB Entry DOI: 10.7270/Q2G1604C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily KQT member 1 (Cavia porcellus) | BDBM50106178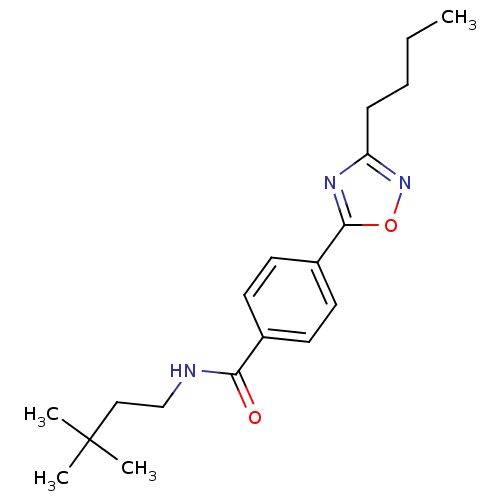 (4-(3-Butyl-[1,2,4]oxadiazol-5-yl)-N-(3,3-dimethyl-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Molar concentration required to inhibit 50% of the activating delayed-rectifier K+ current in isolated guinea pig ventricularmyocytes | J Med Chem 44: 3764-7 (2001) BindingDB Entry DOI: 10.7270/Q2G1604C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily KQT member 1 (Cavia porcellus) | BDBM50106168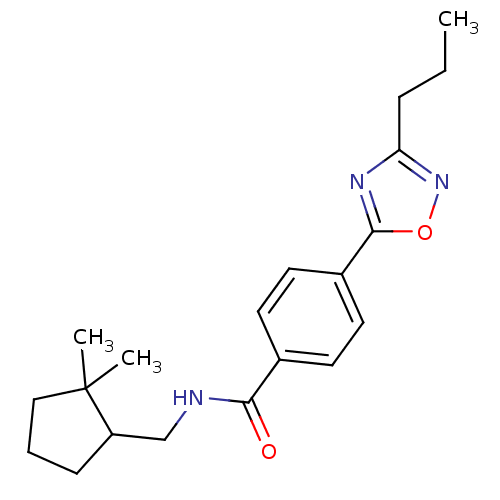 (CHEMBL124448 | Enantiomer-N-(2,2-Dimethyl-cyclopen...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Molar concentration required to inhibit 50% of the activating delayed-rectifier K+ current in isolated guinea pig ventricularmyocytes | J Med Chem 44: 3764-7 (2001) BindingDB Entry DOI: 10.7270/Q2G1604C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily KQT member 1 (Cavia porcellus) | BDBM50106168 (CHEMBL124448 | Enantiomer-N-(2,2-Dimethyl-cyclopen...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Bindind affinity value obtained by measuring the displacement of radioligand [3H]-(-)-cytisine from whole rat brain Nicotinic acetylcholine receptor | J Med Chem 44: 3764-7 (2001) BindingDB Entry DOI: 10.7270/Q2G1604C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily KQT member 1 (Cavia porcellus) | BDBM50061218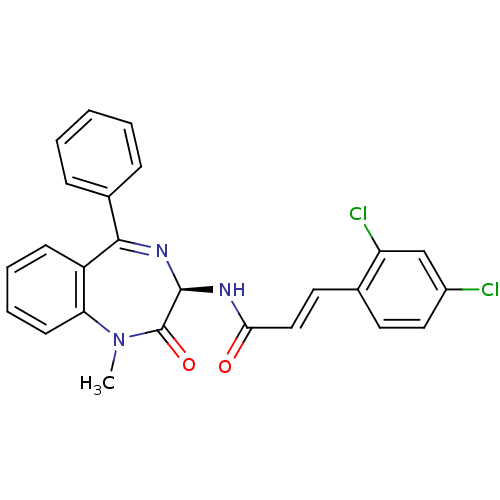 ((E)-3-(2,4-Dichloro-phenyl)-N-((R)-1-methyl-2-oxo-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Molar concentration required to inhibit 50% of the activating delayed-rectifier K+ current in isolated guinea pig ventricularmyocytes | J Med Chem 44: 3764-7 (2001) BindingDB Entry DOI: 10.7270/Q2G1604C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily KQT member 1 (Cavia porcellus) | BDBM50106176 (CHEMBL340681 | Enantiomer-N-(2,2-Dimethyl-cyclopen...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Molar concentration required to inhibit 50% of the activating delayed-rectifier K+ current in isolated guinea pig ventricularmyocytes | J Med Chem 44: 3764-7 (2001) BindingDB Entry DOI: 10.7270/Q2G1604C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily KQT member 1 (Cavia porcellus) | BDBM50106176 (CHEMBL340681 | Enantiomer-N-(2,2-Dimethyl-cyclopen...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Bindind affinity values obtained by measuring the displacement of radioligand [3H]-(-)-cytisine from a preparation of whole rat brain | J Med Chem 44: 3764-7 (2001) BindingDB Entry DOI: 10.7270/Q2G1604C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent T-type calcium channel subunit alpha-1H (Homo sapiens (Human)) | BDBM50373617 (CHEMBL403689) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R& D Curated by ChEMBL | Assay Description Antagonist activity at human alpha1H T-type calcium channel expressed in HEK293 cells by patch clamp technique | Bioorg Med Chem Lett 18: 474-8 (2008) Article DOI: 10.1016/j.bmcl.2007.11.103 BindingDB Entry DOI: 10.7270/Q2C53MQR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily KQT member 1 (Cavia porcellus) | BDBM50106182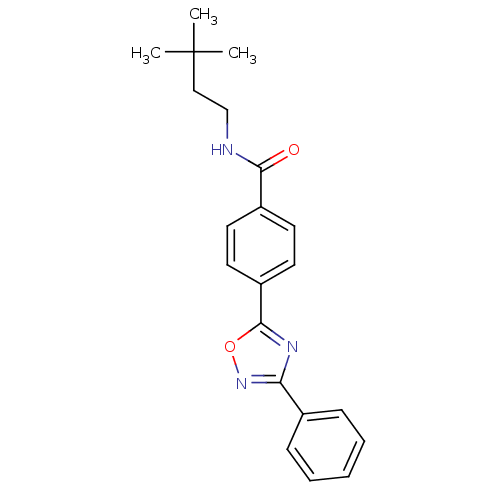 (CHEMBL123085 | N-(3,3-Dimethyl-butyl)-4-(3-phenyl-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Molar concentration required to inhibit 50% of the activating delayed-rectifier K+ current in isolated guinea pig ventricularmyocytes | J Med Chem 44: 3764-7 (2001) BindingDB Entry DOI: 10.7270/Q2G1604C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent T-type calcium channel subunit alpha-1H (Homo sapiens (Human)) | BDBM50117922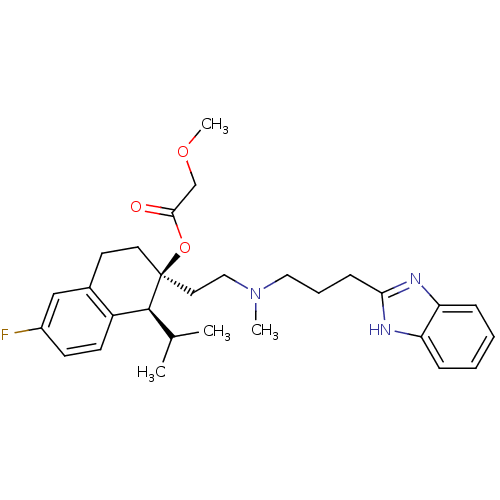 ((1S,2S)-2-(2-((3-(1H-benzo[d]imidazol-2-yl)propyl)...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R& D Curated by ChEMBL | Assay Description Antagonist activity at human alpha1H T-type calcium channel expressed in HEK293 cells by patch clamp technique | Bioorg Med Chem Lett 18: 474-8 (2008) Article DOI: 10.1016/j.bmcl.2007.11.103 BindingDB Entry DOI: 10.7270/Q2C53MQR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent T-type calcium channel subunit alpha-1H (Homo sapiens (Human)) | BDBM50373614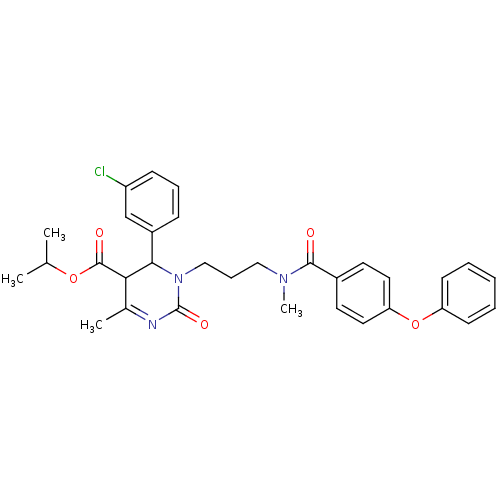 (CHEMBL257288) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R& D Curated by ChEMBL | Assay Description Antagonist activity at human alpha1H T-type calcium channel expressed in HEK293 cells by patch clamp technique | Bioorg Med Chem Lett 18: 474-8 (2008) Article DOI: 10.1016/j.bmcl.2007.11.103 BindingDB Entry DOI: 10.7270/Q2C53MQR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent T-type calcium channel subunit alpha-1H (Homo sapiens (Human)) | BDBM50373620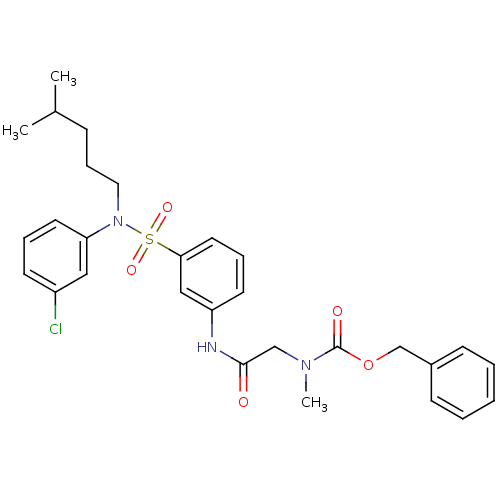 (CHEMBL269980) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R& D Curated by ChEMBL | Assay Description Antagonist activity at human alpha1H T-type calcium channel expressed in HEK293 cells by patch clamp technique | Bioorg Med Chem Lett 18: 474-8 (2008) Article DOI: 10.1016/j.bmcl.2007.11.103 BindingDB Entry DOI: 10.7270/Q2C53MQR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent T-type calcium channel subunit alpha-1H (Homo sapiens (Human)) | BDBM50373616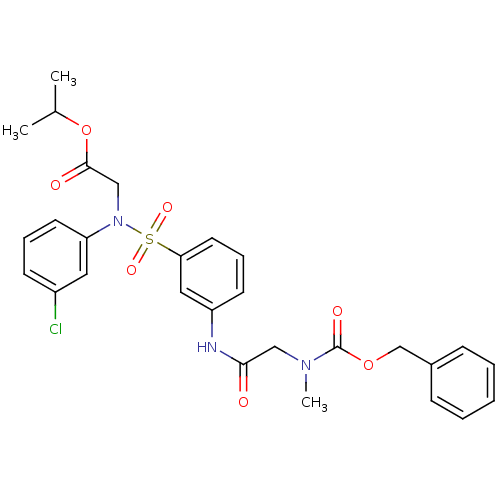 (CHEMBL272128) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R& D Curated by ChEMBL | Assay Description Antagonist activity at human alpha1H T-type calcium channel expressed in HEK293 cells by patch clamp technique | Bioorg Med Chem Lett 18: 474-8 (2008) Article DOI: 10.1016/j.bmcl.2007.11.103 BindingDB Entry DOI: 10.7270/Q2C53MQR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent T-type calcium channel subunit alpha-1H (Homo sapiens (Human)) | BDBM50373614 (CHEMBL257288) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R& D Curated by ChEMBL | Assay Description Antagonist activity at human alpha1H T-type calcium channel expressed in HEK293 cells by patch clamp technique | Bioorg Med Chem Lett 18: 474-8 (2008) Article DOI: 10.1016/j.bmcl.2007.11.103 BindingDB Entry DOI: 10.7270/Q2C53MQR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily KQT member 1 (Cavia porcellus) | BDBM50106166 (CHEMBL122341 | N-(3,3-Dimethyl-butyl)-4-hexyloxy-b...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Molar concentration required to inhibit 50% of the activating delayed-rectifier K+ current in isolated guinea pig ventricularmyocytes | J Med Chem 44: 3764-7 (2001) BindingDB Entry DOI: 10.7270/Q2G1604C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily KQT member 1 (Cavia porcellus) | BDBM50106177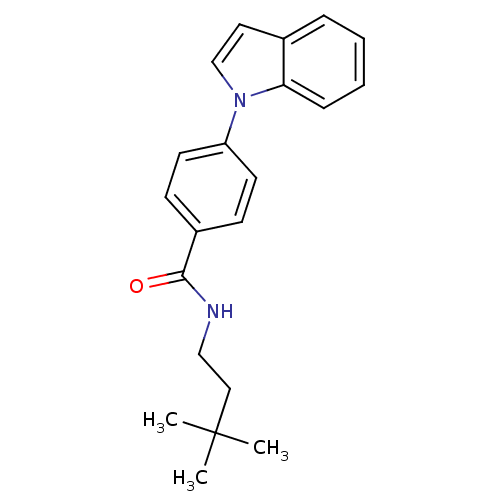 (CHEMBL431682 | N-(3,3-Dimethyl-butyl)-4-indol-1-yl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Molar concentration required to inhibit 50% of the activating delayed-rectifier K+ current in isolated guinea pig ventricularmyocytes | J Med Chem 44: 3764-7 (2001) BindingDB Entry DOI: 10.7270/Q2G1604C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily KQT member 1 (Cavia porcellus) | BDBM50106184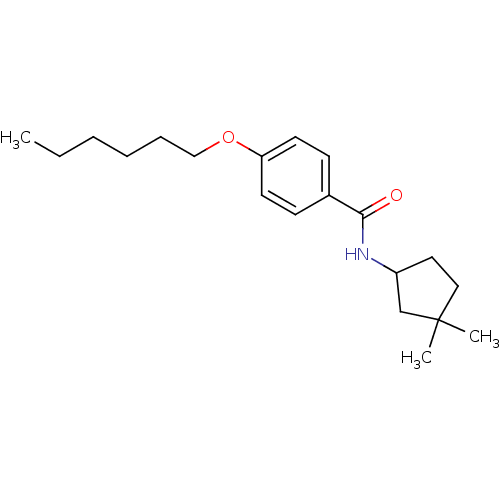 (CHEMBL121751 | N-(3,3-Dimethyl-cyclopentyl)-4-hexy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Molar concentration required to inhibit 50% of the activating delayed-rectifier K+ current in isolated guinea pig ventricularmyocytes | J Med Chem 44: 3764-7 (2001) BindingDB Entry DOI: 10.7270/Q2G1604C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily KQT member 1 (Cavia porcellus) | BDBM50106180 (CHEMBL124595 | N-(3,3-Dimethyl-cyclohexylmethyl)-4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Molar concentration required to inhibit 50% of the activating delayed-rectifier K+ current in isolated guinea pig ventricularmyocytes | J Med Chem 44: 3764-7 (2001) BindingDB Entry DOI: 10.7270/Q2G1604C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent T-type calcium channel subunit alpha-1H (Homo sapiens (Human)) | BDBM50373619 (CHEMBL272127) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R& D Curated by ChEMBL | Assay Description Antagonist activity at human alpha1H T-type calcium channel expressed in HEK293 cells by patch clamp technique | Bioorg Med Chem Lett 18: 474-8 (2008) Article DOI: 10.1016/j.bmcl.2007.11.103 BindingDB Entry DOI: 10.7270/Q2C53MQR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 66 total ) | Next | Last >> |