

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholine-binding protein (Lymnaea stagnalis) | BDBM50382466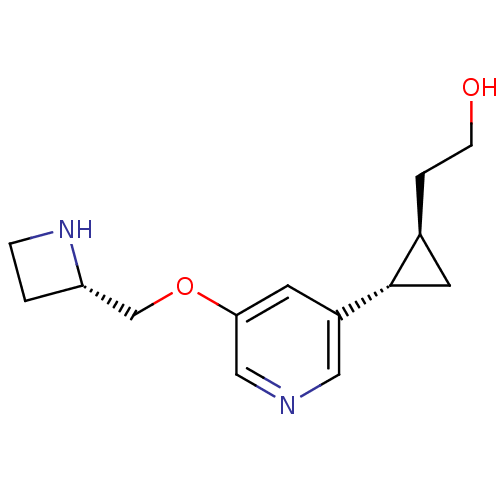 (CHEMBL2024087) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 12.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from Lymnaea stagnalis AChBP | J Med Chem 55: 8028-37 (2012) Article DOI: 10.1021/jm3008739 BindingDB Entry DOI: 10.7270/Q2NC62BW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholine-binding protein (Lymnaea stagnalis) | BDBM50382467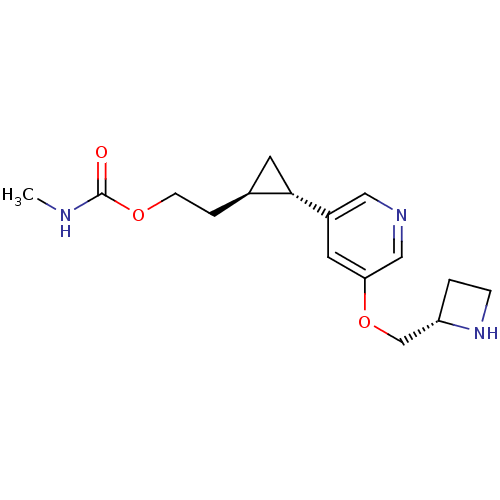 (CHEMBL2024089) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from Lymnaea stagnalis AChBP | J Med Chem 55: 8028-37 (2012) Article DOI: 10.1021/jm3008739 BindingDB Entry DOI: 10.7270/Q2NC62BW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholine-binding protein (Lymnaea stagnalis) | BDBM82070 (CAS_29790-52-1 | NICOTINE-L (BASE) | Nicotine-D sa...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 496 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from Lymnaea stagnalis AChBP | J Med Chem 55: 8028-37 (2012) Article DOI: 10.1021/jm3008739 BindingDB Entry DOI: 10.7270/Q2NC62BW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Soluble acetylcholine receptor (Aplysia Californica) | BDBM82070 (CAS_29790-52-1 | NICOTINE-L (BASE) | Nicotine-D sa...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 598 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from Aplysia californica AChBP | J Med Chem 55: 8028-37 (2012) Article DOI: 10.1021/jm3008739 BindingDB Entry DOI: 10.7270/Q2NC62BW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Soluble acetylcholine receptor (Aplysia Californica) | BDBM50382466 (CHEMBL2024087) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 979 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from Aplysia californica AChBP | J Med Chem 55: 8028-37 (2012) Article DOI: 10.1021/jm3008739 BindingDB Entry DOI: 10.7270/Q2NC62BW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Soluble acetylcholine receptor (Aplysia Californica) | BDBM50382467 (CHEMBL2024089) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from Aplysia californica AChBP | J Med Chem 55: 8028-37 (2012) Article DOI: 10.1021/jm3008739 BindingDB Entry DOI: 10.7270/Q2NC62BW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50579160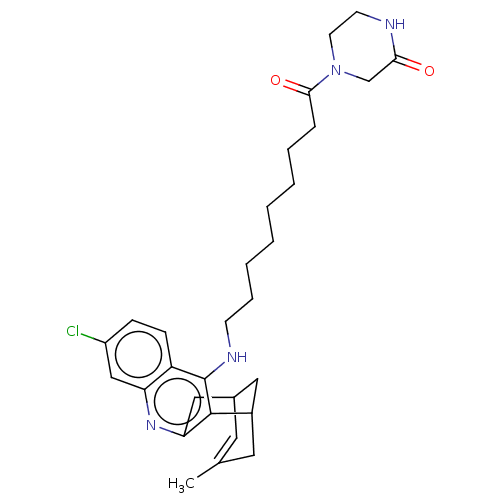 (CHEMBL4854913) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as a substrate preincubated for 20 mins followed by substrate addition by spectro... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113779 BindingDB Entry DOI: 10.7270/Q2TB1BQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50579161 (CHEMBL4848527) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as a substrate preincubated for 20 mins followed by substrate addition by spectro... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113779 BindingDB Entry DOI: 10.7270/Q2TB1BQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10592 (7-chloro-15-methyl-10-azatetracyclo[11.3.1.0^{2,11...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as a substrate preincubated for 20 mins followed by substrate addition by spectro... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113779 BindingDB Entry DOI: 10.7270/Q2TB1BQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50579162 (CHEMBL4872514) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as a substrate preincubated for 20 mins followed by substrate addition by spectro... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113779 BindingDB Entry DOI: 10.7270/Q2TB1BQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50579158 (CHEMBL4866930) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as a substrate preincubated for 20 mins followed by substrate addition by spectro... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113779 BindingDB Entry DOI: 10.7270/Q2TB1BQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50579156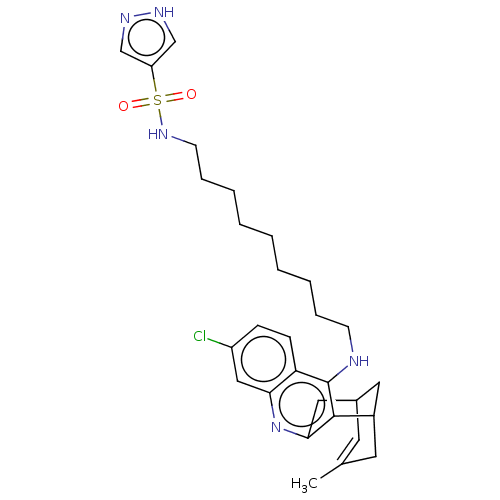 (CHEMBL4862716) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as a substrate preincubated for 20 mins followed by substrate addition by spectro... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113779 BindingDB Entry DOI: 10.7270/Q2TB1BQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50579159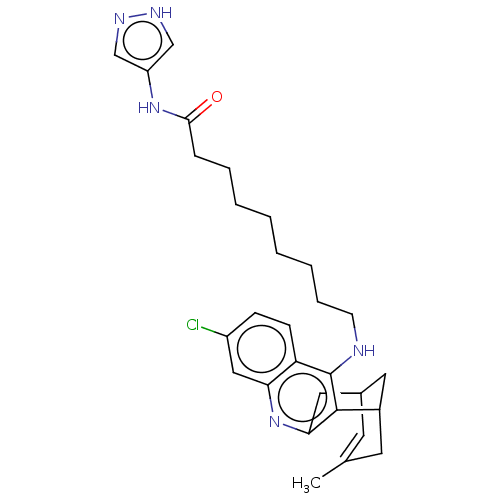 (CHEMBL4863615) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as a substrate preincubated for 20 mins followed by substrate addition by spectro... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113779 BindingDB Entry DOI: 10.7270/Q2TB1BQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50579157 (CHEMBL4859103) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as a substrate preincubated for 20 mins followed by substrate addition by spectro... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113779 BindingDB Entry DOI: 10.7270/Q2TB1BQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM202363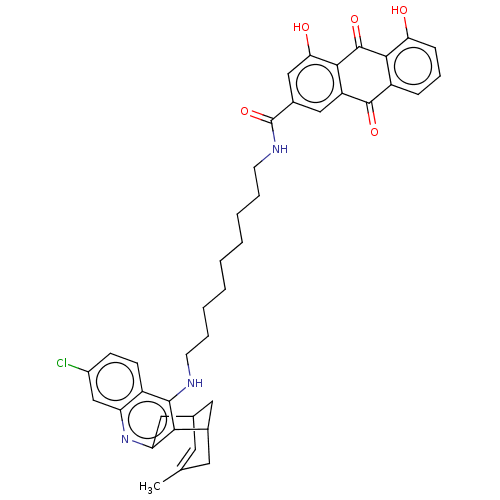 (US9238626, (+/-)-(Ib) HCl) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as a substrate preincubated for 20 mins followed by substrate addition by spectro... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113779 BindingDB Entry DOI: 10.7270/Q2TB1BQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Homo sapiens (Human)) | BDBM50398321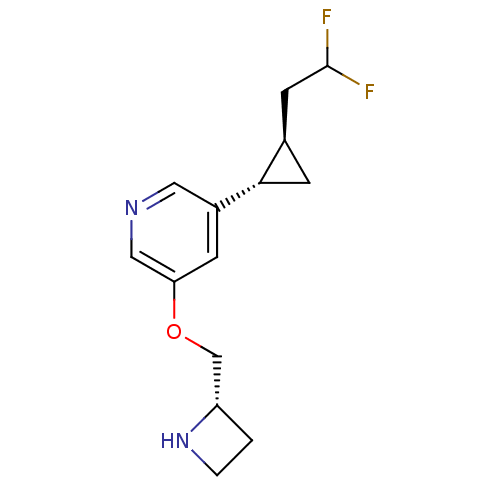 (CHEMBL2177344) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Antagonist activity at high sensitivity and low sensitivity alpha4beta2-nAChR expressed in human SH-EP1 cells assessed as inhibition of carbamylcholi... | J Med Chem 55: 8028-37 (2012) Article DOI: 10.1021/jm3008739 BindingDB Entry DOI: 10.7270/Q2NC62BW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Homo sapiens (Human)) | BDBM50398320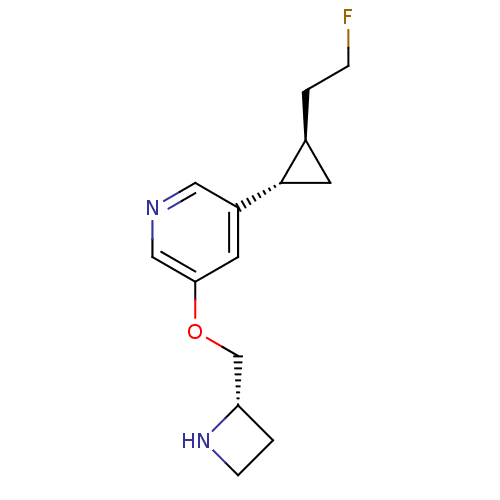 (CHEMBL2177345) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10.2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Antagonist activity at high sensitivity and low sensitivity alpha4beta2-nAChR expressed in human SH-EP1 cells assessed as inhibition of carbamylcholi... | J Med Chem 55: 8028-37 (2012) Article DOI: 10.1021/jm3008739 BindingDB Entry DOI: 10.7270/Q2NC62BW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Homo sapiens (Human)) | BDBM50398319 (CHEMBL2177346) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22.2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Antagonist activity at high sensitivity and low sensitivity alpha4beta2-nAChR expressed in human SH-EP1 cells assessed as inhibition of carbamylcholi... | J Med Chem 55: 8028-37 (2012) Article DOI: 10.1021/jm3008739 BindingDB Entry DOI: 10.7270/Q2NC62BW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as a substrate preincubated for 20 mins followed by substrate addition by spectro... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113779 BindingDB Entry DOI: 10.7270/Q2TB1BQF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50579158 (CHEMBL4866930) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human serum BChE using butyrylthiocholine iodide as a substrate preincubated for 20 mins followed by substrate addition by spectrophoto... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113779 BindingDB Entry DOI: 10.7270/Q2TB1BQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50579157 (CHEMBL4859103) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human serum BChE using butyrylthiocholine iodide as a substrate preincubated for 20 mins followed by substrate addition by spectrophoto... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113779 BindingDB Entry DOI: 10.7270/Q2TB1BQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50579156 (CHEMBL4862716) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human serum BChE using butyrylthiocholine iodide as a substrate preincubated for 20 mins followed by substrate addition by spectrophoto... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113779 BindingDB Entry DOI: 10.7270/Q2TB1BQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50579159 (CHEMBL4863615) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human serum BChE using butyrylthiocholine iodide as a substrate preincubated for 20 mins followed by substrate addition by spectrophoto... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113779 BindingDB Entry DOI: 10.7270/Q2TB1BQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50579160 (CHEMBL4854913) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human serum BChE using butyrylthiocholine iodide as a substrate preincubated for 20 mins followed by substrate addition by spectrophoto... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113779 BindingDB Entry DOI: 10.7270/Q2TB1BQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50579161 (CHEMBL4848527) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human serum BChE using butyrylthiocholine iodide as a substrate preincubated for 20 mins followed by substrate addition by spectrophoto... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113779 BindingDB Entry DOI: 10.7270/Q2TB1BQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50579162 (CHEMBL4872514) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human serum BChE using butyrylthiocholine iodide as a substrate preincubated for 20 mins followed by substrate addition by spectrophoto... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113779 BindingDB Entry DOI: 10.7270/Q2TB1BQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10592 (7-chloro-15-methyl-10-azatetracyclo[11.3.1.0^{2,11...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 181 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human serum BChE using butyrylthiocholine iodide as a substrate preincubated for 20 mins followed by substrate addition by spectrophoto... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113779 BindingDB Entry DOI: 10.7270/Q2TB1BQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM202363 (US9238626, (+/-)-(Ib) HCl) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human serum BChE using butyrylthiocholine iodide as a substrate preincubated for 20 mins followed by substrate addition by spectrophoto... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113779 BindingDB Entry DOI: 10.7270/Q2TB1BQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM202363 (US9238626, (+/-)-(Ib) HCl) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant BACE-1 expressed in Escherichia coli using panvera peptide as a substrate incubated for 1 hr by fluorescence analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113779 BindingDB Entry DOI: 10.7270/Q2TB1BQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50579157 (CHEMBL4859103) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant BACE-1 expressed in Escherichia coli using panvera peptide as a substrate incubated for 1 hr by fluorescence analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113779 BindingDB Entry DOI: 10.7270/Q2TB1BQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM15236 (3,5,7-trihydroxy-2-(3,4,5-trihydroxyphenyl)-4H-chr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 4.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant BACE-1 expressed in Escherichia coli using panvera peptide as a substrate incubated for 1 hr by fluorescence analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113779 BindingDB Entry DOI: 10.7270/Q2TB1BQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 6.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human serum BChE using butyrylthiocholine iodide as a substrate preincubated for 20 mins followed by substrate addition by spectrophoto... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113779 BindingDB Entry DOI: 10.7270/Q2TB1BQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50579156 (CHEMBL4862716) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant BACE-1 expressed in Escherichia coli using panvera peptide as a substrate incubated for 1 hr by fluorescence analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113779 BindingDB Entry DOI: 10.7270/Q2TB1BQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Homo sapiens (Human)) | BDBM50398320 (CHEMBL2177345) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 11.3 | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Agonist activity at high sensitivity and low sensitivity alpha4beta2-nAChR expressed in human SH-EP1 cells by 86Rb+ ion flux assay | J Med Chem 55: 8028-37 (2012) Article DOI: 10.1021/jm3008739 BindingDB Entry DOI: 10.7270/Q2NC62BW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Homo sapiens (Human)) | BDBM50398321 (CHEMBL2177344) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 8.80 | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Agonist activity at high sensitivity and low sensitivity alpha4beta2-nAChR expressed in human SH-EP1 cells by 86Rb+ ion flux assay | J Med Chem 55: 8028-37 (2012) Article DOI: 10.1021/jm3008739 BindingDB Entry DOI: 10.7270/Q2NC62BW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Homo sapiens (Human)) | BDBM50398319 (CHEMBL2177346) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 19.1 | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Agonist activity at high sensitivity and low sensitivity alpha4beta2-nAChR expressed in human SH-EP1 cells by 86Rb+ ion flux assay | J Med Chem 55: 8028-37 (2012) Article DOI: 10.1021/jm3008739 BindingDB Entry DOI: 10.7270/Q2NC62BW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||